Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels
Abstract
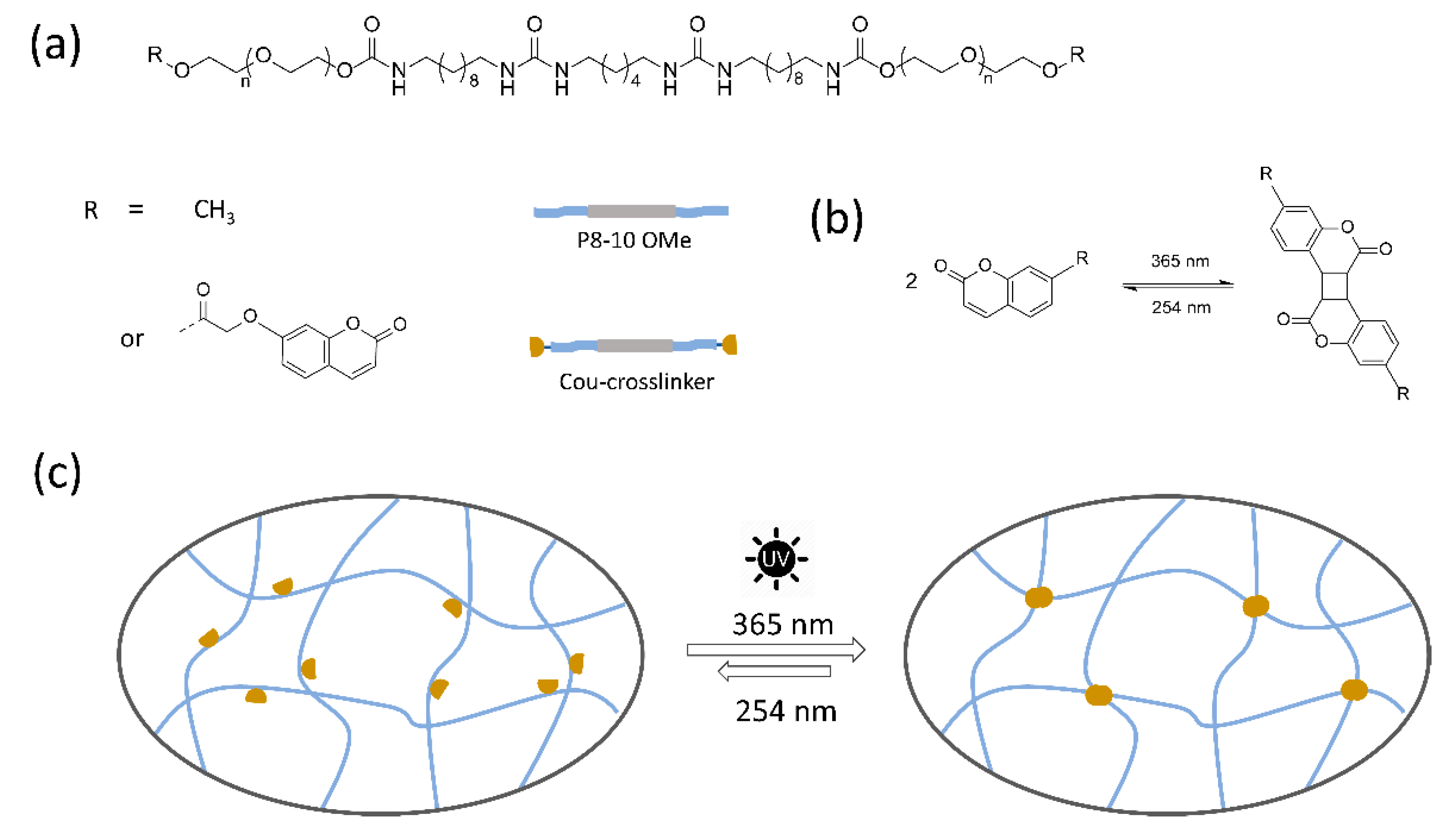
:1. Introduction
2. Results and Discussion
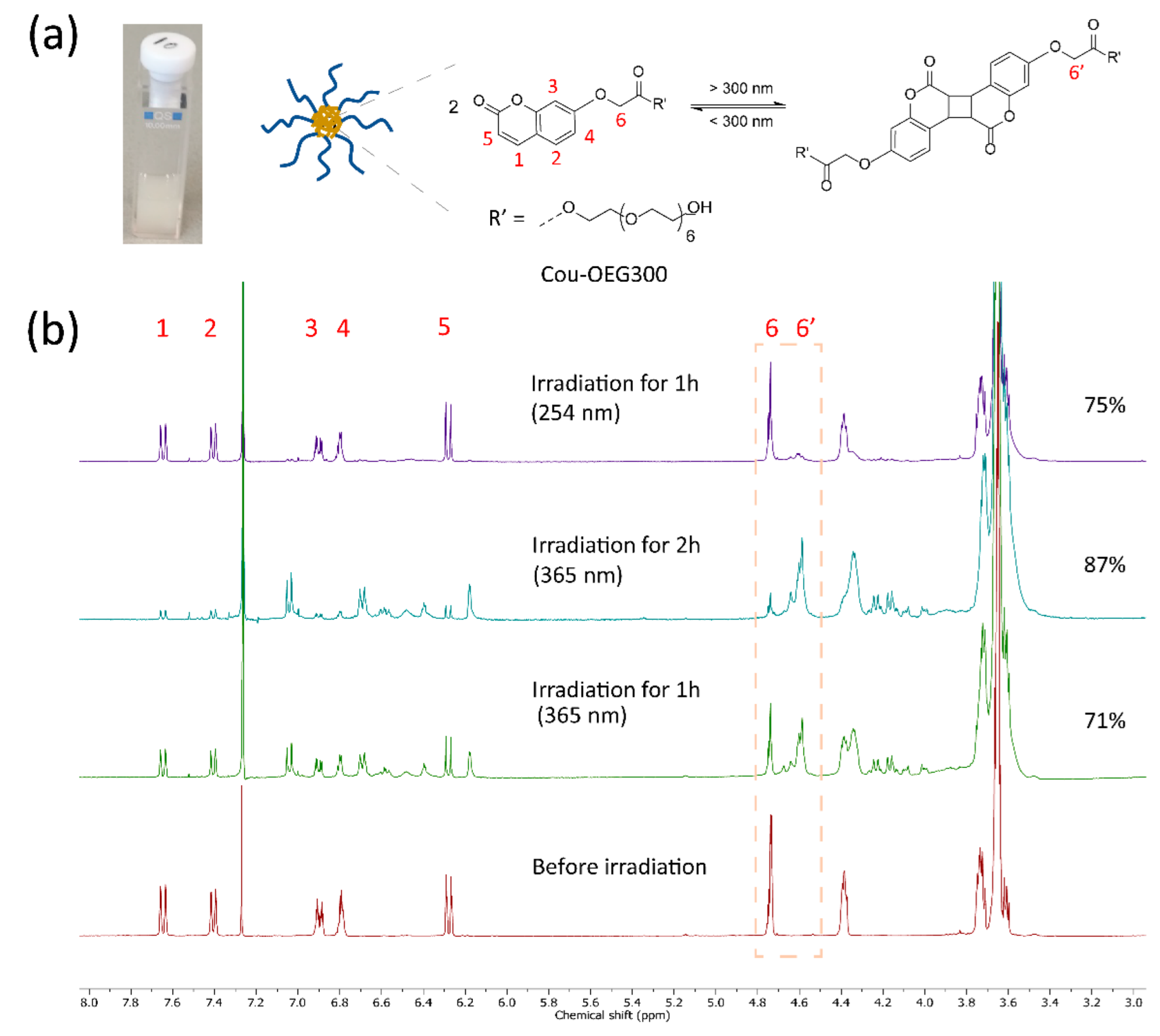
2.1. Model Reaction
2.2. Solution Preparation and UV-Vis Absorption Measurements
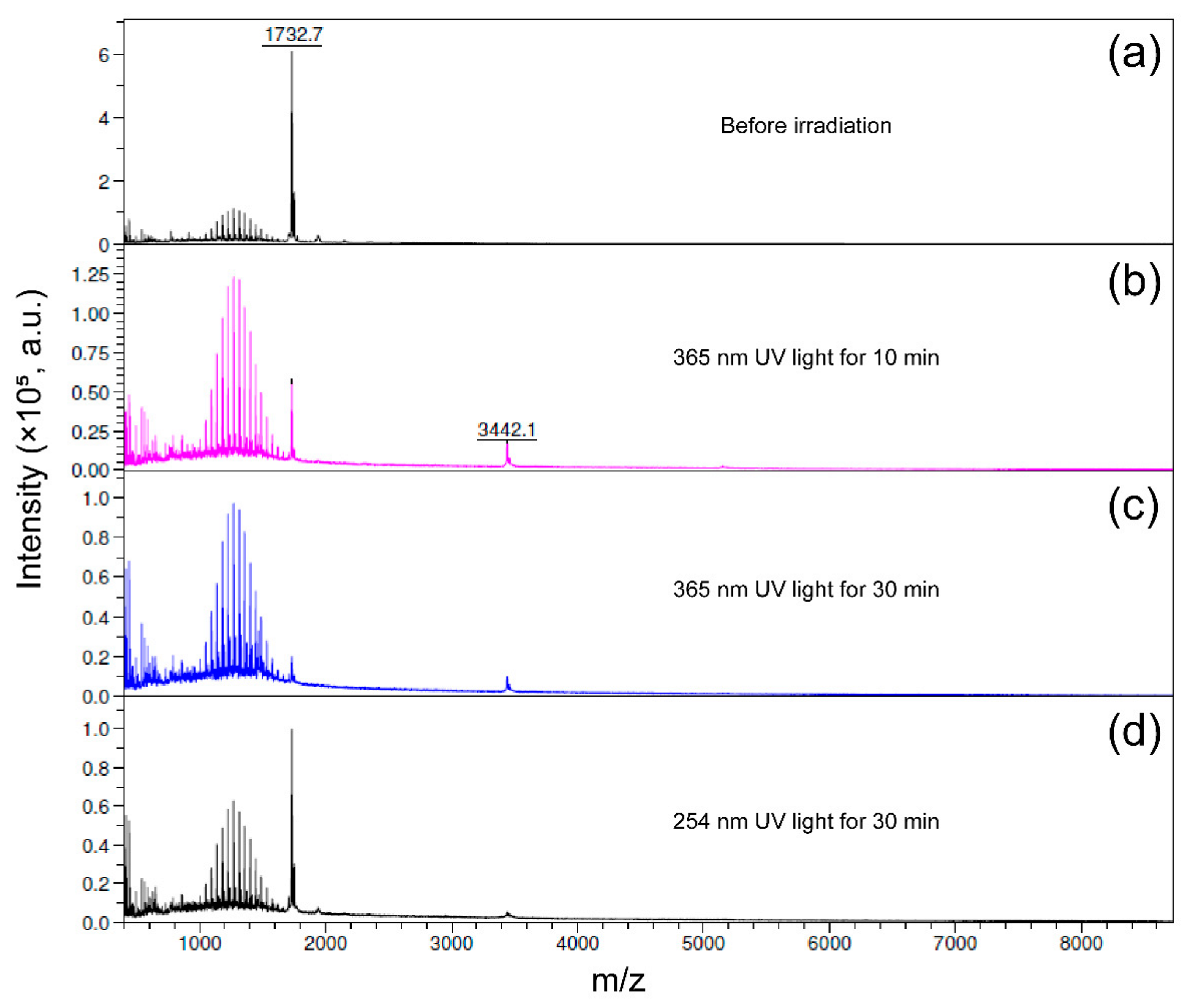
2.3. Mass Spectroscopy
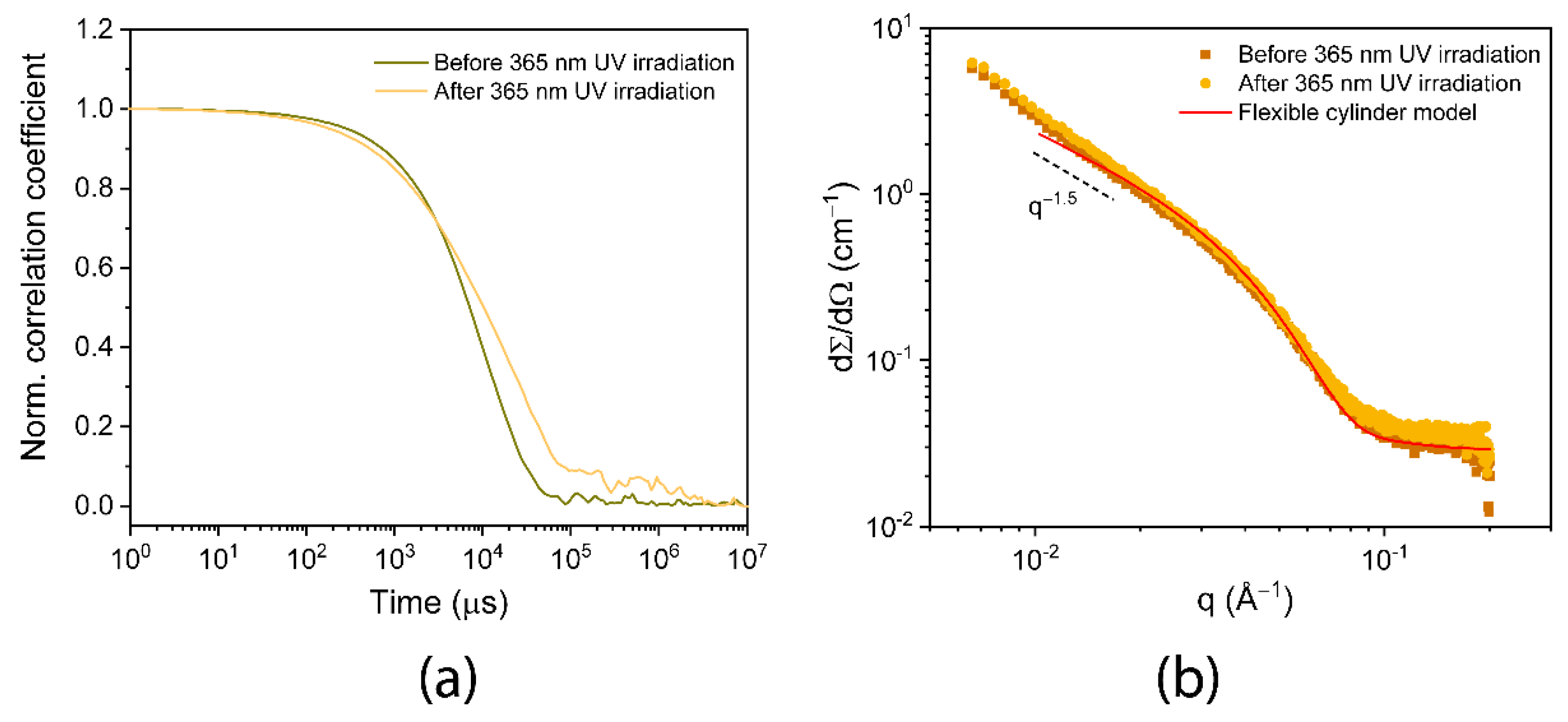
2.4. Dynamic Light Scattering
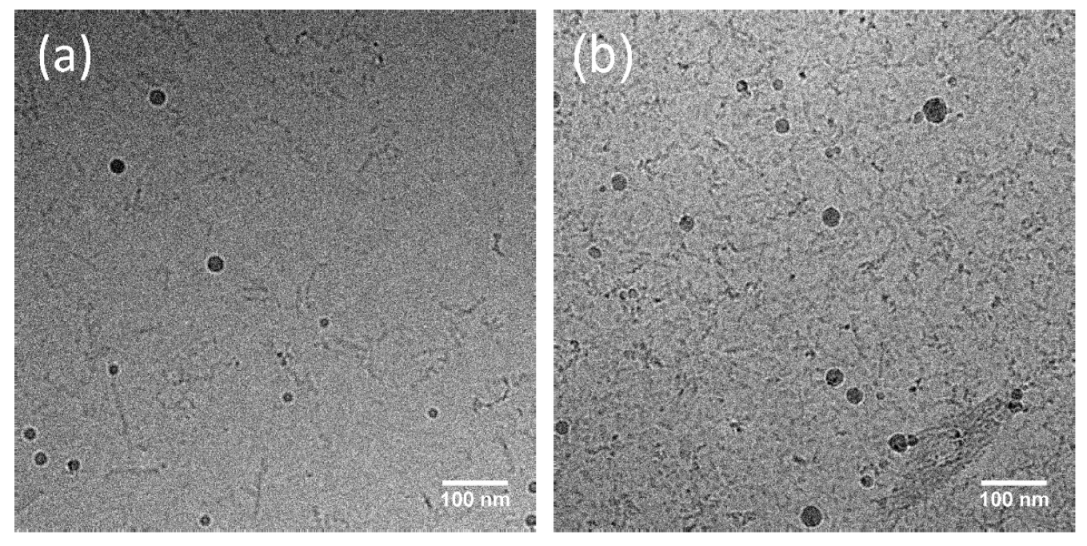
2.5. SAXS and Cryo-TEM Determine Size Change upon Irradiation
2.6. Photo-Induced Gelation and Its Reversibility
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Synthetic Procedures
4.2.2. Sample Preparation Method
4.2.3. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
4.2.4. Small-Angle X-ray Scattering (SAXS)
4.2.5. Rheology
- without UV irradiation.
- irradiated by 365 nm UV light for 30 min.
- initially irradiated by 365 nm UV light for 30 min, followed by irradiation of 254 nm UV light for 30 min.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goor, O.J.G.M.; Hendrikse, S.I.S.; Dankers, P.Y.M.; Meijer, E.W. From Supramolecular Polymers to Multi-Component Biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Dong, Y.; Liu, D. Supramolecular Hydrogels: Mechanical Strengthening with Dynamics. Polymer 2020, 210, 122993. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Gambee, J.E.; Ono, R.N.; Bächinger, H.P.; Sakai, L.Y. Initial Steps in Assembly of Microfibrils: Formation of disulfide-cross-linked multimers containing fibrillin. J. Biol. Chem. 2000, 275, 2205–2210. [Google Scholar] [CrossRef]
- Li, Y.; Qin, M.; Cao, Y.; Wang, W. Designing the Mechanical Properties of Peptide-Based Supramolecular Hydrogels for Biomedical Applications. Sci. China Phys. Mech. Astron. 2014, 57, 849–858. [Google Scholar] [CrossRef]
- Chebotareva, N.; Bomans, P.H.H.; Frederik, P.M.; Sommerdijk, N.A.J.M.; Sijbesma, R.P. Morphological Control and Molecular Recognition by Bis-Urea Hydrogen Bonding in Micelles of Amphiphilic Tri-Block Copolymers. Chem. Commun. 2005, 39, 4967–4969. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Voudouris, P.; Koenigs, M.M.E.; Besenius, P.; Wyss, H.M.; Degirmenci, V.; Sijbesma, R.P. Topochemical Polymerization in Self-Assembled Rodlike Micelles of Bisurea Bolaamphiphiles. Soft Matter 2014, 10, 952–956. [Google Scholar] [CrossRef]
- Liu, J.; Schotman, M.J.G.; Hendrix, M.M.R.M.; Lou, X.; Marín San Román, P.P.; Voets, I.K.; Sijbesma, R.P. Effects of Structural Variation on the Self-Assembly of Bis-Urea Based Bolaamphiphiles. J. Polym. Sci. 2021, 59, 1162–1170. [Google Scholar] [CrossRef]
- Koenigs, M.M.E.; Pal, A.; Mortazavi, H.; Pawar, G.M.; Storm, C.; Sijbesma, R.P. Tuning Cross-Link Density in a Physical Hydrogel by Supramolecular Self-Sorting. Macromolecules 2014, 47, 2712–2717. [Google Scholar] [CrossRef]
- Fernandez-Castano Romera, M.; Lafleur, R.P.M.; Guibert, C.; Voets, I.K.; Storm, C.; Sijbesma, R.P. Strain Stiffening Hydrogels through Self-Assembly and Covalent Fixation of Semi-Flexible Fibers. Angew. Chem. Int. Ed. 2017, 56, 8771–8775. [Google Scholar] [CrossRef]
- Fernández-Castaño Romera, M.; Lou, X.; Schill, J.; ter Huurne, G.; Fransen, P.-P.K.H.; Voets, I.K.; Storm, C.; Sijbesma, R.P. Strain-Stiffening in Dynamic Supramolecular Fiber Networks. J. Am. Chem. Soc. 2018, 140, 17547–17555. [Google Scholar] [CrossRef]
- Rosales, A.M.; Anseth, K.S. The Design of Reversible Hydrogels to Capture Extracellular Matrix Dynamics. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels–Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef]
- Kalayci, K.; Frisch, H.; Truong, V.X.; Barner-Kowollik, C. Green Light Triggered [2 + 2] Cycloaddition of Halochromic Styrylquinoxaline—Controlling Photoreactivity by PH. Nat. Commun. 2020, 11, 4193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, Q.; Han, Y.; Tan, Y. Boronic Ester-Based Self-Healing Hydrogels Formed by Using Intermolecular B-N Coordination. Polymer 2020, 202, 122624. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Grijpma, D.W. Photo-Crosslinked Synthetic Biodegradable Polymer Networks for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Kabb, C.P.; O’Bryan, C.S.; Deng, C.C.; Angelini, T.E.; Sumerlin, B.S. Photoreversible Covalent Hydrogels for Soft-Matter Additive Manufacturing. ACS Appl. Mater. Interfaces 2018, 10, 16793–16801. [Google Scholar] [CrossRef]
- Chung, J.W.; Lee, K.; Neikirk, C.; Nelson, C.M.; Priestley, R.D. Photoresponsive Coumarin-Stabilized Polymeric Nanoparticles as a Detectable Drug Carrier. Small 2012, 8, 1693–1700. [Google Scholar] [CrossRef]
- Cazin, I.; Rossegger, E.; Guedes de la Cruz, G.; Griesser, T.; Schlögl, S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers 2021, 13, 56. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in Polymers: From Light Harvesting to Photo-Cross-Linkable Tissue Scaffolds. Chem. Rev. 2004, 104, 3059–3078. [Google Scholar] [CrossRef]
- Pradhan, R.; Malhotra, P.; Gupta, G.; Singhal, R.; Sharma, G.D.; Mishra, A. Efficient Fullerene-Free Organic Solar Cells Using a Coumarin-Based Wide-Band-Gap Donor Material. ACS Appl. Mater. Interfaces 2020, 12, 41869–41876. [Google Scholar] [CrossRef]
- Beninatto, R.; Barbera, C.; De Lucchi, O.; Borsato, G.; Serena, E.; Guarise, C.; Pavan, M.; Luni, C.; Martewicz, S.; Galesso, D.; et al. Photocrosslinked Hydrogels from Coumarin Derivatives of Hyaluronic Acid for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 96, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wang, H.; Jie, K.; Huang, F. Taco Complex-Templated Highly Regio- and Stereo-Selective Photodimerization of a Coumarin-Containing Crown Ether. Chem. Commun. 2017, 53, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, N.; Inacker, S.; Hampp, N. Cycloreversion Performance of Coumarin and Hetero-Coumarin Dimers under Aerobic Conditions: Unexpected Behavior Triggered by UV-A Light. Phys. Chem. Chem. Phys. 2021, 23, 17703–17712. [Google Scholar] [CrossRef]
- Pal, A.; Karthikeyan, S.; Sijbesma, R.P. Coexisting Hydrophobic Compartments through Self-Sorting in Rod-like Micelles of Bisurea Bolaamphiphiles. J. Am. Chem. Soc. 2010, 132, 7842–7843. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castaño Romera, M.; Göstl, R.; Shaikh, H.; ter Huurne, G.; Schill, J.; Voets, I.K.; Storm, C.; Sijbesma, R.P. Mimicking Active Biopolymer Networks with a Synthetic Hydrogel. J. Am. Chem. Soc. 2019, 141, 1989–1997. [Google Scholar] [CrossRef]
- Fu, Q.; Cheng, L.; Zhang, Y.; Shi, W. Preparation and Reversible Photo-Crosslinking/Photo-Cleavage Behavior of 4-Methylcoumarin Functionalized Hyperbranched Polyester. Polymer 2008, 49, 4981–4988. [Google Scholar] [CrossRef]
- Korchia, L.; Bouilhac, C.; Aubert, A.; Robin, J.-J.; Lapinte, V. Light-Switchable Nanoparticles Based on Amphiphilic Diblock, Triblock and Heterograft Polyoxazoline. RSC Adv. 2017, 7, 42690–42698. [Google Scholar] [CrossRef]
- Azagarsamy, M.A.; McKinnon, D.D.; Alge, D.L.; Anseth, K.S. Coumarin-Based Photodegradable Hydrogel: Design, Synthesis, Gelation, and Degradation Kinetics. ACS Macro Lett. 2014, 3, 515–519. [Google Scholar] [CrossRef]
- Genovese, S.; Taddeo, V.A.; Epifano, F.; Fiorito, S.; Bize, C.; Rives, A.; de Medina, P. Characterization of the Degradation Profile of Umbelliprenin, a Bioactive Prenylated Coumarin of a Ferulago Species. J. Nat. Prod. 2017, 80, 2424–2431. [Google Scholar] [CrossRef]
- Anastasio, R. UV-Cured Polymer Networks: From Processing to Properties. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2019. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lou, X.; Schotman, M.J.G.; Marín San Román, P.P.; Sijbesma, R.P. Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels. Gels 2022, 8, 615. https://doi.org/10.3390/gels8100615
Liu J, Lou X, Schotman MJG, Marín San Román PP, Sijbesma RP. Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels. Gels. 2022; 8(10):615. https://doi.org/10.3390/gels8100615
Chicago/Turabian StyleLiu, Jie, Xianwen Lou, Maaike J. G. Schotman, Patricia P. Marín San Román, and Rint P. Sijbesma. 2022. "Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels" Gels 8, no. 10: 615. https://doi.org/10.3390/gels8100615
APA StyleLiu, J., Lou, X., Schotman, M. J. G., Marín San Román, P. P., & Sijbesma, R. P. (2022). Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels. Gels, 8(10), 615. https://doi.org/10.3390/gels8100615





