A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation
Abstract
1. Introduction
2. Results and Discussion
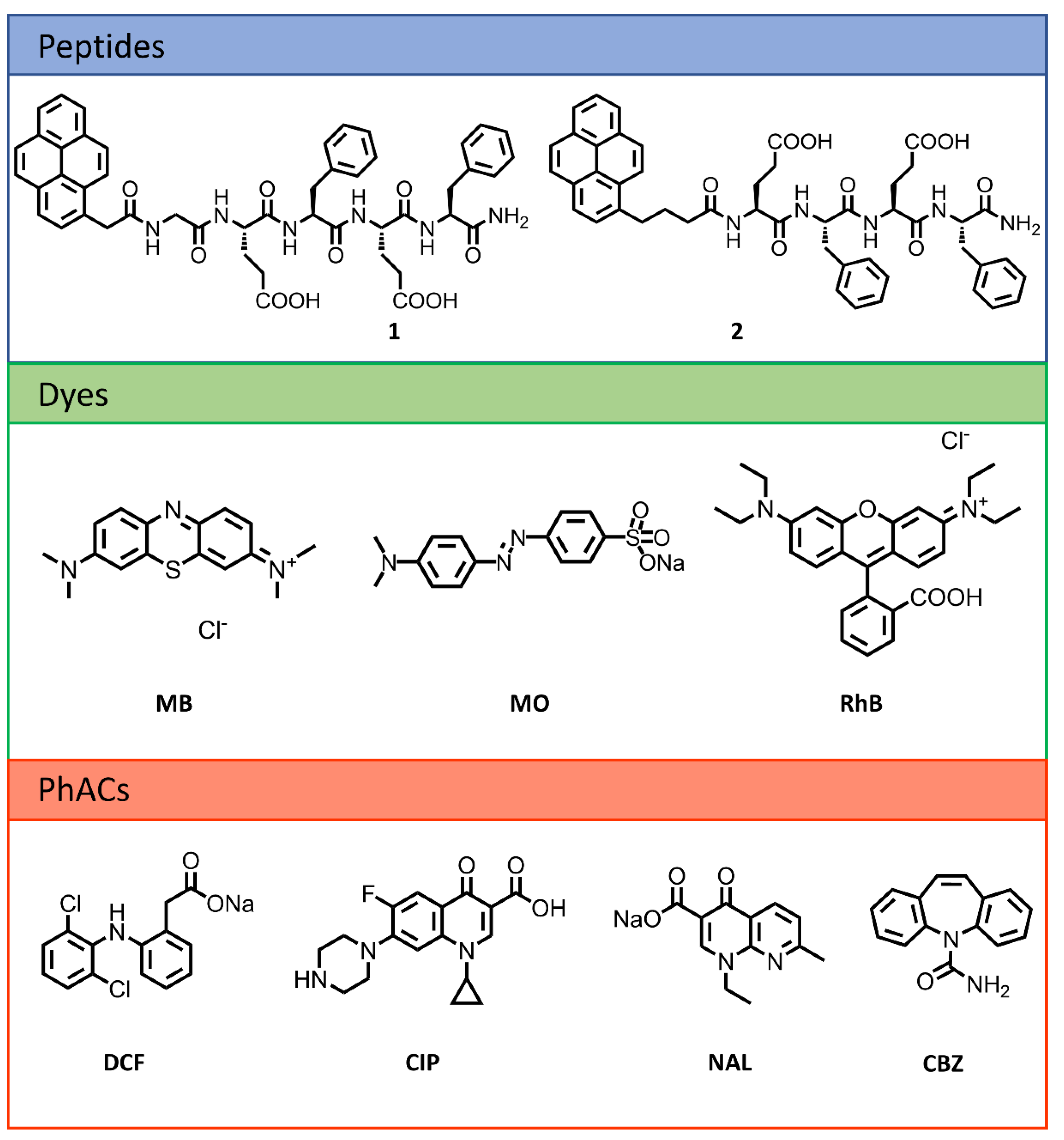
2.1. Peptide Design and Synthesis
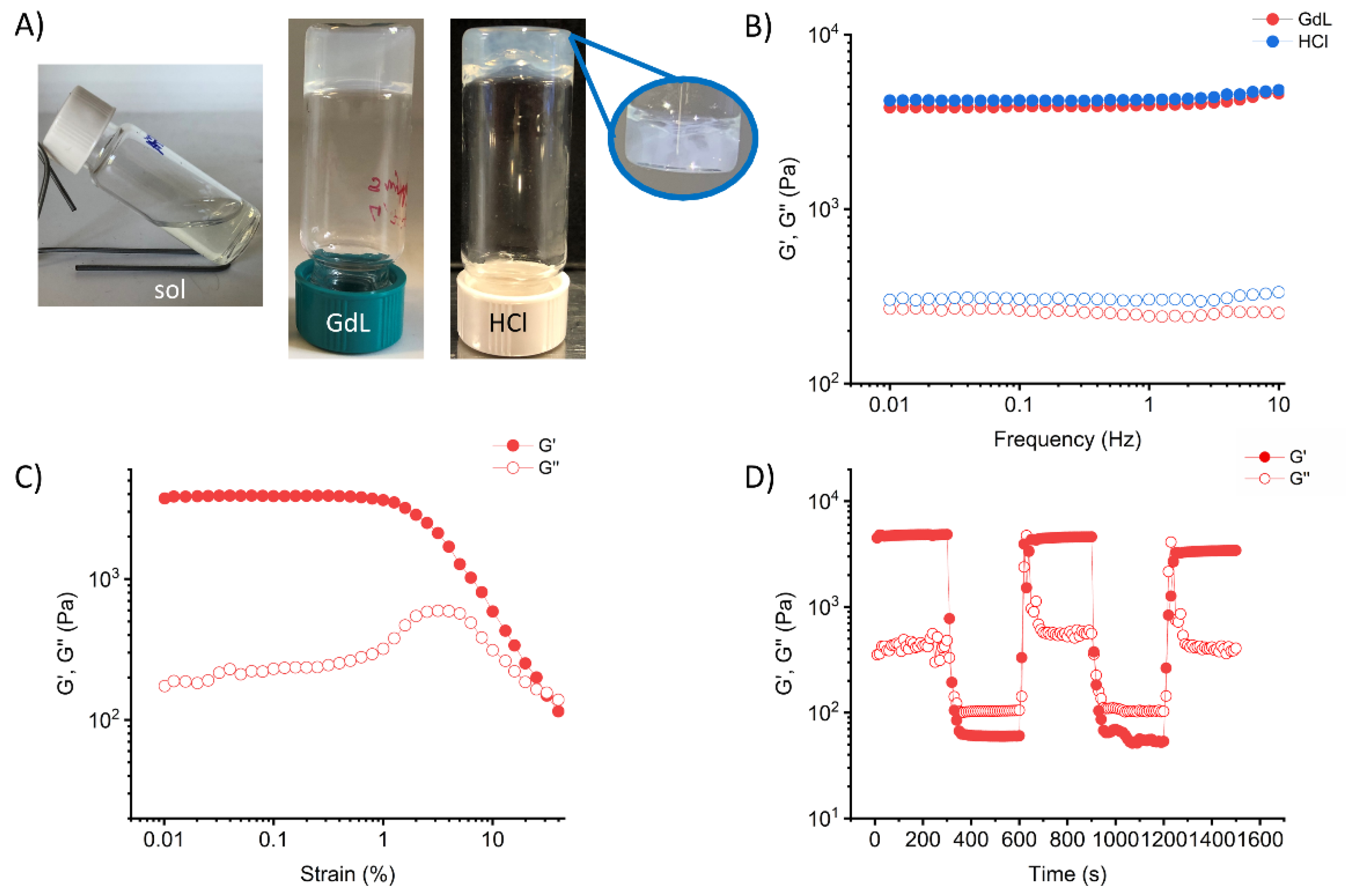
2.2. Gelation Ability
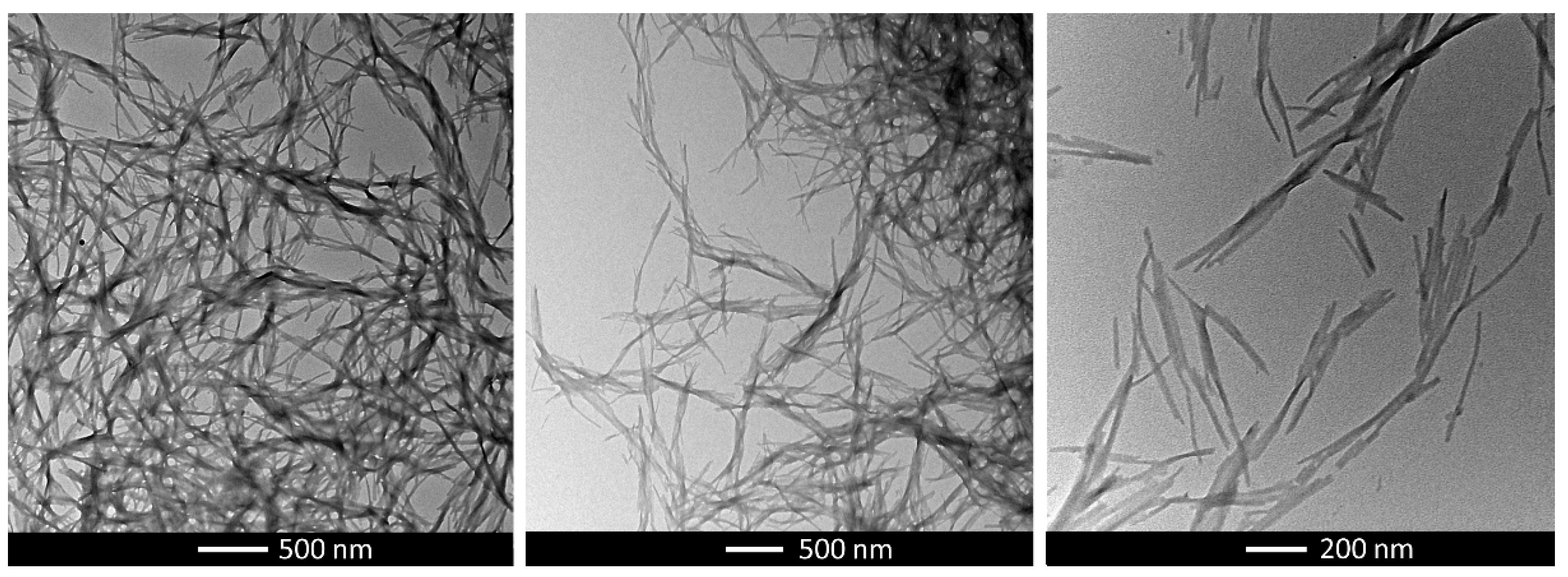
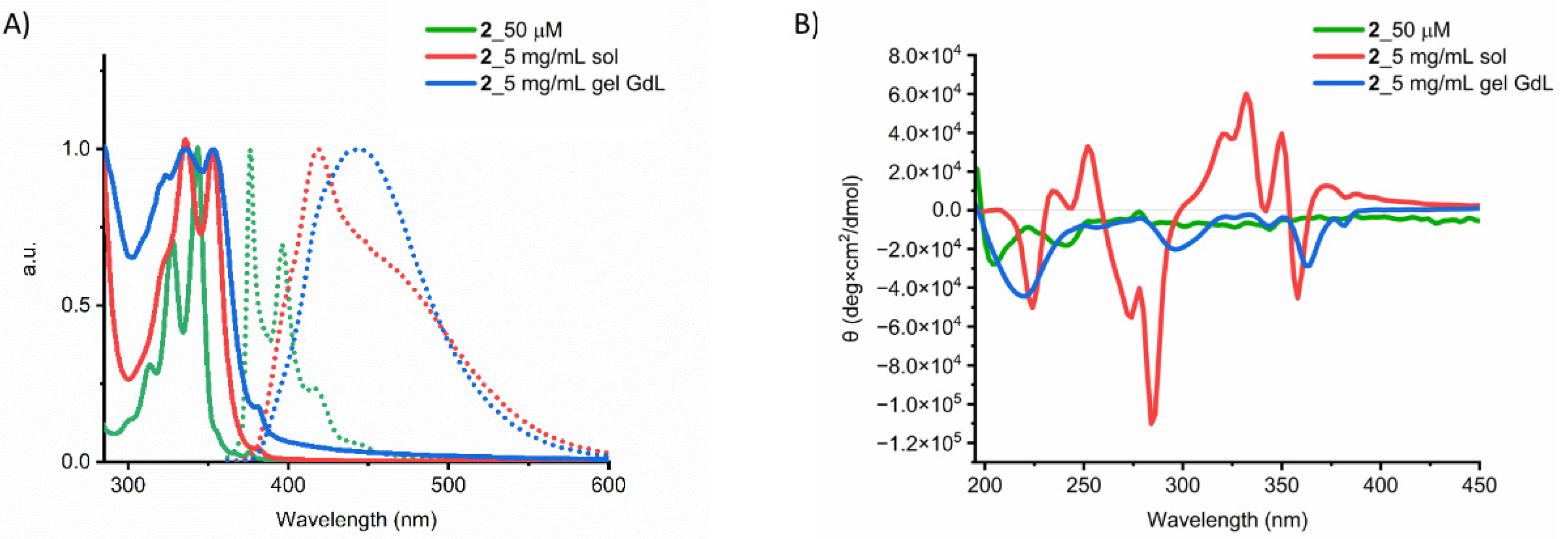
2.3. Gel Characterization
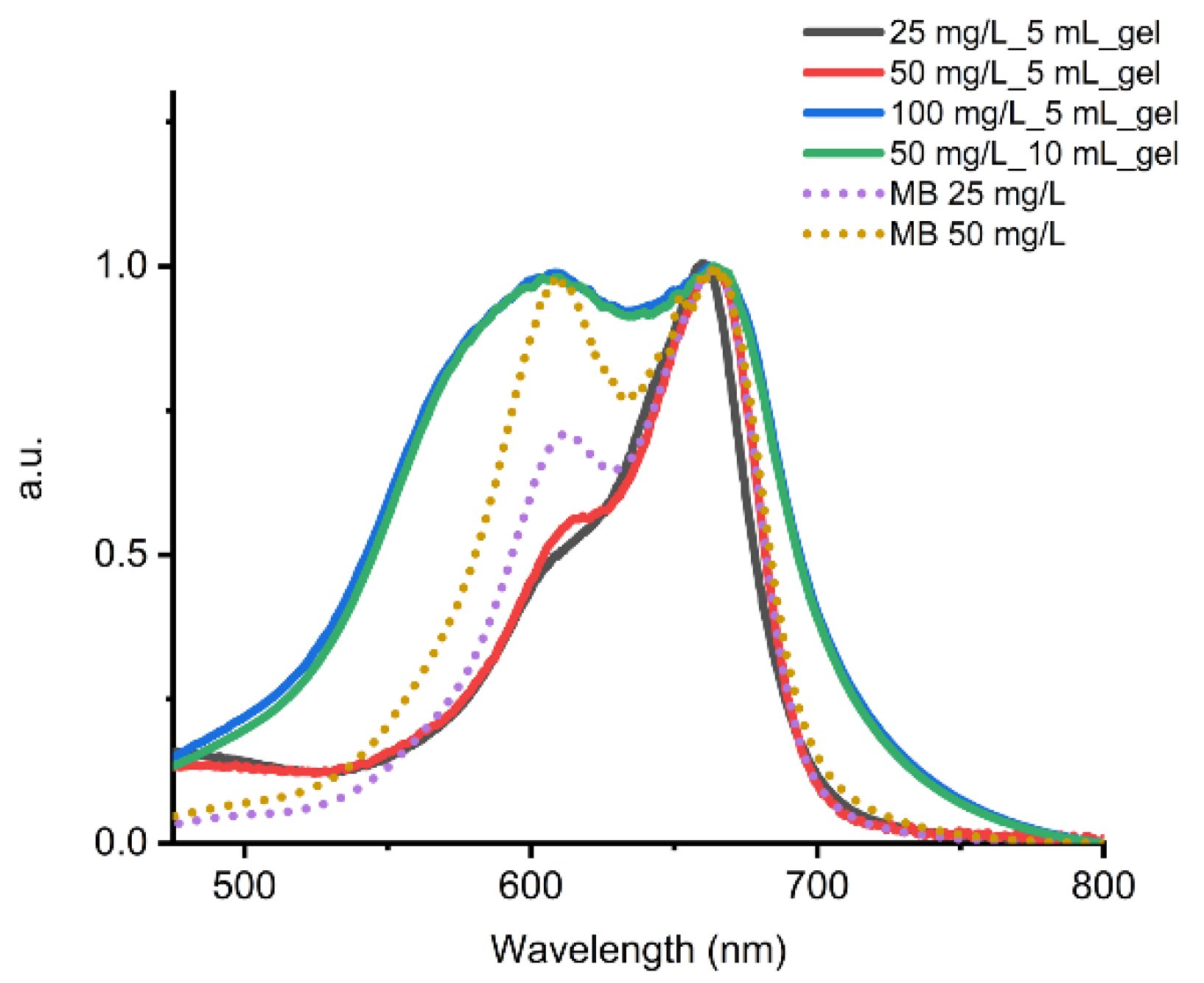
2.4. Adsorption of Dyes
2.5. Adsorption of PhACs
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Synthesis of 2
4.3. Gel Preparation
4.4. Gel Preparation in Syringes
4.5. Adsorption Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Glassmeyer, S.T.; Hinchey, E.K.; Boehme, S.E.; Daughton, C.G.; Ruhoy, I.S.; Conerly, O.; Daniels, R.L.; Lauer, L.; McCarthy, M.; Nettesheim, T.G.; et al. Disposal practices for unwanted residential medications in the United States. Environ. Int. 2009, 35, 566–572. [Google Scholar] [CrossRef]
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef]
- Dolar, D.; Ignjatić Zokić, T.; Košutić, K.; Ašperger, D.; Mutavdžić Pavlović, D. RO/NF membrane treatment of veterinary pharmaceutical wastewater: Comparison of results obtained on a laboratory and a pilot scale. Environ. Sci. Pollut. Res. 2012, 19, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Kaya, S.I.; Gumus, E.; Cetinkaya, A.; Zor, E.; Ozkan, S.A. Trends in on-site removal, treatment, and sensitive assay of common pharmaceuticals in surface waters. TrAC Trends Anal. Chem. 2022, 149, 116556. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2011, 4, 22–26. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System. Membranes 2021, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants-Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Godiya, C.B.; Ruotolo, L.A.M.; Cai, W.Q. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H. A global review on short peptides: Frontiers and perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Das, R.; Gayakvad, B.; Shinde, S.D.; Rath, J.; Jain, A.; Sahu, B. Ultrashort Peptides-A Glimpse into the Structural Modifications and Their Applications as Biomaterials. ACS Appl. Bio Mater. 2020, 3, 5474–5499. [Google Scholar] [CrossRef]
- Hu, X.; Liao, M.; Gong, H.; Zhang, L.; Cox, H.; Waigh, T.A.; Lu, J.R. Recent advances in short peptide self-assembly: From rational design to novel applications. Curr. Opin. Colloid Interface Sci. 2020, 45, 1–13. [Google Scholar] [CrossRef]
- Ekiz, M.S.; Cinar, G.; Khalily, M.A.; Guler, M.O. Self-assembled peptide nanostructures for functional materials. Nanotechnology 2016, 27, 402002. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef] [PubMed]
- Kurbasic, M.; Garcia, A.M.; Viada, S.; Marchesan, S. Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis. Molecules 2021, 26, 173. [Google Scholar] [CrossRef]
- Lee, J.; Kim, C. Peptide Materials for Smart Therapeutic Applications. Macromol. Res. 2021, 29, 2–14. [Google Scholar] [CrossRef]
- Das, S.; Das, D. Rational design of peptide-based smart hydrogels for therapeutic applications. Front. Chem. 2021, 9, 770102. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Ardoña, H.A.M.; Tovar, J.D. Peptide π-electron conjugates: Organic electronics for biology? Bioconjugate Chem. 2015, 26, 2290–2302. [Google Scholar] [CrossRef]
- Panda, S.S.; Katz, H.E.; Tovar, J.D. Solid-state electrical applications of protein and peptide based nanomaterials. Chem. Soc. Rev. 2018, 47, 3640–3658. [Google Scholar] [CrossRef]
- Tena-Solsona, M.; Nanda, J.; Díaz-Oltra, S.; Chotera, A.; Ashkenasy, G.; Escuder, B. Emergent Catalytic Behavior of Self-Assembled Low Molecular Weight Peptide-Based Aggregates and Hydrogels. Chem. Eur. J. 2016, 22, 6687–6694. [Google Scholar] [CrossRef]
- Conejero-Muriel, M.; Contreras-Montoya, R.; Díaz-Mochón, J.J.; de Cienfuegos, L.Á.; Gavira, J.A. Protein crystallization in short-peptide supramolecular hydrogels: A versatile strategy towards biotechnological composite materials. CrystEngComm 2015, 17, 8072–8078. [Google Scholar] [CrossRef]
- Bachl, J.; Oehm, S.; Mayr, J.; Cativiela, C.; Marrero-Tellado, J.J.; Díaz Díaz, D. Supramolecular Phase-Selective Gelation by Peptides Bearing Side-Chain Azobenzenes: Effect of Ultrasound and Potential for Dye Removal and Oil Spill Remediation. Int. J. Mol. Sci. 2015, 16, 11766–11784. [Google Scholar] [CrossRef]
- Chetia, M.; Debnath, S.; Chowdhury, S.; Chatterjee, S. Self-assembly and multifunctionality of peptide organogels: Oil spill recovery, dye absorption and synthesis of conducting biomaterials. RSC Adv. 2020, 10, 5220–5233. [Google Scholar] [CrossRef]
- Basak, S.; Nanda, J.; Banerjee, A. A new aromatic amino acid based organogel for oil spill recovery. J. Mater. Chem. 2012, 22, 11658–11664. [Google Scholar] [CrossRef]
- Debnath, S.; Shome, A.; Dutta, S.; Das, P.K. Dipeptide-based low-molecular-weight efficient organogelators and their application in water purification. Chem. Eur. J. 2008, 14, 6870–6881. [Google Scholar] [CrossRef]
- Mondal, B.; Bairagi, D.; Nandi, N.; Hansda, B.; Das, K.S.; Edwards-Gayle, C.J.C.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Peptide-Based Gel in Environmental Remediation: Removal of Toxic Organic Dyes and Hazardous Pb2+ and Cd2+ Ions from Wastewater and Oil Spill Recovery. Langmuir 2020, 36, 12942–12953. [Google Scholar] [CrossRef]
- Wood, D.M.; Greenland, B.W.; Acton, A.L.; Rodríguez-Llansola, F.; Murray, C.A.; Cardin, C.J.; Miravet, J.F.; Escuder, B.; Hamley, I.W.; Hayes, W. pH-Tunable Hydrogelators for Water Purification: Structural Optimisation and Evaluation. Chem. Eur. J. 2012, 18, 2692–2699. [Google Scholar] [CrossRef]
- Roy, K.; Chetia, M.; Sarkar, A.K.; Chatterjee, S. Co-assembly of charge complementary peptides and their applications as organic dye/heavy metal ion (Pb2+, Hg2+) absorbents and arsenic(iii/v) detectors. RSC Adv. 2020, 10, 42062–42075. [Google Scholar] [CrossRef]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutant Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. Chemnanomat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption. Gels 2021, 7, 85. [Google Scholar] [CrossRef]
- Bowerman, C.J.; Nilsson, B.L. Review self-assembly of amphipathic β-sheet peptides: Insights and applications. Pept. Sci. 2012, 98, 169–184. [Google Scholar] [CrossRef]
- Winnik, F.M. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure–Function Correlation. Chem. Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Lee, N.R.; Bowerman, C.J.; Nilsson, B.L. Effects of Varied Sequence Pattern on the Self-Assembly of Amphipathic Peptides. Biomacromolecules 2013, 14, 3267–3277. [Google Scholar] [CrossRef]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Garifullin, R.; Guler, M.O. Supramolecular chirality in self-assembled peptide amphiphile nanostructures. Chem. Commun. 2015, 51, 12470–12473. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Marbán, G. Visible Light Spectroscopic Analysis of Methylene Blue in Water; What Comes after Dimer? ACS Omega 2020, 5, 29801–29815. [Google Scholar] [CrossRef]
- Avena, M.J.; Valenti, L.E.; Pfaffen, V.; De Pauli, C.P. Methylene Blue Dimerization Does Not Interfere in Surface-Area Measurements of Kaolinite and Soils. Clays Clay Miner. 2001, 49, 168–173. [Google Scholar] [CrossRef]
- Liu, B.; Wen, L.; Nakata, K.; Zhao, X.; Liu, S.; Ochiai, T.; Murakami, T.; Fujishima, A. Polymeric Adsorption of Methylene Blue in TiO2 Colloids—Highly Sensitive Thermochromism and Selective Photocatalysis. Chem. Eur. J. 2012, 18, 12705–12711. [Google Scholar] [CrossRef]
- Dolia, V.; James, A.L.; Chakrabarty, S.; Jasuja, K. Dissimilar adsorption of higher-order aggregates compared with monomers and dimers of methylene blue on graphene oxide: An optical spectroscopic perspective. Carbon Trends 2021, 4, 100066. [Google Scholar] [CrossRef]
- Bergmann, K.; O’Konski, C.T. A Spectroscopic Study of Methylene Blue Monomer, Dimer, and Complexes with Montmorillonite. J. Phys. Chem. 1963, 67, 2169–2177. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]

| Entry | Dye | C0 (mg/L) | V (mL) | mdye (μg) | RE 1 (%) |
|---|---|---|---|---|---|
| 1 | MO | 50 | 5 | 250 | 16 |
| 2 | RhB | 50 | 5 | 250 | 50 |
| 3 | MB | 50 | 5 | 250 | 90 |
| 4 | MB | 25 | 5 | 125 | 100 |
| 5 | MB | 100 | 5 | 500 | 91 |
| 6 | MB | 50 | 2.5 | 125 | 99.5 |
| 7 | MB | 50 | 10 | 500 | 90 |
| Entry | Dye | C0 (mg/L) | V (mL) | mPhAC (μg) | RE 1 (%) |
|---|---|---|---|---|---|
| 1 | NAL | 50 | 5 | 250 | 21 |
| 2 | CBZ | 50 | 5 | 250 | 24 |
| 3 | CIP | 50 | 5 | 250 | 40 |
| 4 | DCF | 50 | 5 | 250 | 75 |
| 5 | DCF | 25 | 5 | 125 | 59 |
| 6 | DCF | 100 | 5 | 500 | 89 |
| 7 | DCF | 50 | 2.5 | 125 | 83 |
| 8 | DCF | 50 | 10 | 500 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. https://doi.org/10.3390/gels8100672
Fortunato A, Mba M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels. 2022; 8(10):672. https://doi.org/10.3390/gels8100672
Chicago/Turabian StyleFortunato, Anna, and Miriam Mba. 2022. "A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation" Gels 8, no. 10: 672. https://doi.org/10.3390/gels8100672
APA StyleFortunato, A., & Mba, M. (2022). A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels, 8(10), 672. https://doi.org/10.3390/gels8100672










