Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. BCP-Hydrogel Inks Composition and Formulation
2.2. Shear Viscosity Tests and Effect of Particle Loading
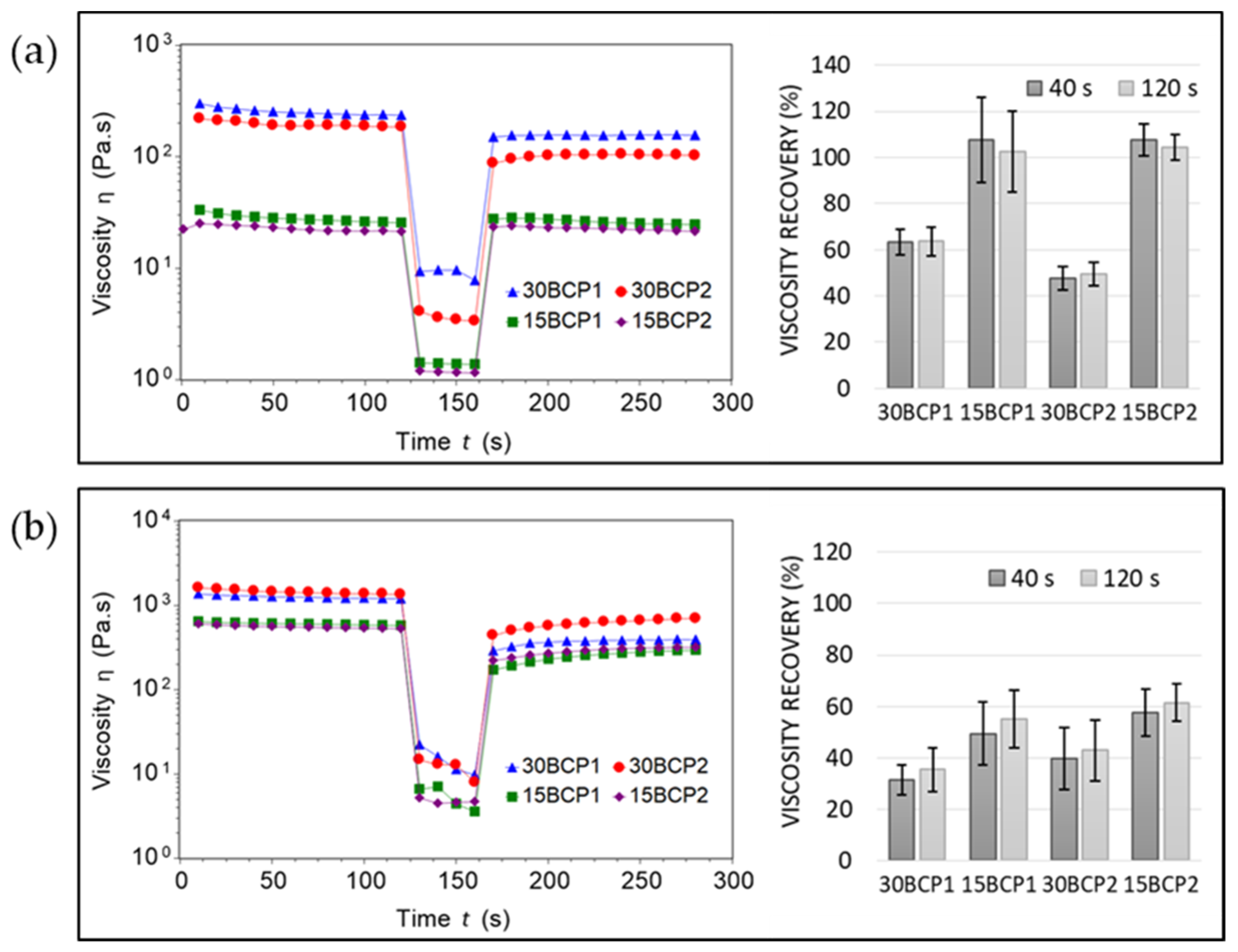
2.3. Thixotropy and Viscosity Recovery Analysis
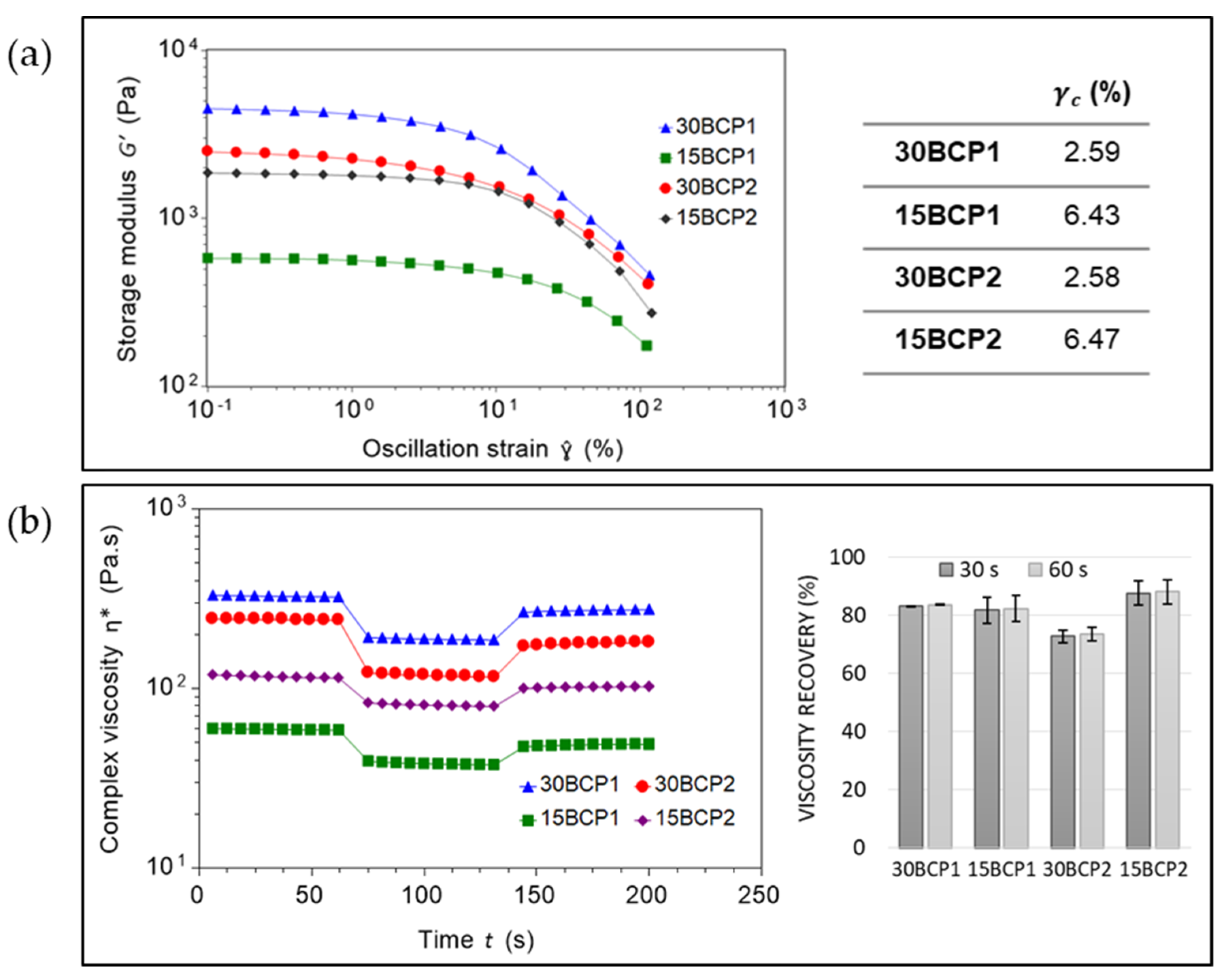
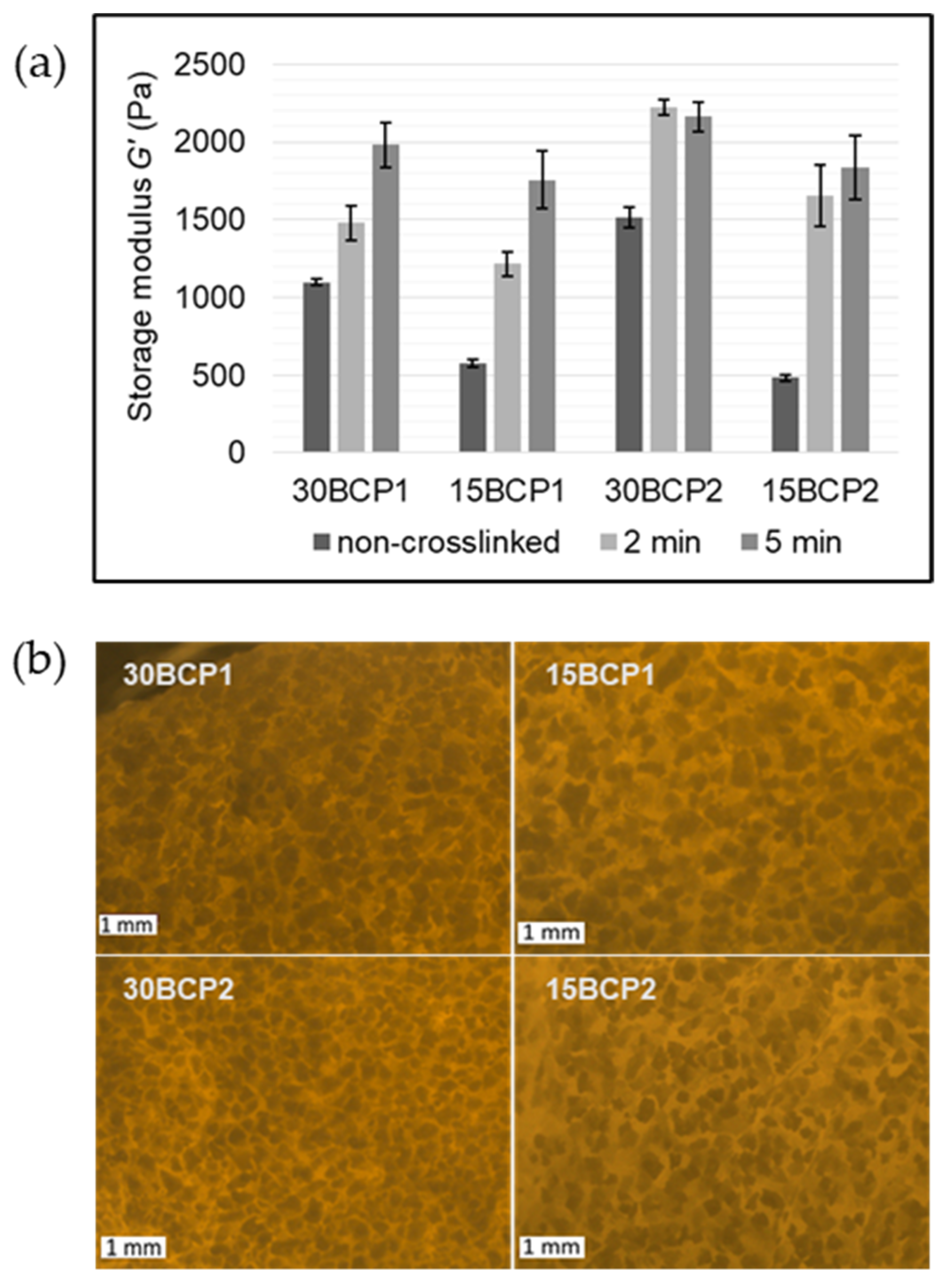
2.4. Evaluation of Ink Stiffness and Effect of CaCl2 Crosslinking
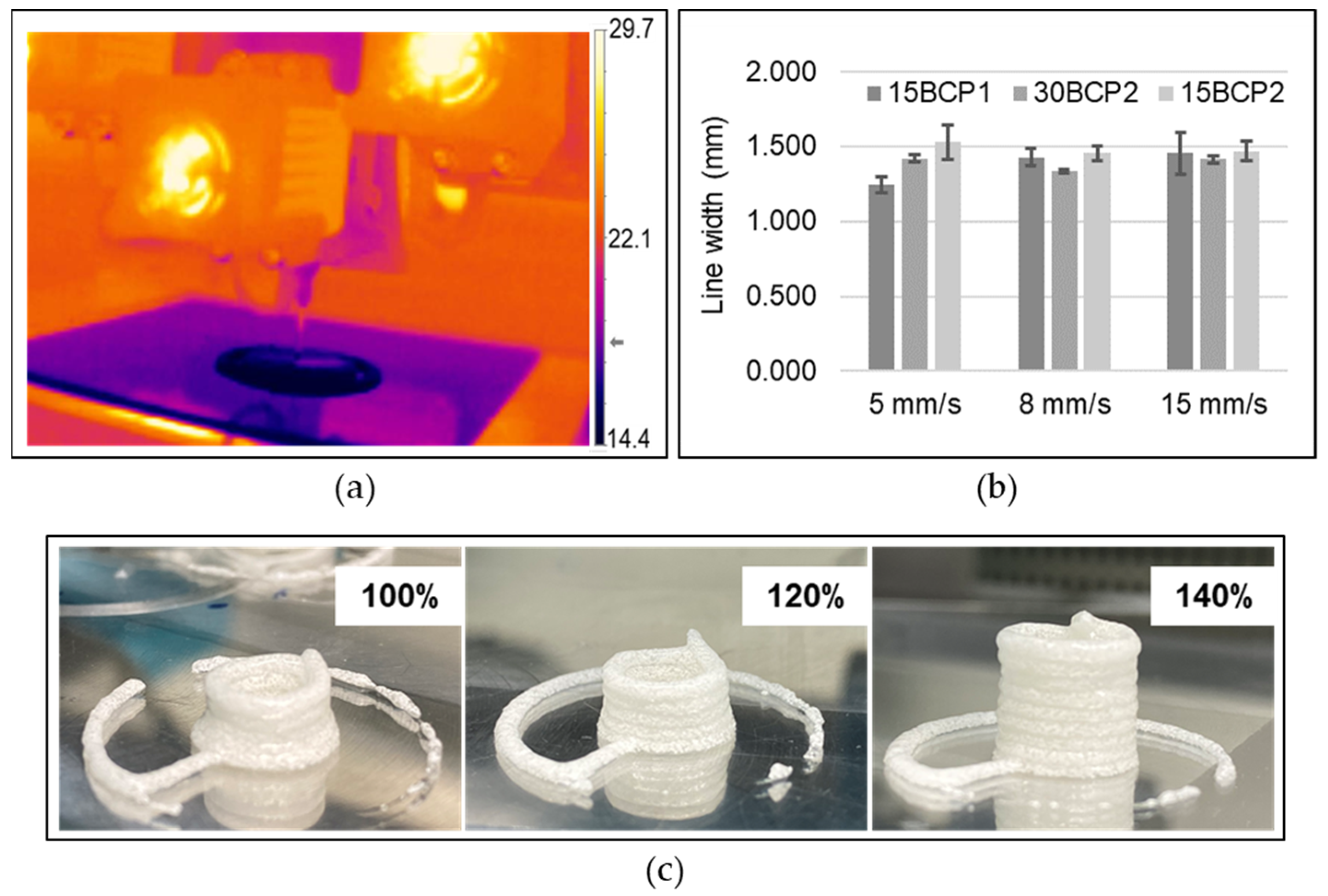
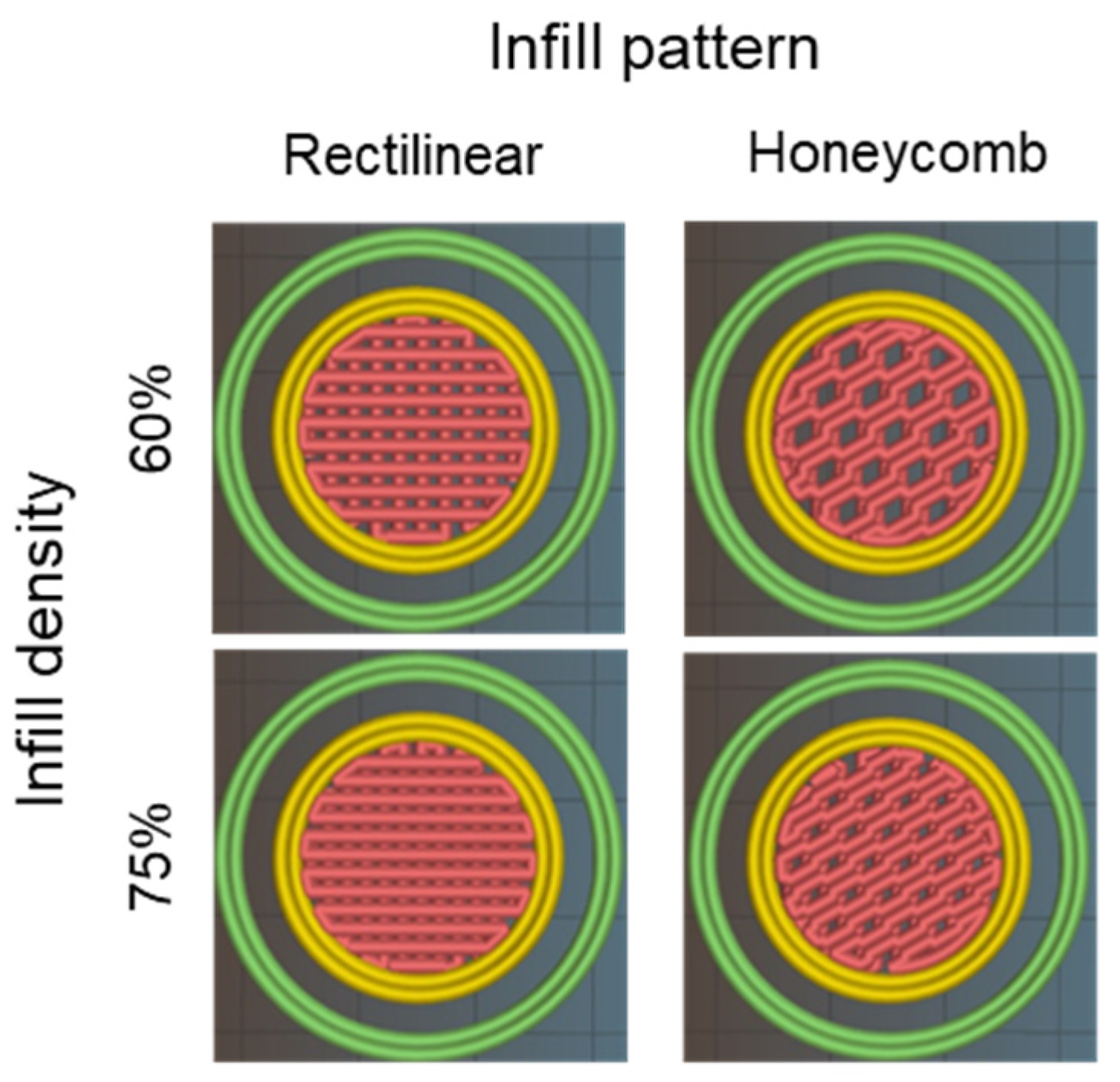
2.5. Printing Settings
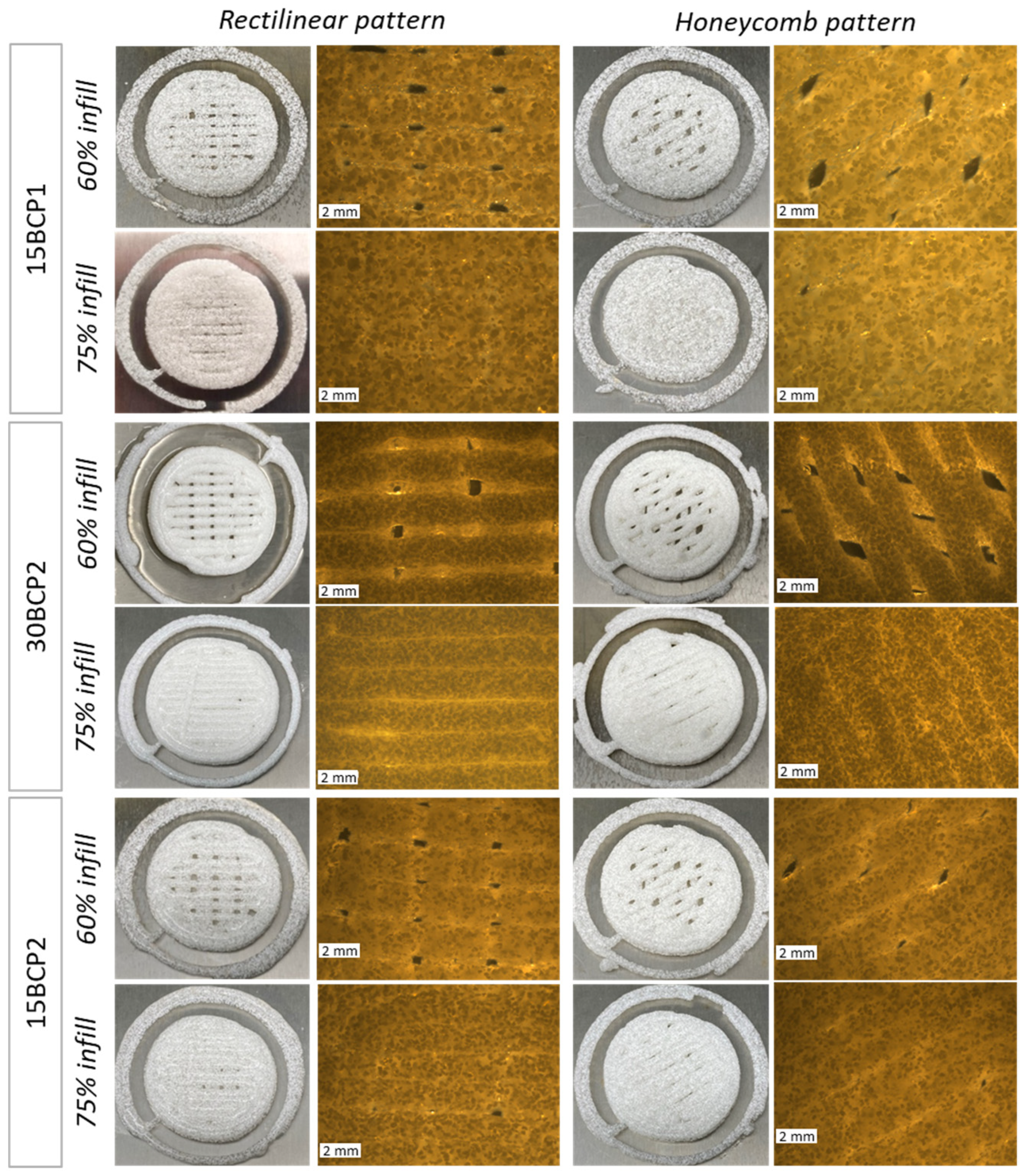
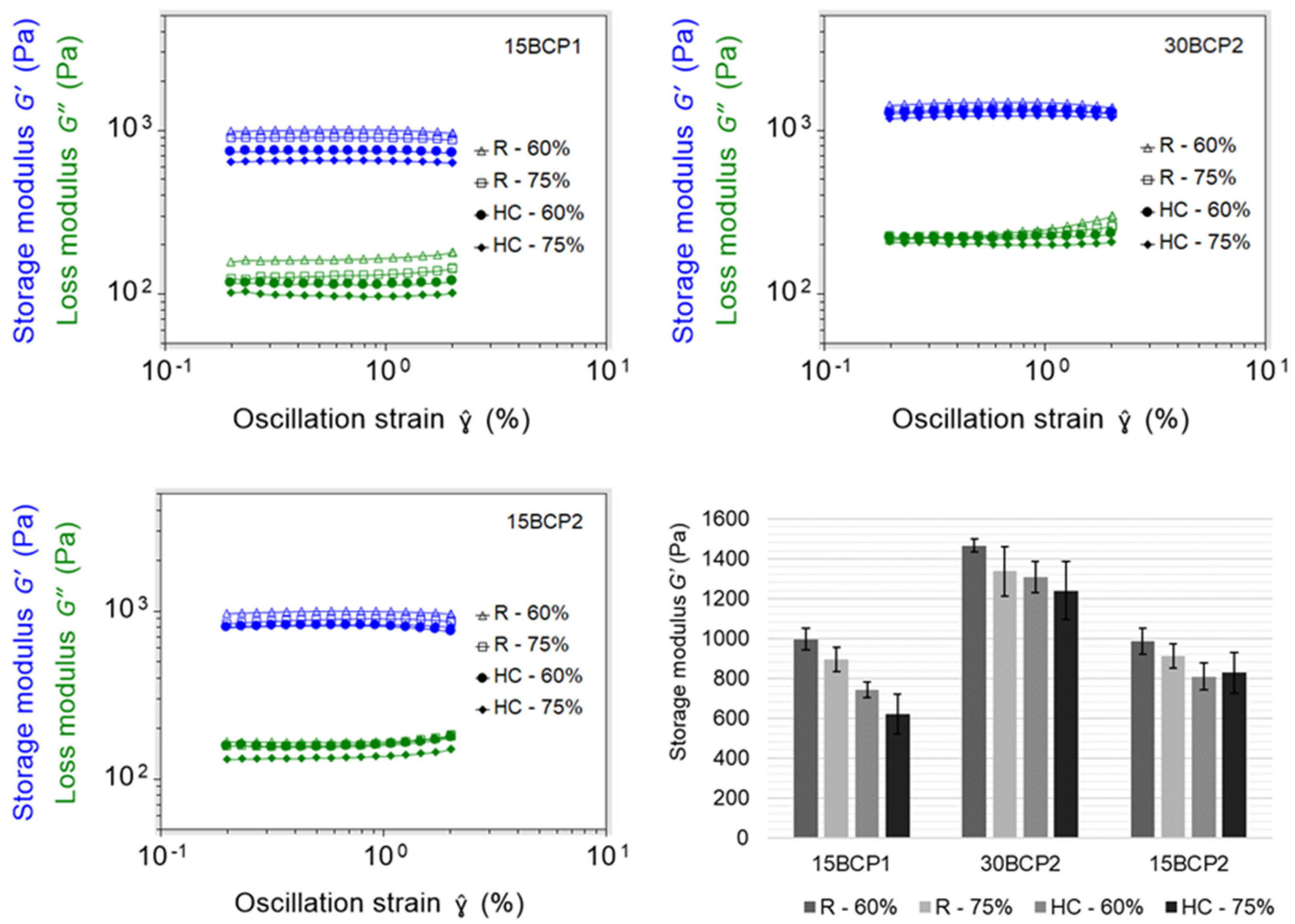
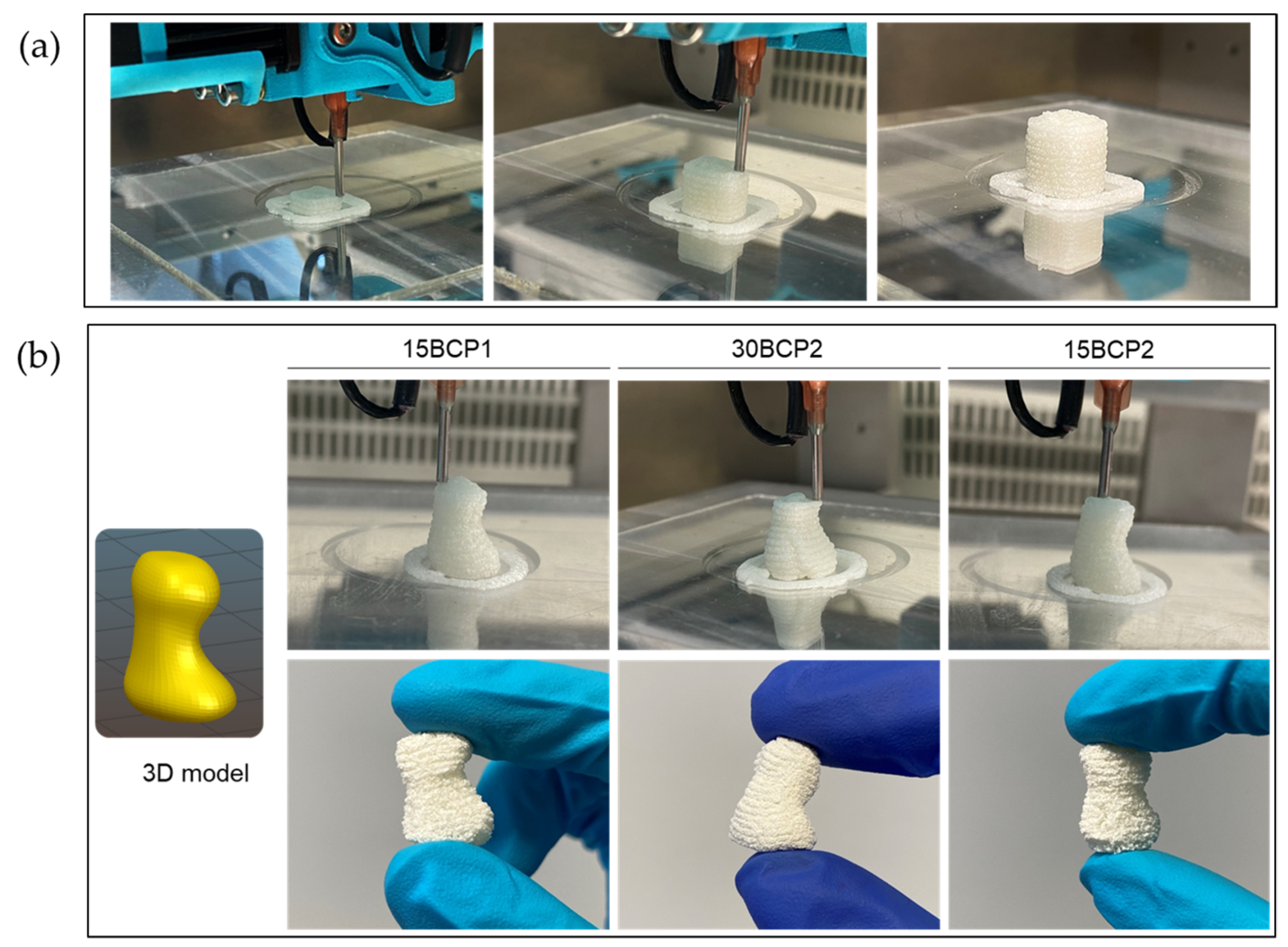
2.6. 3D-Printed Disks: Interplay between Stiffness, Ink Composition, and Printing Process Parameters
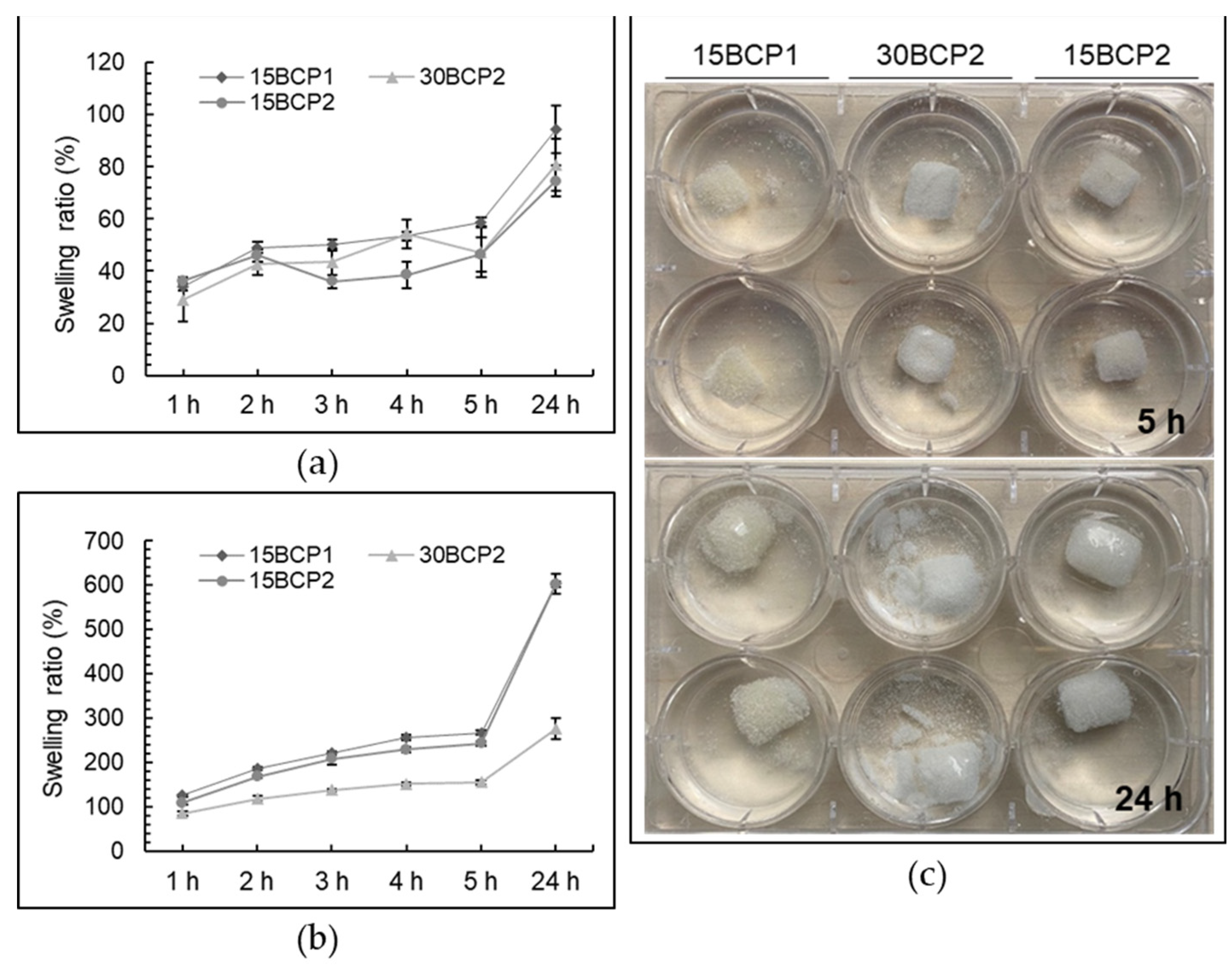
2.7. Swelling Behavior of Printed 3D Structures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. BCP-Hydrogel Preparation
4.3. Rheological Analysis and Printability Assessment
4.3.1. Flow Behavior
4.3.2. Thixotropy and Viscosity Recovery
4.4. Effect of CaCl2 Crosslinking on BCP-Hydrogel Matrix Stiffness
4.5. 3D-Printing Settings
4.5.1. Printing Speed Selection
4.5.2. Extrusion Width Calibration
4.6. Stiffness of Figures with Different Structural Configurations
4.6.1. 3D Model Setup
4.6.2. Stiffness Measurement of the Printed Figures
4.7. Swelling Degree of Fresh and Dehydrated 3D-Printed Cubes
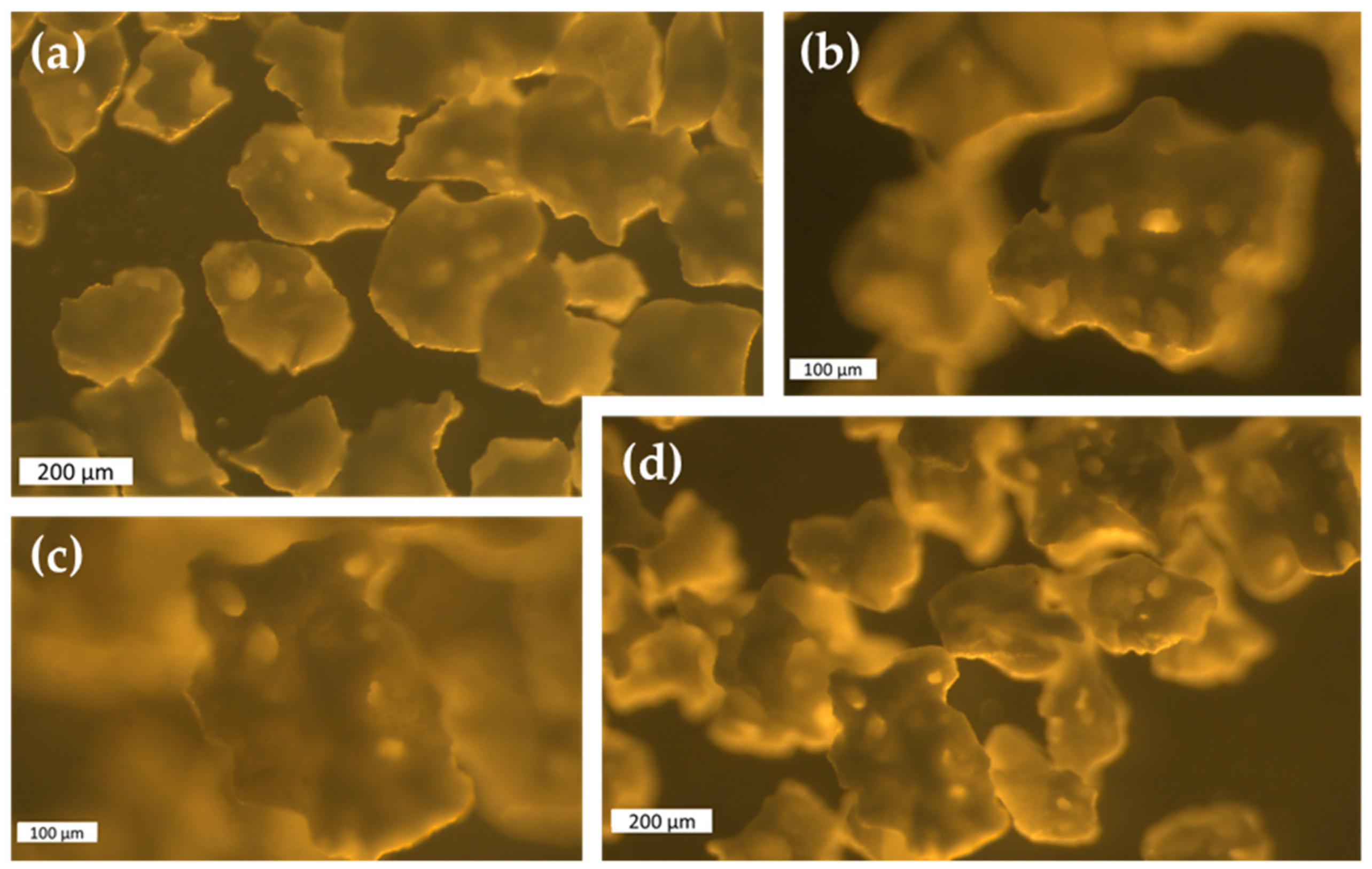
4.8. Microscopy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Sun, H.; Hu, C.; Zhou, C.; Wu, L.; Sun, J.; Zhou, X.; Xing, F.; Long, C.; Kong, Q.; Liang, J.; et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 108540. [Google Scholar] [CrossRef]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, L.; de Groot, F.; Yuan, H.; Campion, C.; Patel, A.; Poelstra, K.; de Bruijn, J. From benchtop to clinic: A translational analysis of the immune response to submicron topography and its relevance to bone healing. Eur. Cells Mater. 2021, 41, 756–773. [Google Scholar] [CrossRef] [PubMed]
- Systematic Review Centre Benefits and associated risks of using allograft, autograft and synthetic bone fusion material for patients and service providers—A Systematic Review. JBI Libr. Syst. Rev. 2010, 8, 303–327.
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Li, N.; Guo, R.; Zhang, Z.J. Bioink Formulations for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 44. [Google Scholar] [CrossRef]
- Ratheesh, G.; Vaquette, C.; Xiao, Y. Patient-Specific Bone Particles Bioprinting for Bone Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 2001323. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Luo, Y.; Schumacher, M.; Nies, B.; Lode, A.; Gelinsky, M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 2015, 27, 264–274. [Google Scholar] [CrossRef]
- Shao, H.; He, J.; Lin, T.; Zhang, Z.; Zhang, Y.; Liu, S. 3D gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceram. Int. 2019, 45, 1163–1170. [Google Scholar] [CrossRef]
- Lin, K.-F.; He, S.; Song, Y.; Wang, C.-M.; Gao, Y.; Li, J.-Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.-X. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 6905–6916. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Kim, G. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Zhang, J.; Barbieri, D.; ten Hoopen, H.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. Microporous calcium phosphate ceramics driving osteogenesis through surface architecture. J. Biomed. Mater. Res. Part A 2015, 103, 1188–1199. [Google Scholar] [CrossRef]
- Duan, R.; van Dijk, L.; Barbieri, D.; de Groot, F.; Yuan, H.; de Bruijn, J. Accelerated bone formation by biphasic calcium phosphate with a novel sub-micron surface topography. Eur. Cells Mater. 2019, 37, 60–73. [Google Scholar] [CrossRef]
- Vu, A.A.; Burke, D.A.; Bandyopadhyay, A.; Bose, S. Effects of surface area and topography on 3D printed tricalcium phosphate scaffolds for bone grafting applications. Addit. Manuf. 2021, 39, 101870. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.M.R.; Zhang, M.; Mujumdar, A.S.; Yang, C. Study on 3D printing of orange concentrate and material characteristics. J. Food Process Eng. 2018, 41, e12689. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Lin, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprinting 2016, 2, 10–12. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking rheology and printability of a multicomponent gel system of carrageenan-xanthan-starch in extrusion based additive manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Qiao, F.; Song, C.; Lv, Y. Matrix stiffness regulates bone repair by modulating 12-lipoxygenase-mediated early inflammation. Mater. Sci. Eng. C 2021, 128, 112359. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.W.; Wenger, A.C.; Tam, K.C.; Tang, X.S. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Xia, J.; Mi, S.; Sun, W. Mussel-Inspired Naturally Derived Double-Network Hydrogels and Their Application in 3D Printing: From Soft, Injectable Bioadhesives to Mechanically Strong Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-S.; Natale, G.; Virgilio, N.; Heuzey, M.-C. Synergistic gelation of gelatin B with xanthan gum. Food Hydrocoll. 2016, 60, 374–383. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Herrada-Manchón, H.; Celada, L.; Rodríguez-González, D.; Alejandro Fernández, M.; Aguilar, E.; Chiara, M.-D. Three-dimensional bioprinted cancer models: A powerful platform for investigating tunneling nanotube-like cell structures in complex microenvironments. Mater. Sci. Eng. C 2021, 128, 112357. [Google Scholar] [CrossRef] [PubMed]
- HadjSadok, A.; Moulai-Mostefa, N.; Rebiha, M. Rheological Properties and Phase Separation of Xanthan-Sodium Caseinate Mixtures Analyzed by a Response Surface Method. Int. J. Food Prop. 2010, 13, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Rodd, A.B.; Dunstan, D.E.; Boger, D.V. Characterisation of xanthan gum solutions using dynamic light scattering and rheology. Carbohydr. Polym. 2000, 42, 159–174. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Thorpe, A.; Mantovani, D.; Popat, K.C.; Moraes, Â.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. [Google Scholar] [CrossRef]
- Izawa, H.; Nishino, S.; Maeda, H.; Morita, K.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Kadokawa, J. Mineralization of hydroxyapatite upon a unique xanthan gum hydrogel by an alternate soaking process. Carbohydr. Polym. 2014, 102, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int. J. Nanomedicine 2013, 8, 47–59. [Google Scholar] [CrossRef]
- Freyer, J.P.; Fillak, D.; Jett, J.H. Use of xantham gum to suspend large particles during flow cytometric analysis and sorting. Cytometry 1989, 10, 803–806. [Google Scholar] [CrossRef]
- Sarker, D. Strange but true: The physics of glass, gels and jellies is all related through rheology. Sch. Sci. Rev. 2017, 99, 102–113. [Google Scholar]
- Mueller, S.; Llewellin, E.W.; Mader, H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 1201–1228. [Google Scholar] [CrossRef] [Green Version]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: Cambridge, UK, 2011; ISBN 978051197. [Google Scholar]
- Han, D.; Kim, J.H.; Lee, J.H.; Kang, S.-T. Critical Grain Size of Fine Aggregates in the View of the Rheology of Mortar. Int. J. Concr. Struct. Mater. 2017, 11, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Kreimendahl, F.; Köpf, M.; Thiebes, A.L.; Duarte Campos, D.F.; Blaeser, A.; Schmitz-Rode, T.; Apel, C.; Jockenhoevel, S.; Fischer, H. Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type I Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng. Part C Methods 2017, 23, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, T.; Madsen, C. Relative Stiffness Measurements of Cell-embedded Hydrogels by Shear Rheology in vitro. Bio-Protocol 2017, 7, e2101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.-H.; Rubert, M.; Müller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470. [Google Scholar] [CrossRef] [Green Version]
- Naghieh, S.; Sarker, M.D.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Kankala, R.; Lu, F.-J.; Liu, C.-G.; Zhang, S.-S.; Chen, A.-Z.; Wang, S.-B. Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials 2018, 11, 1390. [Google Scholar] [CrossRef] [Green Version]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
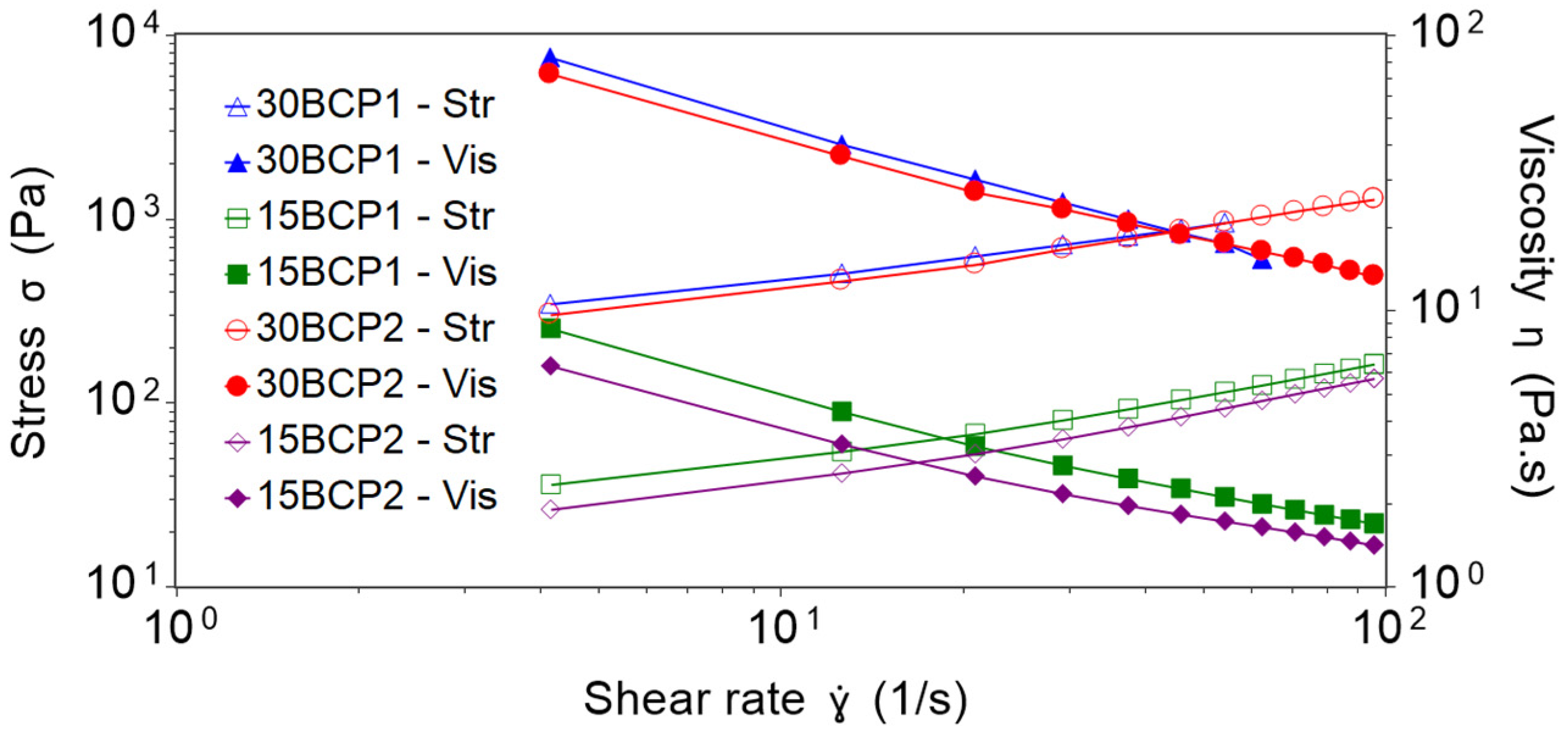
| (Pa.s) | R² | |||
|---|---|---|---|---|
| 30BCP1 | 142.08 | 93.25 | 0.54 | 0.9999 |
| 15BCP1 | 21.92 | 4.98 | 0.73 | 1.0000 |
| 30BCP2 | 125.46 | 70.38 | 0.62 | 0.9993 |
| 15BCP2 | 15.38 | 3.66 | 0.77 | 0.9999 |
| Wi (g) ± SD | Wf (g) ± SD | Weight lost (%) ± SD | Hi (mm) ± SD | Hf (mm) ± SD | Height lost (%) ± SD | |
|---|---|---|---|---|---|---|
| 15BCP1 | 1.380 ± 0.01 | 0.286 ± 0.01 | 79.09 ± 0.66 | 101.00 ± 1.00 | 69.67 ± 0.58 | 31.03 ± 0.34 |
| 30BCP2 | 1.484 ± 0.06 | 0.522 ± 0.02 | 64.36 ± 0.76 | 100.33 ± 0.58 | 93.67 ± 1.15 | 6.00 ± 1.49 |
| 15BCP2 | 1.350 ± 0.01 | 0.285 ± 0.01 | 78.87 ± 0.40 | 101.00 ± 1.00 | 68.0 ± 0.00 | 32.67 ± 0.67 |
| wt % | ||||
|---|---|---|---|---|
| 30BCP1 | 15BCP1 | 30BCP2 | 15BCP2 | |
| Xanthan gum | 3 | 3 | 3 | 3 |
| Sodium alginate | 2 | 2 | 2 | 2 |
| Gelatin | 2 | 2 | 2 | 2 |
| BCP1 (150–500 µm) | 30 | 15 | - | - |
| BCP2 (150–250 µm) | - | - | 30 | 15 |
| Deionized water | 63 | 78 | 63 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Kucko, N.W.; Barrère-de Groot, F.; Aguilar, E. Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels. Gels 2022, 8, 28. https://doi.org/10.3390/gels8010028
Herrada-Manchón H, Rodríguez-González D, Fernández MA, Kucko NW, Barrère-de Groot F, Aguilar E. Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels. Gels. 2022; 8(1):28. https://doi.org/10.3390/gels8010028
Chicago/Turabian StyleHerrada-Manchón, Helena, David Rodríguez-González, Manuel Alejandro Fernández, Nathan William Kucko, Florence Barrère-de Groot, and Enrique Aguilar. 2022. "Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels" Gels 8, no. 1: 28. https://doi.org/10.3390/gels8010028
APA StyleHerrada-Manchón, H., Rodríguez-González, D., Fernández, M. A., Kucko, N. W., Barrère-de Groot, F., & Aguilar, E. (2022). Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels. Gels, 8(1), 28. https://doi.org/10.3390/gels8010028








