Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review
Abstract
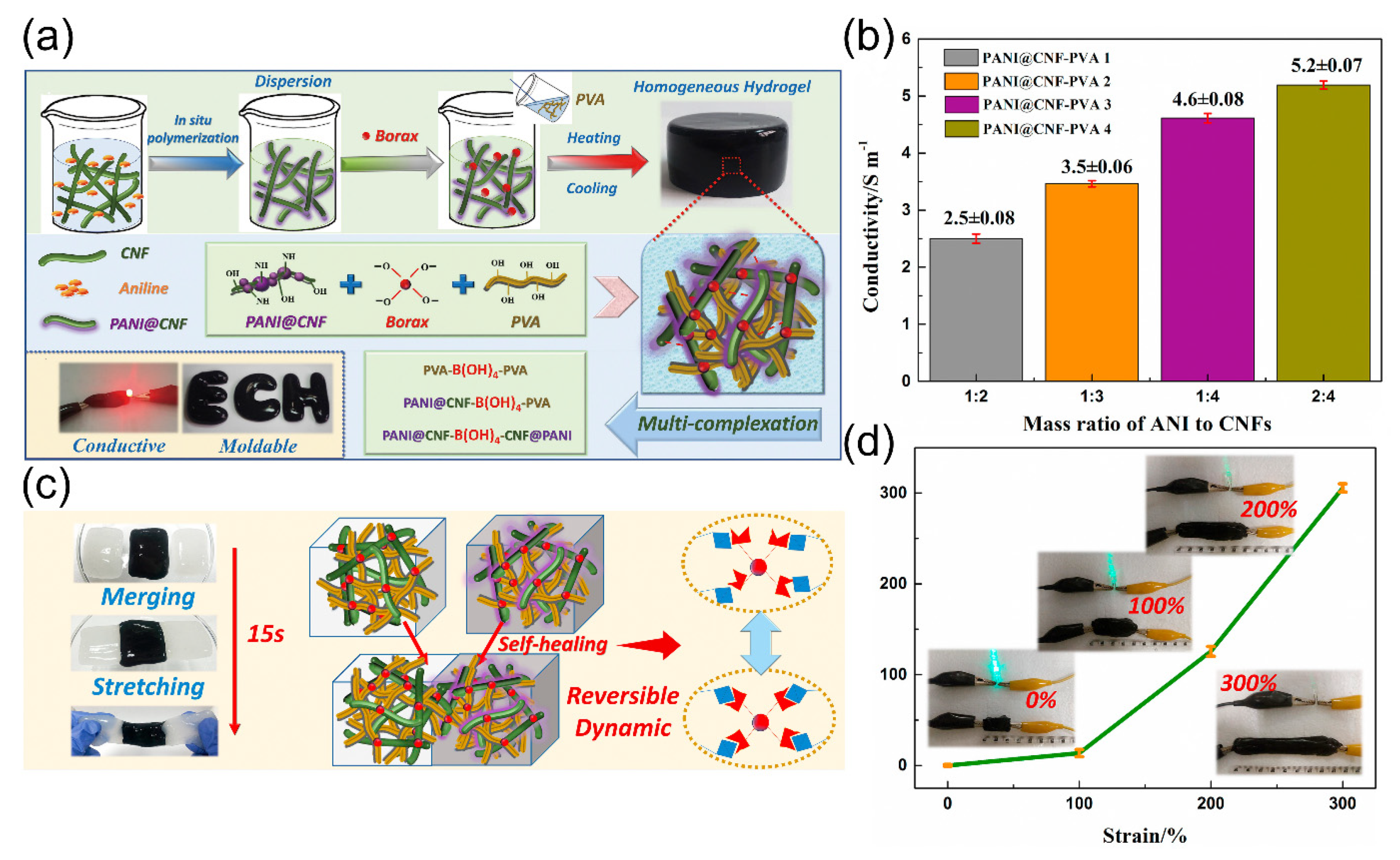
:1. Introduction
2. Self-Healing Mechanism of Hydrogel
2.1. Noncovalent Interactions
2.1.1. Hydrophobic Associations
2.1.2. Hydrogen Bond
2.1.3. Host–Guest Interaction
2.1.4. Metal Coordination
2.2. Dynamic Covalent Bonds
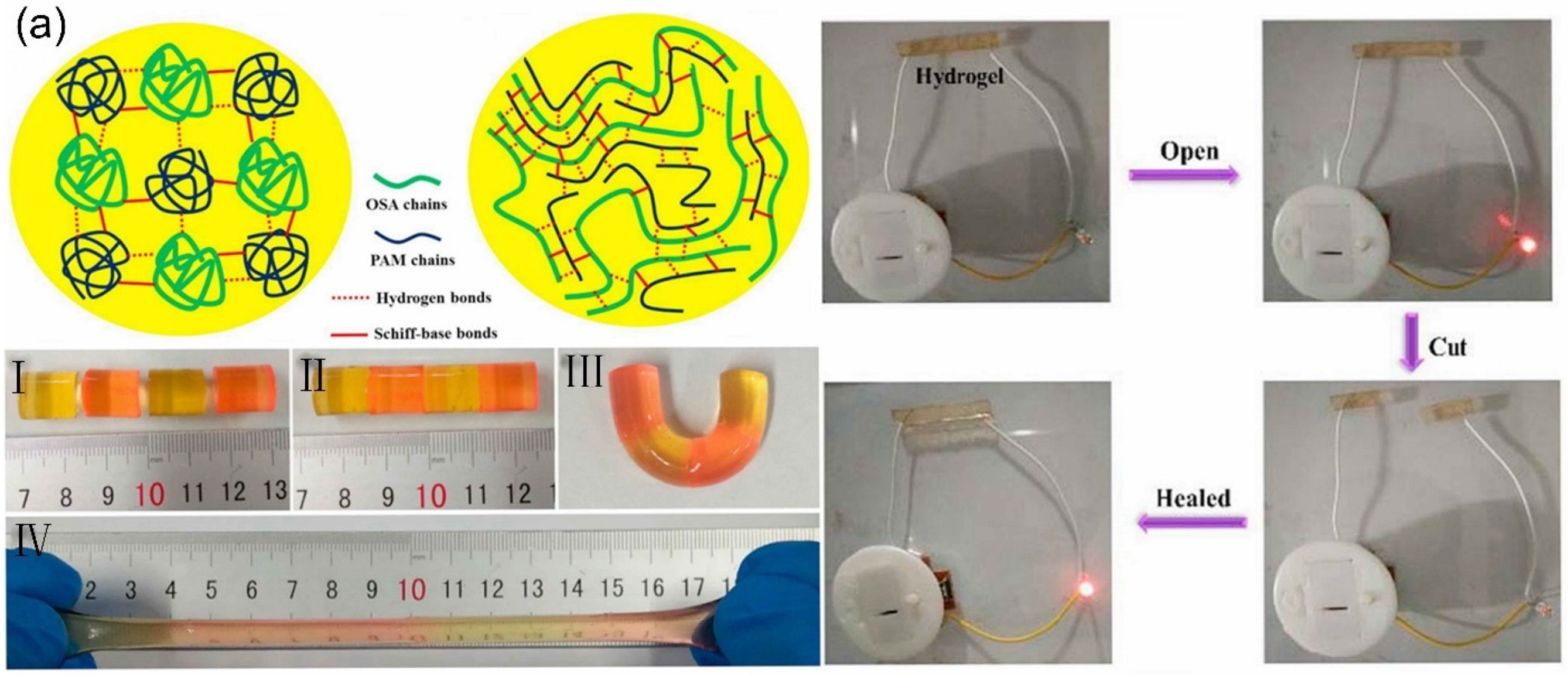
2.2.1. Schiff Base Linkage
2.2.2. Disulfide Bond
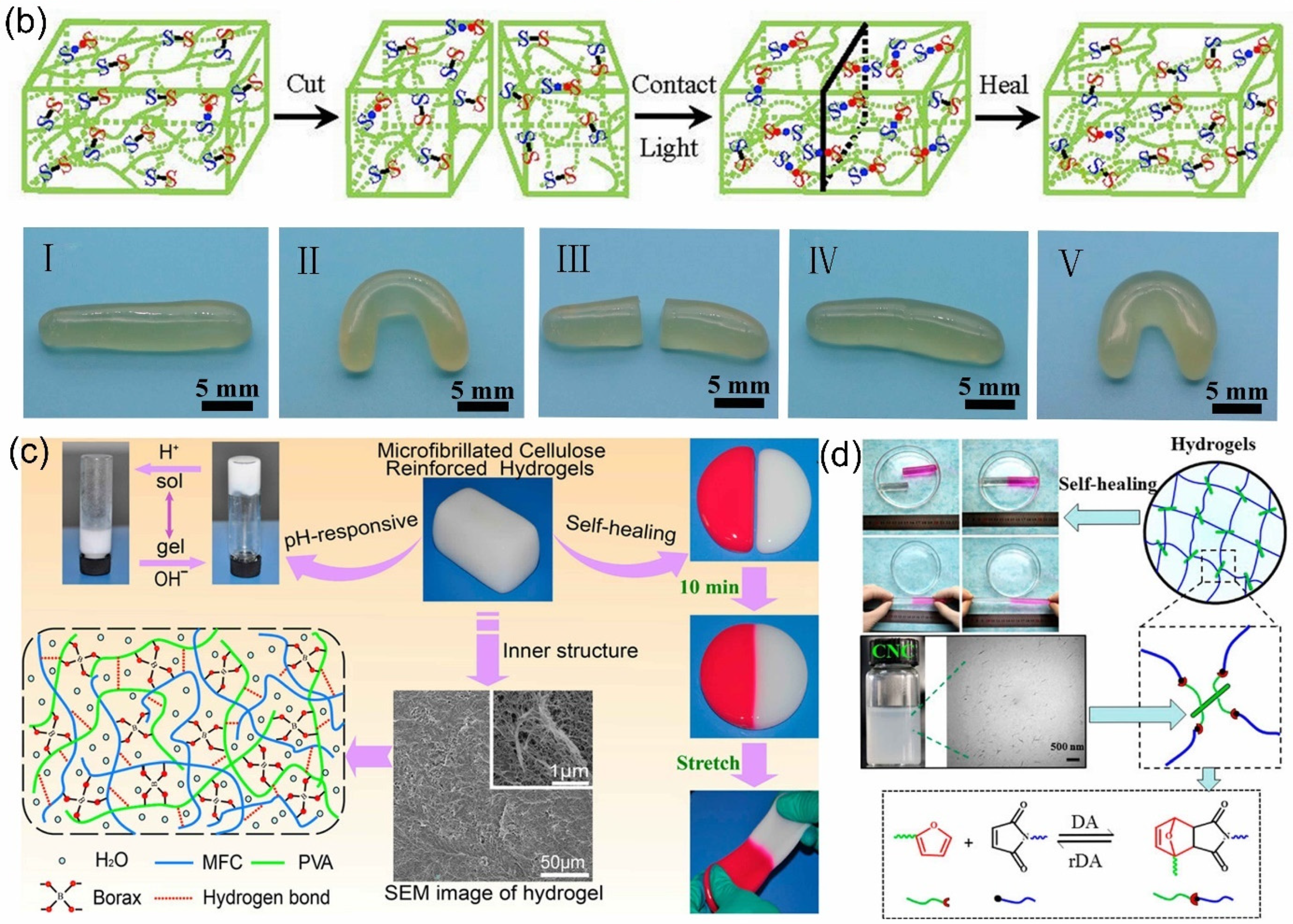
2.2.3. Boronic/Boronate Ester Bond
2.2.4. Diels–Alder Reaction
3. Conductive Categories of Self-Healing Hydrogel for Flexible Sensors
3.1. Self-Healing Hydrogel with Conductive Fillers
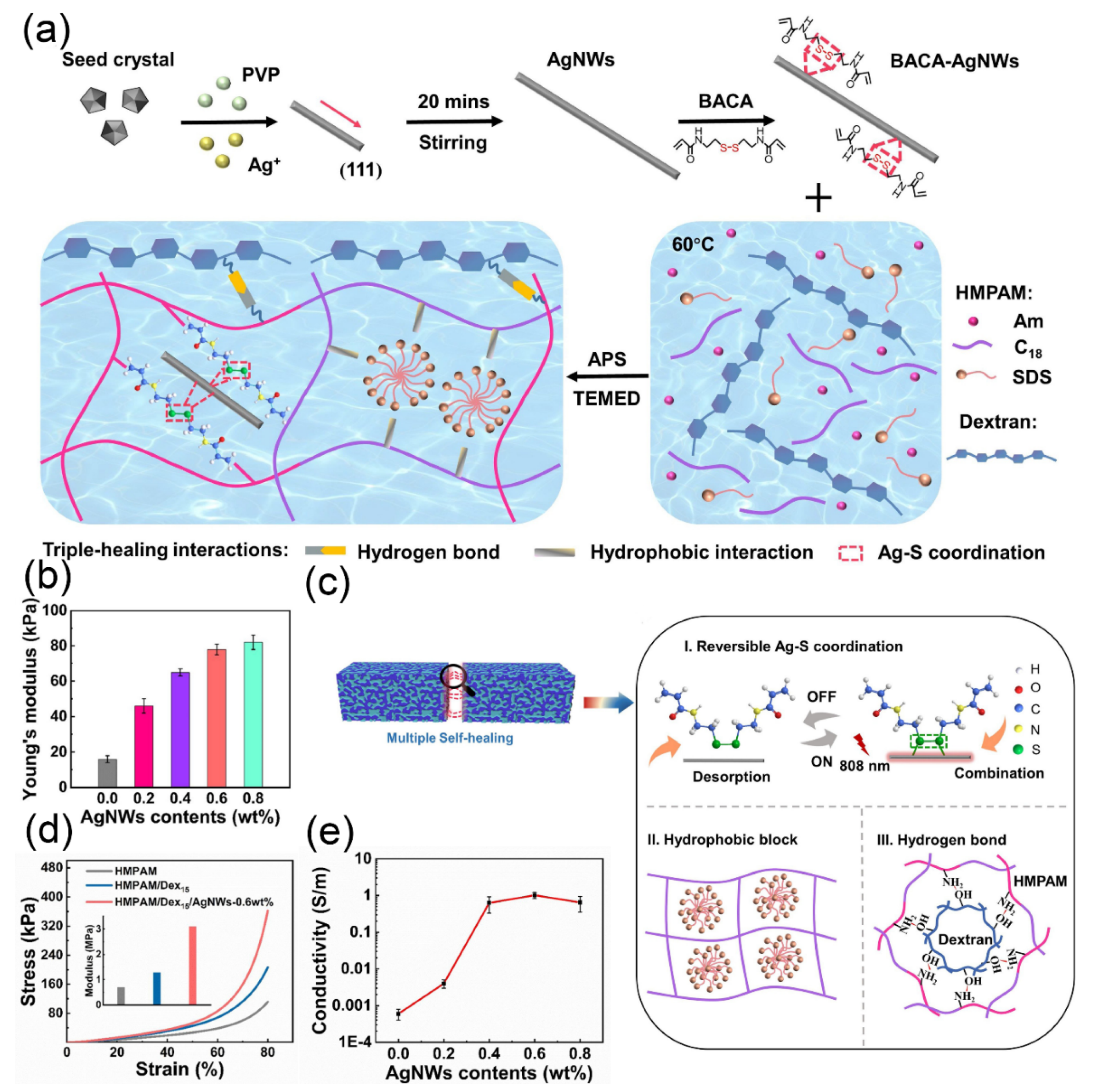
3.1.1. Metal-Based Nanomaterials
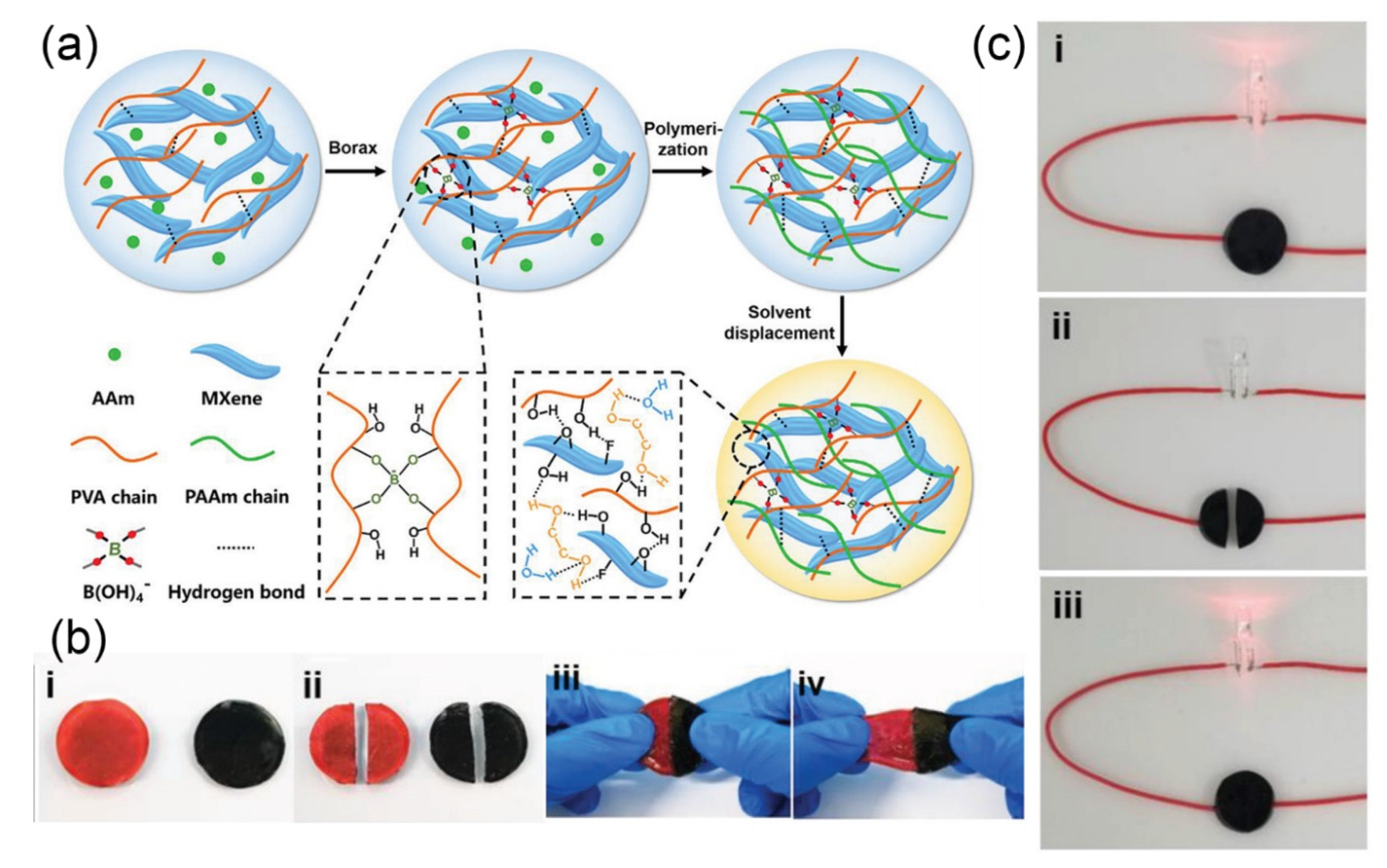
3.1.2. MXene-Based Nanomaterials
3.1.3. Carbon-Based Nanomaterials
| Hydrogel Materials | Conductive Type | Self-Healing Mechanism | Gauge Factor | Conductivity | Application | Ref |
|---|---|---|---|---|---|---|
| PC/rGO/PVA | Electron | cross-linked bonds | 14.14 | Wearable E-skin | [178] | |
| PVA/PDA/pRGO | Electron | hydrogen bonds | 2.7 S cm−1 | soft strain sensor | [179] | |
| PVA/CNTs/ graphene | Electron/ion | hydrogen bonds, borate ester bond | 52.7 | electronic device | [180] | |
| TOCNF/GN/PAA | Electron/ion | hydrogen bonds, metal-ligand interactions | 5.8 | 2.5 S m−1 | soft sensor devices | [181] |
| P(DMA-co-PFPA)/ SWCNTs/PVA | Electron/ion | dynamic boronate ester bonds | 1.27 S m−1 | electronic skins | [182] | |
| rGO–SAP | Electron/ion | hydrogen bonds | 1500 ΩM | soft sensor devices | [183] | |
| PVA/PDAP/ MWCNT | Electron | borate bonds, hydrogen bonds | wearable electronics | [184] | ||
| PAA/CS/GO/Gly | Electron/ion | hydrogen bonds, electrostatic interaction | 1.138 | 5.6 × 10−3 S cm−1 | wearable sensor | [176] |
| PAA-GO | Electron/ion | coordination crosslinking, hydrogen bonds | 0.46 | wearable sensor | [162] | |
| EW/CNT | Electron/ion | hydrogen bonds, hydrophobic interactions | 87.8 kΩ | epidermal sensors | [185] | |
| CS/ZnPcTa | Electron | Schiff-base linkage | 0.0029 S cm−1 | biomedical applications | [186] | |
| PVA/Gly/CB/CNT | Electron | hydrogen bonds | 2.1 | wearable sensor | [187] | |
| Poly(NIPAM-co-β-CD)/CNT/PPY | Electron | Host−Guest Interactions | 34.93 S m−1. | wearable sensors | [188] | |
| CS/DA/GO | Electron | hydrogen bonds, π-π stacking | 1.2 × 10−3 S cm−1 | engineering applications | [189] | |
| rGO/AM | Electron | covalent bonds hydrogen bonds | 27.2 S m−1 | artificial skin, soft robotics | [190] | |
| PNIPAM/Laponite/CNT | Electron | electrostatic interaction, hydrogen bonds | 0.17 S m−1 | wearable sensor | [191] | |
| PAM/MWCNTs | Electron | hydrophobic interactions, hydrogen bonds | 5.6 | 0.5 S m−1 | Wearable medical monitoring | [192] |
| GOxSPNB | Electron/ion | electrostatic interaction, hydrogen bonds | 10.5 mS dm−1 | conductive adhesive materials | [177] | |
| PAA/GO/Ca2+ | Electron/ion | Hydrogel bonds, ionic interactions | 257.31 kΩ | wearable biosensors | [193] | |
| AlgPBA/PVA/ PAM/rGO | Electron | covalent ester bonds, hydrogen bonds | 0.0525 S m−1 | E- skins, healthcare monitoring, | [194] | |
| PVA/FSWCNT/ PDA | Electron/ion | Hydrogen bonds, π–π stacking, | wearable sensors. | [195] |
3.2. Self-Healing Hydrogel with Conductive Polymers
3.3. Ionic Self-Healing Hydrogel
4. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Hussain, I.; Kang, M.; Li, K.; Yao, F.; Liu, S.; Fu, G. Self-recoverable and mechanical-reinforced hydrogel based on hydrophobic interaction with self-healable and conductive properties. Chem. Eng. J. 2018, 353, 900–910. [Google Scholar] [CrossRef]
- Huang, S.; Su, S.; Gan, H.; Wu, L.; Lin, C.; Xu, D.; Zhou, H.; Lin, X.; Qin, Y. Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, Y.; Tang, P.; Gan, D.; Xing, W.; Hou, Y.; Wang, K.; Xie, C.; Lu, X. Conductive, Tough, Transparent, and Self-Healing Hydrogels Based on Catechol-Metal Ion Dual Self-Catalysis. Chem. Mater. 2019, 31, 5625–5632. [Google Scholar] [CrossRef]
- Chen, W.; Bu, Y.; Li, D.; Liu, Y.; Chen, G.; Wan, X.; Li, N. Development of high-strength, tough, and self-healing carboxymethyl guar gum-based hydrogels for human motion detection. J. Mater. Chem. C 2020, 8, 900–908. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, X.; Lv, Q.; Shen, Y.; Liang, H. Fully physically cross-linked hydrogel as highly stretchable, tough, self-healing and sensitive strain sensors. Polymer 2020, 210, 123039. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, S.; Li, Q.; Ren, Y.; Ding, Y.; Wu, H.; He, X.; Shang, Y. High strength zwitterionic nano-micelle hydrogels with superior self-healing, adhesive and ion conductive properties. Eur. Polym. J. 2020, 133, 109761. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Wang, Y.; Guo, L.; Yin, C.; Zhang, X.; Hao, J.; Zhang, G.; Chen, L. Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules 2019, 52, 2531–2541. [Google Scholar] [CrossRef]
- Kang, M.; Liu, S.; Oderinde, O.; Yao, F.; Fu, G.; Zhang, Z. Template method for dual network self-healing hydrogel with conductive property. Mater. Des. 2018, 148, 96–103. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Z.; Zhang, X.; Li, Z.; Xiao, H.; Zhang, M.; Liu, K.; Ni, Y.; Huang, L.; Chen, L.; et al. A self-healing, stretchable, and conductive Poly(N-vinylpyrrolidone)/gallic acid composite hydrogel formed via hydrogen bonding for wearable electronic sensors. Compos. Sci. Technol. 2020, 198, 108294. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Fu, G. Facile and cost-effective synthesis of glycogen-based conductive hydrogels with extremely flexible, excellent self-healing and tunable mechanical properties. Int. J. Biol. Macromol. 2018, 118, 1463–1469. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Oderinde, O.; Kang, M.; Yao, F.; Fu, G. Enhancing the mechanical properties and self-healing efficiency of hydroxyethyl cellulose-based conductive hydrogels via supramolecular interactions. Eur. Polym. J. 2018, 105, 85–94. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Ma, X.; Cao, S.; Ni, Y. Ultrafast gelling using sulfonated lignin-Fe3+ chelates to produce dynamic crosslinked hydrogel/coating with charming stretchable, conductive, self-healing, and ultraviolet-blocking properties. Chem. Eng. J. 2020, 396, 125341. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Healing, flexible, high thermal sensitive dual-network ionic conductive hydrogels for 3D linear temperature sensor. Mater. Sci. Eng. C 2020, 107, 110310. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, L.; Xu, Y.; Wu, M.; Wang, M.; Liu, Y.; Yu, S.; Li, L. Skin-inspired cellulose conductive hydrogels with integrated self-healing, strain, and thermal sensitive performance. Carbohydr. Polym. 2020, 240, 116360. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, S.; Qu, C.; Wang, D.; Liu, C.; Wang, Y.; Fan, X.; Xiao, W.; Li, H.; Zhao, D.; et al. Simple and environmentally friendly approach for preparing high-performance polyimide precursor hydrogel with fully aromatic structures for strain sensor. Eur. Polym. J. 2019, 114, 346–352. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, X.; Wang, S.; Ding, J.; Yuan, N. A self-healing conductive and stretchable aligned carbon nanotube/hydrogel composite with a sandwich structure. Nanoscale 2018, 10, 19360–19366. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Xu, H.; Wu, Q.; Liu, C.; Yang, B.-R.; Gui, X.; Xie, X.; Tao, K.; Shen, Y.; et al. An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater. Horiz. 2019, 6, 595–603. [Google Scholar] [CrossRef]
- Wang, S.; Dai, S.; Yan, H.; Ding, J.; Yuan, N. Conductive double-crosslinked network hydrogel with superior stretchability and self-healing ability. Mater. Res. Express 2019, 6, 105712. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, Y.; Yu, W.; Zhang, H. Highly Stretchable and Self-Healing Strain Sensor Based on Gellan Gum Hybrid Hydrogel for Human Motion Monitoring. ACS Appl. Polym. Mater. 2020, 2, 1325–1334. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.A.; Tian, J.; Lu, F.; He, M. Highly Stretchable and Compressible Cellulose Ionic Hydrogels for Flexible Strain Sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, M.; Zhang, H.; Song, Y.; Jiang, X.; Yu, A.; Jiang, L.; Su, B. Healable green hydrogen bonded networks for circuit repair, wearable sensor and flexible electronic devices. J. Mater. Chem. A 2017, 5, 13138–13144. [Google Scholar] [CrossRef]
- Gunal, G.; Okan, M.; Gokcen, D.; Caykara, T.; Aydin, H.M. Microwave-Assisted Synthesis of Stretchable and Transparent Poly(Ethyleneglycol-Sebacate) Elastomers with Autonomous Self-Healing and Capacitive Properties. Soft Rob. 2021, 8, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, B.; Lyu, Q.; Jia, L.; Tan, H.; Xie, Z.; Xiong, B.; Xue, Z.; Zhang, L.; Zhu, J. Self-healing and recyclable photonic elastomers based on a water soluble supramolecular polymer. Mater. Chem. Front. 2019, 3, 2707–2715. [Google Scholar] [CrossRef]
- Nurhamiyah, Y.; Amir, A.; Finnegan, M.; Themistou, E.; Edirisinghe, M.; Chen, B. Wholly Biobased, Highly Stretchable, Hydrophobic, and Self-healing Thermoplastic Elastomer. ACS Appl. Mater. Interfaces 2021, 13, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.S.; Arshad, M.; Qureshi, A.; Ullah, A. Fabrication of a Self-Healing, 3D Printable, and Reprocessable Biobased Elastomer. ACS Appl. Mater. Interfaces 2020, 12, 51927–51939. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Fan, D.; Cao, J.; Huang, X.; Zheng, Z.; Zhang, X. Scalable manufacturing of real-time self-healing strain sensors based on brominated natural rubber. Chem. Eng. J. 2020, 389, 124448. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhang, L.; Lai, X.; Zeng, X. Highly stretchable, transparent and room-temperature self-healable polydimethylsiloxane elastomer for bending sensor. J. Colloid Interface Sci. 2020, 570, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Y.; Wang, Z.; Song, C.; Gao, C.; Wu, Y. A type of self-healable, dissoluble and stretchable organosilicon elastomer for flexible electronic devices. Eur. Polym. J. 2020, 134, 109857. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Song, J.; Gao, C.; Wang, Z.; Song, C.; Wu, Y.; Liu, Y. Self-Healing Ti3C2 MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interfaces 2020, 12, 45306–45314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, S.; Jin, X.; Niu, P.; Zhang, G.; Wu, Y.; Li, H. Tough, self-healable and conductive elastomers based on freezing-thawing strategy. Chem. Eng. J. 2020, 402, 125421. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, C.; Zhang, Y.; Chen, X.; Yang, B.; Xia, J.; Bian, L. Mussel cuticle-mimetic ultra-tough, self-healing elastomers with double-locked nanodomains exhibit fast stimuli-responsive shape transformation. J. Mater. Chem. A 2020, 8, 12463–12471. [Google Scholar] [CrossRef]
- Ding, L.; Chen, L.; Hu, L.; Feng, X.; Mao, Z.; Xu, H.; Wang, B.; Sui, X. Self-healing and acidochromic polyvinyl alcohol hydrogel reinforced by regenerated cellulose. Carbohydr. Polym. 2021, 255, 117331. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, L.; Yang, S.; Qin, W.; Feng, Y.; Liu, Y.; Zhou, Y.; Yu, G.; Li, J. Highly stretchable and tough alginate-based cyclodextrin/Azo-polyacrylamide interpenetrating network hydrogel with self-healing properties. Carbohydr. Polym. 2021, 256, 117595. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Feng, J.; Shi, J.; He, L.; Guo, P.; Guan, S.; Fu, H.; Ao, Y. Ultra-stretchable, self-recovering, self-healing cationic guar gum/poly(stearyl methacrylate-co-acrylic acid) hydrogels. Carbohydr. Polym. 2021, 256, 117563. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.S.; Jung, Y.C.; Oh, J.-W.; Na, Y.H. Development of a Tough, Self-Healing Polyampholyte Terpolymer Hydrogel Patch with Enhanced Skin Adhesion via Tuning the Density and Strength of Ion-Pair Associations. ACS Appl. Mater. Interfaces 2021, 13, 8889–8900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, C.-W.; Chang, S.-W.; Hsu, S.-H. An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater. 2021, 122, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ren, X.; Bai, Y.; Liu, L.; Wu, G. Adhesive and tough hydrogels promoted by quaternary chitosan for strain sensor. Carbohydr. Polym. 2021, 254, 117298. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Park, W.H. Dual-crosslinked, self-healing and thermo-responsive methylcellulose/chitosan oligomer copolymer hydrogels. Carbohydr. Polym. 2021, 258, 117705. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ren, Z.; Liu, X.; Ling, Q.; Li, Z.; Gu, H. A Multifunctional, Self-Healing, Self-Adhesive, and Conductive Sodium Alginate/Poly(vinyl alcohol) Composite Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 11344–11355. [Google Scholar] [CrossRef]
- Kakati, A.; Das, S. Self-transformed parallel structures in strain sensitive Au thin film micropattern embedded on soft elastomer. J. Micromech. Microeng. 2020, 30, 115004. [Google Scholar] [CrossRef]
- McGhee, J.R.; Sagu, J.S.; Southee, D.J.; Evans, P.S.A.; Wijayantha, K.G.U. Printed, Fully Metal Oxide, Capacitive Humidity Sensors Using Conductive Indium Tin Oxide Inks. ACS Appl. Electron. Mater. 2020, 2, 3593–3600. [Google Scholar] [CrossRef]
- Toral, V.; Loghin, F.C.; Rodriguez-Dieguez, A.; Lapresta-Fernandez, A.; Morales, D.P.; Rivadeneyra, A.; Salinas-Castillo, A. Optimization of Cost-Effective and Reproducible Flexible Humidity Sensors Based on Metal-Organic Frameworks. Sensors 2020, 20, 6981. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shen, Y.; Tian, L.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C.-P. A flexible, ultra-highly sensitive and stable capacitive pressure sensor with convex microarrays for motion and health monitoring. Nano Energy 2020, 70, 104436. [Google Scholar] [CrossRef]
- La Malfa, F.; Puce, S.; Rizzi, F.; De Vittorio, M. A Flexible Carbon Nanotubes-Based Auxetic Sponge Electrode for Strain Sensors. Nanomaterials 2020, 10, 2365. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, N.; Navaraj, W.T.; Gupta, S.; Liu, F.; Vinciguerra, V.; Lorenzelli, L.; Dahiya, R. Piezoelectric graphene field effect transistor pressure sensors for tactile sensing. Appl. Phys. Lett. 2018, 113, 014102. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, B.; Wei, G.; Wu, J.M.; Han, W.; Yang, Y. Polyimide/Graphene Nanocomposite Foam-Based Wind-Driven Triboelectric Nanogenerator for Self-Powered Pressure Sensor. Adv. Mater. Technol. 2019, 4, 1800723. [Google Scholar] [CrossRef]
- Ke, K.; McMaster, M.; Christopherson, W.; Singer, K.D.; Manas-Zloczower, I. Highly sensitive capacitive pressure sensors based on elastomer composites with carbon filler hybrids. Compos. Part A 2019, 126, 105614. [Google Scholar] [CrossRef]
- Wei, P.; Guo, X.; Qiu, X.; Yul, D. Flexible capacitive pressure sensor with sensitivity and linear measuring range enhanced based on porous composite of carbon conductive paste and polydimethylsiloxane. Nanotechnology 2019, 30, 455501. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kwon, D.; Kim, K.; Park, J.; Del Orbe, D.; Gu, J.; Ahn, J.; Cho, I.; Jeong, Y.; Oh, Y.S.; et al. Synergetic Effect of Porous Elastomer and Percolation of Carbon Nanotube Filler towards High Performance Capacitive Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Gao, Y.; Yu, G.; Lu, C.; Tan, J.; Xuan, F. Flexible pressure sensor using carbon nanotube-wrapped polydimethylsiloxane microspheres for tactile sensing. Sens. Actuators A 2018, 284, 260–265. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zeng, X.; Fu, X.; Hu, Y. Expandable microsphere-based triboelectric nanogenerators as ultrasensitive pressure sensors for respiratory and pulse monitoring. Nano Energy 2019, 59, 295–301. [Google Scholar] [CrossRef]
- Wang, J.; Suzuki, R.; Shao, M.; Gillot, F.; Shiratori, S. Capacitive Pressure Sensor with Wide-Range, Bendable, and High Sensitivity Based on the Bionic Komochi Konbu Structure and Cu/Ni Nanofiber Network. ACS Appl. Mater. Interfaces 2019, 11, 11928–11935. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Tran Quang, T.; Lee, N.-E. Recent Progress on Stretchable Electronic Devices with Intrinsically Stretchable Components. Adv. Mater. 2017, 29, 1603167. [Google Scholar]
- Xu, W.; Wang, W.; Chen, S.; Zhang, R.; Wang, Y.; Zhang, Q.; Yuwen, L.; Yang, W.J.; Wang, L. Molybdenum disulfide (MoS2) nanosheets-based hydrogels with light-triggered self-healing property for flexible sensors. J. Colloid Interface Sci. 2021, 586, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bai, Y.; Sun, J.; Lv, K.; Lei, S.; Qiu, J. Tough and self-healing hydrophobic association hydrogels with cationic surfactant. J. Appl. Polym. Sci. 2021, 138, 50645. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Li, Y.; Wang, X.; Yang, W.; Ren, J. Polypyrrole-Doped Conductive Self-Healing Composite Hydrogels with High Toughness and Stretchability. Biomacromolecules 2021, 22, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Li, J.; Li, K.; Yu, F.; Ma, J. Highly flexible, self-healable and conductive poly(vinyl alcohol)/Ti3C2Tx MXene film and it’s application in capacitive deionization. Chem. Eng. J. 2021, 408, 127256. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, F.; Cai, H.; Li, X.; Sun, J.; Wu, Y.; Wang, N.; Zhu, Y. Robust versatile nanocellulose/polyvinyl alcohol/carbon dot hydrogels for biomechanical sensing. Carbohydr. Polym. 2021, 259, 117753. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, H.; Zhang, Z.; Yao, Y.; Bao, X.; Hu, Q. Ultrastretchable, self-adhesive, strain-sensitive and self-healing GO@DA/Alginate/P(AAc-co-AAm) multifunctional hydrogels via mussel-inspired chemistry. Carbohydr. Polym. 2021, 254, 117316. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Hauck, M.; Wacker, I.; Zeller-Plumhoff, B.; Rasch, F.; Taale, M.; Nia, A.S.; Feng, X.; Adelung, R.; Schroeder, R.R.; et al. Microengineered Hollow Graphene Tube Systems Generate Conductive Hydrogels with Extremely Low Filler Concentration. Nano Lett. 2021, 21, 3690–3697. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, Z.; Liu, F.; Zhao, L.; Ling, Q.; Gu, H. Multifunctional Self-Healing Dual Network Hydrogels Constructed via Host-Guest Interaction and Dynamic Covalent Bond as Wearable Strain Sensors for Monitoring Human and Organ Motions. ACS Appl. Mater. Interfaces 2021, 13, 14625–14635. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Lee, W.; Kim, S.-M.; Lee, M.; Koo, J.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Ion-conductive self-healing hydrogels based on an interpenetrating polymer network for a multimodal sensor. Chem. Eng. J. 2019, 371, 452–460. [Google Scholar] [CrossRef]
- Tie, J.; Rong, L.; Liu, H.; Wang, B.; Mao, Z.; Zhang, L.; Zhong, Y.; Feng, X.; Sui, X.; Xu, H. An autonomously healable, highly stretchable and cyclically compressible, wearable hydrogel as a multimodal sensor. Polym. Chem. 2020, 11, 1327–1336. [Google Scholar] [CrossRef]
- Soltani, S.; Emadi, R.; Javanmard, S.H.; Kharaziha, M.; Rahmati, A. Shear-thinning and self-healing nanohybrid alginate-graphene oxide hydrogel based on guest-host assembly. Int. J. Biol. Macromol. 2021, 180, 311–323. [Google Scholar] [CrossRef]
- Wang, H.-J.; Chu, Y.-Z.; Chen, C.-K.; Liao, Y.-S.; Yeh, M.-Y. Preparation of conductive self-healing hydrogels via an interpenetrating polymer network method. RSC Adv. 2021, 11, 6620–6627. [Google Scholar] [CrossRef]
- Xiao, G.; Fu, S.; Lucia, L.A. Poly(aminobenzeneboronic acid)-mediated rapid self-healing and shape memory cellulose crystal nanohydrogels. Carbohydr. Polym. 2021, 255, 117495. [Google Scholar] [CrossRef]
- Hussain, I.; Ma, X.; Luo, Y.; Luo, Z. Fabrication and characterization of glycogen-based elastic, self-healable, and conductive hydrogels as a wearable strain-sensor for flexible e-skin. Polymer 2020, 210, 122961. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hussain, I.; Oderinde, O.; Yao, F.; Zhang, J.; Fu, G. A Conductive Self-Healing Double Network Hydrogel with Toughness and Force Sensitivity. Chem. Eur. J. 2018, 24, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Kim, Y.; Kweon, O.Y.; Won, Y.; Oh, J.H. Deformable and Stretchable Electrodes for Soft Electronic Devices. Macromol. Res. 2019, 27, 625–639. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Dong, Y.; Ma, S.; Ren, J.; Yang, X.; Wang, Y.; Lu, S. Superstretching MXene Composite Hydrogel as a Bidirectional Stress Response Thixotropic Sensor. ACS Appl. Mater. Interfaces 2021, 13, 13629–13636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Lv, X.; Sun, S. A highly sensitive strain sensor based on a silica@polyaniline core-shell particle reinforced hydrogel with excellent flexibility, stretchability, toughness and conductivity. Soft Matter 2021, 17, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int. J. Biol. Macromol. 2021, 170, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Gong, Q.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Facile preparation of stretchable and self-healable conductive hydrogels based on sodium alginate/polypyrrole nanofibers for use in flexible supercapacitor and strain sensors. Int. J. Biol. Macromol. 2021, 172, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, S.; Peng, Z.; Shi, W.; Liu, Z.; Shi, H.; Luo, K.; Wei, G.; Mo, H.; Li, B.; et al. Topologically Enhanced Dual-Network Hydrogels with Rapid Recovery for Low-Hysteresis, Self-Adhesive Epidemic Electronics. ACS Appl. Mater. Interfaces 2021, 13, 12531–12540. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Q.; Liu, Y.; Wang, S.; Wang, J.; Liu, X. Enhanced strength and toughness of kappa-carrageenan/polyacrylic acid physical double-network hydrogels by dual cross-linking of the first network. Eur. Polym. J. 2020, 124, 109474. [Google Scholar] [CrossRef]
- Gao, Z.J.; Kong, L.S.; Jin, R.N.; Liu, X.; Hu, W.; Gao, G.H. Mechanical, adhesive and self-healing ionic liquid hydrogels for electrolytes and flexible strain sensors. J. Mater. Chem. C 2020, 8, 11119–11127. [Google Scholar] [CrossRef]
- Hua, D.W.; Gao, S.T.; Zhang, M.J.; Ma, W.J.; Huang, C.B. A novel xanthan gum-based conductive hydrogel with excellent mechanical, biocompatible, and self-healing performances. Carbohydr. Polym. 2020, 247, 116743. [Google Scholar] [CrossRef]
- Sun, X.X.; Luo, C.H.; Luo, F.L. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar] [CrossRef]
- Tong, X.; Du, L.; Xu, Q. Tough, adhesive and self-healing conductive 3D network hydrogel of physically linked functionalized-boron nitride/clay/poly(N-isopropylacrylamide). J. Mater. Chem. A 2018, 6, 3091–3099. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Biofunctional hydrogels based on host-guest interactions. Polym. J. 2020, 52, 839–859. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive Self-Healing Hybrid Gel Enabled by Metal-Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.Y.; Zou, Y.; Wu, S.P.; Zou, X.B. Self-healing and tough hydrogels with conductive properties prepared through an interpenetrating polymer network strategy. Polymer 2020, 206, 122907. [Google Scholar] [CrossRef]
- Liu, S.L.; Kang, M.M.; Li, K.W.; Yao, F.; Oderinde, O.; Fu, G.D.; Xu, L.Q. Polysaccharide-templated preparation of mechanically-tough, conductive and self-healing hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Dang, C.; Wang, M.; Yu, J.; Chen, Y.; Zhou, S.; Feng, X.; Liu, D.; Qi, H. Transparent, Highly Stretchable, Rehealable, Sensing, and Fully Recyclable Ionic Conductors Fabricated by One-Step Polymerization Based on a Small Biological Molecule. Adv. Funct. Mater. 2019, 29, 1902467. [Google Scholar] [CrossRef]
- Wu, G.; Jin, K.; Liu, L.; Zhang, H. A rapid self-healing hydrogel based on PVA and sodium alginate with conductive and cold-resistant properties. Soft Matter 2020, 16, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A Self-Healing Cellulose Nanocrystal-Poly(ethylene glycol) Nanocomposite Hydrogel via Diels-Alder Click Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Chen, H.; Hao, B.; Ge, P.; Chen, S. Highly stretchable, self-healing, and 3D printing prefabricatable hydrophobic association hydrogels with the assistance of electrostatic interaction. Polym. Chem. 2020, 11, 4741–4748. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Gao, G.-R.; Du, G.-L.; Cheng, Y.-J.; Fu, J. Super Tough, Ultrastretchable, and Thermoresponsive Hydrogels with Functionalized Triblock Copolymer Micelles as Macro-Cross-Linkers. ACS Macro Lett. 2014, 3, 496–500. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Sahin, M.; Sari, M.; Okay, O. Structure optimization of self-healing hydrogels formed via hydrophobic interactions. Polymer 2012, 53, 5513–5522. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Shang, X.; Hu, W.; Gao, G.; Duan, L. Bio-inspired adhesive and self-healing hydrogels as flexible strain sensors for monitoring human activities. Mater. Sci. Eng. C 2020, 106, 110168. [Google Scholar] [CrossRef]
- Luo, C.; Wei, N.; Fu, W. A highly elastic and sensitive sensor based on GSP/HPAM composited hydrogel. J. Appl. Polym. Sci. 2021, 138, 50192. [Google Scholar] [CrossRef]
- Yang, W.; Shao, B.; Liu, T.; Zhang, Y.; Huang, R.; Chen, F.; Fuo, Q. Robust and Mechanically and Electrically Self-Healing Hydrogel for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 8245–8257. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.F.; Zhang, B.; Wan, K.N.; Zhu, J.X.; Xu, J.S.; Zhang, C.; Liu, T.X. Hydrogen-bonded network enables semi-interpenetrating ionic conductive hydrogels with high stretchability and excellent fatigue resistance for capacitive/resistive bimodal sensors. Chem. Eng. J. 2021, 411, 128506. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Gao, J.; Wei, Z.; Zhou, J.; Chen, Y.M. Facile fabrication of self-healing carboxymethyl cellulose hydrogels. Eur. Polym. J. 2015, 72, 514–522. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wu, K.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Synthesis of cellulose-based double-network hydrogels demonstrating high strength, self-healing, and antibacterial properties. Carbohydr. Polym. 2017, 168, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Peng, Q.Y.; Thundat, T.; Zeng, H.B. Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Liu, T.; Zou, S.S.; Hang, C.; Li, J.; Di, X.; Li, X.H.; Wu, Q.; Wang, F.F.; Sun, P.C. Mechanically strong and tough hydrogels with pH-triggered self-healing and shape memory properties based on a dual physically crosslinked network. Polym. Chem. 2020, 11, 1906–1918. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Shi, J.; Wu, W.; Debeli, D.K.; Pan, P.; Shan, G. Fast photothermal poly(NIPAM-co-beta-cyclodextrin) supramolecular hydrogel with self-healing through host-guest interaction for intelligent light-controlled switches. Soft Matter 2020, 16, 10558–10566. [Google Scholar] [CrossRef]
- Itami, T.; Hashidzume, A.; Kamon, Y.; Yamaguchi, H.; Harada, A. The macroscopic shape of assemblies formed from microparticles based on host-guest interaction dependent on the guest content. Sci. Rep. 2021, 11, 6320. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Long, Y.; Li, L.; Du, K.; Hou, K.; Ji, J.; Yang, C.; Zhong, K.; Cai, H.; et al. A supramolecular photonic crystal hydrogel based on host-guest interactions for organic molecule recognition. J. Mater. Chem. C 2020, 8, 14718–14722. [Google Scholar] [CrossRef]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Miao, W. The preparation of pH-sensitive hydrogel based on host-guest and electrostatic interactions and its drug release studies in vitro. J. Polym. Res. 2018, 25, 215. [Google Scholar] [CrossRef]
- Lee, H.; Ha, Y.-M.; Lee, S.H.; Ko, Y.-i.; Muramatsu, H.; Kim, Y.A.; Park, M.; Jung, Y.C. Spontaneously restored electrical conductivity of bioactive gel comprising mussel adhesive protein-coated carbon nanotubes. RSC Adv. 2016, 6, 87044–87048. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Yao, F.; Oderinde, O.; Fu, G. Hydroxyethyl cellulose-based self-healing hydrogels with enhanced mechanical properties via metal-ligand bond interactions. Eur. Polym. J. 2018, 100, 219–227. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- An, S.Y.; Noh, S.M.; Oh, J.K. Multiblock Copolymer-Based Dual Dynamic Disulfide and Supramolecular Crosslinked Self-Healing Networks. Macromol. Rapid Commun. 2017, 38, 1600777. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, S.; Zhao, M.; Lin, X.; Zhang, M.; Xiao, H.; Liu, K.; Huang, L.; Chen, L.; Ouyang, X.; et al. Self-Healing Cellulose Nanocrystals-Containing Gels via Reshuffling of Thiuram Disulfide Bonds. Polymers 2018, 10, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.; Wang, X.; Li, X.; An, H.; Qin, J. Self-Activated Healable Hydrogels with Reversible Temperature Responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 25544–25551. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Feng, W.; Guan, S.; Guo, P. Self-healing hydroxypropyl guar gum/poly(acrylamide-co-3-acrylamidophenyl boronic acid) composite hydrogels with yield phenomenon based on dynamic PBA ester bonds and H-bond. Colloids Surf. A 2019, 561, 325–331. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-Pot Assembly of Microfibrillated Cellulose Reinforced PVA-Borax Hydrogels with Self-Healing and pH-Responsive Properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, L.; Cheng, Q.; Zhang, T.; Jiang, B.; Song, Y.; Huang, Y. Self-Healing Polysiloxane Elastomer Based on Integration of Covalent and Reversible Networks. Ind. Eng. Chem. Res. 2019, 58, 21504–21512. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Ge, W.; Cao, S.; Yang, Y.; Rojas, O.J.; Wang, X. Nanocellulose/LiCl systems enable conductive and stretchable electrolyte hydrogels with tolerance to dehydration and extreme cold conditions. Chem. Eng. J. 2021, 408, 127306. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. A Powder Self-Healable Hydrogel Electrolyte for Flexible Hybrid Supercapacitors with High Energy Density and Sustainability. Small 2021, 17, 2006807. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Z.Y.; Piao, J.H.; Chen, X.F.; Lou, Y.X.; Li, S.H. Electrical behavior of carbon black-filled polymer composites—Effect of interaction between filler and matrix. J. Appl. Polym. Sci. 1994, 51, 1159–1164. [Google Scholar] [CrossRef]
- Jager, K.M.; McQueen, D.H.; Tchmutin, I.A.; Ryvkina, N.G.; Kluppel, M. Electron transport and ac electrical properties of carbon black polymer composites. J. Phys. D Appl. Phys. 2001, 34, 2699–2707. [Google Scholar] [CrossRef]
- Guillet, J.-F.; Valdez-Nava, Z.; Golzio, M.; Flahaut, E. Electrical properties of double-wall carbon nanotubes nanocomposite hydrogels. Carbon 2019, 146, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, Y.; Mensaha, A.; Li, D.; Wang, Q.; Wei, Q. A plant-inspired long-lasting adhesive bilayer nanocomposite hydrogel based on redox-active Ag/Tannic acid-Cellulose nanofibers. Carbohydr. Polym. 2021, 255, 117508. [Google Scholar] [CrossRef] [PubMed]
- Chitra, G.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. Noncytotoxic silver and gold nanocomposite hydrogels with enhanced antibacterial and wound healing applications. Polym. Eng. Sci. 2018, 58, 2133–2142. [Google Scholar] [CrossRef]
- Dai, X.; Wang, J.; Teng, F.; Shao, Z.; Huang, X. Zr(IV)-Crosslinked Polyacrylamide/Polyanionic Cellulose Composite Hydrogels with High Strength and Unique Acid Resistance. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 981–991. [Google Scholar] [CrossRef]
- Espinoza-Ibarra, P.A.; Sanchez-Valdes, S.; Yanez-Flores, I.G.; Graciano-Verdugo, A.Z.; Espinoza-Ibarra, D.F.; Fernandez-Tavizon, S.; Ledezma-Perez, A.S.; Espinoza-Martinez, A.B.; Rodriguez-Fernandez, O.S.; Betancourt-Galindo, R.; et al. Preparation and characterization of cotton fibers coated with AA-IA hydrogel containing silver/graphene or graphene oxide nanoparticles. Polym. Plast. Technol. Mater. 2019, 58, 753–764. [Google Scholar] [CrossRef]
- Youssef, A.M.; Hasanin, M.S.; Abd El-Aziz, M.E.; Turky, G.M. Conducting chitosan/hydroxylethyl cellulose/polyaniline bionanocomposites hydrogel based on graphene oxide doped with Ag-NPs. Int. J. Biol. Macromol. 2021, 167, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, C.; Wang, M.; Zhang, Y.; Liu, L.; Yang, W. An Electrically and Mechanically Autonomic Self-healing Hybrid Hydrogel with Tough and Thermoplastic Properties. ACS Appl. Mater. Interfaces 2017, 9, 11134–11143. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, L.; Zhang, L.; Wang, X.; Li, Y.; Yang, W. Electroconductive and free-shapeable nanocomposite hydrogels with an ultrafast self-healing property and high stretchability performance. Soft Matter 2020, 16, 8422–8431. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qiao, Z.; Zhang, Y.; Wei, D.; Chen, S.; Tang, J.; Chen, L.; Wei, D.; Sun, J.; Fan, H. NIR-responsive multi-healing HMPAM/dextran/AgNWs hydrogel sensor with recoverable mechanics and conductivity for human-machine interaction. Carbohydr. Polym. 2020, 247, 116686. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, S.Y.; Lin, J.; Wu, Z.L.; Yin, J.; Qian, J.; Qu, S.; Zheng, Q. Integrated multifunctional flexible electronics based on tough supramolecular hydrogels with patterned silver nanowires. J. Mater. Chem. C 2020, 8, 7688–7697. [Google Scholar] [CrossRef]
- Han, X.; Lv, Z.; Ran, F.; Dai, L.; Li, C.; Si, C. Green and stable piezoresistive pressure sensor based on lignin-silver hybrid nanoparticles/polyvinyl alcohol hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef]
- Rahman, A.; Solaiman; Foyez, T.; Susan, M.A.B.H.; Imran, A.B. Self-Healable and Conductive Double-Network Hydrogels with Bioactive Properties. Macromol. Chem. Phys. 2020, 221, 2000207. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Mariano, M.; Mashtalir, O.; Antonio, F.Q.; Ryu, W.-H.; Deng, B.; Xia, F.; Gogotsi, Y.; Taylor, A.D. Solution-processed titanium carbide MXene films examined as highly transparent conductors. Nanoscale 2016, 8, 16371–16378. [Google Scholar] [CrossRef]
- Shang, T.; Lin, Z.; Qi, C.; Liu, X.; Li, P.; Tao, Y.; Wu, Z.; Li, D.; Simon, P.; Yang, Q.-H. 3D Macroscopic Architectures from Self-Assembled MXene Hydrogels. Adv. Funct. Mater. 2019, 29, 1903960. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Pan, X.; Wang, X.; Gao, H.; Chen, Y.; Chen, L.; Ni, Y.; Cao, S.; Ma, X. Spider web-inspired ultra-stable 3D Ti3C2TX (MXene) hydrogels constructed by temporary ultrasonic alignment and permanent in-situ self-assembly fixation. Compos. Part B 2020, 197, 108187. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Y.; Li, X.; Zhang, C.; Cao, M.; Chen, W.; Luo, S.; Zhu, M.; Gao, Y. Flexible and high-sensitivity piezoresistive sensor based on MXene composite with wrinkle structure. Ceram. Int. 2020, 46, 23592–23598. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareefit, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, 0098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Wei, Y.; Xiang, L.; Ou, H.; Li, F.; Zhang, Y.; Qian, Y.; Hao, L.; Diao, J.; Zhang, M.; Zhu, P.; et al. MXene-Based Conductive Organohydrogels with Long-Term Environmental Stability and Multifunctionality. Adv. Funct. Mater. 2020, 30, 2005135. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.-Z.; Zhang, W.; Yuan, W.; El-Demellawi, J.K.; Zhang, P.; Di Fabrizio, E.; Dong, X.; Alshareef, H.N. Ti3C2Tx MXene-Activated Fast Gelation of Stretchable and Self-Healing Hydrogels: A Molecular Approach. ACS Nano 2021, 15, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, X.; Wang, X.; Cao, S.; Chen, L.; Ma, X.; Huang, L.; Ni, Y. Fabrication strategies and application fields of novel 2D Ti3C2Tx (MXene) composite hydrogels: A mini-review. Ceram. Int. 2021, 47, 4398–4403. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Guo, J.; Zhang, Z.; Zhang, X.; Zhao, Y. Bio-Inspired Stretchable, Adhesive, and Conductive Structural Color Film for Visually Flexible Electronics. Adv. Funct. Mater. 2020, 30, 2000151. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Fan, M.; Tang, P.; Yang, S.; Pan, L.; Wang, H.; Bin, Y. Strong and tough PVA/PAA hydrogel fiber with highly strain sensitivity enabled by coating MWCNTs. Compos. Part A 2020, 138, 106050. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Shi, Z.; Santaniello, T.; Lenardi, C.; Huang, J. Mechanical characteristics of tunable uniaxial aligned carbon nanotubes induced by robotic extrusion technique for hydrogel nanocomposite. Compos. Part A 2020, 129, 105707. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, C.; Li, Y.; Xiang, D.; Wang, Z. Highly stretchable, self-healing, and strain-sensitive based on double-crosslinked nanocomposite hydrogel. Compos. Commun. 2020, 17, 22–27. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, X.; Li, W.; Tang, Y.; Bai, Y. Quickly self-healing hydrogel at room temperature with high conductivity synthesized through simple free radical polymerization. J. Appl. Polym. Sci. 2019, 136, 47379. [Google Scholar] [CrossRef]
- Yi, F.-L.; Meng, F.-C.; Li, Y.-Q.; Huang, P.; Hu, N.; Liao, K.; Fu, S.-Y. Highly stretchable CNT Fiber/PAAm hydrogel composite simultaneously serving as strain sensor and supercapacitor. Compos. Part B 2020, 198, 108246. [Google Scholar] [CrossRef]
- Ahn, J.; Pak, S.; Song, Y.; Kim, H. In-situ synthesis of carbon dot at cellulose nanofiber for durable water treatment membrane with high selectivity. Carbohydr. Polym. 2021, 255, 117387. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni-Co-O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Pan, K.; Peng, S.; Chu, Y.; Liang, K.; Wang, C.H.; Wu, S.; Xu, J. Highly sensitive, stretchable and durable strain sensors based on conductive double-network polymer hydrogels. J. Polym. Sci. 2020, 58, 3069–3081. [Google Scholar] [CrossRef]
- Gao, F.; Teng, H.; Song, J.; Xu, G.; Luo, X. A flexible and highly sensitive nitrite sensor enabled by interconnected 3D porous polyaniline/carbon nanotube conductive hydrogels. Anal. Methods 2020, 12, 604–610. [Google Scholar] [CrossRef]
- Han, S.; Liu, C.; Lin, X.; Zheng, J.; Wu, J.; Liu, C. Dual Conductive Network Hydrogel for a Highly Conductive, Self-Healing, Anti-Freezing, and Non-Drying Strain Sensor. ACS Appl. Polym. Mater. 2020, 2, 996–1005. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Yu, Q.; Zhang, H.; Wu, X.; Yao, M.; Liu, W.; Yao, F.; Li, J. Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S. Advanced ferrogels with high magnetic response and wear resistance using carbon nanotubes. J. Alloys Compd. 2020, 848, 156259. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, L.; Qiao, Z.; Wang, J.; Jiang, X.; Zhang, Y.S.; Yang, H. Functionalizing Double-Network Hydrogels for Applications in Remote Actuation and in Low-Temperature Strain Sensing. ACS Appl. Mater. Interfaces 2020, 12, 30247–30258. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, H.; Yue, Y.; Mei, C.; Chen, J.; Huang, C.; Wu, Q.; Xu, X. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhang, Q.; Ji, X.-X.; Liu, L.-B. Highly Stretchable, Compressible, Adhesive, Conductive Self-healing Composite Hydrogels with Sensor Capacity. Chin. J. Polym. Sci. 2020, 38, 1221–1229. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Jia, F.; Gao, G. A flexible, adhesive and self-healable hydrogel-based wearable strain sensor for human motion and physiological signal monitoring. J. Mater. Chem. B 2019, 7, 4638–4648. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Chen, L.; Ouyang, X.; Huang, L.; Wang, H.; Xu, S. A bionic tactile plastic hydrogel-based electronic skin constructed by a nerve-like nanonetwork combining stretchable, compliant, and self-healing properties. Chem. Eng. J. 2020, 379, 122271. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Khan, R.; Liu, H.; Chen, C.; Chen, T.; Zhang, R.; Li, H. A fast self-healing and conductive nanocomposite hydrogel as soft strain sensor. Colloids Surf. A 2019, 567, 139–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, E.; Li, A.; Cui, C.; Guo, R.; Tang, H.; Xiao, H.; Zhou, M.; Qin, W.; Wang, X.; et al. A porous self-healing hydrogel with an island-bridge structure for strain and pressure sensors. J. Mater. Chem. B 2021, 9, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Mutlu, H.; Malik, S.; Theato, P. Conductive hydrogel composites with autonomous self-healing properties. Soft Matter 2020, 16, 10969–10976. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Yu, Y.; Chen, S.; Yang, Y.; Tang, Y. Conductive nanocomposite hydrogels with self-healing property. RSC Adv. 2014, 4, 35149–35155. [Google Scholar] [CrossRef]
- Guan, Q.; Lin, G.; Gong, Y.; Wang, J.; Tan, W.; Bao, D.; Liu, Y.; You, Z.; Sun, X.; Wen, Z.; et al. Highly efficient self-healable and dual responsive hydrogel-based deformable triboelectric nanogenerators for wearable electronics. J. Mater. Chem. A 2019, 7, 13948–13955. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Li, Y.; Gao, G. Highly sensitive and wearable gel-based sensors with a dynamic physically cross-linked structure for strain-stimulus detection over a wide temperature range. J. Mater. Chem. C 2019, 7, 11303–11314. [Google Scholar] [CrossRef]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Raza-Karimi, A.; Khodadadi, A. Mechanically Robust 3D Nanostructure Chitosan-Based Hydrogels with Autonomic Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 27254–27263. [Google Scholar] [CrossRef]
- Gu, J.; Huang, J.; Chen, G.; Hou, L.; Zhang, J.; Zhang, X.; Yang, X.; Guan, L.; Jiang, X.; Liu, H. Multifunctional Poly(vinyl alcohol) Nanocomposite Organohydrogel for Flexible Strain and Temperature Sensor. ACS Appl. Mater. Interfaces 2020, 12, 40815–40827. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host-Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Chang, S.; Wang, B.; Liu, Y.; Li, Z.; Hu, X.; Zhang, X.; Zhang, H. Radiation-assistant preparation of highly conductive, transparent and self-healing hydrogels with triple-network structure. Polymer 2020, 188, 122156. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- An, R.; Zhang, B.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Strain-sensitivity conductive MWCNTs composite hydrogel for wearable device and near-infrared photosensor. J. Mater. Sci. 2019, 54, 8515–8530. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.; Chen, X.; Wang, X.-W.; Zhang, W.-B.; Peng, J.; Li, J.; Zhai, M. Stretchable, Conductive, and Self-Healing Hydrogel with Super Metal Adhesion. Chem. Mater. 2018, 30, 4289–4297. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Zhan, R.; Xu, K.; Wang, Y.; Zhang, X.; Li, B.; Luo, G.; Xing, M.; Zhong, W. Tough but self-healing and 3D printable hydrogels for E-skin, E-noses and laser controlled actuators. J. Mater. Chem. A 2019, 7, 24814–24829. [Google Scholar] [CrossRef]
- Ma, D.; Wu, X.; Wang, Y.; Liao, H.; Wan, P.; Zhang, L. Wearable, Antifreezing, and Healable Epidermal Sensor Assembled from Long-Lasting Moist Conductive Nanocomposite Organohydrogel. ACS Appl. Mater. Interfaces 2019, 11, 41701–41709. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, S.K.; Zhu, S.; Lu, Y.; Yue, Y.; Han, J.; Xu, X.; Wu, Q.; Xiao, H. Self-Healable Electro-Conductive Hydrogels Based on Core-Shell Structured Nanocellulose/Carbon Nanotubes Hybrids for Use as Flexible Supercapacitors. Nanomaterials 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liu, S.; Shi, X.; Han, D.; Liang, F. A thermally responsive host-guest conductive hydrogel with self-healing properties. Mater. Chem. Front. 2018, 2, 2212–2219. [Google Scholar] [CrossRef]
- Han, L.; Cui, S.; Yu, H.-Y.; Song, M.; Zhang, H.; Grishkewich, N.; Huang, C.; Kim, D.; Tam, K.M.C. Self-Healable Conductive Nanocellulose Nanocomposites for Biocompatible Electronic Skin Sensor Systems. ACS Appl. Mater. Interfaces 2019, 11, 44642–44651. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Tong, X.; Dai, K.; Xu, Q. A super-stretchable and tough functionalized boron nitride/PEDOT:PSS/poly(N-isopropylacrylamide) hydrogel with self-healing, adhesion, conductive and photothermal activity. J. Mater. Chem. A 2019, 7, 8204–8209. [Google Scholar] [CrossRef]
- Ding, X.; Jia, R.; Gan, Z.; Du, Y.; Wang, D.; Xu, X. Tough and conductive polymer hydrogel based on double network for photo-curing 3D printing. Mater. Res. Express 2020, 7, 055304. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Huang, T.; Yu, A. A conductive self-healing hydrogel binder for high-performance silicon anodes in lithium-ion batteries. J. Power Sources 2020, 449, 227472. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Wang, S.; Guo, G.; Lu, X.; Ji, S.; Tan, G.; Gao, L. Facile Soaking Strategy Toward Simultaneously Enhanced Conductivity and Toughness of Self-Healing Composite Hydrogels Through Constructing Multiple Noncovalent Interactions. ACS Appl. Mater. Interfaces 2018, 10, 19133–19142. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, P.; Nautiyal, A.; Li, S.; Liu, N.; Yin, J.; Deng, K.; Zhang, X. Tunable Three-Dimensional Nanostructured Conductive Polymer Hydrogels for Energy-Storage Applications. ACS Appl. Mater. Interfaces 2019, 11, 4258–4267. [Google Scholar] [CrossRef]
- Tahir, Z.M.; Alocilja, E.C.; Grooms, D.L. Polyaniline synthesis and its biosensor application. Biosens. Bioelectron. 2005, 20, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.T.; Tan, T.C.; Neoh, K.G.; Ong, Y.K. Halogen-induced charge transfer polymerization of pyrrole in aqueous media. Polymer 1986, 27, 1958–1962. [Google Scholar] [CrossRef]
- Qian, R.Y.; Pei, Q.B.; Huang, Z.T. The role of H+ ions in the electrochemical polymerization of pyrrole. Makromol. Chem. Macromol. Chem. Phys. 1991, 192, 1263–1273. [Google Scholar] [CrossRef]
- Omastova, M.; Trchova, M.; Kovarova, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, W.; Stevens, J.R. Proton transport in polyacrylamide based hydrogels doped with H3PO4 or H2SO4. Polymer 1997, 38, 2057–2065. [Google Scholar] [CrossRef]
- Skaarup, S.; Bay, L.; Vidanapathirana, K.; Thybo, S.; Tofte, P.; West, K. Simultaneous anion and cation mobility in polypyrrole. Solid State Ion. 2003, 159, 143–147. [Google Scholar] [CrossRef]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, H.; Zhu, J.; Luo, L.; Huang, H.; Li, L.; Yu, X. Highly Stretchable, Fast Self-Healing, Responsive Conductive Hydrogels for Supercapacitor Electrode and Motion Sensor. Macromol. Mater. Eng. 2020, 305, 2000018. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Thomas, A.K.; Patsis, P.A.; Kurth, T.; Kraeter, M.; Eckert, K.; Bornhaeuser, M.; Zhang, Y. Noncovalently Assembled Electroconductive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 14418–14425. [Google Scholar] [CrossRef]
- Yang, C.; Yin, J.; Chen, Z.; Du, H.; Tian, M.; Zhang, M.; Zheng, J.; Ding, L.; Zhang, P.; Zhang, X.; et al. Highly Conductive, Stretchable, Adhesive, and Self-Healing Polymer Hydrogels for Strain and Pressure Sensor. Macromol. Mater. Eng. 2020, 305, 2000479. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Xu, Y.; Chen, J.; Ning, N.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Highly Stretchable and Conductive Self-Healing Hydrogels for Temperature and Strain Sensing and Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 40990–40999. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Im, K.; Kim, S.W.; Kim, J.; Chung, D.-Y.; Kim, T.-H.; Jo, K.H.; Hahn, J.H.; Bao, Z.; Hwang, S.; et al. Polypyrrole/Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel. ACS Nano 2014, 8, 10066–10076. [Google Scholar] [CrossRef]
- Park, N.; Chae, S.C.; Kim, I.T.; Hur, J. Fabrication of Self-Healable and Patternable Polypyrrole/Agarose Hybrid Hydrogels for Smart Bioelectrodes. J. Nanosci. Nanotechnol. 2016, 16, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhou, M.; Fu, H. Study on mussel-inspired tough TA/PANI@CNCs nanocomposite hydrogels with superior self-healing and self-adhesive properties for strain sensors. Compos. Part B 2020, 201, 108356. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Han, J.; Ding, Q.; Mei, C.; Wu, Q.; Yue, Y.; Xu, X. An intrinsically self-healing and biocompatible electroconductive hydrogel based on nanostructured nanocellulose-polyaniline complexes embedded in a viscoelastic polymer network towards flexible conductors and electrodes. Electrochim. Acta 2019, 318, 660–672. [Google Scholar] [CrossRef]
- Song, M.; Yu, H.; Zhu, J.; Ouyang, Z.; Abdalkarim, S.Y.H.; Tam, K.C.; Li, Y. Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem. Eng. J. 2020, 398, 125547. [Google Scholar] [CrossRef]
- Han, L.; Liu, M.; Yan, B.; Li, Y.; Lan, J.; Shi, L.; Ran, R. Polydopamine/polystyrene nanocomposite double-layer strain sensor hydrogel with mechanical, self-healing, adhesive and conductive properties. Mater. Sci. Eng. C 2020, 109, 110567. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.; Ma, Y.; Yan, B.; Pan, M.; Peng, Q.; Wang, W.; Han, L.; Liu, J.; Zeng, H. Ultra elastic, stretchable, self-healing conductive hydrogels with tunable optical properties for highly sensitive soft electronic sensors. J. Mater. Chem. A 2020, 8, 24718–24733. [Google Scholar] [CrossRef]
- Lee, H.-R.; Kim, C.-C.; Sun, J.-Y. Stretchable Ionics—A Promising Candidate for Upcoming Wearable Devices. Adv. Mater. 2018, 30, 1704403. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liang, X.; Wang, Q.; Wang, M.; Li, Z.; Sun, G. A semi-interpenetrating network ionic composite hydrogel with low modulus, fast self-recoverability and high conductivity as flexible sensor. Carbohydr. Polym. 2020, 248, 116797. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Xu, B.; Zhao, J.; Rong, J. Chitosan derivative-based self-healable hydrogels with enhanced mechanical properties by high-density dynamic ionic interactions. Carbohydr. Polym. 2018, 193, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, J.; Liu, J.; Huang, X.; Wang, C. Transparent, highly-stretchable, adhesive, and ionic conductive composite hydrogel for biomimetic skin. J. Mater. Sci. 2021, 56, 2725–2737. [Google Scholar] [CrossRef]
- Huang, J.; Peng, S.; Gu, J.; Chen, G.; Gao, J.; Zhang, J.; Hou, L.; Yang, X.; Jiang, X.; Guan, L. Self-powered integrated system of a strain sensor and flexible all-solid-state supercapacitor by using a high performance ionic organohydrogel. Mater. Horiz. 2020, 7, 2085–2096. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Ren, X.; Wu, G.F. Flexible strain sensors with rapid self-healing by multiple hydrogen bonds. Polymer 2020, 202, 122657. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yu, X.; Sun, X.; Zhu, L.; Qin, G.; Dai, Y.; Chen, Q. Tough and Conductive Dual Physically Cross-Linked Hydrogels for Wearable Sensors. Ind. Eng. Chem. Res. 2019, 58, 17001–17009. [Google Scholar] [CrossRef]
- Lv, R.; Bei, Z.; Huang, Y.; Chen, Y.; Zheng, Z.; You, Q.; Zhu, C.; Cao, Y. Mussel-Inspired Flexible, Wearable, and Self-Adhesive Conductive Hydrogels for Strain Sensors. Macromol. Rapid Commun. 2020, 41, 1900450. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Lu, X.; Han, S.; Yang, B.-R.; Gui, X.; Tao, K.; Miao, J.; Liu, C. Ultrastretchable and Stable Strain Sensors Based on Antifreezing and Self-Healing Ionic Organohydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 9405–9414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, X.; Gao, G.; Duan, L. High strength, anti-freezing and strain sensing carboxymethyl cellulose-based organohydrogel. Carbohydr. Polym. 2019, 223, 115051. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, H.; Tian, Z.; Duan, X.; Chai, Z.; Feng, Y.; Wang, Y.; Fan, Y.; Huang, J. A Solvent Co-cross-linked Organogel with Fast Self-Healing Capability and Reversible Adhesiveness at Extreme Temperatures. ACS Appl. Mater. Interfaces 2020, 12, 29757–29766. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Chen, T.; Chen, G.; Liu, H.; Mugaanire, I.T.; Hou, K.; Zhu, M. Conductive Self-Healing Nanocomposite Hydrogel Skin Sensors with Antifreezing and Thermoresponsive Properties. ACS Appl. Mater. Interfaces 2020, 12, 3068–3079. [Google Scholar] [CrossRef]
- You, Z.; Dong, Y.; Li, X.; Yang, P.; Luo, M.; Zhu, Z.; Wu, L.; Zhou, X.; Chen, M. One-pot synthesis of multi-functional cellulose-based ionic conductive organohydrogel with low-temperature strain sensitivity. Carbohydr. Polym. 2021, 251, 117019. [Google Scholar] [CrossRef] [PubMed]




| Hydrogel Materials | Self-Healing Systems | Self-Healing Time | Mechanical Healing Efficiency | Mechanical Property Recovery | Ref. |
|---|---|---|---|---|---|
| PAAm/SDS/NaCl/C18-C22 | noncovalent | a few seconds | ~100% | break at elongation ratios | [85] |
| κ-CG/PAA | noncovalent | 24 h | 67% | toughness | [86] |
| HPAAN/PDA | noncovalent | 5 h | 49%, 67%, 78% | mechanical strength, tensile strain, modulus | [87] |
| XG/MMT/PAAm | noncovalent | 24 h | 70% | tensile | [88] |
| PVA/Agar/AS | noncovalent | -- | 27.8%, 82.9% | tensile stress, tensile strain | [89] |
| f-BNNS/clay/PNIPAM | noncovalent | 6 h | ~70% | tensile | [90] |
| βCD-Ad | noncovalent | 24 h | 84% | strength | [91] |
| PPy/G-Zn-tpy | noncovalent | 60 s | ~100% | strength | [92] |
| PVA/AMCS7/ADA | covalent | 12 h | -- | -- | [93] |
| OSA/PAM | covalent | 6 h | >70% | tensile strength | [94] |
| LA/PAA/Fe3+ | covalent | 14 h | 86% | fracture stress | [95] |
| PVA/SA/NaCl | covalent | 15 s | well restored | heavy object pull test | [96] |
| CNC/PEG | covalent | 24 h | 78% | tensile strength | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y.; Lei, M.; Li, M.; Li, D.; Zhang, L.; Wu, Y. Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review. Gels 2021, 7, 216. https://doi.org/10.3390/gels7040216
Zhang J, Wang Y, Wei Q, Wang Y, Lei M, Li M, Li D, Zhang L, Wu Y. Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review. Gels. 2021; 7(4):216. https://doi.org/10.3390/gels7040216
Chicago/Turabian StyleZhang, Juan, Yanen Wang, Qinghua Wei, Yanmei Wang, Mingju Lei, Mingyang Li, Dinghao Li, Longyu Zhang, and Yu Wu. 2021. "Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review" Gels 7, no. 4: 216. https://doi.org/10.3390/gels7040216
APA StyleZhang, J., Wang, Y., Wei, Q., Wang, Y., Lei, M., Li, M., Li, D., Zhang, L., & Wu, Y. (2021). Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review. Gels, 7(4), 216. https://doi.org/10.3390/gels7040216






