Abstract
As a novel stimulus, we use high-frequency ultrasonic waves to provide the required energy for breaking hydrogen bonds between Poly(N-isopropylacrylamide) (PNIPAM) and water molecules while the solution temperature is maintained below the volume phase transition temperature (VPTT = 32 °C). Ultrasonic waves propagate through the solution and their energy will be absorbed due to the liquid viscosity. The absorbed energy partially leads to the generation of a streaming flow and the rest will be spent to break the hydrogen bonds. Therefore, the microgels collapse and become insoluble in water and agglomerate, resulting in solution turbidity. We use turbidity to quantify the ultrasound energy absorption and show that the acousto-response of PNIPAM microgels is a temporal phenomenon that depends on the duration of the actuation. Increasing the solution concentration leads to a faster turbidity evolution. Furthermore, an increase in ultrasound frequency leads to an increase in the breakage of more hydrogen bonds within a certain time and thus faster turbidity evolution. This is due to the increase in ultrasound energy absorption by liquids at higher frequencies.
1. Introduction
Microgels are cross-linked polymeric networks, in a size range of nanometer to micrometer. These particles swell in water and according to their environment, their size and density can be adjusted. They are stimuli-responsive due to their ability to undergo a volume phase transition (VPT) subjected to changes in temperature, pH, ionic strength, and so on [1]. These smart polymers have drawn attention for several years since they have promising applications in a wide range of industries from biomedical technologies, cement, ink-jet printing, and oil recovery, etc. [2].
Poly(N-isopropylacrylamide) —known as PNIPAM— is a thermoresponsive microgel having a volume phase transition temperature (VPTT) of 32 °C [3,4]. At room temperature (below VPTT), PNIPAM is fully swollen and surrounded by hydrogen-bonded water molecules—approximately 4–6 hydrogen bonds per NIPAM monomer are formed [5]. By increasing their solution temperature above the VPTT, their hydrogen bonds break, and microgels collapse and become insoluble in water. The required thermal energy for breaking the hydrogen bonds is 2 KBT per NIPAM monomer [5,6].
PNIPAM microgels have been designed to be responsive to other stimuli such as pH [7,8], light [9], magnetic field [10], ionic strength [11,12], or a combination of these parameters [11,13,14] in addition to their natural responsiveness to changes in temperature (thermal energy). PNIPAM-based pH-sensitive microgels have been synthesized by copolymerization of acrylic acid (AAc) with PNIPAM [7]. In addition to pH, these microgels show sensitivity to changes in the electrolyte concentration by increasing the amount of AAc [11]. Furthermore, the sorption of a photosensitive surfactant by PNIPAM microgels leads to the formation of light-sensitive microgels that swell and de-swell by irradiation of UV light and visible light, respectively [9].
In all of these cases, one has to modify the PNIPAM microgel by copolymerization so it becomes responsive to another stimulus. Recently, we have shown that PNIPAM microgels, without any additional modification, are acousto-responsive [15]. Hydrogen bonds between PNIPAM and water molecules will be broken due to the absorption of a certain amount of energy carried by ultrasound waves while the solution temperature is below VPTT. Sound absorption in liquids has been the subject of research for a long time [16]. The intensity of the ultrasound at a distance (x) from the input wave source can be calculated by , where denotes the intensity at and is the wave attenuation/absorption coefficient. Hundreds of years of research reveal that the absorption coefficient is proportional to the liquid viscosity due to the relaxation of translational, vibrational, and rotational degrees of molecular freedom [17]. If the molecules, that are excited vibrationally, return to the equilibrium state in a shorter time than the exciting ultrasound period, the vibrational energy of the molecules returns to the control volume in the same phase as the input waves. As a result, the net energy loss due to vibrational modes will be zero. As the frequency of the actuating ultrasound increases, the energy of vibrating molecules returns out of phase with the input ultrasound period and will be detected as energy loss. Finally, by increasing the wave frequency to a large enough value, there will not be enough time for the molecules to exchange their energy between translational and vibrational modes. In the case that the actuating ultrasonic frequency is comparable to the inverse time required for the energy exchange, the input frequency is called relaxation frequency [18]. The aforementioned theory is important in the analysis of hydrogen bond breakage and turbidity evolution due to the absorption of ultrasound energy at different frequencies.
In this paper, we further elaborate on the acousto-responsiveness of PNIPAM microgels by using the turbidity evolution of PNIPAM solutions subjected to ultrasonic actuation. We measure the ultrasound energy loss in the solutions corresponding to the required energy for breaking hydrogen bonds between PNIPAM microgels and water molecules. As a result, the solution becomes turbid due to the agglomeration of the collapsed microgels. In our recent work [15], we showed that PNIPAM microgels are responsive to ultrasound energy, and by increasing the input power, turbidity evolution becomes faster. The question remains on the effects of input frequency on turbidity evolution (i.e., hydrogen bond breakage) of PNIPAM solutions. Therefore, in the present study, the acousto-responsiveness of the PNIPAM microgels is introduced as a function of ultrasound frequency by keeping the input acoustic pressure constant. We used turbidity to quantify the ultrasound energy absorption by solutions, using image processing.
2. Results and Discussion
2.1. Turbidity Evolution
Ultrasonic waves created by the piezoelectric transducer propagate into the PNIPAM solution and by dissipation of their energy (due to the liquid viscosity) into the solution, the energy that is required to break the hydrogen bonds will be provided. Therefore, the PNIPAM polymers collapse and become insoluble in water, the solution becomes turbid and the agglomeration of polymers can be visualized by a camera. Continuous actuation of the liquid results in the breaking of more hydrogen bonds, and, thus, more turbidity arises until the entire medium becomes fully white. The thermodynamics of hydrogen bond breakage on the PNIPAM phase transition is defined in references [5,6,19]. In order to break the hydrogen bonds with thermal energy, one needs to provide 5 kJ per mol of NIPAM in the water [5,6,19]. Based on these works, calculations in our experiments reveal that approximately 2.2 J, 0.44 J and 0.088 J are required to break all hydrogen bonds inside 1 mL of 5 wt%, 1 wt% and 0.2 wt% solutions, respectively. We use low power ultrasound. Therefore, the temperature measurements (see Section 4) during the ultrasound actuation show that the bulk temperature is not more than 25 °C, far below the VPTT. However, we cannot measure the temperature at the molecular level to see if it is higher than the VPTT in the vicinity of the hydrogen bonds between PNIPAM and the water molecules, as a result of the ultrasound energy absorption. Therefore, the mechanism by which a hydrogen bond breaks due to the absorption of ultrasound energy is unclear.
The turbidity evolution of 1 wt% microgel solution is shown sequentially for 2.5 MHz and 30 mV (150 kPa sound pressure inside the cuvette) of input actuation in Figure 1. In order to break the hydrogen bonds between PNIPAM and water, and thus turbid the medium, the calculated amount of energy is needed as discussed above. This energy is provided by ultrasound waves instead of thermal energy and equals the absorbed energy of the acoustic waves by the liquid and not the total energy possessed by the waves. Continuing the actuation for a certain time collects the absorbed energy from the acoustic field gradually and the expected stored energy for appearing turbidity will be provided. Turbidity starts from the bottom and extends to the whole solution in less than 90 s.

Figure 1.
Acoustic wave energy absorption (turbidity) mechanism during the imposing 2.5 MHz and 30 mV ultrasonic actuation of the 1 wt% microgel solution.
2.2. Effects of Solution Concentration
The kinetics of the energy absorption has been quantified similarly to our previous work [15] using image processing and the absorbed energy over time for different solution concentrations (i.e., 0.2, 1, and 5 wt%) is shown in Figure 2. Increasing the solution concentration increases the ultrasound energy absorption and consequently increases the speed of turbidity evolution. In our previous work [15], it is shown that increasing the solution concentration leads to a greater increase in “bulk viscosity” that reflects the vibrational and rotational degrees of molecular freedom in comparison with the increase in “shear viscosity” that corresponds to their translational movement. Therefore, in denser solutions, the PNIPAM chains are slower in the structural reorganization of their molecules upon ultrasonic actuation and thus the energy absorption is larger.
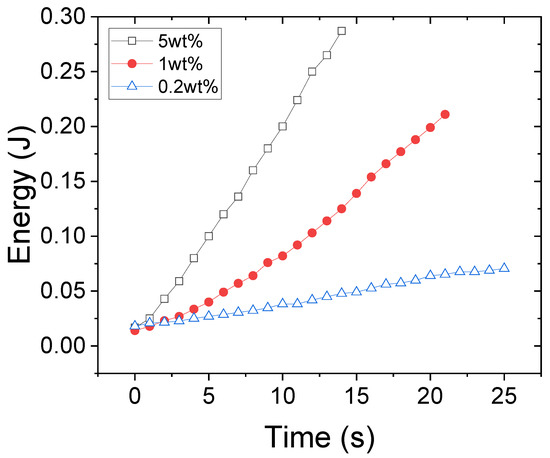
Figure 2.
The energy associated with the broken hydrogen bonds due to 2.5 MHz ultrasound waves in solution concentrations of 0.2, 1, and 5 wt%.
2.3. Effects of Frequency of Ultrasound
In addition to solution concentration, the effect of ultrasound frequency was investigated at a fixed input ultrasound pressure. The absorbed ultrasound energy in 1 wt% PNIPAM solution versus time due to the actuation frequency, ranging from 40 kHz to 5 MHz, is shown in Figure 3. According to Figure 3, low-frequency actuation leads to lower energy absorption and therefore a longer turbidity evolution, ultimately a frequency exists (i.e., 40 kHz) in which the turbidity does not occur even after 10 min of actuation. This is because at the 40 kHz actuation the liquid does not absorb enough ultrasound energy and the absorbed energy is spent solely on acoustic streaming. Furthermore, the streaming flow helps the microgels to regain their bonding strength during the mixing. In contrast, when the ultrasound frequency is in the MHz range, the entire solution becomes turbid in less than two minutes. The total absorbed energy divided by the required time to break all hydrogen bonds within the solution is calculated for all frequency cases to define a new parameter as ‘absorbed power’ for different frequencies. The result (Figure 4) is consistent with the theory and experiments stating that liquids absorb more power from ultrasound waves that have higher frequencies [20]. From Figure 3, one can infer that the relaxation frequency for the fixed input ultrasound power falls between 40 kHz and 260 kHz, where the hydrogen bonds between PNIPAM and water can regain their strength in the period of the input ultrasound waves.
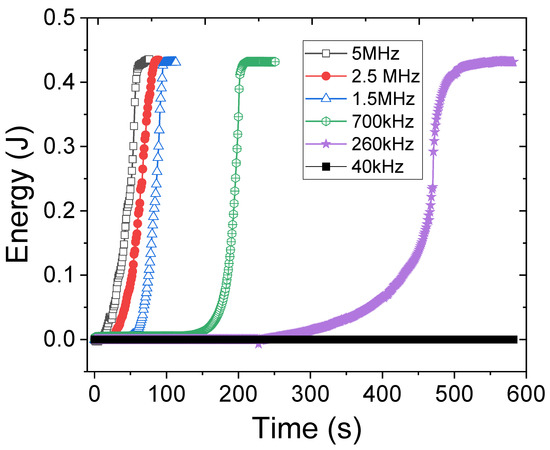
Figure 3.
Ultrasound energy absorption during actuation of 1 wt% PNIPAM solution in different frequencies.
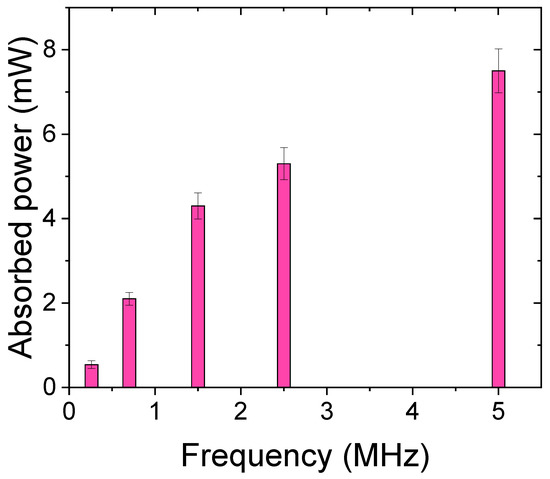
Figure 4.
Acoustic power absorption by 1 wt% PNIPAM solution subjected to ultrasound waves in different frequencies.
According to the literature [21], imposing ultrasound waves on a liquid generates translational movement of the liquid molecules which is called acoustic streaming. The question is how the turbidity evolution can be related to this acoustic streaming. Do all hydrogen bonds break at the bottom of the glass cuvette and will all the collapsed microgels be transferred to the rest of the cuvette due to the streaming? In order to address this question, we added polystyrene particles to the solutions to visualize the particle trajectory before the turbidity evolution starts. If the particle trajectory is the same as the turbidity evolution pattern, we can conclude that the microgels collapse at the bottom and travel to the rest of the media. Figure 5 shows the image of particle trajectory and hence the streaming pattern merged with the turbidity evolution image of 1 wt% PNIPAM solution, at 65 s of actuation, subjected to 2.5 MHz ultrasound waves. The red arrows in Figure 5 show a clear distinction between the streaming pattern and the turbidity evolution pattern. Therefore, we can conclude that hydrogen bonds break not only at the bottom of the cuvette but wherever the required energy for breaking them is provided by the complex acoustic field due to the reflection of waves on the walls of the cuvette. On the other hand, we cannot claim that the turbidity evolution is only due to the breaking of hydrogen bonds locally since the results, shown in Figure 5, prove the existence of the streaming inside the solution and the green arrows show a partial congruency of turbidity with the streaming. Therefore, the turbidity evolution pattern is complex and consists of both streaming flow and hydrogen bond breakage in the entire solution.

Figure 5.
The streaming pattern inside the 1 wt% PNIPAM solution due to 2.5 MHz ultrasound waves, visualized by the polystyrene particles’ trajectory. The red arrows show the discrepancy between the turbidity evolution pattern (at 65 s) and the trajectory of particles (before the turbidity starts), whereas the green arrows show their congruency.
By exposing a PNIPAM solution to ultrasonic waves, the liquid absorbs enough energy to start the translational movement of molecules, and the additional absorbed energy will be spent to break the hydrogen bonds. In addition, all of the hydrogen bond breakages cannot be visualized since microgel agglomeration can only be observed (via turbidity). Therefore, the averaged absorbed energy over time is a more suitable term to compare in different cases (Figure 4).
3. Conclusions
In this paper, we showed that PNIPAM microgels are responsive to ultrasonic actuation and this acousto-response depends on the duration of the actuation in contrast to the thermo-response of PNIPAM microgels. When the solution is subjected to ultrasonic waves, the ultrasound energy will be absorbed by the solution due to its viscosity resulting in the translational, vibrational and rotational relaxation of liquid molecules. The absorbed energy due to the translational relaxation leads to acoustic streaming, and the rest of the absorbed energy provides the required energy for breaking hydrogen bonds between PNIPAM and water molecules. As a result, microgel particles become insoluble in water, agglomerate and can be seen as a turbid medium. Therefore, no matter how much energy the input waves have, the absorbed energy by the solution ultimately accumulates during a certain time, and if the collected absorbed energy reaches a threshold it starts to break the hydrogen bonds. However, this may not lead to hydrogen bond breakage and turbidity in low frequencies, since the absorbed energy is not enough and the mixing of solution due to the acoustic streaming helps microgels to regain their hydrogen bond strength. We used this phenomenon to quantify the ultrasonic energy absorption in PNIPAM solutions. We showed that the solution concentration decreases the time for turbidity evolution since solutions with higher concentrations absorb more ultrasound power. Furthermore, by increasing the ultrasound frequency the solution absorbs more power and turbidity arises faster. Therefore, one can conclude that ultrasound waves having a higher frequency can be a faster stimulus for PNIPAM microgels. The hydrogen bond breakage mechanism at the molecular level due to the absorption of ultrasound energy is still unclear and requires a detailed theoretical and experimental analysis in future works.
4. Materials and Methods
Linear poly(N-isopropylacrylamide) (PNIPAM) () was purchased from Sigma–Aldrich, Darmstadt, Germany. Three different concentrations of 0.2 wt%, 1 wt%, and 5 wt% were prepared by dissolving it in Milli-Q water and stirred over one night. A fresh microgel solution (1 mL) of each sample was poured inside a Quartz SUPRASIL Macro Cell (PerkinElmer, Waltham, MA, USA) cuvette (inside dimensions of ) and attached to the piezoelectric transducer using a two-component latex glue (UHU Endfest Plus300, Bühl, Germany). Piezo-ceramic ultrasonic transducers (STEMINC-PIEZO, Davenport, IA, USA) with a resonance frequency of 5 MHz, 2.5 MHz, 1.5 MHz, 700 kHz, 260 kHz, and 40 kHz were used to transfer acoustic waves into the liquid (Figure 1). A 3D-printed holder was designed and fabricated to hold the transducer with the attached cuvette. A function generator (SDG1062X, SIGLENT, Shenzhen, China) was used to generate RF signals. The signal was amplified by an RF amplifier (VBA100-30, Vectawave, UK) and connected to the transducer. Input voltages were carefully selected to create the same vibration amplitude (or sound pressure) in all frequencies. The sound pressure was measured using a needle-shaped hydrophone (HNR-1000, Onda, CA, USA) at the bottom of the cuvette and was adjusted at kPa in all frequencies. The experiments were carried out at an ambient temperature of around 22 °C. The temperature measurement using thermocouples shows that continuous actuation of transducers does not increase their temperature by more than 3 °C, making sure the PNIPAM solutions maintain below their VPTT = 32 °C. A macro lens (TAMRON, SP 90 mm, F/2.8) mounted on a Nikon D7200 camera was used to visualize the turbidity in the solutions by 60 frames per second (FPS) videos (Figure 6). Then, 1 microgram of 5 polystyrene microparticles (Microparticle GmbH, Berlin, Germany) was added inside the 1 wt% PNIPAM solution to visualize the acoustic streaming before the turbidity starts. Ten particles at different times were randomly selected to track their trajectory using MATLAB image processing code.
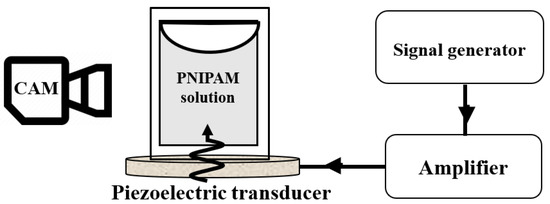
Figure 6.
The experimental setup consists of a signal generator, amplifier, and piezoelectric transducer as well as a camera for visualization of the PNIPAM solution.
Author Contributions
Conceptualization, A.R. (Atieh Razavi), A.R. (Amin Rahimzadeh) and R.v.K.; methodology, A.R. (Atieh Razavi) and A.R. (Amin Rahimzadeh); formal analysis, A.R. (Atieh Razavi); investigation, A.R. (Atieh Razavi), M.R. and S.W.; resources, M.R., S.W. and M.K.; writing—original draft preparation, A.R. (Atieh Razavi) and A.R. (Amin Rahimzadeh); writing—review and editing, A.R. (Atieh Razavi), M.R., S.W., M.K., R.v.K. and A.R. (Amin Rahimzadeh); supervision, M.K. and R.v.K. and A.R. (Amin Rahimzadeh); project administration, M.K. and R.v.K.; funding acquisition, A.R. (Amin Rahimzadeh) and R.v.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG)—Project Number (460540240).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-Isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel Applications and Commercial Considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-Sensitive Aqueous Microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Füllbrandt, M.; Ermilova, E.; Asadujjaman, A.; Hölzel, R.; Bier, F.F.; von Klitzing, R.; Schönhals, A. Dynamics of Linear Poly(N-Isopropylacrylamide) in Water around the Phase Transistion Investigated by Dielectric Relaxation Spectroscopy. J. Phys. Chem. B 2014, 118, 3750–3759. [Google Scholar] [CrossRef]
- Mukherji, D.; Kremer, K. Coil–Globule–Coil Transition of PNIPAm in Aqueous Methanol: Coupling All-Atom Simulations to Semi-Grand Canonical Coarse-Grained Reservoir. Macromolecules 2013, 46, 9158–9163. [Google Scholar] [CrossRef]
- Burmistrova, A.; Richter, M.; Uzum, C.; Klitzing, R.v. Effect of Cross-Linker Density of P(NIPAM-Co-AAc) Microgels at Solid Surfaces on the Swelling/Shrinking Behaviour and the Young’s Modulus. Colloid Polym. Sci. 2011, 289, 613–624. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S. A Novel Approach for Preparation of PH-Sensitive Hydrogels for Enteric Drug Delivery. J. Control. Release 1991, 15, 141–152. [Google Scholar] [CrossRef]
- Zakrevskyy, Y.; Richter, M.; Zakrevska, S.; Lomadze, N.; von Klitzing, R.; Santer, S. Light-Controlled Reversible Manipulation of Microgel Particle Size Using Azobenzene-Containing Surfactant. Adv. Funct. Mater. 2012, 22, 5000–5009. [Google Scholar] [CrossRef]
- Backes, S.; Witt, M.U.; Roeben, E.; Kuhrts, L.; Aleed, S.; Schmidt, A.M.; von Klitzing, R. Loading of PNIPAM Based Microgels with CoFe2O4 Nanoparticles and Their Magnetic Response in Bulk and at Surfaces. J. Phys. Chem. B 2015, 119, 12129–12137. [Google Scholar] [CrossRef]
- Snowden, M.J.; Chowdhry, B.Z.; Vincent, B.; Morris, G.E. Colloidal Copolymer Microgels of N-Isopropylacrylamide and Acrylic Acid: PH, Ionic Strength and Temperature Effects. J. Chem. Soc. Faraday Trans. 1996, 92, 5013–5016. [Google Scholar] [CrossRef]
- Snowden, M.J.; Thomas, D.; Vincent, B. Use of Colloidal Microgels for the Absorption of Heavy Metal and Other Ions from Aqueous Solution. Analyst 1993, 118, 1367–1369. [Google Scholar] [CrossRef]
- Mergel, O.; Wünnemann, P.; Simon, U.; Böker, A.; Plamper, F.A. Microgel Size Modulation by Electrochemical Switching. Chem. Mater. 2015, 27, 7306–7312. [Google Scholar] [CrossRef]
- Kleinen, J.; Klee, A.; Richtering, W. Influence of Architecture on the Interaction of Negatively Charged Multisensitive Poly(N-Isopropylacrylamide)-Co-Methacrylic Acid Microgels with Oppositely Charged Polyelectrolyte: Absorption vs Adsorption. Langmuir 2010, 26, 11258–11265. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, A.; Rutsch, M.; Kupnik, M.; von Klitzing, R. Visualization of Acoustic Energy Absorption in Confined Aqueous Solutions by PNIPAM Microgels: Effects of Bulk Viscosity. Langmuir 2021, 37, 5854–5863. [Google Scholar] [CrossRef]
- Markham, J.J.; Beyer, R.T.; Lindsay, R.B. Absorption of Sound in Fluids. Rev. Mod. Phys. 1951, 23, 353–411. [Google Scholar] [CrossRef]
- Temkin, S. Elements of Acoustics, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1981. [Google Scholar]
- Mason, T.J.; Lorimer, J.P. Sonochemistry: Theory, Applications and Uses of Ultrasound in Chemistry; Ellis Horwood Series in Physical Chemistry; Ellis Horwood: Chichester, UK, 1988; ISBN 9780745802404. [Google Scholar]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. On the Molecular Origin of the Cooperative Coil-to-Globule Transition of Poly(N-Isopropylacrylamide) in Water. Phys. Chem. Chem. Phys. 2018, 20, 9997–10010. [Google Scholar] [CrossRef] [PubMed]
- Dukhin, A.S.; Goetz, P.J. Bulk Viscosity and Compressibility Measurement Using Acoustic Spectroscopy. J. Chem. Phys. 2009, 130, 124519. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of Acoustic Streaming in Microfluidic Devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).