Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Coatings by the Sol-Gel Method
2.2. Samples Characterization Techniques
3. Results and Discussion
3.1. Samples Preparation
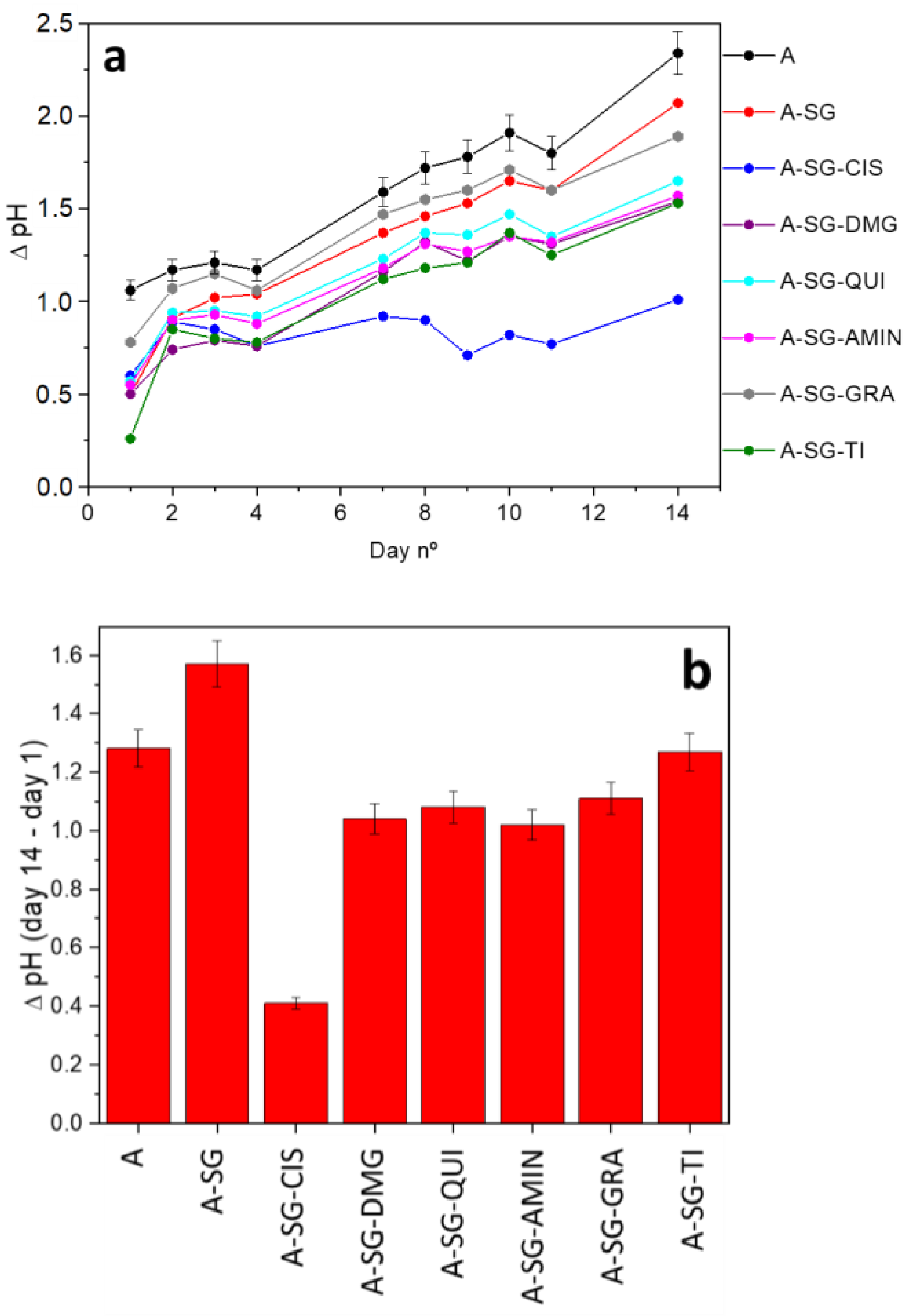
3.2. Weathering Test
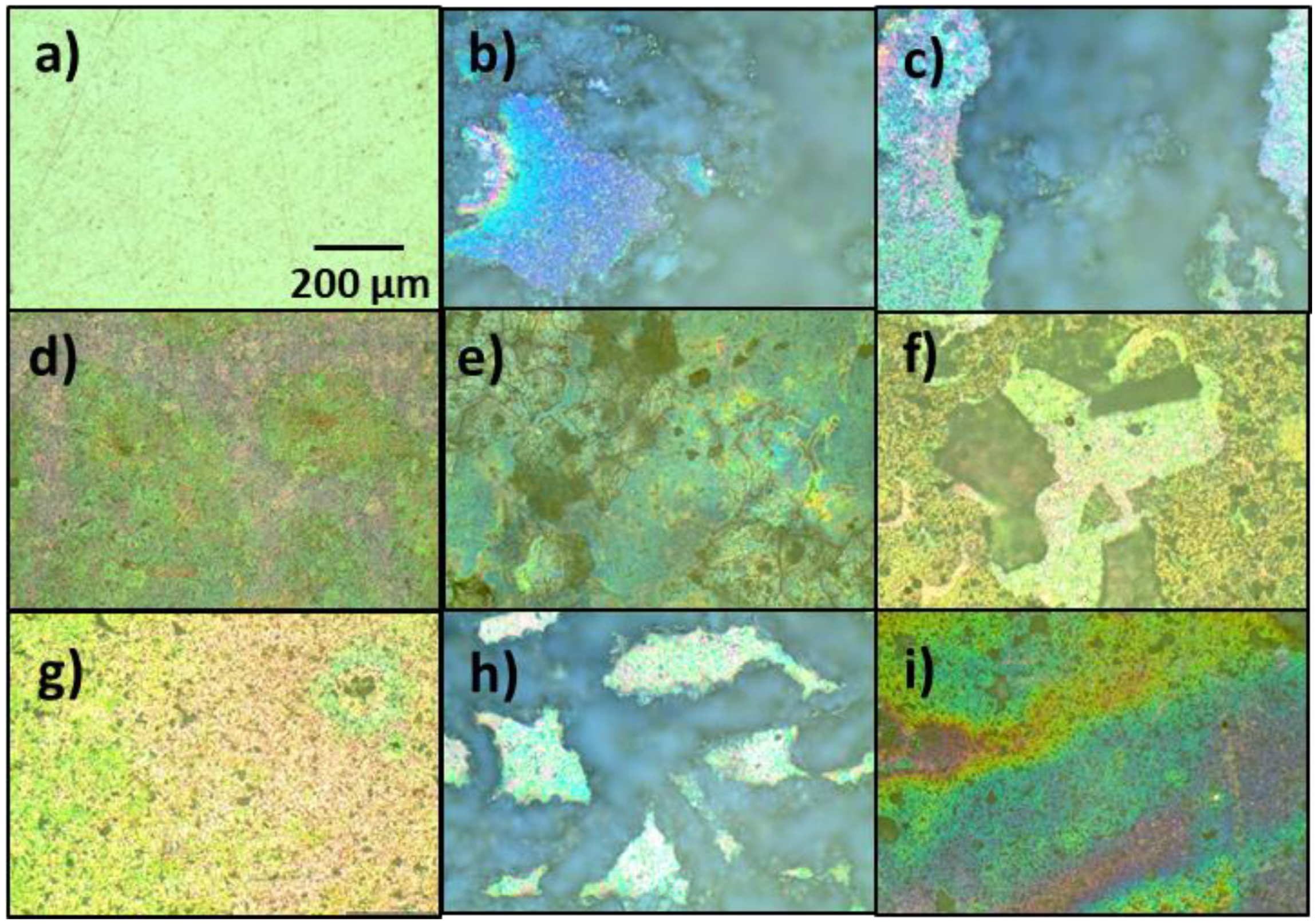
3.3. Optical Microscopy Characterization
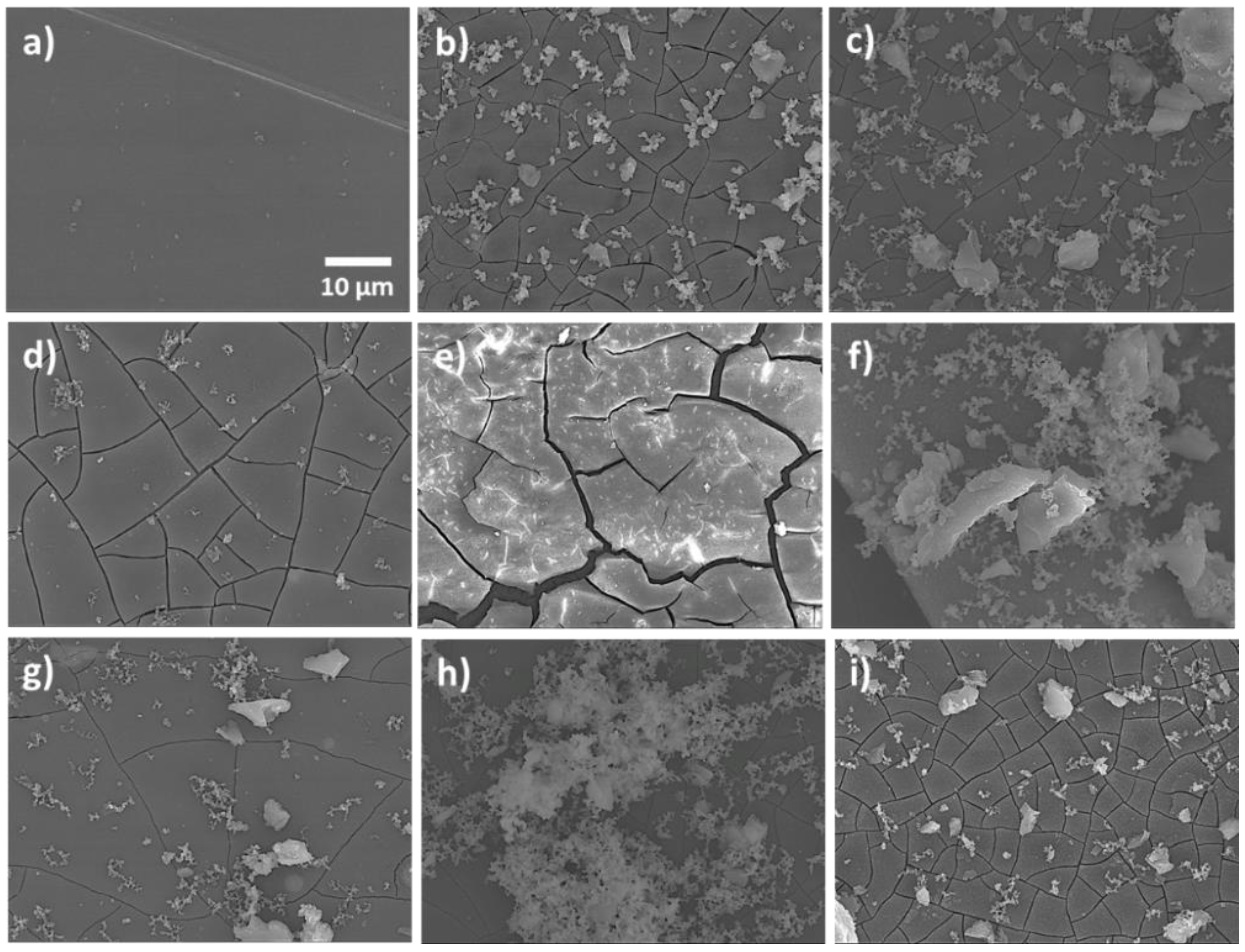
3.4. SEM Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, C.; Fones, A.; Hamilton, H.G.C.; Adkins, N.J.E.; Attallah, M. A new approach to develop palladium-modified Ti-based alloys for biomedical application. Mater. Des. 2016, 109, 98–111. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- El Hadad, A.A.; García-Galván, F.R.; Mezour, M.A.; Hickman, G.J.; Soliman, I.E.; Jiménez-Morales, A.; Barranco, V.; Galván, J.C.; Perry, C.C. Organic-inorganic hybrid coatings containing phosphorus precursors prepared by sol–gel on Ti6Al4V alloy: Electrochemical and in-vitro biocompatibility evaluation. Prog. Org. Coat. 2020, 148, 105834. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Wang, S.; Qiao, L.; Sun, D. In-situ XAFS and SERS study of self-healing of passive film on Ti in Hank’s physiological solution. Appl. Surf. Sci. 2019, 496, 143657. [Google Scholar] [CrossRef]
- García-Galván, F.R.; Fajardo, S.; Barranco, V.; Feliu, S., Jr. Experimental apparent stern–geary coefficients for AZ31B Mg alloy in physiological body fluids for accurate corrosion rate determination. Metals 2021, 11, 391. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Sumayli, A. Recent trends on bioimplant materials: A review. Mater. Today Proc. 2021, 46, 2726–2731. [Google Scholar] [CrossRef]
- Kumar, K.; Das, A.; Prasad, S.B. Recent developments in biodegradable magnesium matrix composites for orthopaedic applications: A review based on biodegradability, mechanical and biocompatibility perspective. Mater. Today Proc. 2021, 44, 2038–2042. [Google Scholar] [CrossRef]
- Feliu Jr, S.; Samaniego, A.; Barranco, V.; El-Hadad, A.A.; Llorente, I.; Serra, C.; Galván, J.C. A study on the relationships between corrosion properties and chemistry of thermally oxidised surface films formed on polished commercial magnesium alloys AZ31 and AZ61. Appl. Surf. Sci. 2014, 295, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Pacha-Olivenza, M.A.; Galván, J.C.; Porro, J.A.; Lieblich, M.; Díaz, M.; Angulo, I.; Cordovilla, F.; García-Galván, F.R.; Fernández-Calderón, M.C.; González-Martín, M.L.; et al. Efficacy of laser shock processing of biodegradable Mg and Mg-1Zn alloy on their in vitro corrosion and bacterial response. Surf. Coat. Technol. 2020, 384, 125320. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Rivero, P.J.; Redin, D.M.; Rodríguez, R.J. Electrospinning: A powerful tool to improve the corrosion resistance of metallic surfaces using nanofibrous coating. Metals 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Kokub, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Podbielska, H.; Ulatowska-Jarza, A. Sol-gel technology for biomedical engineering. Bull. Pol. Acad. Sci. Tech. Sci. 2005, 53, 261–271. [Google Scholar]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Carbonell, D.J.; García-Casas, A.; Izquierdo, J.; Souto, R.M.; Galván, J.G.; Jiménez-Morales, A. Scanning electrochemical microscopy characterization of sol-gel coatings applied on AA2024-T3 substrate for corrosion protection. Corros. Sci. 2016, 111, 625–636. [Google Scholar] [CrossRef]
- Abuín, M.; Serrano, A.; Llopis, J.; García, M.A.; Carmona, N. Silica doped with lanthanum sol-gel thin films for corrosion protection. Thin Solid Films 2012, 520, 5267–5271. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galván, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Hernández, L.; Veleva, L.; García-Galván, F.R.; Galván, J.C. Effect of ZrO2 and L-Cys nanoparticles as dopants in sol-gel of mesoporous silica coating for corrosion protection of AZ61 magnesium alloy. Rev. De Metal. 2019, 55, e155. [Google Scholar] [CrossRef]
- Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings 2017, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Twite, R.L.; Bierwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminium aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Claesson, P.M.; Sundell, P.-E.; Tyrode, E.; Pan, J. Active corrosion protection by conductive composites of polyaniline in a UV-cured polyester acrylate coating. Prog. Org. Coat. 2016, 90, 154–162. [Google Scholar] [CrossRef]
- Bazlia, L.; Yusuf, M.; Farahani, A.; Kiamarzi, M.; Seyedhosseini, Z.; Nezhadmansari, M.; Aliasghari, M.; Iranpoor, M. Application of composite conducting polymers for improving the corrosion behavior of various substrates: A Review. J. Compos. Compd. 2020, 2, 228–240. [Google Scholar] [CrossRef]
- Siva, T.; Ramadoss, A.; Sathiyanarayanan, S. Emerging Action of Corrosion Prevention Based on Sustained Self-healing Coatings. Surf. Interfaces 2021, 26, 101440. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Yoysefi, N. Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy. Prog. Org. Coat. 2019, 136, 105296. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef]
- Liu, J.; Lou, Y.; Zhang, C.; Yin, S.; Li, H.; Sun, D.; Sun, X. Improved corrosion resistance and antibacterial properties of composite arch-wires by N-doped TiO2 coating. RSC Adv. 2017, 7, 43938–43949. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Liu, J.; Li, Y.; Li, S.; Yu, M. Enhancing corrosion protection properties of sol-gel coating by pH-responsive amino-silane functionalized graphene oxide-mesoporous silica nanosheets. Prog. Org. Coat. 2019, 135, 228–239. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodríguez, R. Effect of Graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Matatagui, D.; López-Sánchez, J.; Peña, A.; Serrano, A.; del Campo, A.; de la Fuente, O.R.; Carmona, N.; Navarro, E.; Marín, P.; Horrillo, M.C. Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous Graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Palanisamy, G. Corrosion Inhibitors; Ambrish, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Tkacz, J.; Sloukova, K.; Minda, J.; Drabikova, J.; Fintova, S.; Dolezal, P.; Wasserbauer, J. Influence of the composition of the Hank’s Balanced Salt Solution on the corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef] [Green Version]
- ASTM Standard G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals (Reapproved 1990); Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2004; p. 302.
- García, M.A.; Paje, S.; Llopis, J.; Villegas, M.A. Influencia de las condiciones de preparación en la luminiscencia de recubrimientos de sílice pura. Bol. De La Soc. Española De Artic. Cerámica Y Vidr. 2000, 39, 641–646. [Google Scholar] [CrossRef]
- Samaniego, A.; Llorente, I.; Feliu, S. Combined effect of composition and surface condition on corrosion behaviour of magnesium alloys AZ31 and AZ61. Corros. Sci. 2013, 68, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadad, A.A.; Peón, E.; García-Galván, F.R.; Barranco, V.; Parra, J.; Jiménez-Morales, A.; Galván, J.C. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
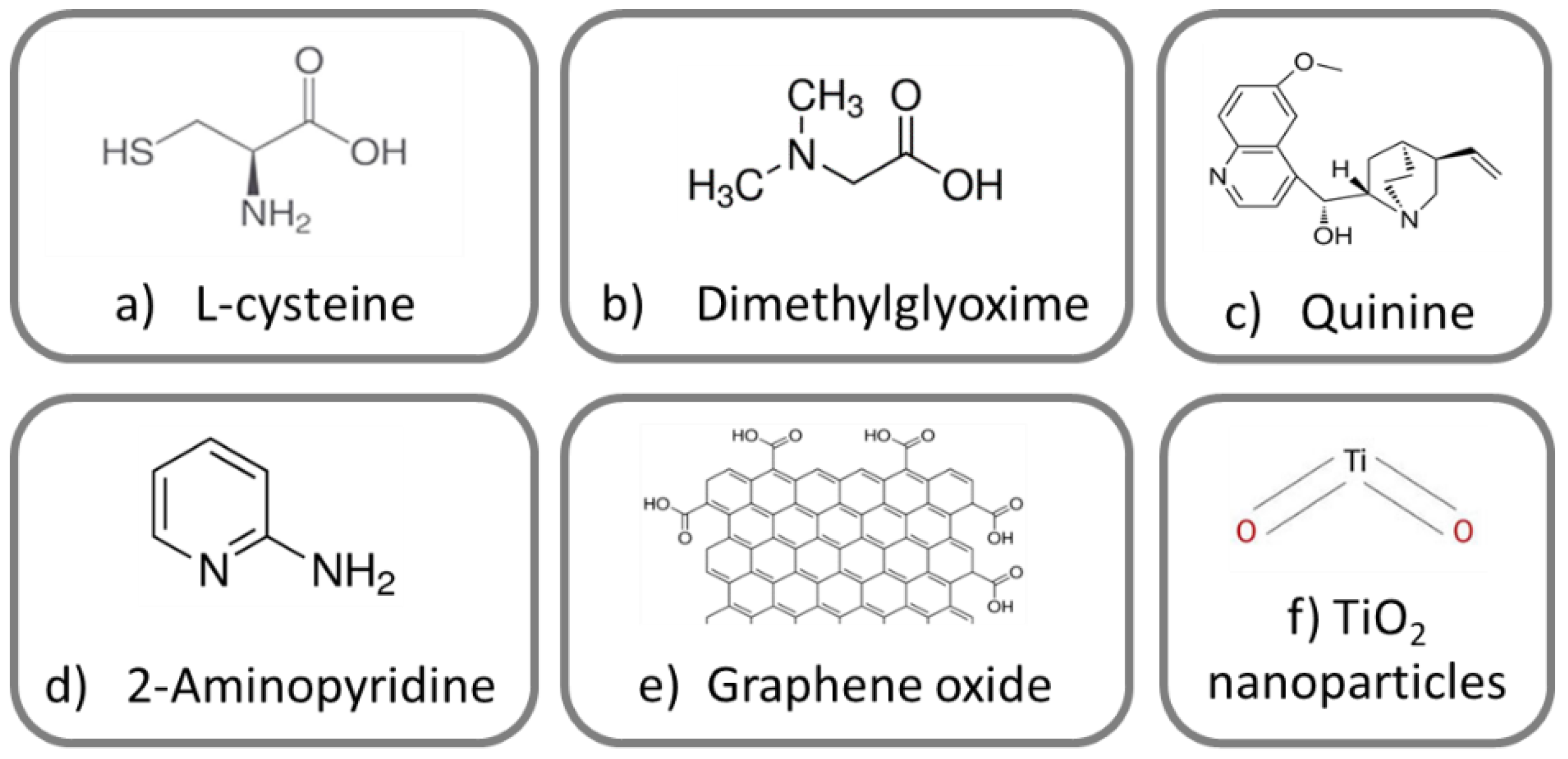

| Sample Name | Sol-Gel Coating at a TEOS:MEMO Ratio of 1:1 (v/v) | Corrosion Inhibitor Added | Corrosion Test Performed |
|---|---|---|---|
| 0 | No | No | No |
| A | No | No | Yes |
| A-SG | Yes | No | Yes |
| A-SG-CIS | Yes | L-cysteine | Yes |
| A-SG-DMG | Yes | Dimethyl glyoxime | Yes |
| A-SG-QUI | Yes | Quinine | Yes |
| A-SG-AMIN | Yes | 2-Aminopyridine | Yes |
| A-SG-GRA | Yes | Graphene oxide | Yes |
| A-SG-TI | Yes | TiO2 nanoparticles | Yes |
| Chemical Composition (wt %) | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | A | A-SG | A-SG-CIS | A-SG-DMG | A-SG-QUI | A-SG-AMIN | A-SG-GRA | A-SG-TI | |
| Mg | 91.6 ± 0.7 | 61.8 ± 1.0 | 51.6 ± 1.4 | 51.6 ± 0.4 | 3.8 ± 0.1 | 27.5 ± 0.4 | 62.9 ± 0.8 | 53.1 ± 0.7 | 50.6 ± 1.0 |
| Al | 5.0 ± 0.4 | 4.7 ± 0.4 | 3.9 ± 0.3 | 5.8 ± 0.1 | 0.3 ± 0.1 | 2.3 ± 0.1 | 4.8 ± 0.2 | 3.5 ± 0.1 | 4.1 ± 0.4 |
| Si | - | - | 0.5 ± 0.2 | 2.5 ± 0.1 | 22.1 ± 0.4 | 4.0 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | - |
| C | - | - | 7.6 ± 2.0 | 5.1 ± 0.6 | 32.3 ± 1.0 | 19.8 ± 1.0 | 5.8 ± 1.0 | 4.7 ± 0.9 | - |
| O | 3.4 ± 0.6 | 26.4 ± 1.0 | 28.7 ± 1.2 | 29.8 ± 0.3 | 40.7 ± 0.8 | 35.4 ± 0.7 | 21.3 ± 0.5 | 26.0 ± 0.5 | 29.9 ± 1.2 |
| Ca | - | 3.2 ± 0.2 | 4.0 ± 0.3 | 1.7 ± 0.1 | 0.8 ± 0.1 | 5.3 ± 0.1 | 1.9 ± 0.1 | 4.4 ± 0.1 | 7.5 ± 0.4 |
| Cl | - | - | - | - | - | - | 0.2 ± 0.1 | - | - |
| P | - | 3.9 ± 0.4 | 3.7 ± 0.4 | 2.9 ± 0.1 | - | 3.4 ± 0.1 | 2.2 ± 0.1 | 4.4 ± 0.1 | 7.9 ± 0.5 |
| Nb | - | - | - | - | - | 2.3 ± 0.4 | - | 2.8 ± 0.4 | - |
| Zn | - | - | - | 0.6 ± 0.1 | - | - | 0.7 ± 0.2 | 0.6 ± 0.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Alonso, L.; López-Sánchez, J.; Serrano, A.; Rodríguez de la Fuente, O.; Galván, J.C.; Carmona, N. Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels 2022, 8, 34. https://doi.org/10.3390/gels8010034
Rodríguez-Alonso L, López-Sánchez J, Serrano A, Rodríguez de la Fuente O, Galván JC, Carmona N. Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels. 2022; 8(1):34. https://doi.org/10.3390/gels8010034
Chicago/Turabian StyleRodríguez-Alonso, Luis, Jesús López-Sánchez, Aida Serrano, Oscar Rodríguez de la Fuente, Juan Carlos Galván, and Noemí Carmona. 2022. "Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy" Gels 8, no. 1: 34. https://doi.org/10.3390/gels8010034
APA StyleRodríguez-Alonso, L., López-Sánchez, J., Serrano, A., Rodríguez de la Fuente, O., Galván, J. C., & Carmona, N. (2022). Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels, 8(1), 34. https://doi.org/10.3390/gels8010034








