Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Characteristics of the Samples
2.2. TEM Study
2.3. EPR Spin Probe Characterization of the Samples
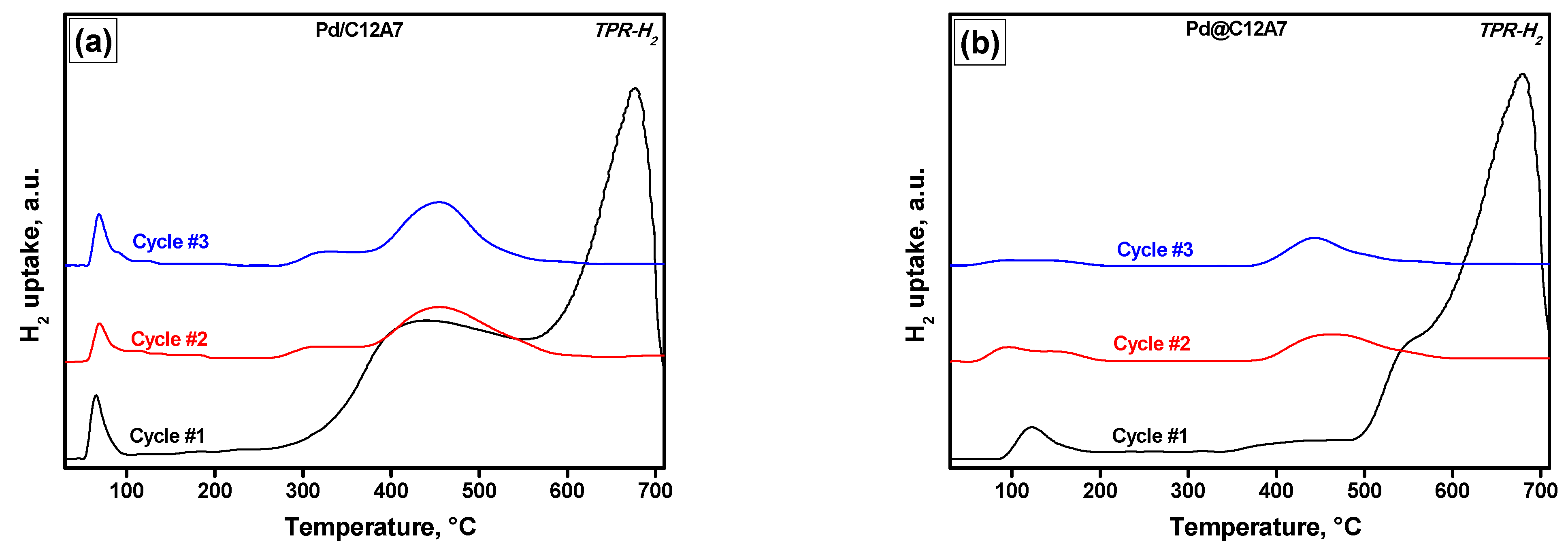
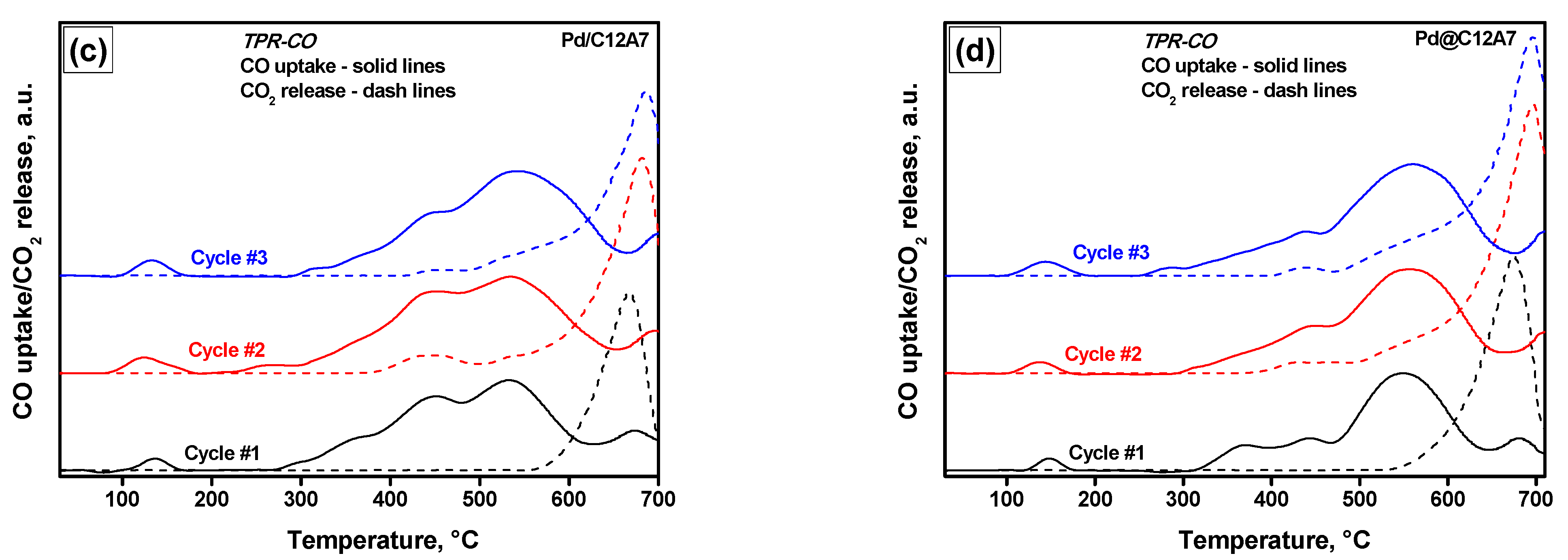
2.4. Temperature-Programmed Reduction Experiments
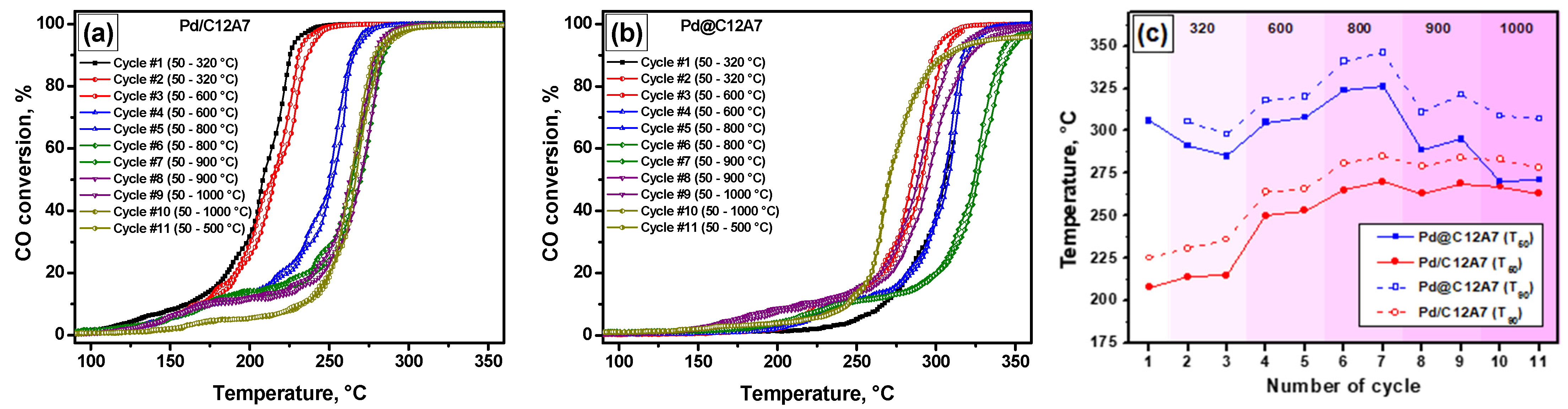
2.5. Catalytic Performance of the Samples
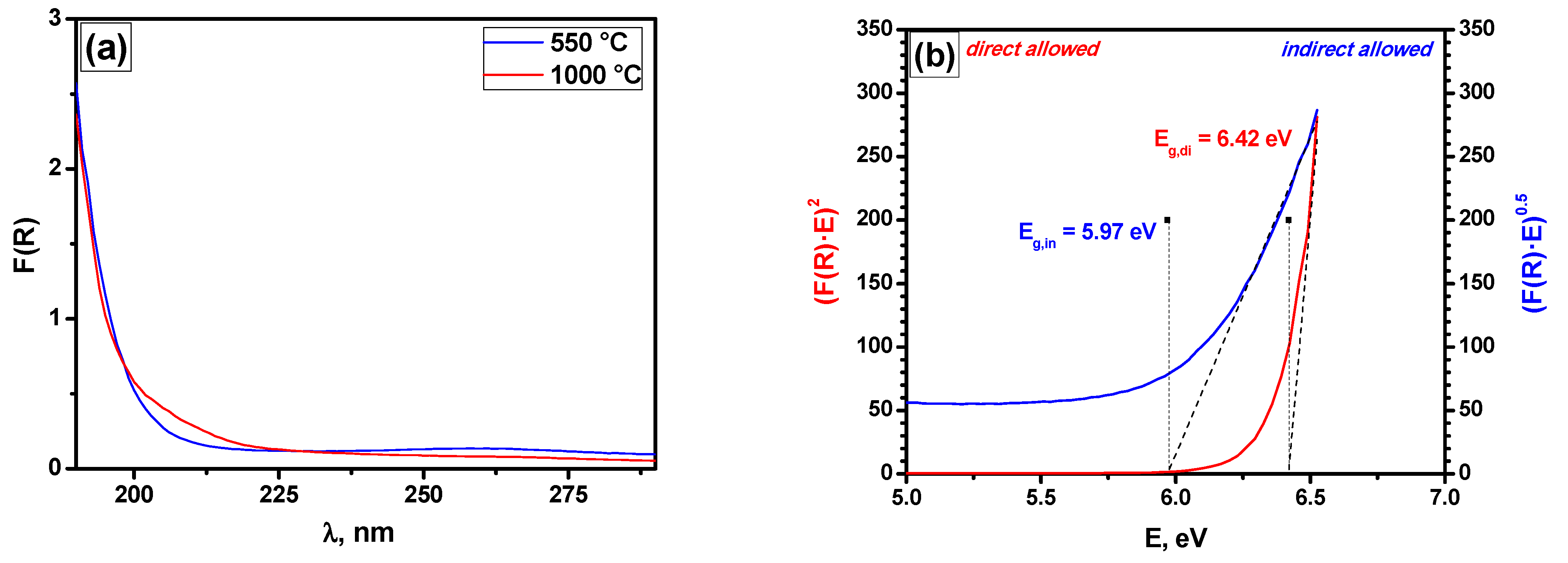
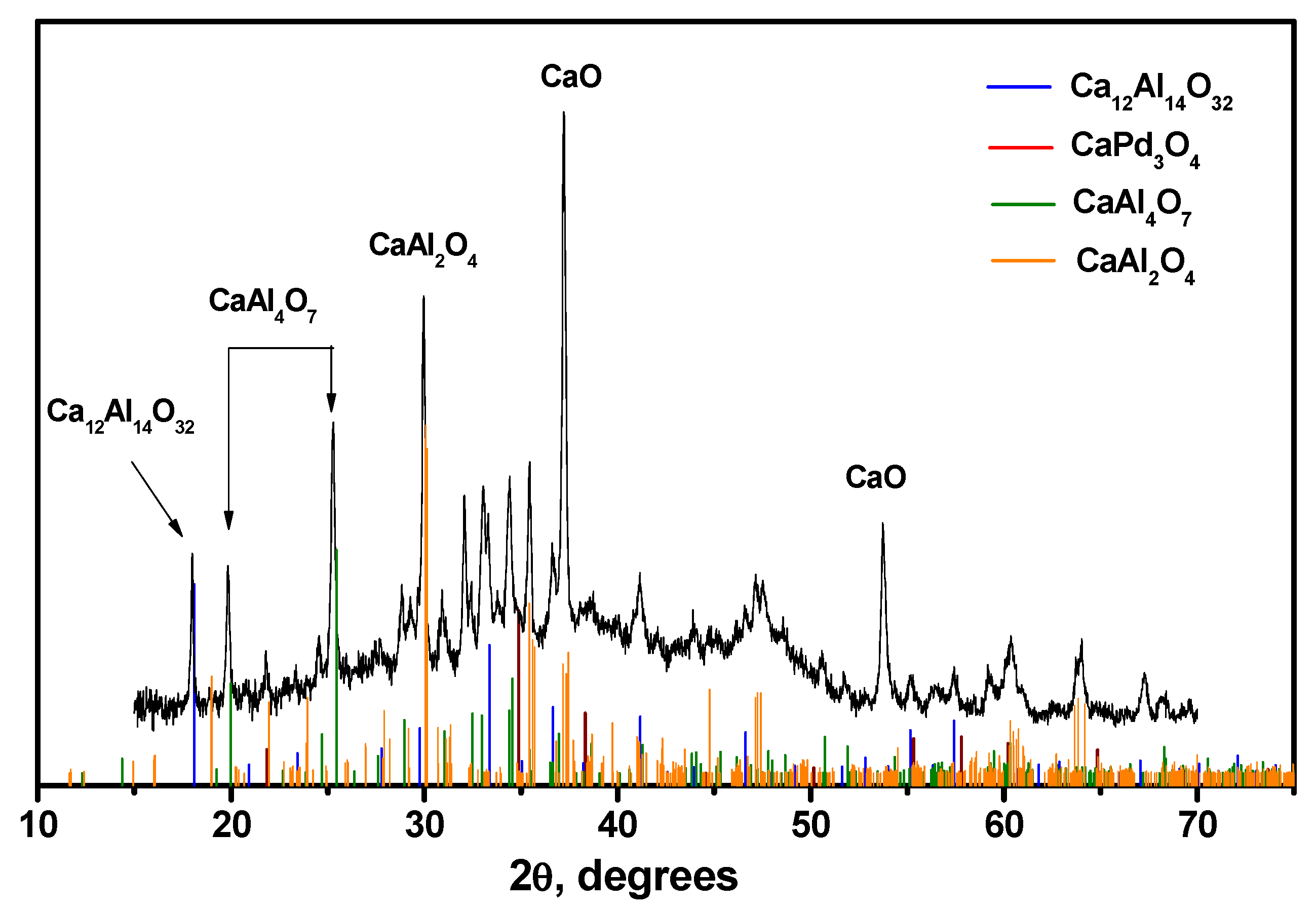
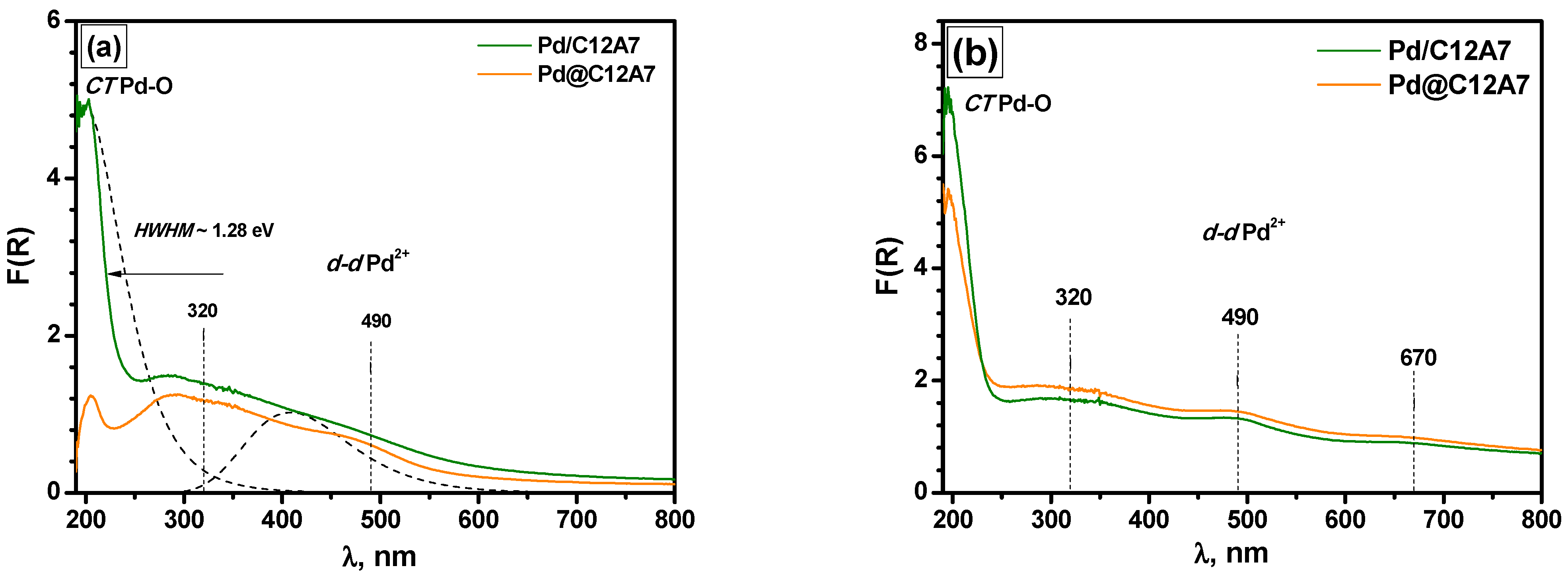
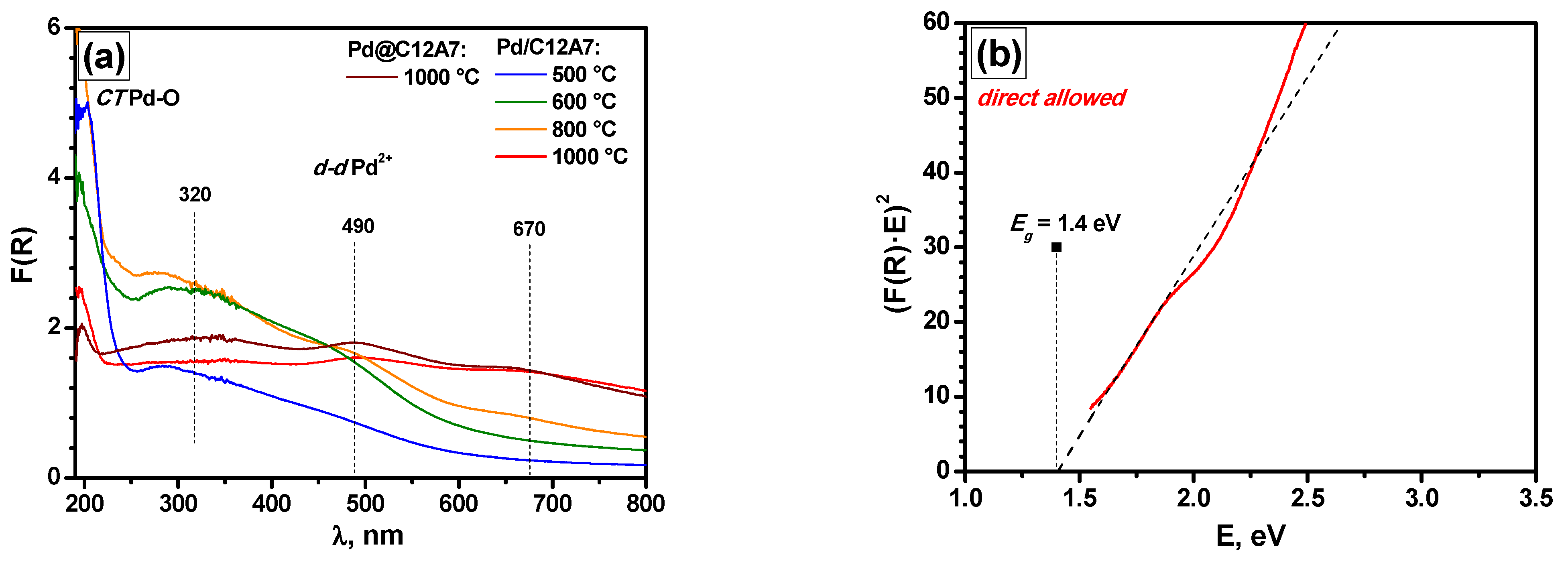
2.6. Diffuse Reflectance UV–Vis Spectroscopy and XRD Data
3. Conclusions
4. Materials and Methods
4.1. Preparation of the Samples
4.2. Characterization of the Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boysen, H.; Lerch, M.; Stys, A.; Senyshyn, A. Structure and oxygen mobility in mayenite (Ca12Al14O33): A high-temperature neutron powder diffraction study. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Ruszak, M.; Witkowski, S.; Sojka, Z. EPR and Raman investigations into anionic redox chemistry of nanoporous 12CaO·7Al2O3 interacting with O2, H2 and N2O. Res. Chem. Intermed. 2007, 33, 689–703. [Google Scholar] [CrossRef]
- Intiso, A.; Rossi, F.; Proto, A.; Cucciniello, R. The fascinating world of mayenite (Ca12Al14O33) and its derivatives. Rend. Lincei Sci. Fis. Nat. 2021, 32, 699–708. [Google Scholar] [CrossRef]
- Schmidt, A.; Boysen, H.; Senyshyn, A.; Lerch, M. New findings on N-mayenite and a new kind of anion substituted mayenite: Ca12Al14O32(NO2)2. Z. Krist. Cryst. Mater. 2014, 229, 427–434. [Google Scholar] [CrossRef]
- Proto, A.; Cucciniello, R.; Genga, A.; Capacchione, C. A study on the catalytic hydrogenation of aldehydes using mayenite as active support for palladium. Catal. Commun. 2015, 68, 41–45. [Google Scholar] [CrossRef]
- Yang, S.; Kondo, J.N.; Hayashi, K.; Hirano, M.; Domen, K.; Hosono, H. Partial oxidation of methane to syngas over promoted C12A7. Appl. Catal. A Gen. 2004, 277, 239–246. [Google Scholar] [CrossRef]
- Ye, T.-N.; Xiao, Z.; Li, J.; Gong, Y.; Abe, H.; Niwa, Y.; Sasase, M.; Kitano, M.; Hosono, H. Stable single platinum atoms trapped in sub-nanometer cavities in 12CaO·7Al2O3 for chemoselective hydrogenation of nitroarenes. Nat. Commun. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Manera, C.; Perondi, D.; Barcellos, T.; Godinho, M. CO2 gasification of elephant grass: Effect of Ni/mayenite catalyst on dry reforming of tar. Biomass Bioenergy 2020, 143, 105829. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. A crucial role of O2− and O22− on mayenite structure for biomass tar steam reforming over Ni/Ca12Al14O33. Appl. Catal. B Environ. 2009, 88, 351–360. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Gallucci, K.; Foscolo, P.U.; Courson, C. Effect of Ni precursor salts on Ni-mayenite catalysts for steam methane reforming and on Ni-CaO-mayenite materials for sorption enhanced steam methane reforming. Int. J. Hydrog. Energy 2019, 44, 6461–6480. [Google Scholar] [CrossRef]
- Bracciale, M.P.; de Caprariis, B.; De Filippis, P.; Hernandez, A.D.; Scarsella, M. New synthetic route for the production of mayenite support to enhance Ni resistance to coke deposition in the reforming of tar model compounds. Appl. Catal. A Gen. 2019, 574, 48–59. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of tar contained in a raw fuel gas from biomass gasification using nickel-mayenite catalyst. Int. J. Hydrog. Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Girr, J.; Massacesi, R.; Gallucci, K.; Courson, C. Sorption enhanced steam methane reforming by Ni–CaO materials supported on mayenite. Int. J. Hydrogen Energy 2017, 42, 13661–13680. [Google Scholar] [CrossRef]
- Savuto, E.; Navarro, R.M.; Mota, N.; Di Carlo, A.; Bocci, E.; Carlini, M.; Fierro, J.L.G. Steam reforming of tar model compounds over Ni/Mayenite catalysts: Effect of Ce addition. Fuel 2018, 224, 676–686. [Google Scholar] [CrossRef]
- Peng, R.; Chen, Y.; Zhang, B.; Li, Z.; Cui, X.; Guo, C.; Zhao, Y.; Zhang, J. Tailoring the stability of Ni-Fe/mayenite in methane—Carbon dioxide reforming. Fuel 2021, 284, 118909. [Google Scholar] [CrossRef]
- Gong, J.; Wang, D.; Li, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. Experimental and kinetic modeling of degreened and aged three-way catalysts: Aging impact on oxygen storage capacity and catalyst performance. SAE Tech. Pap. 2018. [Google Scholar] [CrossRef]
- Duprez, D.; Descorme, C.; Birchem, T.; Rohart, E. Oxygen storage and mobility on model three-way catalysts. Top. Catal. 2001, 16, 49–56. [Google Scholar] [CrossRef]
- Mc Grane, L.; Douglas, R.; Elliott, M.; Irwin, K.; Woods, A.; Pedlow, A. OSC modelling of 3-way automotive catalysts to understand the effect of latent OSC on dynamic OSC performance. SAE Tech. Pap. 2022. [Google Scholar] [CrossRef]
- Intiso, A.; Martinez-Triguero, J.; Cucciniello, R.; Rossi, F.; Palomares, A.E. Influence of the synthesis method on the catalytic activity of mayenite for the oxidation of gas-phase trichloroethylene. Sci. Rep. 2019, 9, 425. [Google Scholar] [CrossRef]
- Cucciniello, R.; Intiso, A.; Siciliano, T.; Palomares, A.E.; Martínez-Triguero, J.; Cerrillo, J.L.; Proto, A.; Rossi, F. Oxidative degradation of trichloroethylene over Fe2O3-doped mayenite: Chlorine poisoning mitigation and improved catalytic performance. Catalysts 2019, 9, 747. [Google Scholar] [CrossRef]
- Teusner, M.; De Souza, R.A.; Krause, H.; Ebbinghaus, S.G.; Martin, M. Oxygen transport in undoped and doped mayenite. Solid State Ionics 2016, 284, 25–27. [Google Scholar] [CrossRef]
- Yang, S.; Kondo, J.N.; Hayashi, K.; Hirano, M.; Domen, K.; Hosono, H. Formation and desorption of oxygen species in nanoporous crystal 12CaO•7Al2O3. Chem. Mater. 2004, 16, 104–110. [Google Scholar] [CrossRef]
- Bedilo, A.; Klabunde, K. Synthesis of high surface area zirconia aerogels using high temperature supercritical drying. Nanostruct. Mater. 1997, 8, 119–135. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Cherepanova, S.V.; Nadeev, A.N.; Bedilo, A.F.; Klabunde, K.J. Synthesis and characterization of mesoporous VOx/MgO aerogels with high surface area. Microporous Mesoporous Mater. 2012, 160, 32–40. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for preparation of catalytic materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Gerus, Y.Y.; Cherepanova, S.V.; Bedilo, A.F. Synthesis of C12A7 calcium aluminate aerogels. Mater. Lett. 2021, 293, 129699. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Bedilo, A.F.; Cherepanova, S.V.; Gerus, Y.Y.; Shuvarakova, E.I.; Vedyagin, A.A. Aerogel synthesis of calcium aluminates with varied stoichiometry. J. Sol-Gel Sci. Technol. 2022, 104, 259–266. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Ilyina, E.V.; Bedilo, A.F.; Vedyagin, A.A. Nanocrystalline aerogel VOx/MgO as a catalyst for oxidative dehydrogenation of propane. React. Kinet. Catal. Lett. 2009, 97, 355–361. [Google Scholar] [CrossRef]
- Kapishnikov, A.V.; Kenzhin, R.M.; Koskin, A.P.; Volodin, A.M.; Geydt, P.V. Mayenite synthesis from hydroxide precursors: Structure formation and active sites on its surface. Materials 2022, 15, 778. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Karnaukhov, T.M.; Krivoshapkina, E.F.; Ilyina, E.V.; Maksimova, T.A.; Cherepanova, S.V.; Krivoshapkin, P.V. Sol–gel synthesis and characterization of two-component systems based on MgO. J. Sol-Gel Sci. Technol. 2017, 82, 611–619. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Rybinskaya, A.A.; Kenzhin, R.M.; Volodin, A.M.; Bedilo, A.F. Characterization of electron donor sites on Al2O3 surface. Phys. Chem. Chem. Phys. 2012, 14, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Volodin, M.; Bedilo, A.F.; Stoyanovskii, V.O.; Zaikovskii, V.I. High-temperature synthesis of finely dispersed oxide materials and C12A7: E electrides in carbon nanoreactor conditions. Nanosyst. Phys. Chem. Math. 2018, 9, 558–567. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Shuvarakova, E.I.; Rybinskaya, A.A.; Medvedev, D.A. Characterization of electron-donor and electron-acceptor sites on the surface of sulfated alumina using spin probes. J. Phys. Chem. C 2014, 118, 15779–15794. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl. Catal. B Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Shuvarakova, E.I.; Ilyina, E.V.; Stoyanovskii, V.O.; Veselov, G.B.; Bedilo, A.F.; Vedyagin, A.A. Exploration of Optical, Redox, and Catalytic Properties of Vanadia-Mayenite Nanocomposites. J. Compos. Sci. 2022, 6, 308. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Tsyrulnikov, P.G. The features of the CO disproportionation reaction over iron-containing catalysts prepared by different methods. React. Kinet. Mech. Catal. 2016, 117, 35–46. [Google Scholar] [CrossRef]
- Xiao, X.; Sichen, D.; Seetharaman, S.; Sohn, H. Determination of kinetic parameters using differential thermal analysis—Application to the decomposition of CaCO3. Metall. Mater. Trans. B 1997, 28, 1157–1164. [Google Scholar] [CrossRef]
- Mandapaka, R.K.; Madras, G. Kinetics of CO oxidation on palladium using microkinetics coupled with reaction route analysis. Chem. Eng. Sci. 2015, 131, 271–281. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Chesnokov, V.V.; Mishakov, I.V. CO oxidation over Pd/ZrO2 catalysts: Role of support′ s donor sites. Molecules 2016, 21, 1289. [Google Scholar] [CrossRef]
- Lieske, H.; Voelter, J. Palladium redispersion by spreading of palladium(II) oxide in oxygen treated palladium/alumina. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Homeyer, S.T.; Sachtler, W.M.H. Oxidative redispersion of palladium and formation of PdO particles in NaY—An application of high-precision TPR. Appl. Catal. 1989, 54, 189–202. [Google Scholar] [CrossRef]
- Ali, A.; Chin, Y.H.; Resasco, D.E. Redispersion of Pd on acidic supports and loss of methane combustion activity during the selective reduction of NO by CH4. Catal. Lett. 1998, 56, 111–117. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Dahlberg, K.A.; Seo, C.Y.; Fisher, G.B.; Peczonczyk, S.L.; Rhodes, K.; Jagner, M.J.; Haack, L.P. Pd model catalysts: Effect of aging environment and lean redispersion. Appl. Catal. B Environ. 2016, 183, 343–360. [Google Scholar] [CrossRef]
- Seo, C.Y.; Chen, X.Y.; Sun, K.; Allard, L.F.; Fisher, G.B.; Schwank, J.W. Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure. Catal. Commun. 2018, 108, 73–76. [Google Scholar] [CrossRef]
- Gladysheva, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Korenev, S.V. New complex salts as precursors of porous Pd–Ir–Rh nanoalloys. Russ. J. Inorg. Chem. 2022, 67, 1135–1143. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Shubin, Y.V.; Kenzhin, R.M.; Plyusnin, P.E.; Stoyanovskii, V.O. The attractiveness of the ternary Rh-Pd-Pt alloys for CO oxidation process. Processes 2020, 8, 928. [Google Scholar] [CrossRef]
- Filatova, E.O.; Konashuk, A.S. Interpretation of the changing the band gap of Al2O3 depending on its crystalline form: Connection with different local symmetries. J. Phys. Chem. C 2015, 119, 20755–20761. [Google Scholar] [CrossRef]
- Lushchik, C.; Feldbach, E.; Frorip, A.; Kirm, M.; Lushchik, A.; Maaroos, A.; Martinson, I. Multiplication of electronic excitations in CaO and YAlO3 crystals with free and self-trapped excitons. J. Phys. Conden. Matt. 1994, 6, 11177–11187. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Dieguez, L.C. Dispersion stability and methylcyclopentane hydrogenolysis in Pd/Al2O3 catalysts. Appl. Catal. A Gen. 2000, 201, 241–251. [Google Scholar] [CrossRef]
- Tessier, D.; Rakai, A.; Bozon-Verduraz, F. Spectroscopic study of the interaction of carbon monoxide with cationic and metallic palladium in palladium–alumina catalysts. J. Chem. Soc. Faraday Trans. 1992, 88, 741–749. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Rogov, V.A.; Medvedev, D.A.; Mishakov, I.V. Characterization and study on the thermal aging behavior of palladium–alumina catalysts. J. Therm. Anal Calorim. 2017, 130, 1865–1874. [Google Scholar] [CrossRef]
- Nilsson, P.O. Optical properties of PdO in the range of 0.5–5.4 eV. J. Phys. C 1979, 12, 1423–1427. [Google Scholar] [CrossRef]
- Okamoto, H.; Asô, T. Formation of thin films of PdO and their electric properties. Jpn. J. Appl. Phys. 1967, 6, 779. [Google Scholar] [CrossRef]
- Wnuk, R.C.; Touw, T.R.; Post, B. The crystal structure of CaPd3O4. IBM J. Res. Dev. 1964, 8, 185–186. [Google Scholar] [CrossRef]
- Waser, J.; Levy, H.A.; Peterson, S.W. The structure of PdO. Acta Crystallogr. 1953, 6, 661–663. [Google Scholar] [CrossRef]
- Lamontagne, L.K.; Laurita, G.; Knight, M.; Yusuf, H.; Hu, J.; Seshadri, R.; Page, K. The Role of Structural and Compositional Heterogeneities in the Insulator-to-Metal Transition in Hole-Doped APd3O4 (A = Ca, Sr). Inorg. Chem. 2017, 56, 5158–5164. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Shubin, Y.V.; Plyusnin, P.E.; Mishakov, I.V. Effect of metal-metal and metal-support interaction on activity and stability of Pd-Rh/alumina in CO oxidation. Catal. Today 2017, 293–294, 73–81. [Google Scholar] [CrossRef]
- Poojitha, B.; Reddy, B.H.; Joshi, A.; Kumar, A.; Ali, A.; Singh, R.S.; Saha, S. Electron–phonon coupling in APd3O4: A = Ca, Sr, and Sr0.85Li0.15. J. Phys. Conden. Matt. 2020, 33, 105601. [Google Scholar] [CrossRef]
- Shuvarakova, E.I.; Bedilo, A.F.; Kenzhin, R.M.; Ilyina, E.V.; Gerus, Y.Y. Synthesis and investigation of finely dispersed calcium aluminates and catalysts based on them. Russ. J. Phys. Chem. B 2022, 16, 411–420. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Knözinger, H. Nature and estimation of functional groups on solid surfaces. In Catalysis; Springer: Berlin/Heidelberg, Germany, 1983; pp. 39–207. [Google Scholar]
- Tauc, J. Optical Properties of Amorphous Semiconductors. In Amorphous and Liquid Semiconductors; Tauc, J., Ed.; Springer: Boston, MA, USA, 1974; pp. 159–220. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
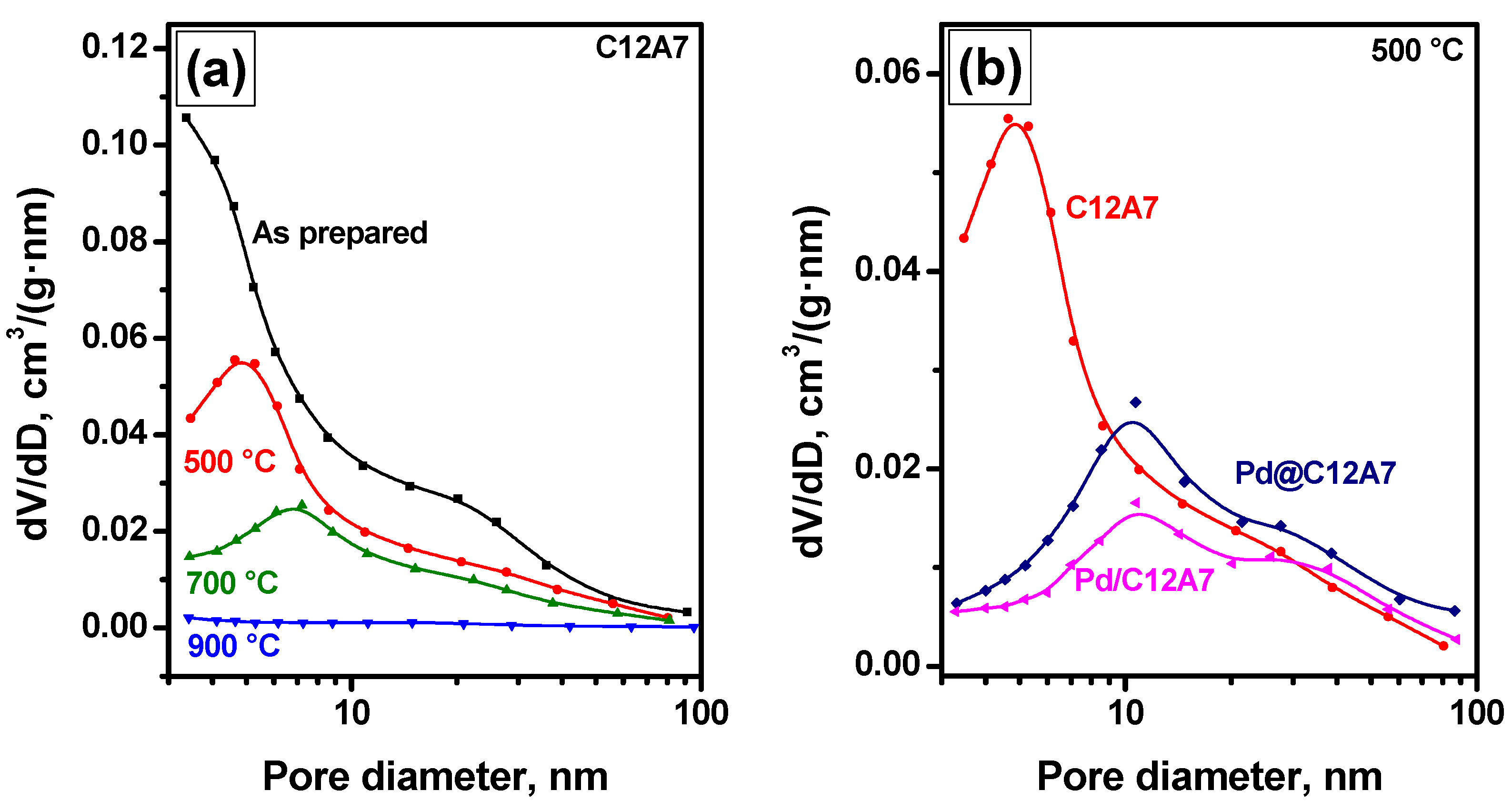
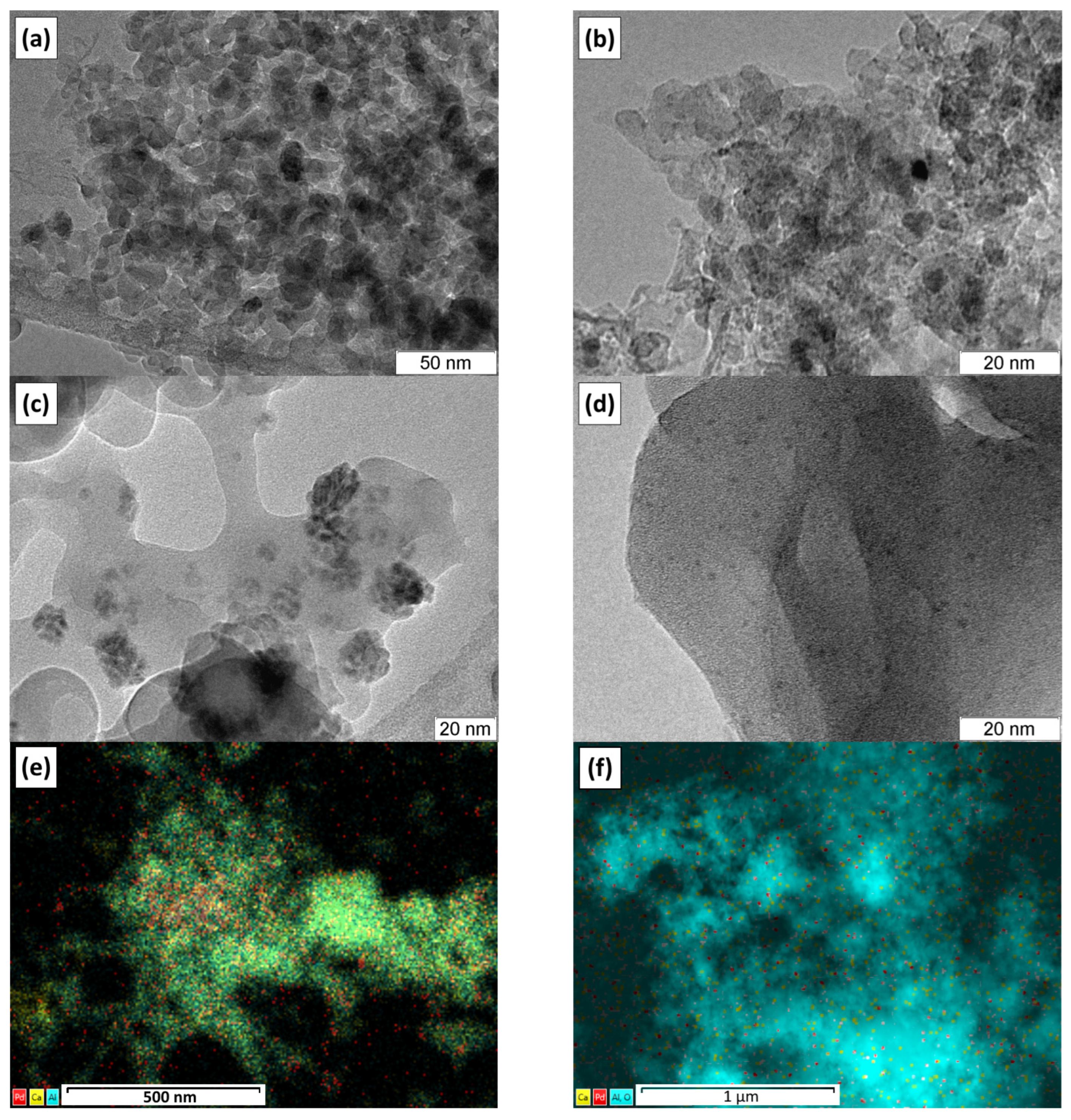
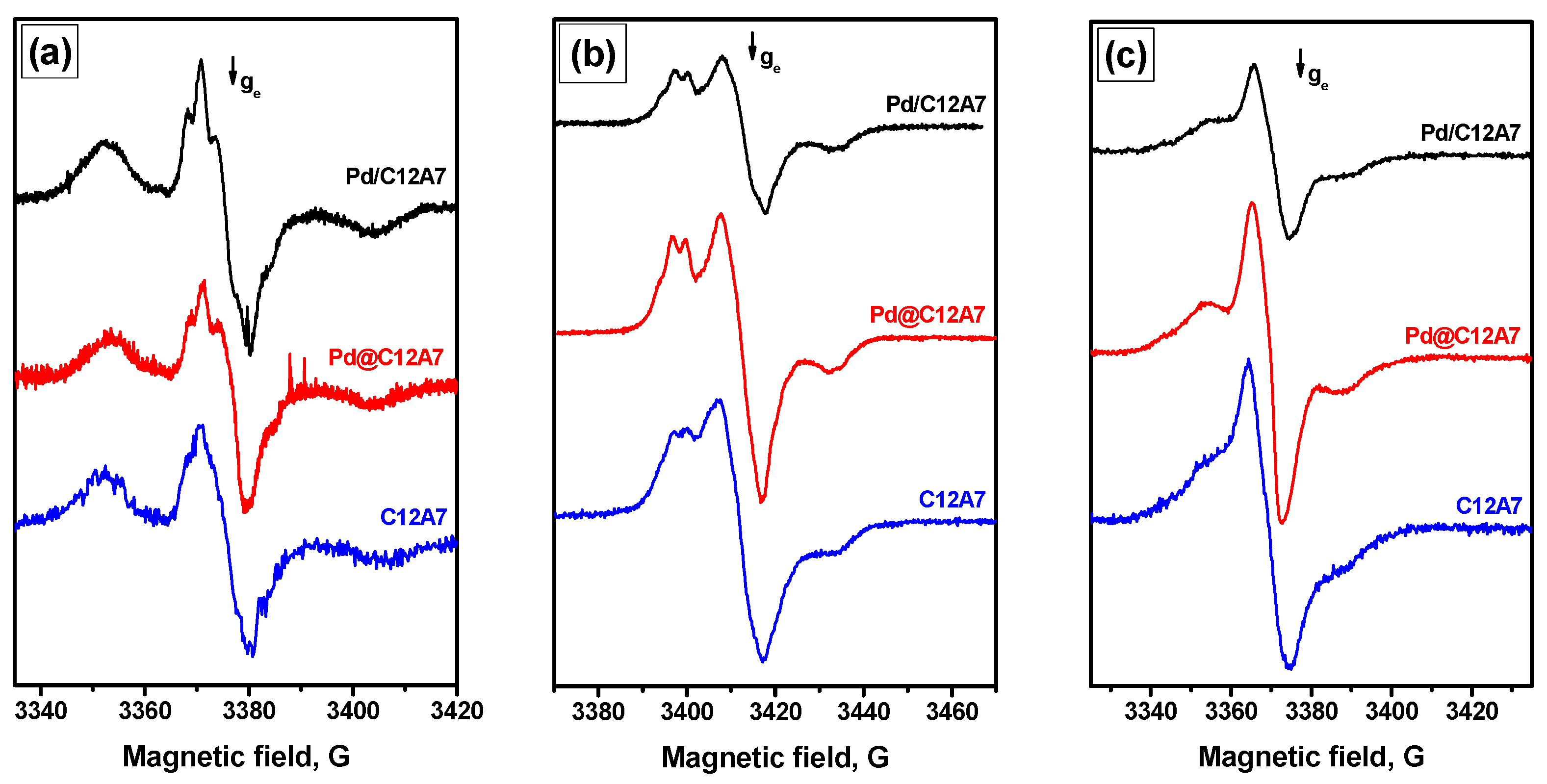
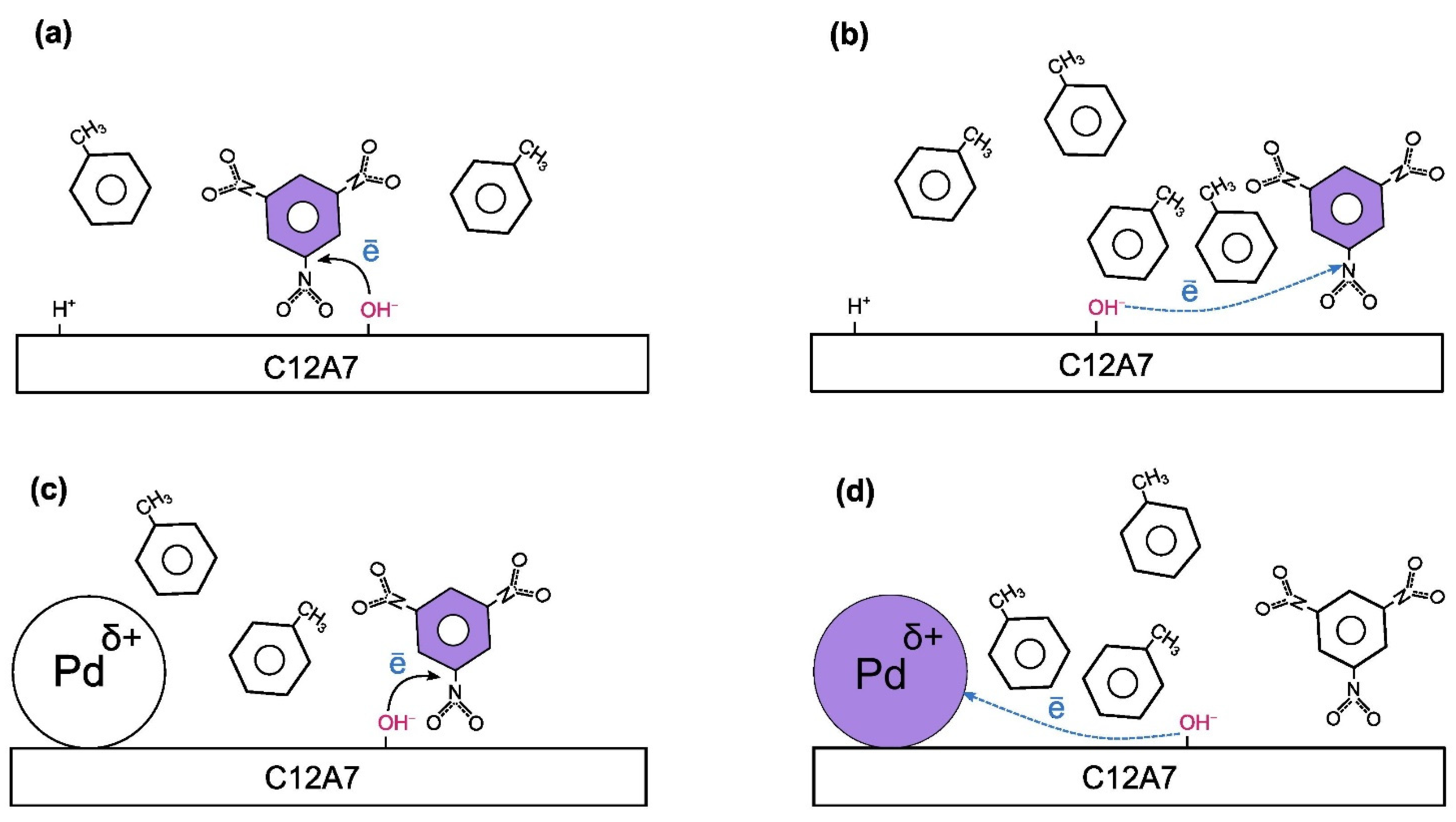
| Sample | Calcination Temperature, °C | SSA, m2/g | Vpore, cm3/g |
|---|---|---|---|
| C12A7 | As-prepared | 327 | 1.41 |
| 500 | 247 | 0.95 | |
| 700 | 147 | 0.61 | |
| 900 | 18 | 0.06 | |
| Pd/C12A7 | 500 | 200 | 1.0 |
| Pd@C12A7 | 180 | 1.1 |
| Sample | Diphenylamine | Phenothiazine | 1,3,5-Trinitrobenzene | |||
|---|---|---|---|---|---|---|
| After Activation at 500 °C | After Heating at 80 °C for 18 h | After Activation at 500 °C | After Heating at 80 °C for 18 h | After Activation at 500 °C | After Heating at 80 °C for 18 h | |
| C12A7 | 2.45 | - | 3.55 | 33 | 0.83 | 8.13 |
| Pd/C12A7 | 1.06 | - | 0.85 | 1.58 | 0.54 | 0.76 |
| Pd@C12A7 | 1.48 | - | 1.34 | 1.44 | 0.44 | 0.46 |
| Sample | Cycle Number | TPR-H2 | TPR-CO | |||
|---|---|---|---|---|---|---|
| H2 Uptake, µmol/g | CO Uptake, µmol/g | CO2 Released, µmol/g | ||||
| <200 °C | >200 °C | <200 °C | >200 °C | |||
| Pd/C12A7 | 1 | 71 | 3539 | 23 | 1322 | 666 |
| 2 | 83 | 423 | 51 | 1521 | 1103 | |
| 3 | 71 | 433 | 37 | 1509 | 942 | |
| Pd@C12A7 | 1 | 79 | 2624 | 22 | 1024 | 924 |
| 2 | 81 | 209 | 24 | 1344 | 1476 | |
| 3 | 32 | 152 | 42 | 1501 | 1315 | |
| Sample | Cycle Number | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Pd/C12A7 | 208 | 214 | 215 | 250 | 253 | 265 | 270 | This work |
| Pd@C12A7 | 225 | 231 | 236 | 264 | 266 | 281 | 285 | This work |
| Pd0.6(Ir0.2Rh0.8)/Al2O3 | 219 | 256 | 260 | 271 | 272 | 277 | 278 | [45] |
| Pt/Al2O3 | 286 | 281 | 279 | 278 | 275 | 265 | 262 | [46] |
| Rh0.4Pt0.6/Al2O3 | 278 | 276 | 274 | 274 | 273 | 262 | 261 | [46] |
| Rh0.4(Pd0.75Pt0.25)0.6/Al2O3 | 208 | 240 | 243 | 256 | 258 | 266 | 265 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyina, E.V.; Bedilo, A.F.; Veselov, G.B.; Gerus, Y.Y.; Shuvarakova, E.I.; Stoyanovskii, V.O.; Vedyagin, A.A. Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches. Gels 2022, 8, 809. https://doi.org/10.3390/gels8120809
Ilyina EV, Bedilo AF, Veselov GB, Gerus YY, Shuvarakova EI, Stoyanovskii VO, Vedyagin AA. Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches. Gels. 2022; 8(12):809. https://doi.org/10.3390/gels8120809
Chicago/Turabian StyleIlyina, Ekaterina V., Alexander F. Bedilo, Grigory B. Veselov, Yuri Y. Gerus, Ekaterina I. Shuvarakova, Vladimir O. Stoyanovskii, and Aleksey A. Vedyagin. 2022. "Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches" Gels 8, no. 12: 809. https://doi.org/10.3390/gels8120809
APA StyleIlyina, E. V., Bedilo, A. F., Veselov, G. B., Gerus, Y. Y., Shuvarakova, E. I., Stoyanovskii, V. O., & Vedyagin, A. A. (2022). Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches. Gels, 8(12), 809. https://doi.org/10.3390/gels8120809









