Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms
Abstract
1. Introduction
2. Results and Discussion
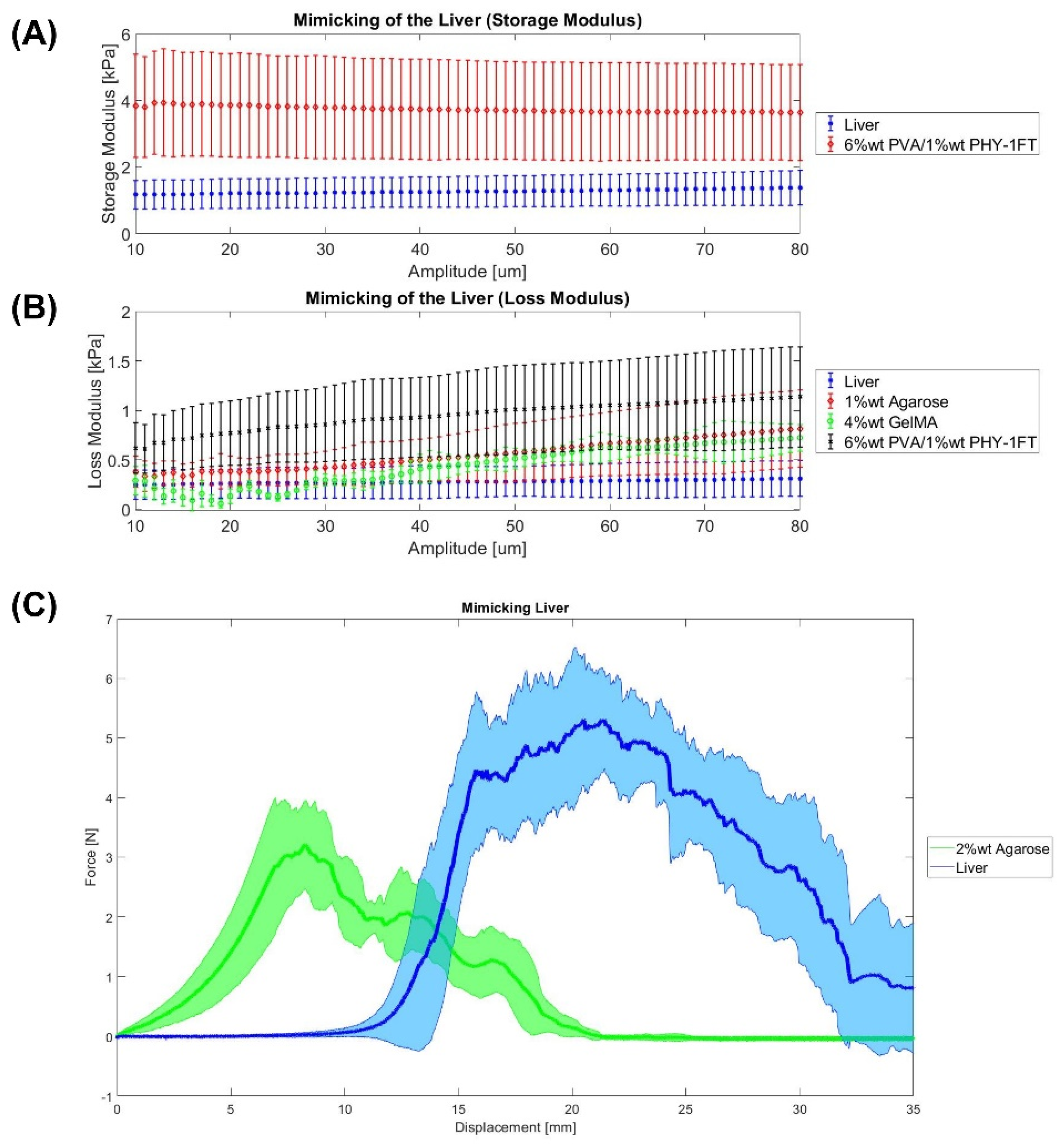
2.1. Liver
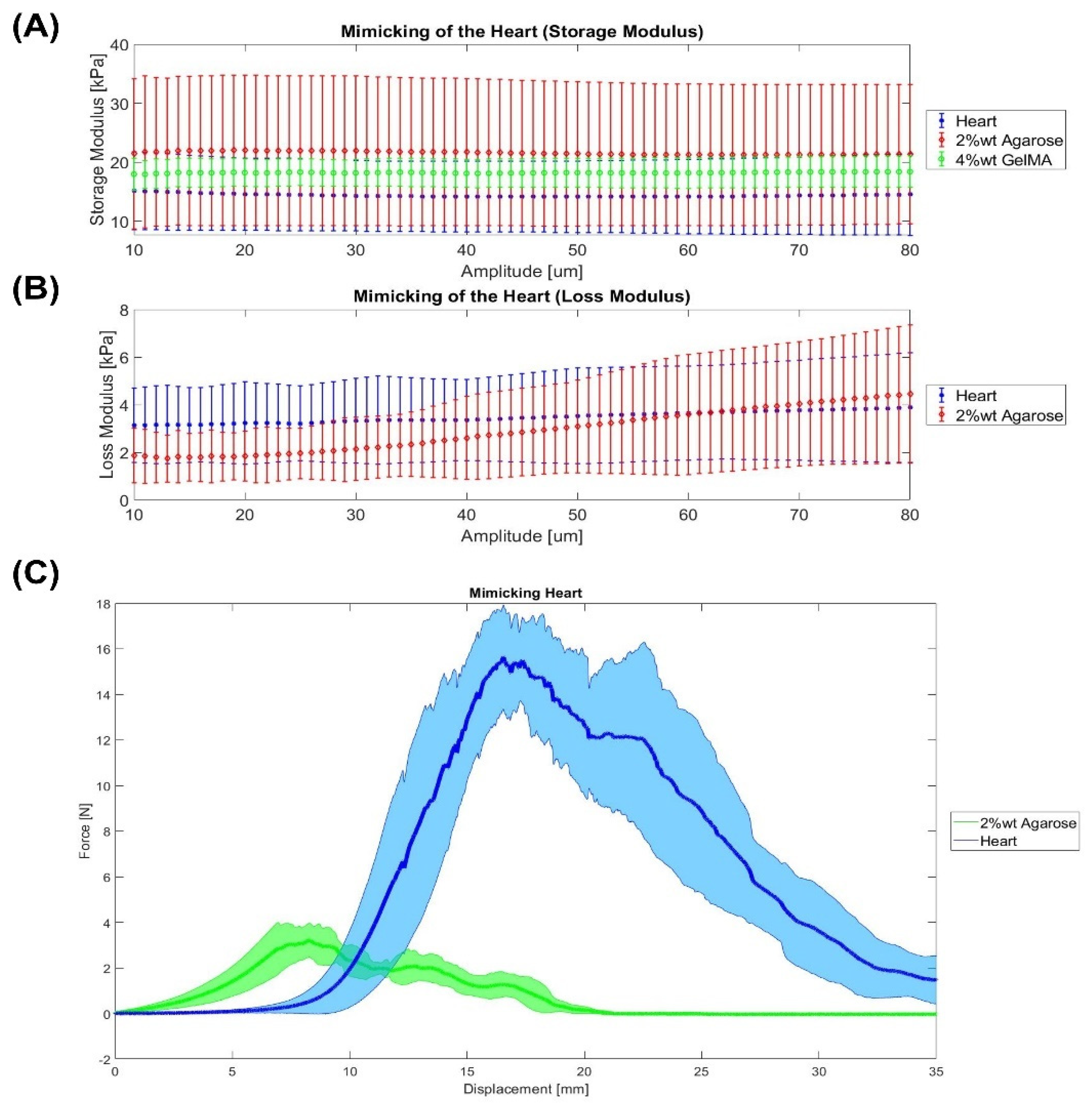
2.2. Heart
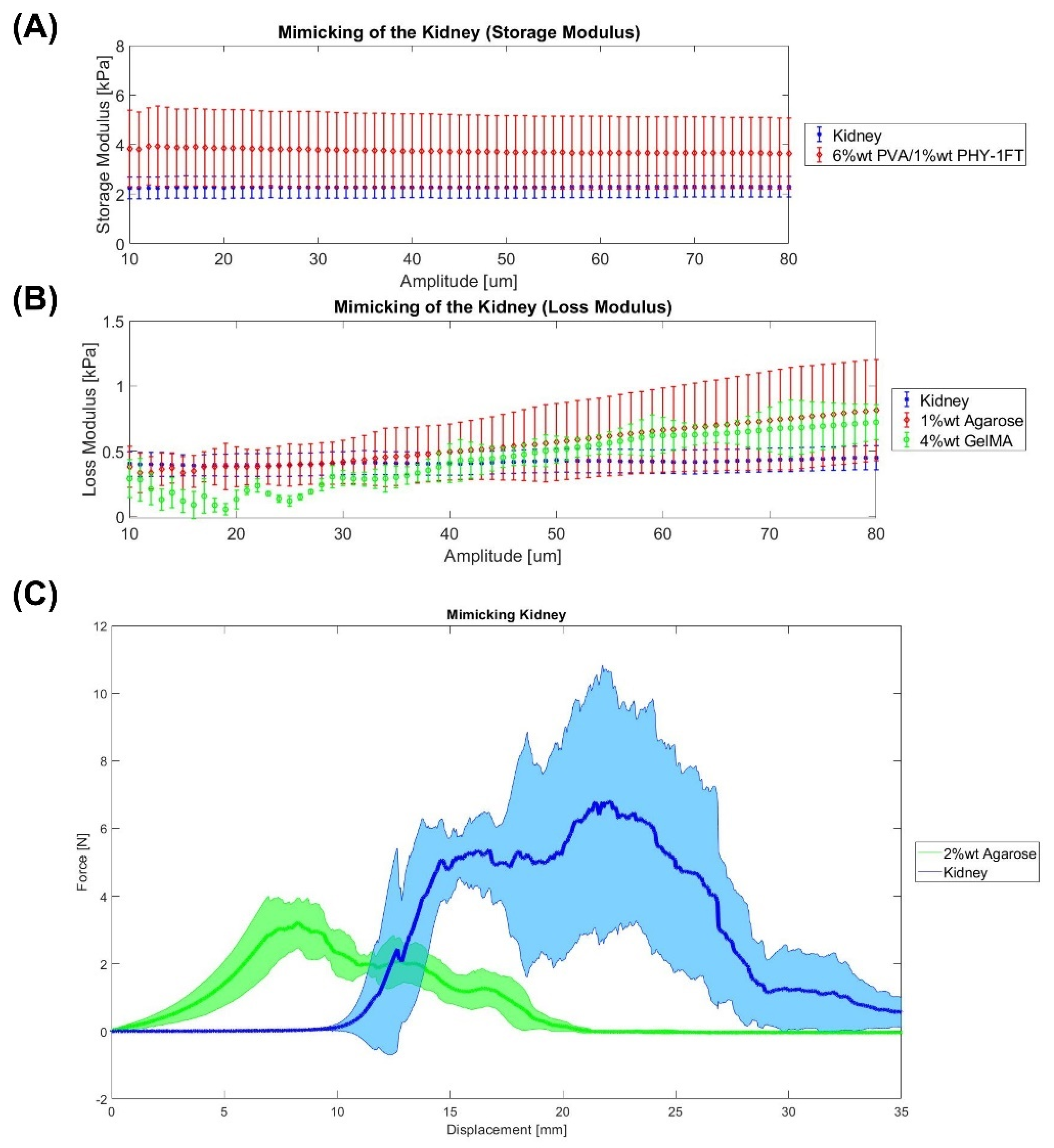
2.3. Kidney
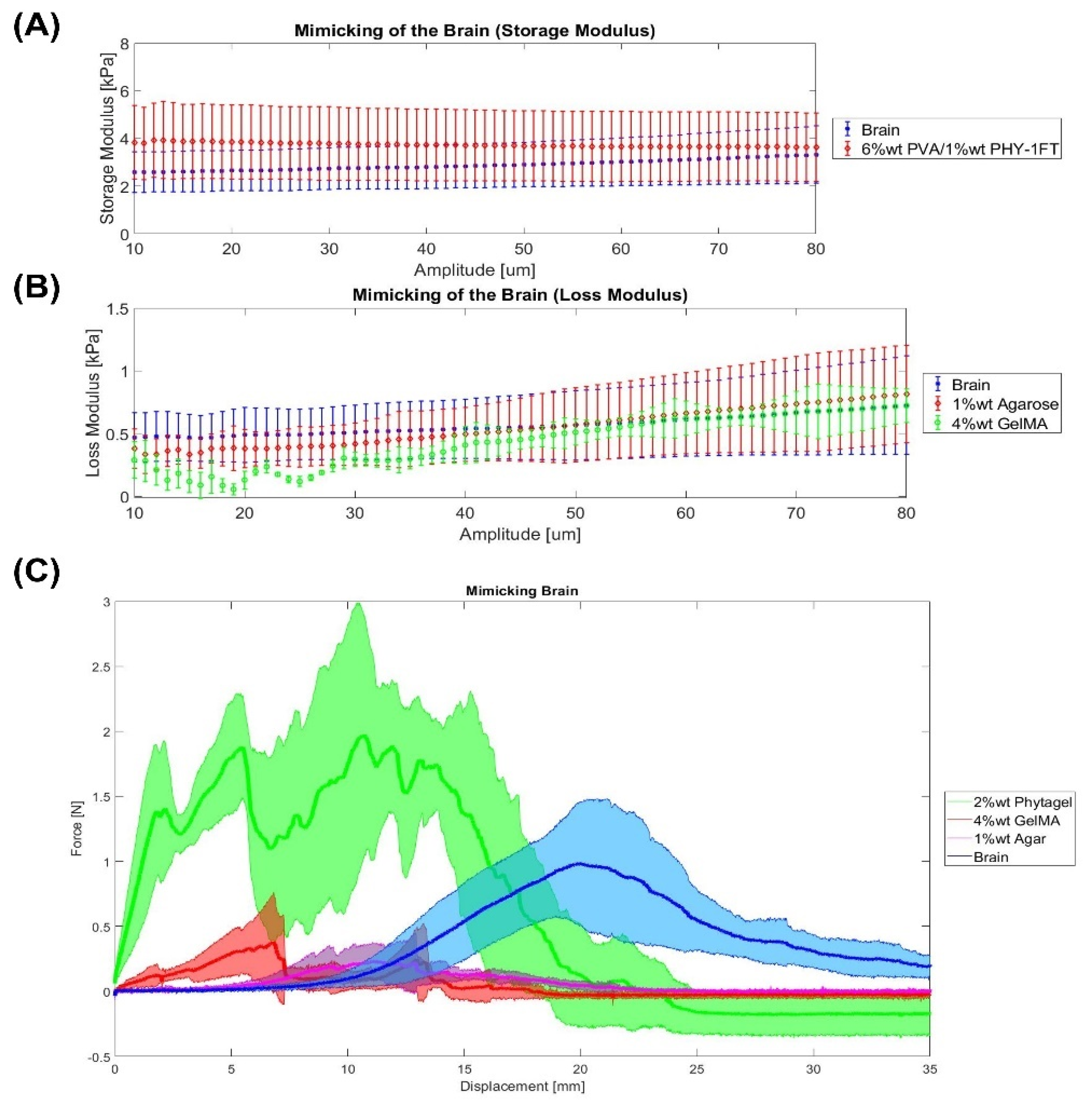
2.4. Brain
2.5. Qualitative Summary
3. Conclusions
4. Materials and Methods
4.1. Biological Tissue Sample Preparation
4.2. Hydrogels Sample Preparation
4.2.1. Agarose Gels
4.2.2. GelMA
4.2.3. PHY Gels
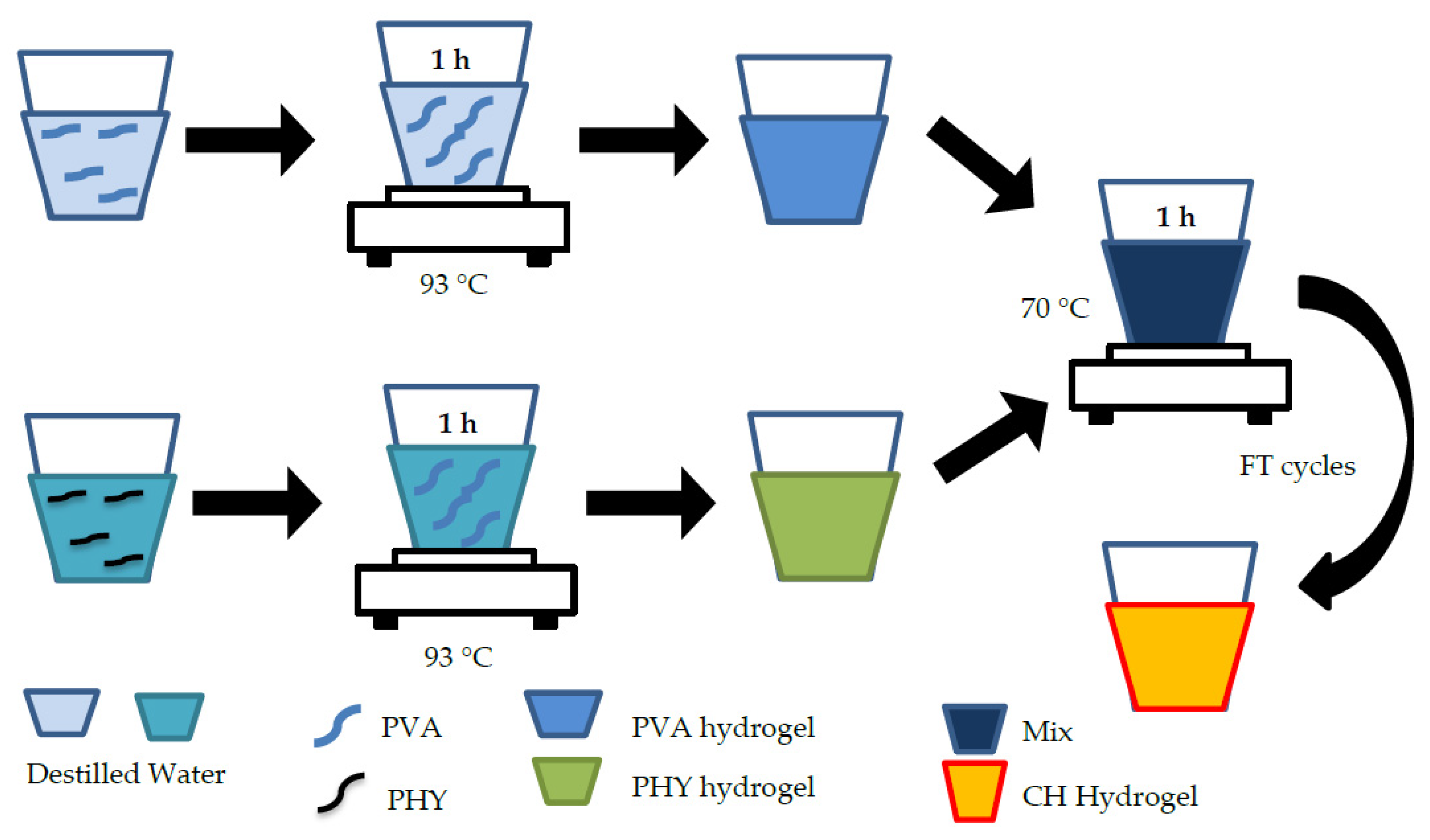
4.2.4. PVA/PHY Composite Hydrogel (CH)
4.3. Parameters for the Mimicking of Soft Tissues
4.3.1. Dynamic Mechanical Analysis (DMA)
4.3.2. Shore Hardness Test
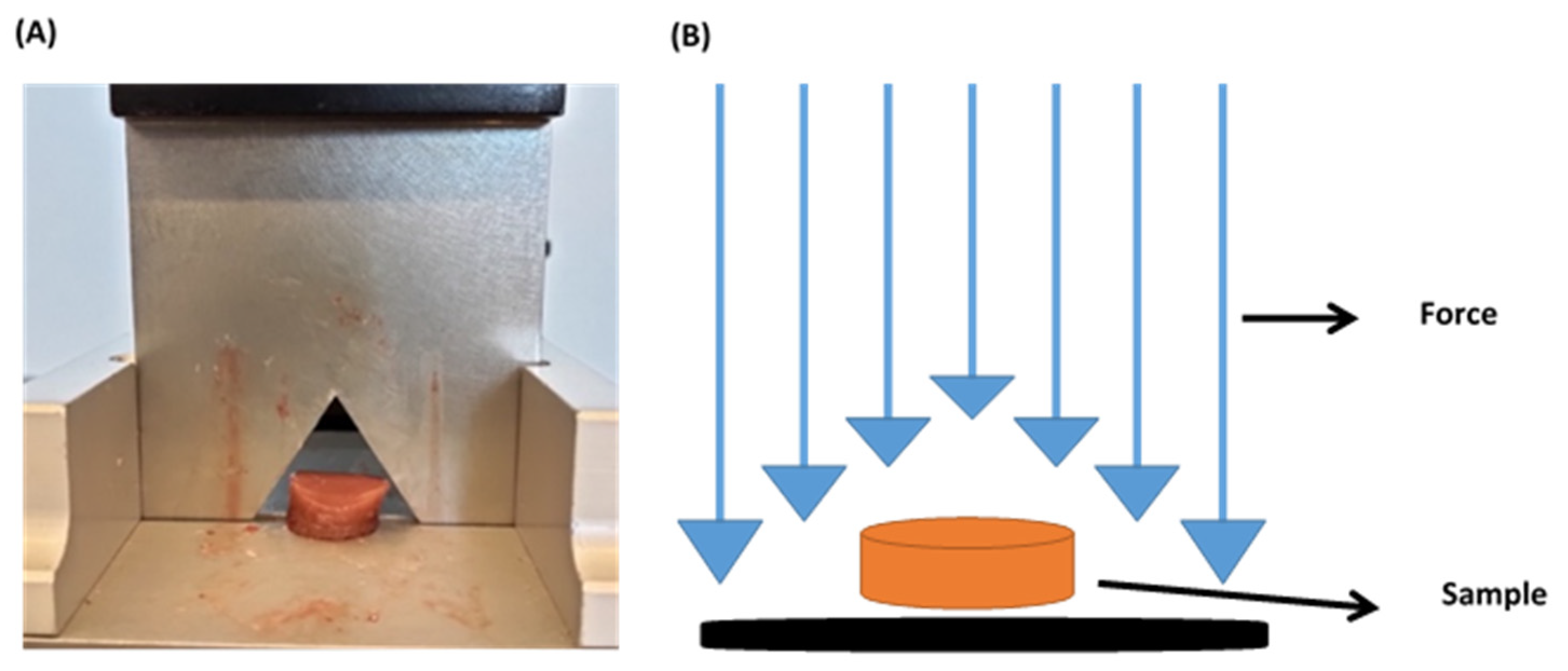
4.3.3. Warner–Bratzler Shear Test
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derkus, B.; Okesola, B.O.; Barrett, D.W.; D’Este, M.; Chowdhury, T.T.; Eglin, D.; Mata, A. Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater. 2020, 109, 82–94. [Google Scholar] [CrossRef]
- Gupta, S.; Teotia, A.K.; Qayoom, I.; Shiekh, P.A.; Andrabi, S.M.; Kumar, A. Periosteum-Mimicking Tissue-Engineered Composite for Treating Periosteum Damage in Critical-Sized Bone Defects. Biomacromolecules 2021, 22, 3237–3250. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 338, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Lustig-Gainza, P.; Fenollosa-Artés, F.; Valls, A.; Krauel, L.; Buj-Corral, I. 3D printed soft surgical planning prototype for a biliary tract rhabdomyosarcoma. J. Mech. Behav. Biomed. Mater. 2020, 109, 103844. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Dini, D.; Rodriguez y Baena, F.; Forte, A.E. Composite hydrogel: A high fidelity soft tissue mimic for surgery. Mater. Des. 2018, 160, 886–894. [Google Scholar] [CrossRef]
- Chikwe, J.; de Souza, A.C.; Pepper, J.R. No time to train the surgeons. BMJ 2004, 328, 418–419. [Google Scholar] [CrossRef]
- Sekhar, A.; Sun, M.R.; Siewert, B. A tissue phantom model for training residents in ultrasound-guided liver biopsy. Acad. Radiol. 2014, 21, 902–908. [Google Scholar] [CrossRef]
- Adams, F.; Qiu, T.; Mark, A.; Fritz, B.; Kramer, L.; Schlager, D.; Wetterauer, U.; Miernik, A.; Fischer, P. Soft 3D-Printed Phantom of the Human Kidney with Collecting System. Ann. Biomed. Eng. 2017, 45, 963–972. [Google Scholar] [CrossRef]
- de Jong, T.L.; Pluymen, L.H.; van Gerwen, D.J.; Kleinrensink, G.J.; Dankelman, J.; van den Dobbelsteen, J.J. PVA matches human liver in needle-tissue interaction. J. Mech. Behav. Biomed. Mater. 2017, 69, 223–228. [Google Scholar] [CrossRef]
- van Oosten, A.S.G.; Chen, X.; Chin, L.K.; Cruz, K.; Patteson, A.E.; Pogoda, K.; Shenoy, V.B.; Janmey, P.A. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 2019, 573, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Falland-Cheung, L.; Scholze, M.; Hammer, N.; Waddell, J.N.; Tong, D.C.; Brunton, P.A. Elastic behavior of brain simulants in comparison to porcine brain at different loading velocities. J. Mech. Behav. Biomed. Mater. 2018, 77, 609–615. [Google Scholar] [CrossRef]
- Oflaz, H.; Baran, O. A new medical device to measure a stiffness of soft materials. Acta Bioeng. Biomech. 2014, 16, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Meisel, N.A.; Dillard, D.A.; Williams, C.B. Impact of material concentration and distribution on composite parts manufactured via multi-material jetting. Rapid Prototyp. J. 2018, 24, 872–879. [Google Scholar] [CrossRef]
- Bezek, L.B.; Cauchi, M.P.; De Vita, R.; Foerst, J.R.; Williams, C.B. 3D printing tissue-mimicking materials for realistic transseptal puncture models. J. Mech. Behav. Biomed. Mater. 2020, 110, 103971. [Google Scholar] [CrossRef]
- Mueller, J.; Courty, D.; Spielhofer, M.; Spolenak, R.; Shea, K. Mechanical Properties of Interfaces in Inkjet 3D Printed Single- and Multi-Material Parts. 3D Print. Addit. Manuf. 2017, 4, 193–199. [Google Scholar] [CrossRef]
- Leibinger, A.; Forte, A.E.; Tan, Z.; Oldfield, M.J.; Beyrau, F.; Dini, D.; Rodriguez y Baena, F. Soft Tissue Phantoms for Realistic Needle Insertion: A Comparative Study. Ann. Biomed. Eng. 2016, 44, 2442–2452. [Google Scholar] [CrossRef]
- Forte, A.E.; Galvan, S.; Manieri, F.; Rodriguez y Baena, F.; Dini, D. A composite hydrogel for brain tissue phantoms. Mater. Des. 2016, 112, 227–238. [Google Scholar] [CrossRef]
- Forte, A.E.; Galvan, S.; Dini, D. Models and tissue mimics for brain shift simulations. Biomech. Model. Mechanobiol. 2018, 17, 249–261. [Google Scholar] [CrossRef]
- Forte, A.E.; Gentleman, S.M.; Dini, D. On the characterization of the heterogeneous mechanical response of human brain tissue. Biomech. Model. Mechanobiol. 2017, 16, 907–920. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Estermann, S.J.; Pahr, D.H.; Reisinger, A. Quantifying tactile properties of liver tissue, silicone elastomers, and a 3D printed polymer for manufacturing realistic organ models. J. Mech. Behav. Biomed. Mater. 2020, 104, 103630. [Google Scholar] [CrossRef]
- Distler, T.; Schaller, E.; Steinmann, P.; Boccaccini, A.R.; Budday, S. Alginate-based hydrogels show the same complex mechanical behavior as brain tissue. J. Mech. Behav. Biomed. Mater. 2020, 111, 103979. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, A.K.; Adnan, A.S. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Hillgärtner, M.; Ganesan, K.; Milow, B.; Itskov, M.; Rege, A. Computational design of biopolymer aerogels and predictive modelling of their nanostructure and mechanical behavior. Sci. Rep. 2021, 11, 10198. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Ritchie, A.C. Biological and mechanical evaluation of mineralized-hydrogel scaffolds for tissue engineering applications. J. Biomater. Appl. 2021, 36, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Chatelin, S.; Constantinesco, A.; Willinger, R. Fifty years of brain tissue mechanical testing: From in vitro to in vivo investigations. Biorheology 2010, 47, 255–276. [Google Scholar] [CrossRef]
- Estermann, S.J.; Pahr, D.H.; Reisinger, A. Hyperelastic and viscoelastic characterization of hepatic tissue under uniaxial tension in time and frequency domain. J. Mech. Behav. Biomed. Mater. 2020, 112, 104038. [Google Scholar] [CrossRef]
- Mattei, G.; Tirella, A.; Gallone, G.; Ahluwalia, A. Viscoelastic characterisation of pig liver in unconfined compression. J. Biomech. 2014, 47, 2641–2646. [Google Scholar] [CrossRef]
- Kiss, M.Z.; Varghese, T.; Hall, T.J. Viscoelastic characterization of in vitro canine tissue. Phys. Med. Biol. 2004, 49, 4207–4218. [Google Scholar] [CrossRef]
- Chatelin, S.; Oudry, J.; Périchon, N.; Sandrin, L.; Allemann, P.; Soler, L.; Willinger, R. In vivo liver tissue mechanical properties by transient elastography: Comparison with dynamic mechanical analysis. Biorheology 2011, 48, 75–88. [Google Scholar] [CrossRef]
- Barnes, S.C. Viscoelastic Properties of the Bladder and Design of a Surgical Instrument for the Removal of Bladder Tumours. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2016; p. 164. [Google Scholar]
- Amador Carrascal, C. Measurement of Kidney Viscoelasticity with Shearwave Dispersion Ultrasound Vibrometry. Ph.D. Thesis, College of Medicine-Mayo Clinic, Rochester, MN, USA, 2011; p. 167. [Google Scholar]
- Ramadan, S.; Paul, N.; Naguib, H.E. Standardized static and dynamic evaluation of myocardial tissue properties. Biomed. Mater. 2017, 12, 25013. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Lee, J.S.; Park, S.U.; Kwon, J.H.; Hong, T.H.; Kim, D.G. Quantitative assessment of liver fibrosis using shore durometer. Ann. Surg. Treat. Res. 2017, 93, 300. [Google Scholar] [CrossRef][Green Version]
- Foitzik, T.; Gock, M.; Schramm, C.; Prall, F.; Klar, E. Octreotide hardens the pancreas. Langenbeck Arch. Surg. 2006, 391, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Riedle, H.; Molz, P.; Franke, J. Determination of the mechanical properties of cardiac tissue for 3D printed surgical models. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; pp. 171–176. [Google Scholar] [CrossRef]
- Arani, A.; Lanzino, G.; Ehman, R. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery 2015, 77, 653–659. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Lu, R.; Solomon, M.B.; Berry, B.W. Tensile properties and warner-bratzler tenderness measurement of raw and cooked beef. Trans. ASAE 1998, 41, 1431–1439. [Google Scholar] [CrossRef]
- Yoo, S.J.; Spray, T.; Austin, E.H.; Yun, T.J.; van Arsdell, G.S. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J. Thorac. Cardiovasc. Surg. 2017, 153, 1530–1540. [Google Scholar] [CrossRef]
- Riedle, H.; Ghazy, A.; Seufert, A.; Seitz, V.; Dorweiler, B.; Franke, J. Generic design of an anatomical heart model optimized for additive manufacturing with silicone. Rapid Prototyp. J. 2020, 27, 217–222. [Google Scholar] [CrossRef]
- Gustavsson, P.-E. Per-OlofLars Son Journal of Chromatography Library. In Journal of Chromatography Library; Elsevier: Amsterdam, The Netherlands, 1993; Volume 55, pp. 797–800. [Google Scholar]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Khademhosseini, A. 2010 Biomaterials Ali. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2011, 31, 5536–5544. [Google Scholar] [CrossRef]
- Phyto Technology Laboratories ® Product Information Sheet Coconut Powder Phyto Technology Laboratories®. Available online: https://phytotechlab.com/mwdownloads/download/link/id/59/ (accessed on 25 November 2021).
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- ASTM D2240-15e1: Standard Test Method for Rubber Property—Durometer Hardness. Available online: https://www.astm.org/d2240-15e01.html (accessed on 25 November 2021).
| SH | E′ | E″ | WB | |
|---|---|---|---|---|
| 1% wt agarose | 0.044 | X | 0.069 | X |
| 2% wt agarose | X | X | X | X |
| 4% wt GelMA | X | X | 0.01 | X |
| 2% wt PHY | X | X | X | X |
| 6% wt PVA/1% wt PHY-1FT | X | 0.016 | 0.008 | X |
| 6% wt PVA/1% wt PHY-2FT | X | X | X | X |
| SH | E′ | E″ | WB | |
|---|---|---|---|---|
| 1% wt agarose | 0.42 | X | X | X |
| 2% wt agarose | X | 0.28 | 0.98 | X |
| 4% wt GelMA | X | 0.18 | X | X |
| 2% wt PHY | X | X | X | X |
| 6% wt PVA/1% wt PHY-1FT | X | X | X | X |
| 6% wt PVA/1% wt PHY-2FT | X | X | X | X |
| SH | E′ | E″ | WB | |
|---|---|---|---|---|
| 1% wt agarose | X | X | 0.10 | X |
| 2% wt agarose | 0.59 | X | X | 0.02 |
| 4% wt GelMA | 0.42 | X | 0.03 | X |
| 2% wt PHY | X | X | X | X |
| 6% wt PVA/1% wt PHY-1FT | X | 0.02 | X | X |
| 6% wt PVA/1% wt PHY-2FT | X | X | X | X |
| SH | E′ | E″ | WB | |
|---|---|---|---|---|
| 1% wt agarose | X | X | 0.70 | 0.02 |
| 2% wt agarose | X | X | X | X |
| 4% wt GelMA | X | X | 0.61 | 0.12 |
| 2% wt PHY | X | X | X | 3.89 × 10−3 |
| 6% wt PVA/1% wt PHY-1FT | X | 0.34 | X | X |
| 6% wt PVA/1% wt PHY-2FT | X | X | X | X |
| Liver | Heart | Kidney | Brain | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | E′ | E″ | WB | SH | E′ | E″ | WB | SH | E′ | E″ | WB | SH | E′ | E″ | WB | |
| 1% wt Agar | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||
| 2% wt Agar | ✔ | ✔ | ✔ | |||||||||||||
| 4% wt GelMA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||
| 2% wt PHY | ✔ | |||||||||||||||
| 6% wt PVA/1% wt PHY-1FT | ✔ | ✔ | ✔ | ✔ | ||||||||||||
| 6% wt PVA/1% wt PHY-1FT | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels 2022, 8, 40. https://doi.org/10.3390/gels8010040
Tejo-Otero A, Fenollosa-Artés F, Achaerandio I, Rey-Vinolas S, Buj-Corral I, Mateos-Timoneda MÁ, Engel E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels. 2022; 8(1):40. https://doi.org/10.3390/gels8010040
Chicago/Turabian StyleTejo-Otero, Aitor, Felip Fenollosa-Artés, Isabel Achaerandio, Sergi Rey-Vinolas, Irene Buj-Corral, Miguel Ángel Mateos-Timoneda, and Elisabeth Engel. 2022. "Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms" Gels 8, no. 1: 40. https://doi.org/10.3390/gels8010040
APA StyleTejo-Otero, A., Fenollosa-Artés, F., Achaerandio, I., Rey-Vinolas, S., Buj-Corral, I., Mateos-Timoneda, M. Á., & Engel, E. (2022). Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels, 8(1), 40. https://doi.org/10.3390/gels8010040








