Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Diffusion of Silicate Solution in Fiber-like BC Matrix
2.2. Wettability
2.3. Microstructures
2.4. Mechanical Properties
2.5. Thermal Insulation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Bacterial Cellulose Matrix
4.3. Preparation of Sodium Silicate Solution
4.4. Preparation of Silica-Bacterial Cellulose Composite Wet Gel Fibers
4.5. Hydrophobic Modification and Atmospheric Drying of CAFs
4.6. Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-L.; Xie, T. Advances of thermal conductivity models of nanoscale silica aerogel insulation material. Appl. Therm. Eng. 2015, 81, 28–50. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Hilonga, A.; Quang, D.V.; Kim, H.T. Synthesis of hydrophilic and hydrophobic xerogels with superior properties using sodium silicate. Microporous Mesoporous Mater. 2011, 139, 138–147. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Schwan, M.; Rößler, M.; Milow, B.; Ratke, L. From Fragile to Resilient Insulation: Synthesis and Characterization of Aramid-Honeycomb Reinforced Silica Aerogel Composite Materials. Gels 2015, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doneliene, J.; Fataraite-Urboniene, E.; Rudzikas, M.; Pakalka, S.; Danchova, N.; Ulbikas, J. Effect of Precursor Nature and Sol-Gel Synthesis Conditions on TiO2 Aerogel’s Structure. Molecules 2021, 26, 5090. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.; Sawvel, A.M.; Maxwell, R.; Satcher, J.H. Synthesis of High-Surface-Area Alumina Aerogels without the Use of Alkoxide Precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Rostamitabar, M.; Subrahmanyam, R.; Gurikov, P.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Cellulose aerogel micro fibers for drug delivery applications. Mater. Sci. Eng. C-Mater. 2021, 127, 112196. [Google Scholar] [CrossRef]
- Sala, M.R.; Skalli, O.; Leventis, N.; Sabri, F. Nerve Response to Superelastic Shape Memory Polyurethane Aerogels. Polymers 2020, 12, 2995. [Google Scholar] [CrossRef]
- Lin, Y.-Z.; Zhong, L.-B.; Dou, S.; Shao, Z.-D.; Liu, Q.; Zheng, Y.-M. Facile synthesis of electrospun carbon nanofiber/graphene oxide composite aerogels for high efficiency oils absorption. Environ. Int. 2019, 128, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A.; Puntar, N.A.; Özyürek, M.; Güngör, Z.B.; Kucur, M.; Kamış, H.; Dicle, D.A. Ethylenediamine grafted carbon nanotube aerogels modified screen-printed electrode for simultaneous electrochemical immunoassay of multiple tumor markers. J. Electroanal. Chem. 2021, 900, 115700. [Google Scholar] [CrossRef]
- Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.G.; Goiti, E.; Reichenauer, G.; Zhao, S.; Koebel, M.; Barrio, A. Thermal assessment of ambient pressure dried silica aerogel composite boards at laboratory and field scale. Energy Build. 2016, 128, 111–118. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M.; Fan, X.; Ma, M.; Shi, Z.; Guo, S.-W. Ultralight, Recoverable, and High-Temperature-Resistant SiC Nanowire Aerogel. ACS Nano 2018, 12, 3103–3111. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, C.; Chen, J.; Fang, Q.; Chen, W.; He, Z.; Xu, H.; Song, S. A hybrid block consisting of covalent triazine frameworks and GO aerogel with switchable selectivity between adsorption of UV filters and regeneration under sunlight. Chem. Eng. J. 2020, 395, 125074. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Yu, F.; Du, X. Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 2011, 54, 2355–2366. [Google Scholar] [CrossRef]
- Li, M.; Gan, F.; Dong, J.; Fang, Y.; Zhao, X.; Zhang, Q. Facile Preparation of Continuous and Porous Polyimide Aerogel Fibers for Multifunctional Applications. ACS Appl. Mater. Interfaces 2021, 13, 10416–10427. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Liu, Z.; Cheng, H.; Li, C. Continuous, Strong, Porous Silk Firoin-Based Aerogel Fibers toward Textile Thermal Insulation. Polymers 2019, 11, 1899. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Threads for Thermal Insulation in Harsh Environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Chang, T.-F.; Lu, S.-Y.; Chang, Y.-C. Preparation of Monolithic Silica Aerogel of Low Thermal Conductivity by Ambient Pressure Drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Tao, Y.; Mahendran, M. Fire tests and thermal analyses of LSF walls insulated with silica aerogel fibreglass blanket. Fire Saf. J. 2021, 122, 103352. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.-Z.; Wang, X.; Li, Y.-M.; Tao, W.-Q. Thermal conductivity of fiber and opacifier loaded silica aerogel composite. Int. J. Heat Mass Transf. 2017, 115, 21–31. [Google Scholar] [CrossRef]
- Zhang, G.; Dass, A.; Rawashdeh, A.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Gong, L.; Zhang, H. Aramid fibers reinforced silica aerogel composites with low thermal conductivity and improved mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 84, 316–325. [Google Scholar] [CrossRef]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically Strong Aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Zhang, G.; Vassilaras, P.; Johnston, J.C.; Leventis, N. Cross-linking Amine-Modified Silica Aerogels with Epoxies: Mechanically Strong Lightweight Porous Materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.N.; Meador, M.A.; Medoro, A.; Arendt, V.; Randall, J.; McCorkle, L.; Shonkwiler, B. Elastic Behavior of Methyltrimethoxysilane Based Aerogels Reinforced with Tri-Isocyanate. ACS Appl. Mater. Interfaces 2010, 2, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Brovko, O.; Palamarchuk, I.; Bogdanovich, N.; Ivakhnov, A.; Chukhchin, D.; Belousova, M.; Arkhilin, M.; Gorshkova, N. Composite aerogel materials based on lignosulfonates and silica: Synthesis, structure, properties. Mater. Chem. Phys. 2021, 269, 124768. [Google Scholar] [CrossRef]
- Wang, S.; Meng, W.; Lv, H.; Wang, Z.; Pu, J. Thermal insulating, light-weight and conductive cellulose/aramid nanofibers composite aerogel for pressure sensing. Carbohydr. Polym. 2021, 270, 118414. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Chen, G.; Shi, X.; Yang, X.; Dai, H.; Chen, X. Mechanical reinforced fiber needle felt/silica aerogel composite with its flammability. J. Sol-Gel Sci. Technol. 2018, 88, 129–140. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Wang, J.; Liu, Z.; Zhang, K.; Ji, X.; You, Y.; Zhang, X. Reaction-Spun Transparent Silica Aerogel Fibers. ACS Nano 2020, 14, 11919–11928. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, J.; Chen, W.; Wang, X.; Zhu, M. Construction of continuous hollow silica aerogel fibers with hierarchical pores and excellent adsorption performance. Microporous Mesoporous Mater. 2019, 273, 294–296. [Google Scholar] [CrossRef]
- Zhou, J.; Hsieh, Y.-L. Nanocellulose aerogel-based porous coaxial fibers for thermal insulation. Nano Energy 2020, 68, 104305. [Google Scholar] [CrossRef] [Green Version]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.S.; Abdat, M.; Nasir, N.A.M.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R.; et al. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Sai, H.; Zhang, J.; Jin, Z.; Fu, R.; Wang, M.; Wang, Y.; Wang, Y.; Ma, A.L. Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process. Polymers 2020, 12, 807. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, H.; Li, Y.; Zheng, X. Robust Silk Fibroin/Graphene Oxide Aerogel Fiber for Radiative Heating Textiles. ACS Appl. Mater. Interfaces 2020, 12, 15726–15736. [Google Scholar] [CrossRef]
- Yu, H.J.; Tong, Z.W.; Zhang, B.J.; Chen, Z.W.; Li, X.L.; Su, D.; Ji, H.M. Thermal radiation shielded, high strength, fire resistant fiber/nanorod/ aerogel composites fabricated by in-situ growth of TiO2 nanorods for thermal insulation. Chem. Eng. J. 2021, 418, 129342. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Xiong, X.; Ge, P.; Wu, J.; Sun, J.; Wang, J.; Zhuo, Q.; Qin, C.; Dai, L. Strategies for Preparing Continuous Ultraflexible and Ultrastrong Poly(Vinyl Alcohol) Aerogel Fibers with Excellent Thermal Insulation. Macromol. Mater. Eng. 2021, 306, 2100399. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Wang, W.Q.; Zou, L.P.; Lian, Y.; Zhang, Z.H.; Liu, B.; Zhang, F. Robust, Highly Thermally Stable, Core-Shell Nanostructured Metal Oxide Aerogels as High-Temperature Thermal Superinsulators, Adsorbents, and Catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F.; Zhang, T. Flexible aerogels with interpenetrating network structure of bacterial cellulose–silica composite from sodium silicate precursor via freeze drying process. RSC Adv. 2014, 4, 30453–30461. [Google Scholar] [CrossRef]

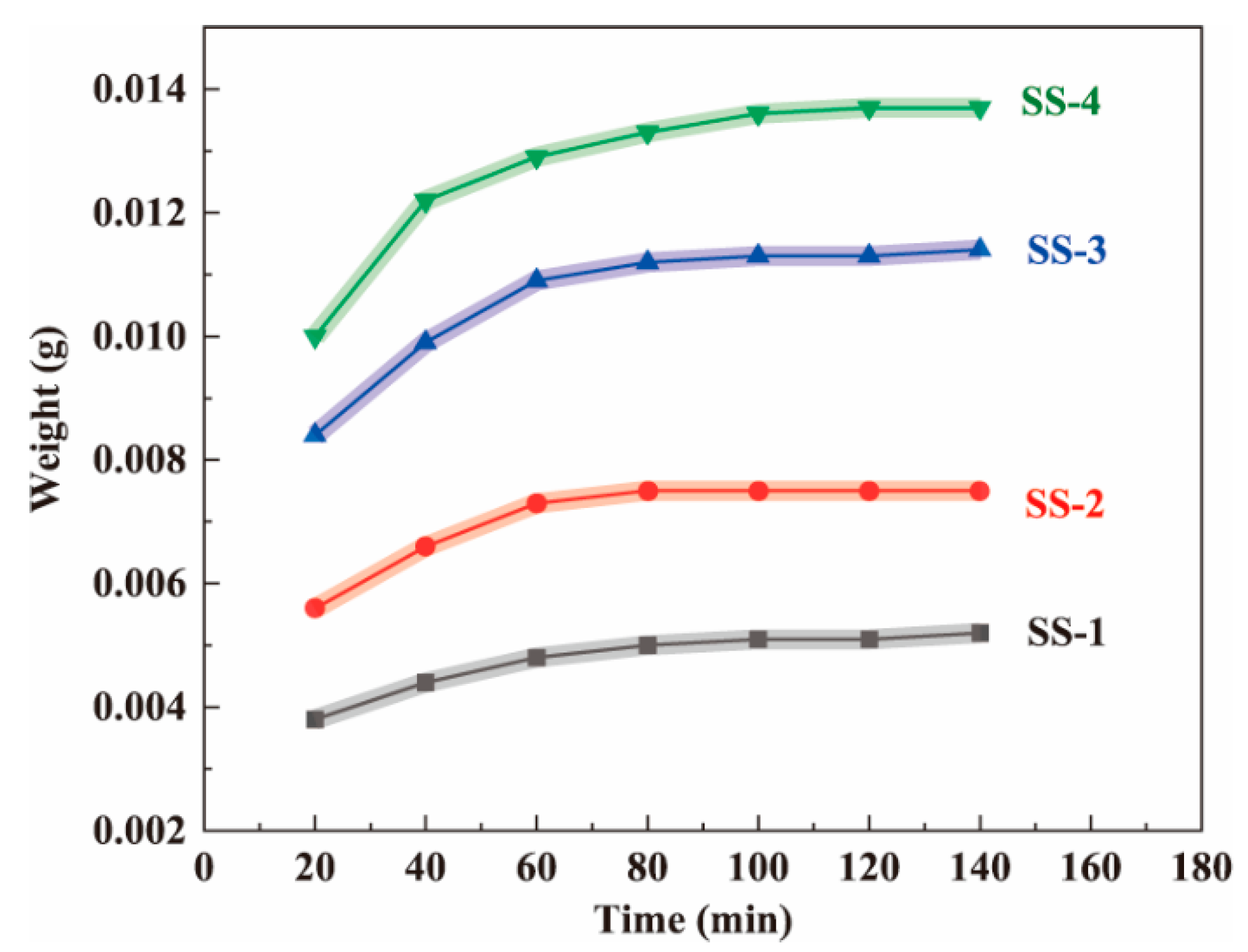
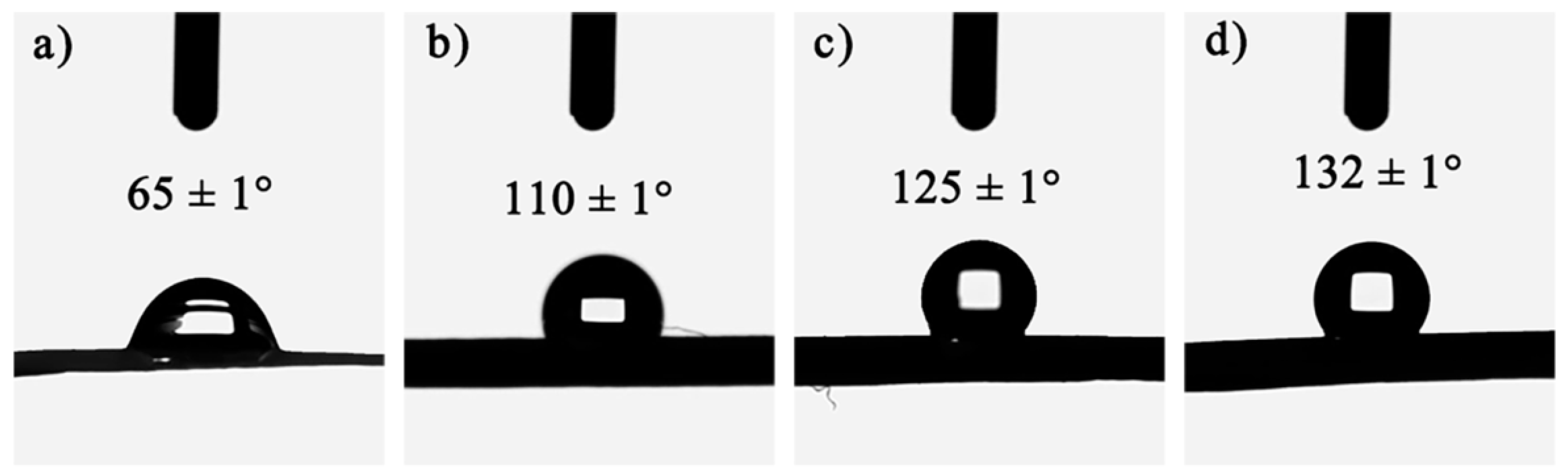


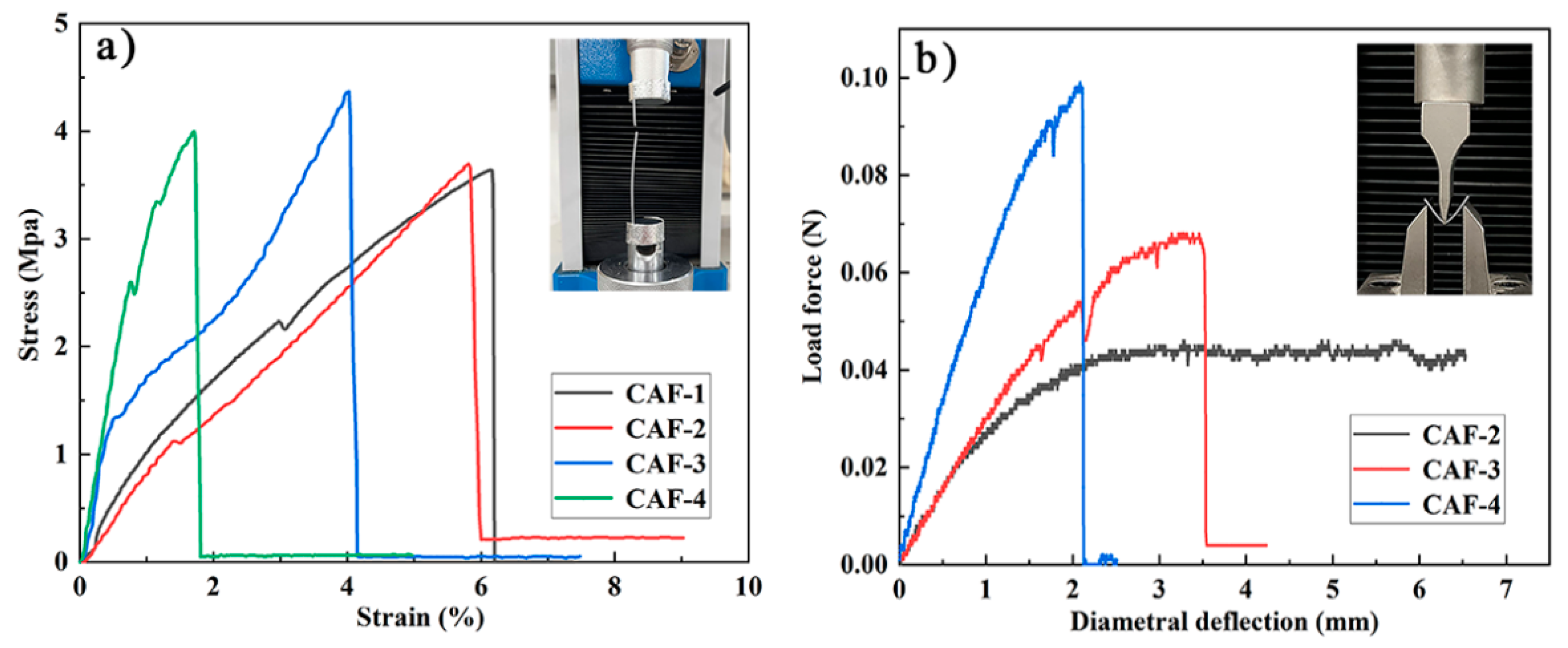
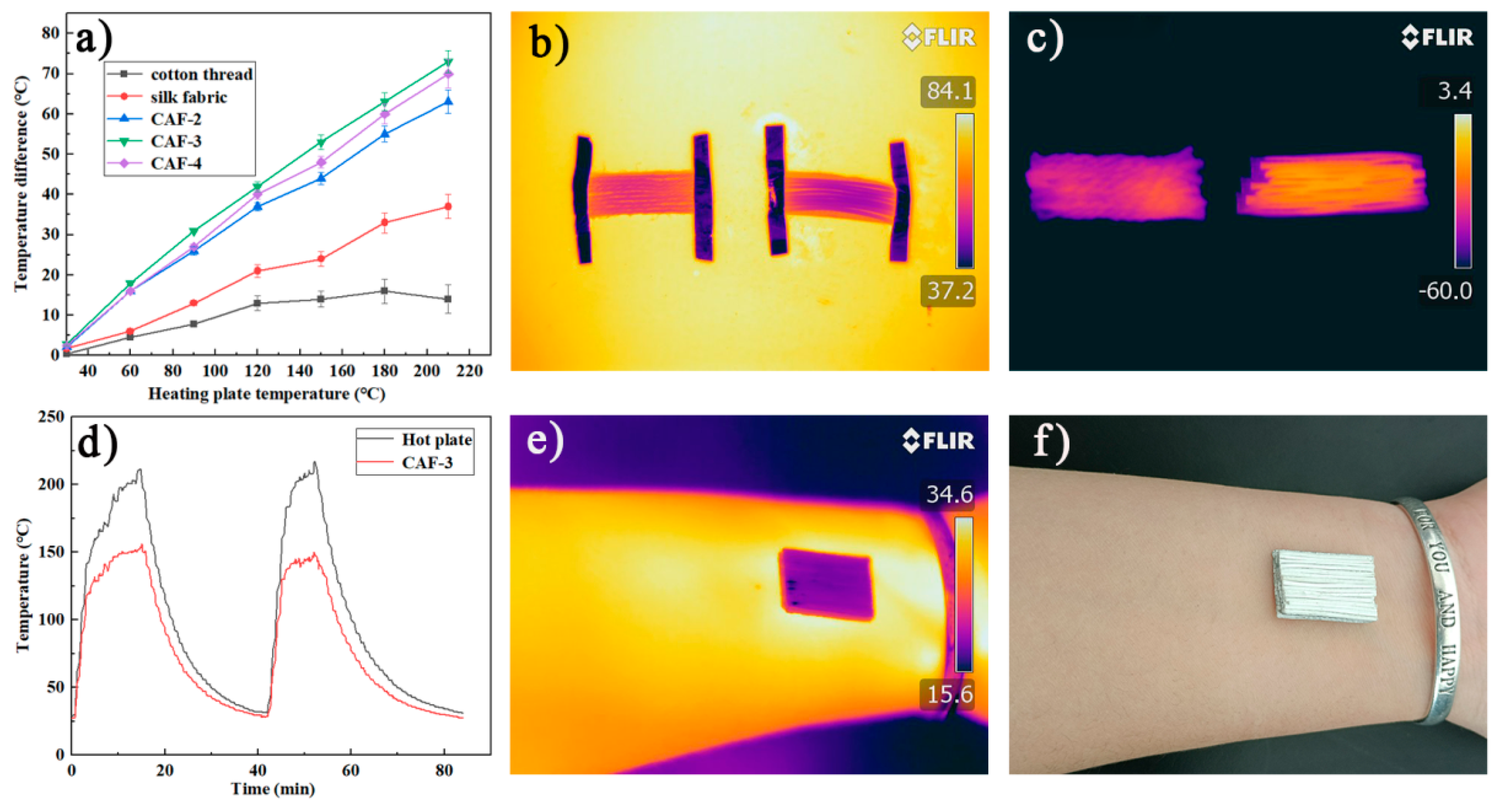

| Samples | SiO2 in Aerogels [% w/w] | Bulk Density [g cm−3] | SBET [m2 g−1] | Pore Size [nm] | Porosity a [%] |
|---|---|---|---|---|---|
| CAF-1 | 22 | 0.080 | 158.5 | 13.2 | 80.3 |
| CAF-2 | 38 | 0.123 | 404.7 | 13.0 | 89.1 |
| CAF-3 | 46 | 0.146 | 548.1 | 13.7 | 90.6 |
| CAF-4 | 55 | 0.154 | 671.3 | 9.5 | 90.2 |
| Materials | Fabrication Method | Tensile Strength | Density | Ref. |
|---|---|---|---|---|
| CA/PAA-SF | wet-spinning | 2.6 ± 0.4 MPa | 0.21 g/cm3 | [19] |
| CA/PAA-SF/GO | coaxial wet-spinning | 3.0 ± 0.2 MPa | [39] | |
| QF/ASA | hydrothermal and Ti-H2O2 | 3.17 ± 0.04 MPa | 0.302 g/cm3 | [40] |
| PVA | freeze-spinning | 8.36 MPa | [41] | |
| SiO2-Cellulose | secondary shaping | 5.4 MPa | 0.164 g/cm3 | [35] |
| SiO2 | reaction-spun | 230 KPa | 0.15–0.2 g/cm3 | [32] |
| Solution | SS-1 | SS-2 | SS-3 | SS-4 |
|---|---|---|---|---|
| Na2O·3SiO2 (g) | 10 | 22 | 30 | 50 |
| H2O (mL) | 170 | 178 | 150 | 180 |
| SiO2 in solution (% w/w) | 4 | 8 | 12 | 16 |
| Corresponding sample number | CAF-1 | CAF-2 | CAF-3 | CAF-4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; Miao, C.; Sai, H.; Gu, J.; Wang, M.; Jiang, P.; Wang, Y.; Fu, R.; Wang, Y. Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels 2022, 8, 17. https://doi.org/10.3390/gels8010017
Song Q, Miao C, Sai H, Gu J, Wang M, Jiang P, Wang Y, Fu R, Wang Y. Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels. 2022; 8(1):17. https://doi.org/10.3390/gels8010017
Chicago/Turabian StyleSong, Qiqi, Changqing Miao, Huazheng Sai, Jie Gu, Meijuan Wang, Pengjie Jiang, Yutong Wang, Rui Fu, and Yaxiong Wang. 2022. "Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor" Gels 8, no. 1: 17. https://doi.org/10.3390/gels8010017
APA StyleSong, Q., Miao, C., Sai, H., Gu, J., Wang, M., Jiang, P., Wang, Y., Fu, R., & Wang, Y. (2022). Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels, 8(1), 17. https://doi.org/10.3390/gels8010017








