Biocompatible Semi-Interpenetrating Materials Based on Poly(3-hydroxyalkanoate)s and Poly(ethyleneglycol) Diacrylate
Abstract
1. Introduction
2. Results and Discussion
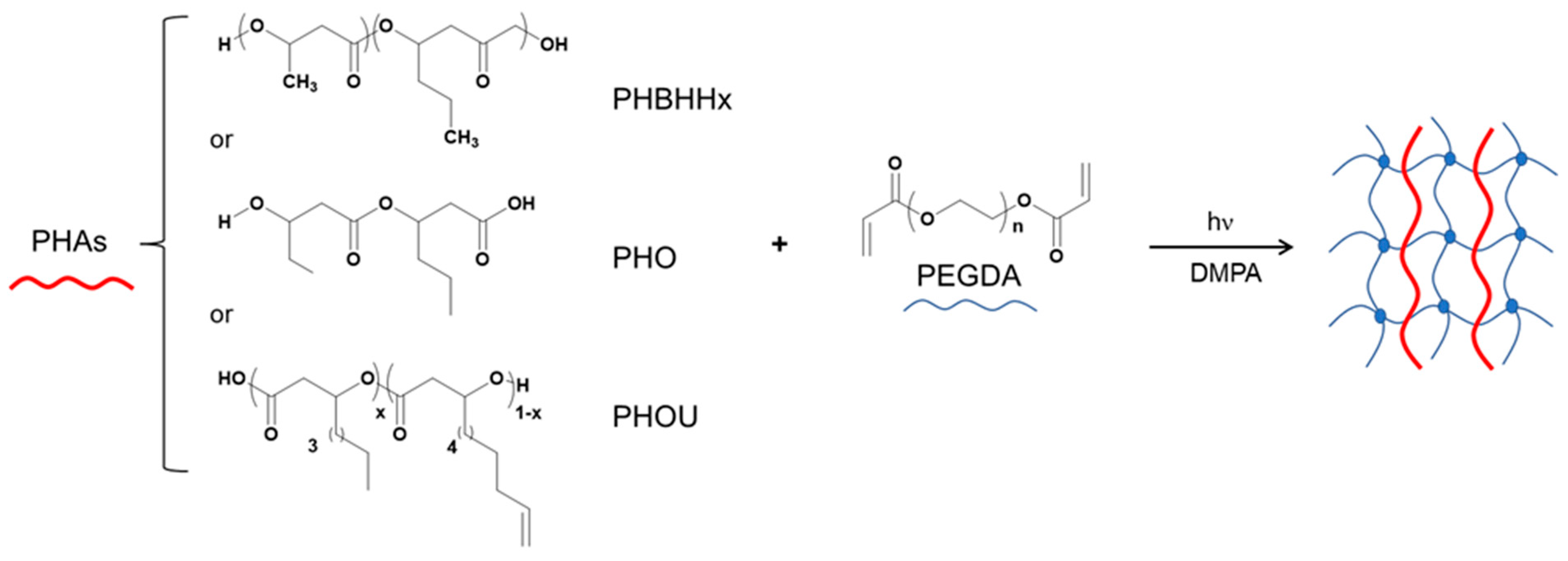
2.1. Preparation of Semi-IPNs Networks PHAxPEGDA1−x
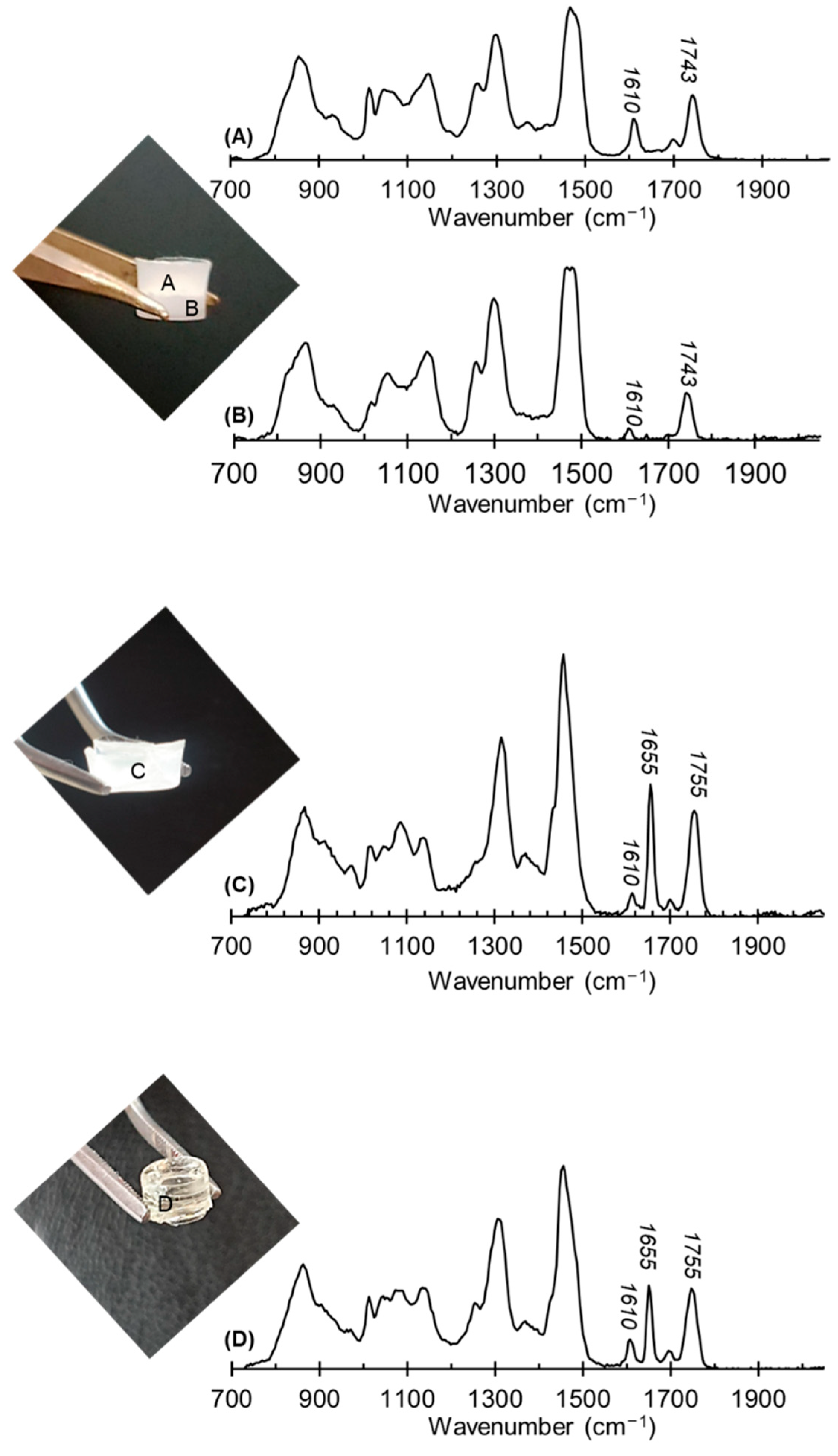
2.2. Characterization of Semi-IPNs, PHAxPEGDA1−x
2.3. Time Domain 1H DQ Solid State NMR
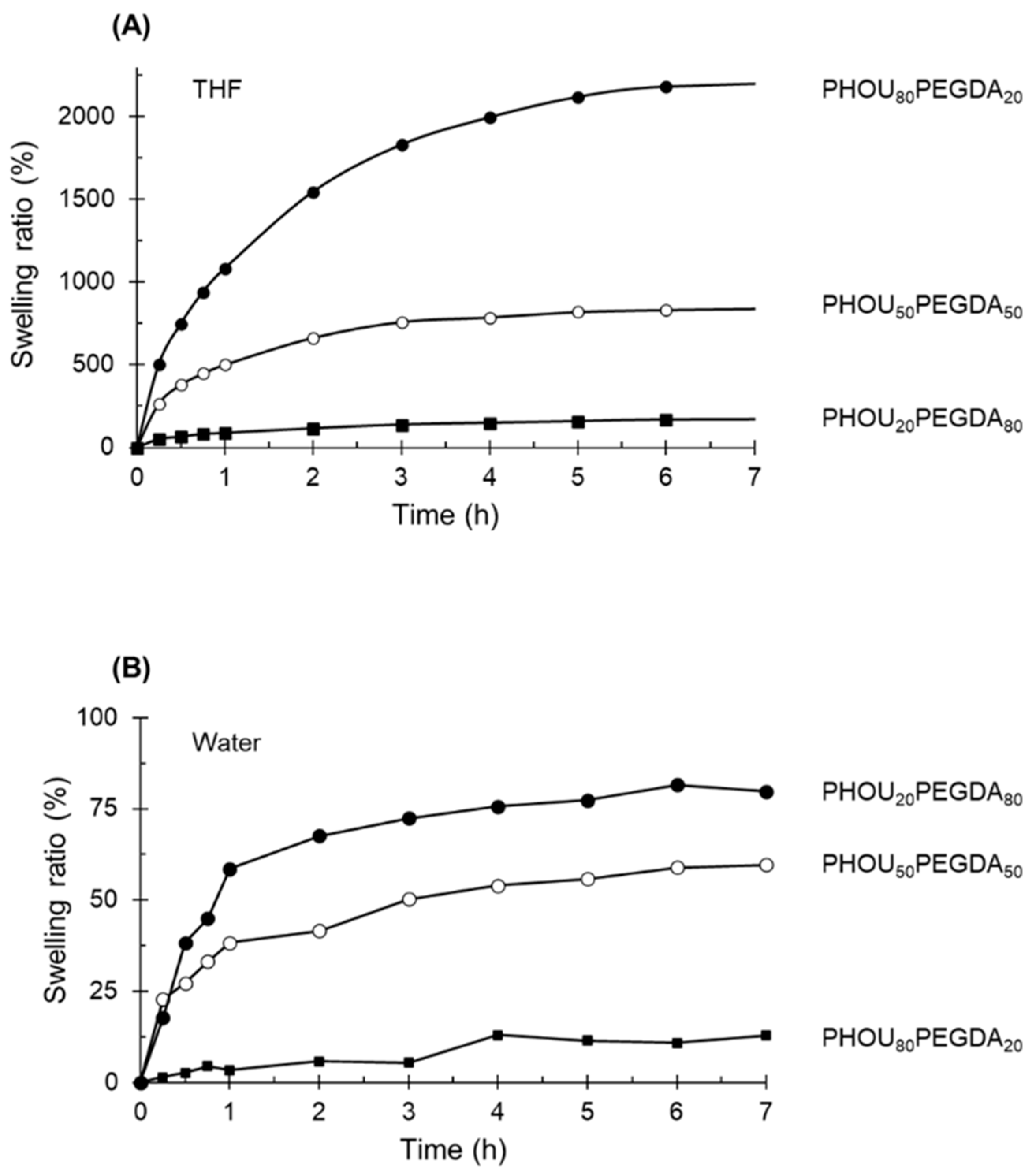
2.4. Rheological Properties and Swelling Behavior of Networks
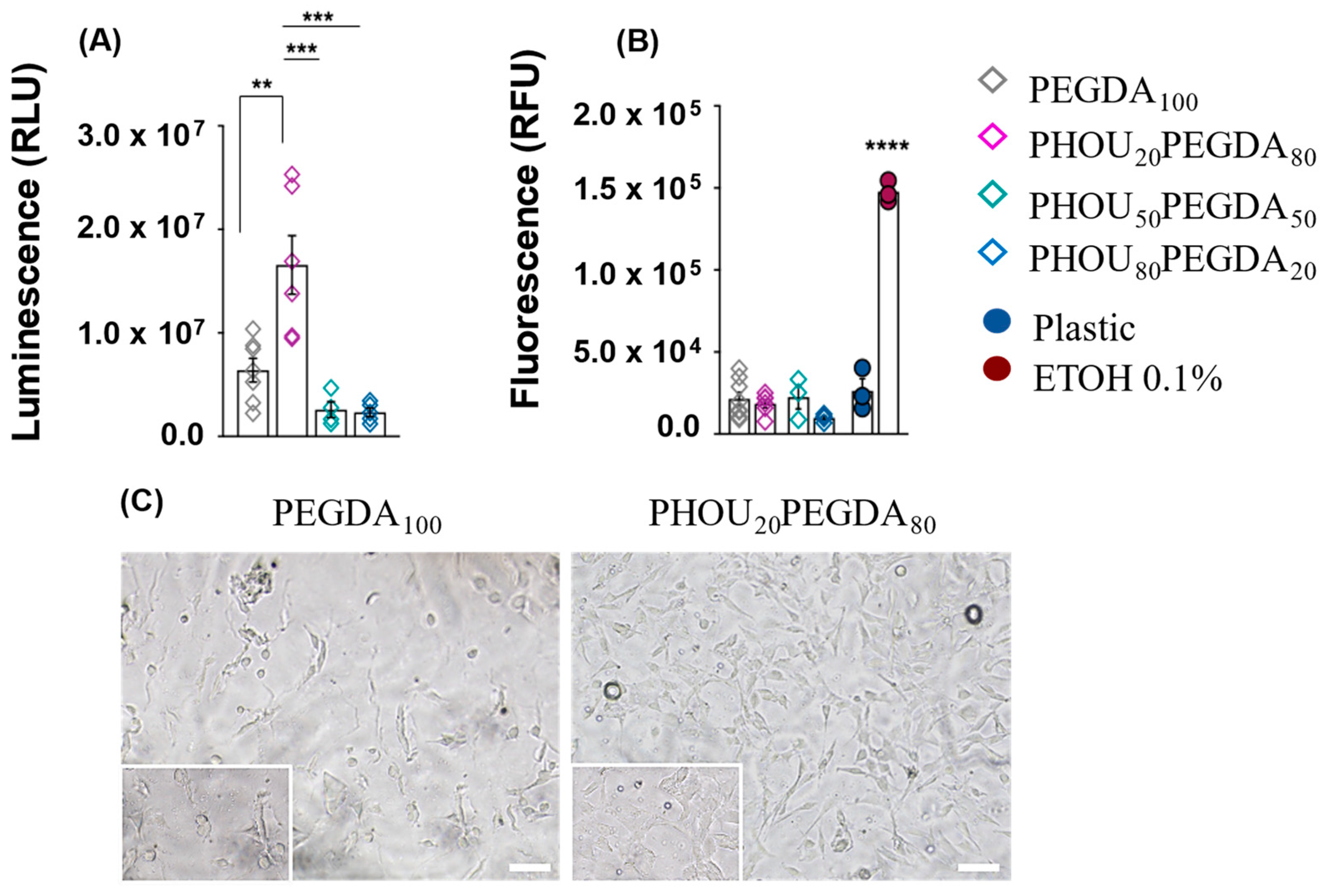
2.5. Biological Test
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PHAx/PEGDAy
4.3. Characterization
4.3.1. Preparation of Semi-IPNS Networks PHAxPEGDA1−x
4.3.2. Thermomechanical Behavior of Networks PHAxPEGDA1−x
4.3.3. Time Domain 1H DQ Solid State NMR
4.3.4. Rheological Properties and Swelling Behavior of Networks PHAxPEGDA1−x
4.4. Biological Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinbüchel, A. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial Polyesters: Biosynthesis, Biodegradable Plastics and Biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; Renard, E.; Langlois, V. Degradation of natural and artificial poly [(R)-3-hydroxyalkanoate]s: From biodegradation to hydrolysis. In Plastics from Bacteria; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 283–321. [Google Scholar]
- Renard, E.; Walls, M.; Guérin, P.; Langlois, V. Hydrolytic degradation of blends of polyhydroxyalkanoates and functionalized polyhydroxyalkanoates. Polym. Degrad. Stab. 2004, 85, 779–787. [Google Scholar] [CrossRef]
- Grassie, N.; Murray, E.J. The Thermal Degradation of Poly(-(D)-fl-Hydroxybutyric Acid): Part 2 Changes in Molecular Weight. Polym. Degrad. Stab. 1984, 6, 95–103. [Google Scholar] [CrossRef]
- Numata, K.; Abe, H.; Iwata, T. Biodegradability of Poly(hydroxyalkanoate) Materials. Materials 2009, 2, 1104–1126. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial Degradation of Polyhydroxyalkanoates with Different Chemical Compositions and Their Biodegradability. Microb. Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Biopolymers Online; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar]
- Ramier, J.; Grande, D.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Albanese, P.; Renard, E. From design of bio-based biocomposite electrospun scaffolds to osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2014, 25, 1563–1575. [Google Scholar] [CrossRef]
- Grande, D.; Ramier, J.; Versace, D.L.; Renard, E.; Langlois, V. Design of functionalized biodegradable PHA-based electrospun scaffolds meant for tissue engineering applications. New Biotechnol. 2017, 37, 129–137. [Google Scholar] [CrossRef]
- Asare, E.; Gregory, D.A.; Fricker, A.; Marcello, E.; Paxinou, A.; Taylor, C.S.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates, their processing and biomedical applications. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 255–284. [Google Scholar]
- Timbart, L.; Renard, E.; Tessier, M.; Langlois, V. Monohydroxylated Poly(3-hydroxyoctanoate) Oligomers and Its Functionalized Derivatives Used as Macroinitiators in the Synthesis of Degradable Diblock Copolyesters. Biomacromolecules 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-P.; Zhang, X.; Wei, D.-X.; Wu, Q.; Jiang, X.-R.; Chen, G.-Q. Highly Efficient Fluorescent Material Based on Rare-Earth-Modified Polyhydroxyalkanoates. Biomacromolecules 2019, 20, 3233–3241. [Google Scholar] [CrossRef]
- Sparks, J.; Scholz, C. Synthesis and Characterization of a Cationic Poly(β-hydroxyalkanoate). Biomacromolecules 2008, 9, 2091–2096. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef]
- Loh, X.J.; Zhang, Z.-X.; Wu, Y.-L.; Lee, T.S.; Li, J. Synthesis of Novel Biodegradable Thermoresponsive Triblock Copolymers Based on Poly[(R)-3-hydroxybutyrate] and Poly(N-isopropylacrylamide) and Their Formation of Thermoresponsive Micelles. Macromolecules 2009, 42, 194–202. [Google Scholar] [CrossRef]
- Andrade, A.P.; Neuenschwander, P.; Hany, R.; Egli, T.; Witholt, B.; Li, Z. Synthesis and Characterization of Novel Copoly(ester−urethane) Containing Blocks of Poly-[(R)-3-hydroxyoctanoate] and Poly-[(R)-3-hydroxybutyrate]. Macromolecules 2002, 35, 4946–4950. [Google Scholar] [CrossRef]
- Renard, E.; Rios, A.; Versace, D.-L.; Langlois, V. The Design of Functionalized PHA-Based Polymeric Materials by Chemical Modifications. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 17–42. [Google Scholar]
- Hazer, B. Amphiphiles from Poly(3-hydroxyalkanoates). In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 43–64. [Google Scholar]
- Jain-Beuguel, C.; Li, X.; Houel-Renault, L.; Modjinou, T.; Simon-Colin, C.; Gref, R.; Renard, E.; Langlois, V. Water-Soluble Poly(3-hydroxyalkanoate) Sulfonate: Versatile Biomaterials Used as Coatings for Highly Porous Nano-Metal Organic Framework. Biomacromolecules 2019, 20, 3324–3332. [Google Scholar] [CrossRef] [PubMed]
- Babinot, J.; Renard, E.; Le Droumaguet, B.; Guigner, J.-M.; Mura, S.; Nicolas, J.; Couvreur, P.; Langlois, V. Facile Synthesis of Multicompartment Micelles Based on Biocompatible Poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 2013, 34, 362–368. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Che, X.; Yu, L.; Jia, W.; Shen, R.; Chen, J.; Ma, Y.; Chen, G.-Q. Synthesis and characterization of polyhydroxyalkanoate organo/hydrogels. Biomacromolecules 2019, 20, 3303. [Google Scholar] [CrossRef]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked poly(ethylene glycol) diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 36. [Google Scholar] [CrossRef]
- Spearman, B.S.; Hodge, A.J.; Porter, J.L.; Hardy, J.G.; Davis, Z.D.; Xu, T.; Zhang, X.; Schmidt, C.E.; Hamilton, M.C.; Lipke, E.A. Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater. 2015, 28, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.; Illikainen, M.; Sain, M.; Oksman, K. Polylactic acid/polyurethane blend reinforced with cellulose nanocrystals with semi-interpenetrating polymer network (S-IPN) structure. Eur. Polym. J. 2017, 86, 188–199. [Google Scholar] [CrossRef]
- Gursel, I.; Balcik, C.; Arica, Y.; Akkus, O.; Akkas, N.; Hasirci, V. Synthesis and mechanical properties of interpenetrating networks of polyhydroxybutyrate-co-hydroxyvalerate and polyhydroxyethyl methacrylate. Biomaterials 1998, 19, 1137–1143. [Google Scholar] [CrossRef]
- Hao, J.; Deng, X. Semi-interpenetrating networks of bacterial poly(3-hydroxybutyrate) with net-poly(ethylene glycol). Polymer 2001, 42, 4091–4097. [Google Scholar] [CrossRef]
- Mangeon, C.; Modjinou, T.; Rios de Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Renewable Semi-Interpenetrating Polymer Networks Based on Vegetable Oils Used as Plasticized Systems of Poly(3-hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2018, 6, 5034–5042. [Google Scholar] [CrossRef]
- Rekowska, N.; Teske, M.; Arbeiter, D.; Brietzke, A.; Konasch, J.; Riess, A.; Mau, R.; Eickner, T.; Seitz, H.; Grabow, N. Biocompatibility and thermodynamic properties of PEGDA and two of its copolymer. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Berlin, Germany, 23–27 July 2019; pp. 1093–1096. [Google Scholar]
- Hanna, K.; Yasar-Inceoglu, O.; Yasar, O. Drug Delivered Poly(ethylene glycol) Diacrylate (PEGDA) Hydrogels and Their Mechanical Characterization Tests for Tissue Engineering Applications. MRS Adv. 2018, 3, 1697–1702. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef]
- Yen, Y.; Pines, A. Multiple-quantum NMR in solids. J. Chem. Phys. 1983, 78, 3579–3582. [Google Scholar] [CrossRef]
- Baum, J.; Munowitz, M.; Garroway, A.N.; Pines, A. Multiple-quantum dynamics in solid state NMR. J. Chem. Phys. 1985, 83, 2015–2025. [Google Scholar] [CrossRef]
- Saalwächter, K. 1H multiple-quantum nuclear magnetic resonance investigations of molecular order in polymer networks. II. Intensity decay and restricted slow dynamics. J. Chem. Phys. 2004, 120, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Lorthioir, C.; Randriamahefa, S.; Deloche, B. Some aspects of the orientational order distribution of flexible chains in a diblock mesophase. J. Chem. Phys. 2013, 139, 224903. [Google Scholar] [CrossRef]
- Vieyres, A.; Per, R.; Long, D.R.; Sotta, P. Sulfur-Cured Natural Rubber Elastomer Networks: Correlating Cross- Link Density, Chain Orientation, and Mechanical Response by Combined Techniques. Macromolecules 2013, 46, 889–899. [Google Scholar] [CrossRef]
- Rios de Anda, A.; Sotta, P.; Modjinou, T.; Langlois, V.; Versace, D.-L.; Renard, E. Multiscale Structural Characterization of Biobased Diallyl–Eugenol Polymer Networks. Macromolecules 2020, 53, 2187–2197. [Google Scholar] [CrossRef]
- Grogan, B.F.; Hsu, J.R. Volumetric Muscle Loss. Am. Acad. Orthop. Surg. 2011, 19, S35–S37. [Google Scholar] [CrossRef] [PubMed]



| Compositions | Aspect | Soluble Extract (%) |
|---|---|---|
| PEGDA100 | Solid | 0 |
| PHBHHx20 PEGDA80 | Solid | 30.0 ± 5.0 |
| PHBHHx50 PEGDA50 | Liquid | nd |
| PHO20PEGDA80 | Solid | 24.0 ± 3.0 |
| PHO50PEGDA50 | Liquid | nd |
| PHOU20 PEGDA80 | Solid | 4.9 ± 0.5 |
| PHOU50 PEGDA50 | Solid | 4.1 ± 0.2 |
| PHOU80 PEGDA20 | Solid | 6.9 ± 3.7 |
| PHOU100 | Liquid | nd |
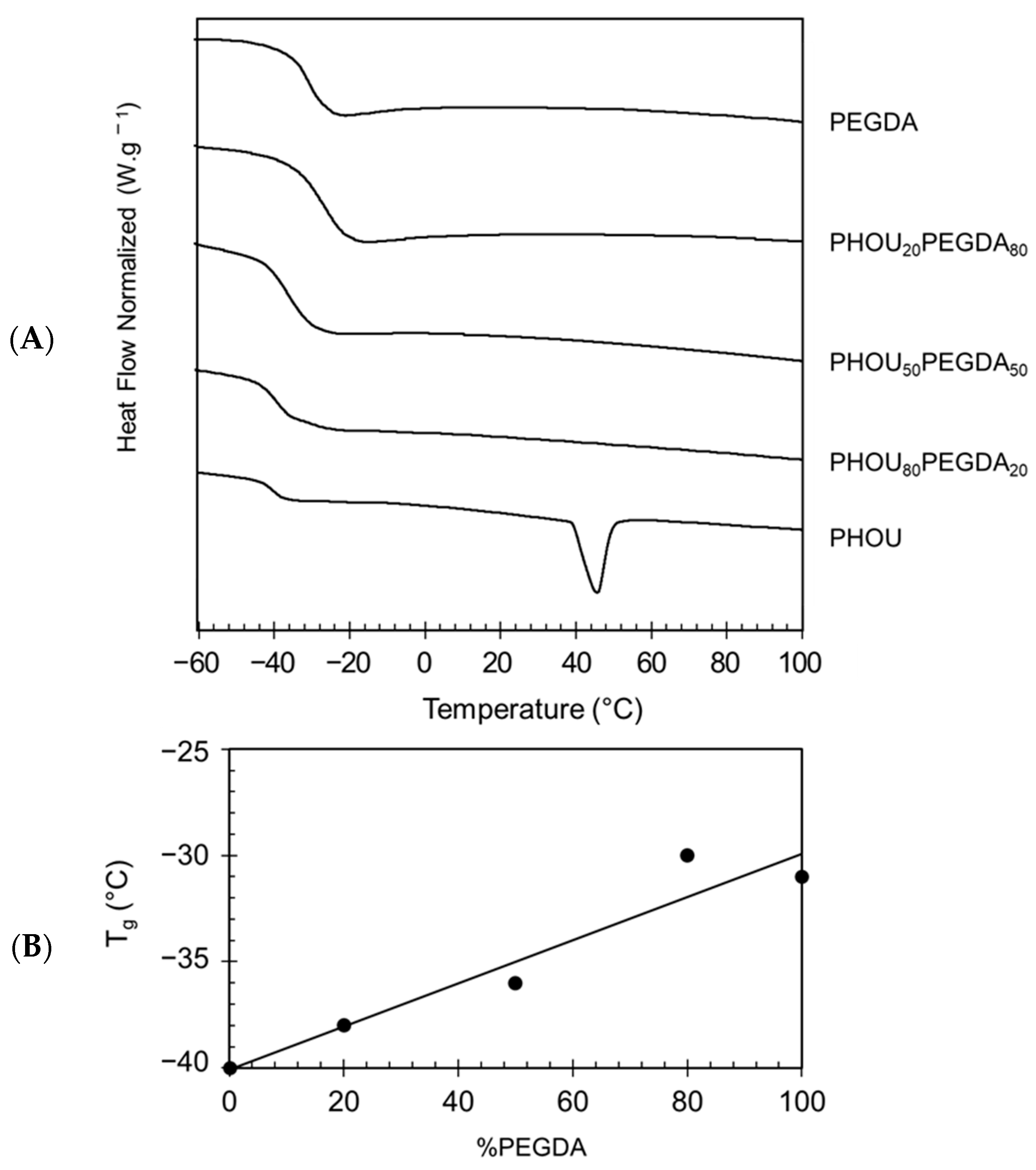
| Samples | Tconversion C=C (a) (%) | Tg (b) (°C) | Tm (b) (°C) | Td30 (c) (°C) |
|---|---|---|---|---|
| PEGDA100 | 99 ± 1 | −30 | - | 371 |
| PHOU20 PEGDA80 | 85 ± 8 | −28 | - | 370 |
| PHOU50 PEGDA50 | 70 ± 2 | −36 | - | 301 |
| PHOU80 PEGDA20 | 80 ± 2 | −38 | - | 279 |
| PHOU100 | 3 ± 1 | −40 | 46 | 279 |
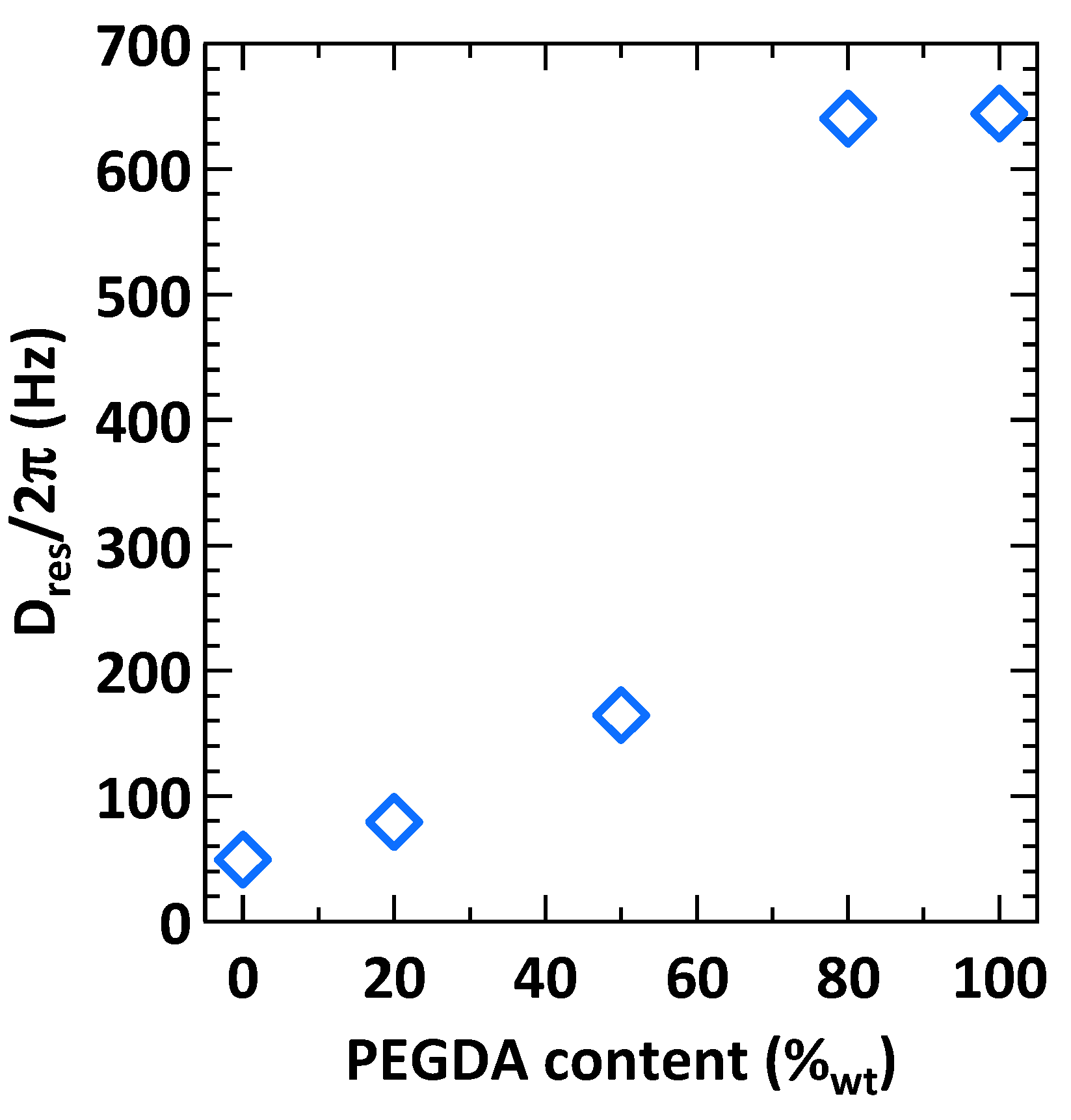
| Samples | Dres/2π (Hz) | N |
|---|---|---|
| PHOU100PEGDA0 | 49.3 | 1.68 |
| PHOU80PEGDA20 | 79.5 | 1.55 |
| PHOU50PEGDA50 | 164.4 | 1.57 |
| PHOU20PEGDA80 | 640.1 | 1.32 |
| PHOU0PEGDA100 | 644.2 | 1.25 |
| Samples | G′ (Pa) | G″ (Pa) |
|---|---|---|
| PHOU20PEGDA80 | 8853 | 388 |
| PHOU50PEGDA50 | 7602 | 442 |
| PHOU80PEGDA20 | 1088 | 145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brelle, L.; Rios de Anda, A.; Ozturk, T.; Didier, N.; Renard, E.; Langlois, V. Biocompatible Semi-Interpenetrating Materials Based on Poly(3-hydroxyalkanoate)s and Poly(ethyleneglycol) Diacrylate. Gels 2022, 8, 632. https://doi.org/10.3390/gels8100632
Brelle L, Rios de Anda A, Ozturk T, Didier N, Renard E, Langlois V. Biocompatible Semi-Interpenetrating Materials Based on Poly(3-hydroxyalkanoate)s and Poly(ethyleneglycol) Diacrylate. Gels. 2022; 8(10):632. https://doi.org/10.3390/gels8100632
Chicago/Turabian StyleBrelle, Laura, Agustin Rios de Anda, Teoman Ozturk, Nathalie Didier, Estelle Renard, and Valérie Langlois. 2022. "Biocompatible Semi-Interpenetrating Materials Based on Poly(3-hydroxyalkanoate)s and Poly(ethyleneglycol) Diacrylate" Gels 8, no. 10: 632. https://doi.org/10.3390/gels8100632
APA StyleBrelle, L., Rios de Anda, A., Ozturk, T., Didier, N., Renard, E., & Langlois, V. (2022). Biocompatible Semi-Interpenetrating Materials Based on Poly(3-hydroxyalkanoate)s and Poly(ethyleneglycol) Diacrylate. Gels, 8(10), 632. https://doi.org/10.3390/gels8100632







