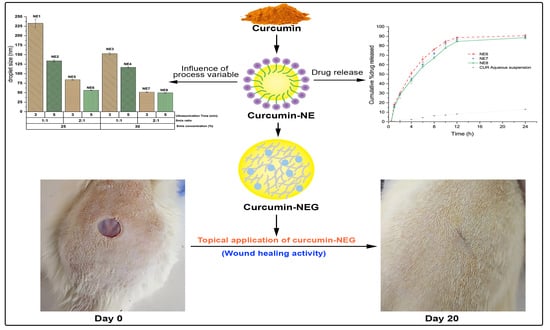
Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of NE Components
2.2. Preparation of NE through Ultrasonication
2.2.1. Influence of Smix Ratio and Smix Concentration on Droplet Size
2.2.2. Influence of Ultrasonication Time on Droplet Size
2.3. Characterization of NE
2.3.1. Analysis of Thermodynamic Stability and Zeta Potential
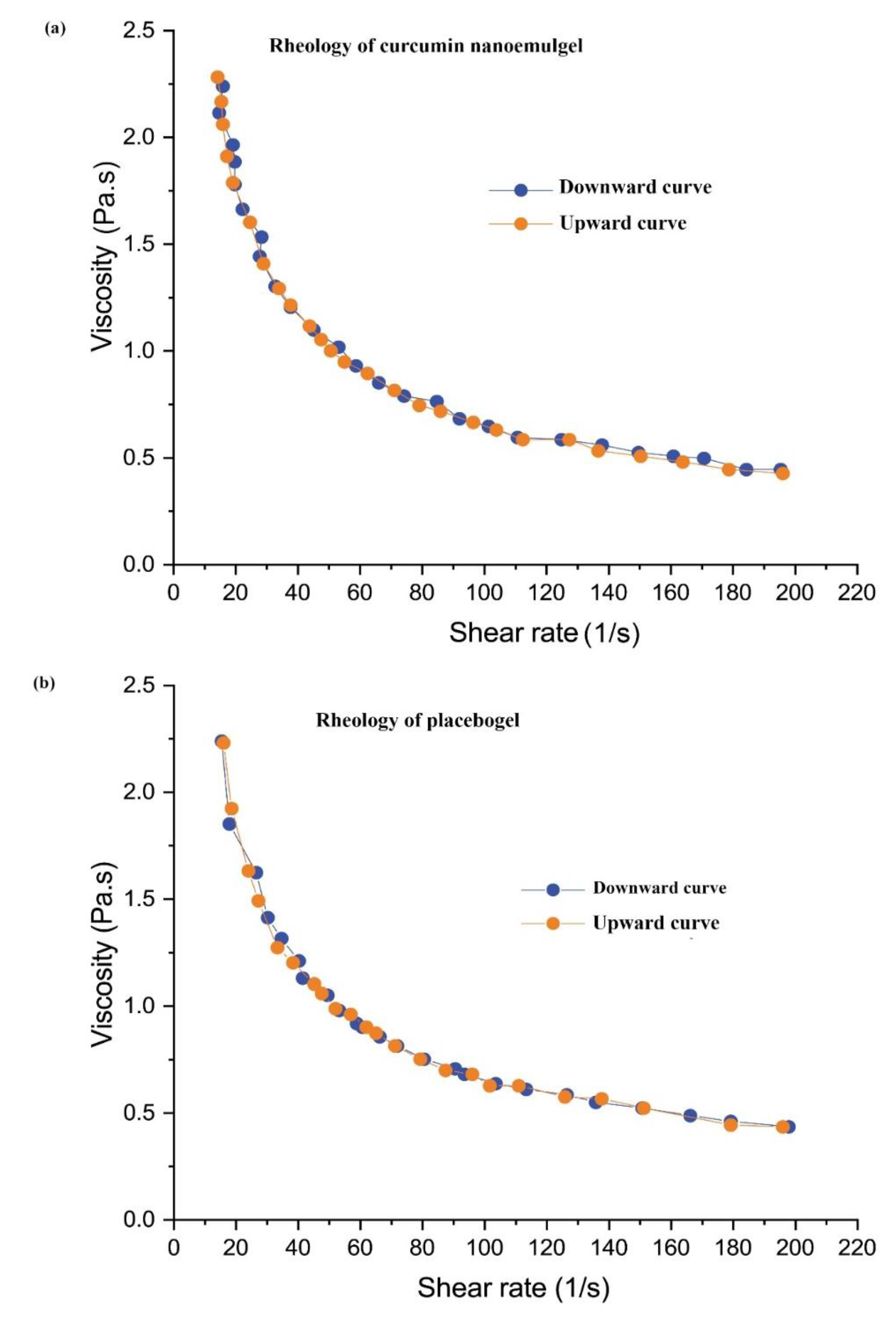
2.3.2. Viscosity
2.3.3. Analysis of Drug Content
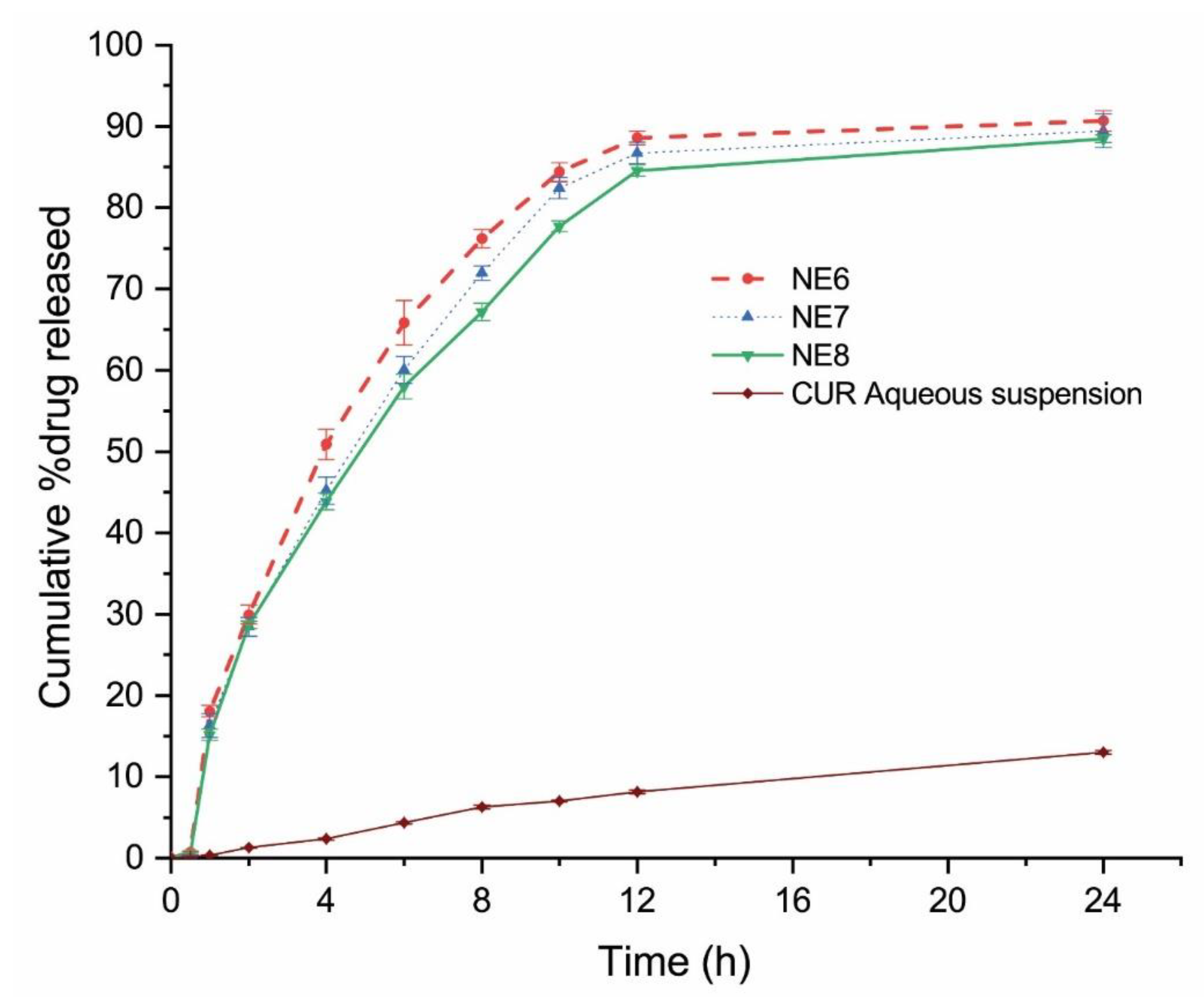
2.4. In Vitro Drug Release
2.5. Preparation and Characterization of Nanoemulgel
2.6. Ex-Vivo Skin Permeability Study
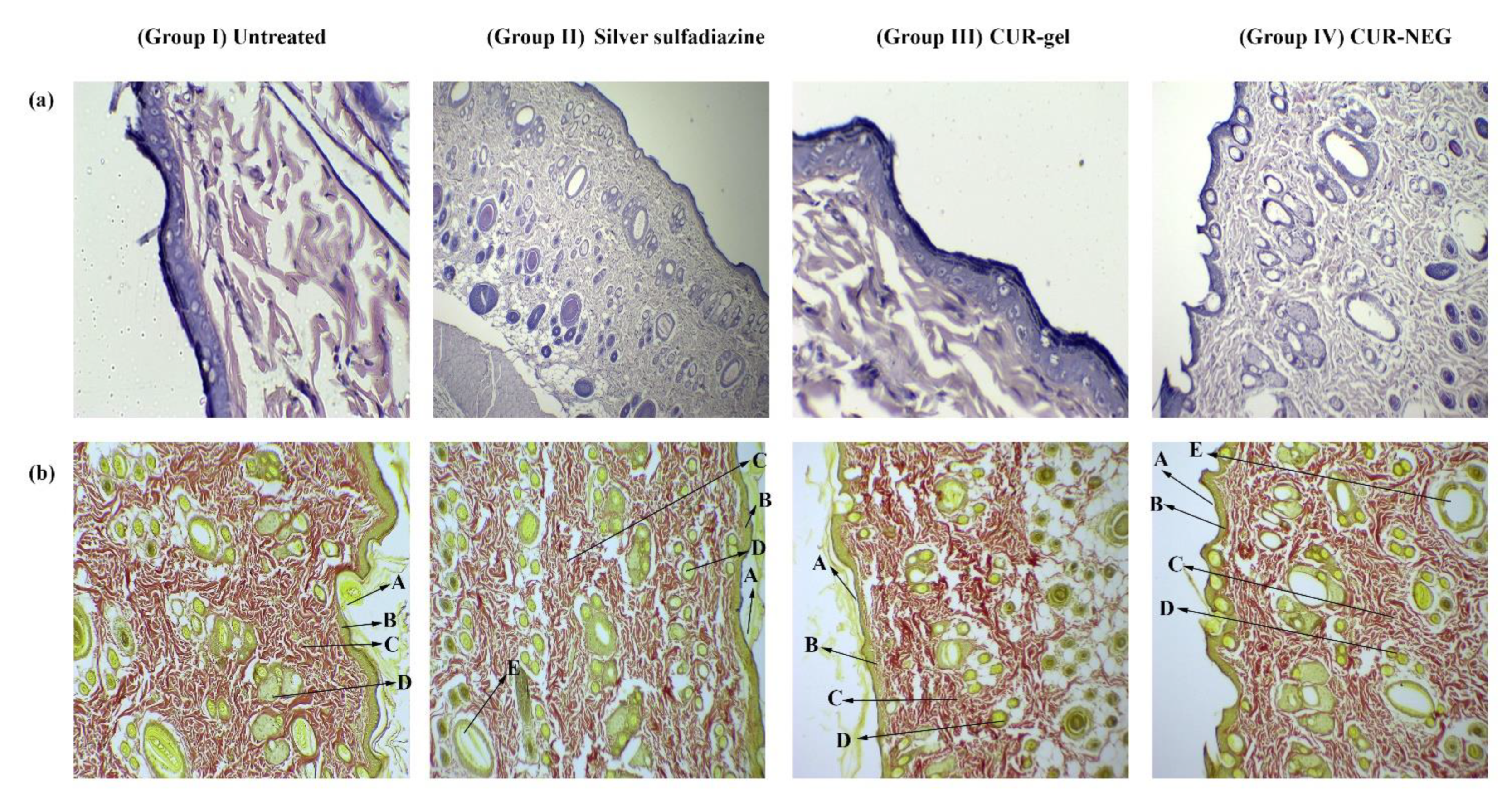
2.7. In-Vivo Wound Healing Activity
3. Conclusions
4. Materials and Methodology
4.1. Materials
4.2. Screening of NE Components
4.3. Preparation of Curcumin NE through High-Energy Emulsification
4.4. Characterization of Curcumin NE
4.4.1. Thermodynamic Stability
4.4.2. Droplet Size, Polydispersity Index, and Zeta Potential
4.4.3. Determination of Curcumin NE Viscosity
4.4.4. Analysis of the Drug Content
4.5. In-Vitro Drug Release
4.6. Preparation and Characterization of Curcumin Nanoemulgel
4.7. Ex-Vivo Skin Permeability
4.8. In-Vivo Study
4.8.1. Experimental Protocol
4.8.2. Evaluation of Wound Healing Area
4.8.3. Histopathology
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Govindarajan, R.; Nath, V.; Rawat, A.K.S.; Mehrotra, S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. et Lind. J. Ethnopharmacol. 2006, 107, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, R.; Saurabh, M.K.; Thakur, G.S.; Saurabh, A. Study of formulation, characterisation and wound healing potential of transdermal patches of curcumin. Asian J. Pharm. Clin. Res. 2012, 5, 225–230. [Google Scholar]
- Boateng, J.S.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskalova, A.; Almquist, B.D. Using biomaterials to rewire the process of wound repair. Biomater. Sci. 2017, 5, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Ahmed, M.; Gomathi, K.; Chitra, K.; Sehgal, P.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef]
- Farooq, U.; Rasul, A.; Zafarullah, M.; Abbas, G.; Rasool, M.; Ali, F.; Ahmed, S.; Javaid, Z.; Abid, Z.; Riaz, H.; et al. Nanoemulsions as novel nanocarrieres for drug delivery across the skin: In-vitro, in-vivo evaluation of miconazole nanoemulsions for treatment of Candidiasis albicans. Des. Monomers Polym. 2021, 24, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Peltola, S.; Saarinen-Savolainen, P.; Kiesvaara, J.; Suhonen, T.; Urtti, A. Microemulsions for topical delivery of estradiol. Int. J. Pharm. 2003, 254, 99–107. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; AlGahtani, M.S.; Ahmad, J.; Kohli, K.; Shafiq-Un-Nabi, S.; Warsi, M.H.; Ahmad, M.Z. Formulation design and evaluation of aceclofenac nanogel for topical application. Ther. Deliv. 2020, 11, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Algahtani, M.; Ahmad, M.; Shaikh, I.; Abdel-Wahab, B.; Nourein, I.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, R.; Arpita; Goel, S.; Uppal, S.; Bhatia, A.; Mehta, S.K. Physiochemical and cytotoxicity study of TPGS stabilized nanoemulsion designed by ultrasonication method. Ultrason. Sonochem. 2017, 34, 173–182. [Google Scholar] [CrossRef]
- Li, P.-H.; Chiang, B.-H. Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Rodrigues, C.F.; Peron, G.; Dall’Acqua, S.; Sharifi-Rad, J.; Azmi, L.; Shukla, I.; Singh Baghel, U.; Prakash Mishra, A.; Elissawy, A.M.; et al. Curcumin nanoformulations for antimicrobial and wound healing purposes. Phytother. Res. 2021, 35, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artigas, M.A.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Campos, F.F.; Campmany, A.C.C.; Delgado, G.R.; Serrano, O.L.; Naveros, B.C. Development and Characterization of a Novel Nystatin-Loaded Nanoemulsion for the Buccal Treatment of Candidosis: Ultrastructural Effects and Release Studies. J. Pharm. Sci. 2012, 101, 3739–3752. [Google Scholar] [CrossRef] [PubMed]
- Garduño-Ramírez, M.L.; Clares, B.; Domínguez-Villegas, V.; Peraire, C.; Ruiz, M.A.; García, M.L.; Calpena, A.C. Skin Permeation of Cacalol, Cacalone and 6-epi-Cacalone Sesquiterpenes from a Nanoemulsion. Nat. Prod. Commun. 2012, 7, 821–823. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv. Pharm. 2014, 2014, 468456. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.; Khan, H.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration Enhancing Effect of Polysorbate 20 and 80 on the In Vitro Percutaneous Absorption of LAscorbic Acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Valenti, D.; Lai, F.; Fadda, A.M. Niosomes as carriers for tretinoin: III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int. J. Pharm. 2006, 311, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; FNabavi, S.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; MNabavi, S. Wound healing effects of curcumin: A short review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.Y.; Zheng, Y. Formulation Development and Bioavailability Evaluation of a Self-Nanoemulsified Drug Delivery System of Oleanolic Acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Ntimenou, V.; Fahr, A.; Antimisiaris, S. Elastic vesicles for transdermal drug delivery of hydrophilic drugs: A comparison of important physicochemical characteristics of different vesicle types. J. Biomed. Nanotechnol. 2012, 8, 613–623. [Google Scholar] [CrossRef]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug. Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.K.; Srivastava, A.K.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 2016, 9249040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro Souza, J.D.; Estevão, L.R.; Baratella-Evêncio, L.; Vieira, M.G.; Simões, R.S.; Florencio-Silva, R.; Evêncio-Luz, L.; Evêncio-Neto, J. Mast cell concentration and skin wound contraction in rats treated with Ximenia americana L1. Acta Cir. Bras. 2017, 32, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
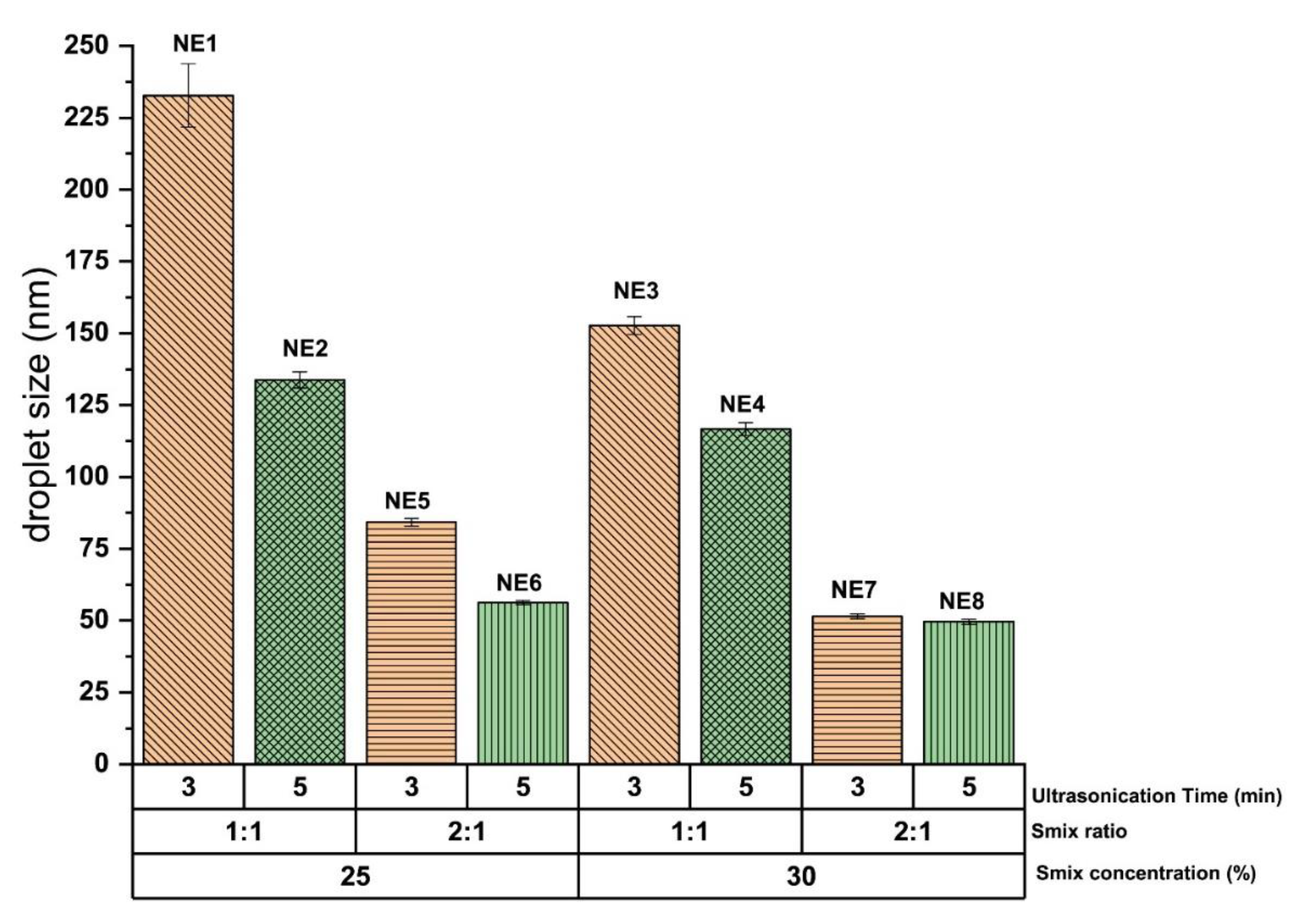

| Formulation Code | Oil% | Smix% | Water% | Smix Ratio | Ultrasonication Time (Min) | Mean Droplet Size (Nm) | Polydispersity Index |
|---|---|---|---|---|---|---|---|
| NE1 | 20.0 | 25.0 | 55.0 | 1:1 | 3.0 | 232.80 ± 11.04 | 0.23 ± 0.16 |
| NE2 | 20.0 | 25.0 | 55.0 | 1:1 | 5.0 | 133.76 ± 2.84 | 0.29 ± 0.11 |
| NE3 | 20.0 | 30.0 | 50.0 | 1:1 | 3.0 | 152.70 ± 3.08 | 0.20 ± 0.04 |
| NE4 | 20.0 | 30.0 | 50.0 | 1:1 | 5.0 | 116.63 ± 2.22 | 0.14 ± 0.12 |
| NE5 | 20.0 | 25.0 | 55.0 | 2:1 | 3.0 | 84.23 ± 1.33 | 0.23 ± 0.05 |
| NE6 | 20.0 | 25.0 | 55.0 | 2:1 | 5.0 | 56.25 ± 0.69 | 0.05 ± 0.01 |
| NE7 | 20.0 | 30.0 | 50.0 | 2:1 | 3.0 | 51.47 ± 0.71 | 0.13 ± 0.04 |
| NE8 | 20.0 | 30.0 | 50.0 | 2:1 | 5.0 | 49.61 ± 0.53 | 0.10 ± 0.01 |
| Formulation Code | Thermodynamic Stability | Mean Droplet Size (nm) | Polydis-persity Index | Zeta Potential (mV) | Drug Content (%) | Viscosity (mPas) | ||
|---|---|---|---|---|---|---|---|---|
| Heating Cooling Cycle | Centrifugation Study | Freeze-Thaw Cycle | ||||||
| NE5 | √ | √ | √ | 84.23 ± 1.33 | 0.23 ± 0.05 | −15.96 ± 0.55 | 98.86 ± 0.58 | 89.82 ± 1.27 |
| NE6 | √ | √ | √ | 56.25 ± 0.69 | 0.05 ± 0.01 | −20.26 ± 0.65 | 99.23 ± 0.28 | 83.74 ± 1.92 |
| NE7 | √ | √ | √ | 51.47 ± 0.71 | 0.13 ± 0.04 | −19.26 ± 0.20 | 99.16 ± 0.15 | 85.05 ± 2.30 |
| NE8 | √ | √ | √ | 49.61 ± 0.53 | 0.10 ± 0.01 | −19.6 ± 0.41 | 99.03 ± 0.41 | 87.37 ± 0.66 |
| Formulation | Cumulative Amount of Drug Permeated (µg/cm2) | Drug Deposited in Skin (µg/cm2) | Lag Time (h) | Flux (µg/cm2.h) | Permeability Coefficient (K × 10−3) | ER | Local Accumulation Efficiency (LAE) |
|---|---|---|---|---|---|---|---|
| CUR-NEG | 773.82 ± 1.08 | 1161.54 ± 2.78 | 0.75 ± 0.03 | 13.74 ± 1.08 | 5.49 ± 0.67 | 6.27 ± 0.77 | 1.50 ± 0.01 |
| CUR-gel | 156.90 ± 0.95 | 179.47 ± 1.56 | 2.37 ± 0.09 | 2.19 ± 0.10 | 0.876 ± 0.01 | - | 1.14 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels 2021, 7, 213. https://doi.org/10.3390/gels7040213
Algahtani MS, Ahmad MZ, Nourein IH, Albarqi HA, Alyami HS, Alyami MH, Alqahtani AA, Alasiri A, Algahtani TS, Mohammed AA, et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels. 2021; 7(4):213. https://doi.org/10.3390/gels7040213
Chicago/Turabian StyleAlgahtani, Mohammed S., Mohammad Zaki Ahmad, Ihab Hamed Nourein, Hassan A. Albarqi, Hamad S. Alyami, Mohammad H. Alyami, Abdulsalam A. Alqahtani, Ali Alasiri, Thamer S. Algahtani, Abdul Aleem Mohammed, and et al. 2021. "Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation" Gels 7, no. 4: 213. https://doi.org/10.3390/gels7040213
APA StyleAlgahtani, M. S., Ahmad, M. Z., Nourein, I. H., Albarqi, H. A., Alyami, H. S., Alyami, M. H., Alqahtani, A. A., Alasiri, A., Algahtani, T. S., Mohammed, A. A., & Ahmad, J. (2021). Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels, 7(4), 213. https://doi.org/10.3390/gels7040213