Luminescent Hydrogel Based on Silver Nanocluster/Malic Acid and Its Composite Film for Highly Sensitive Detection of Fe3+
Abstract
:1. Introduction
2. Results and Discussion
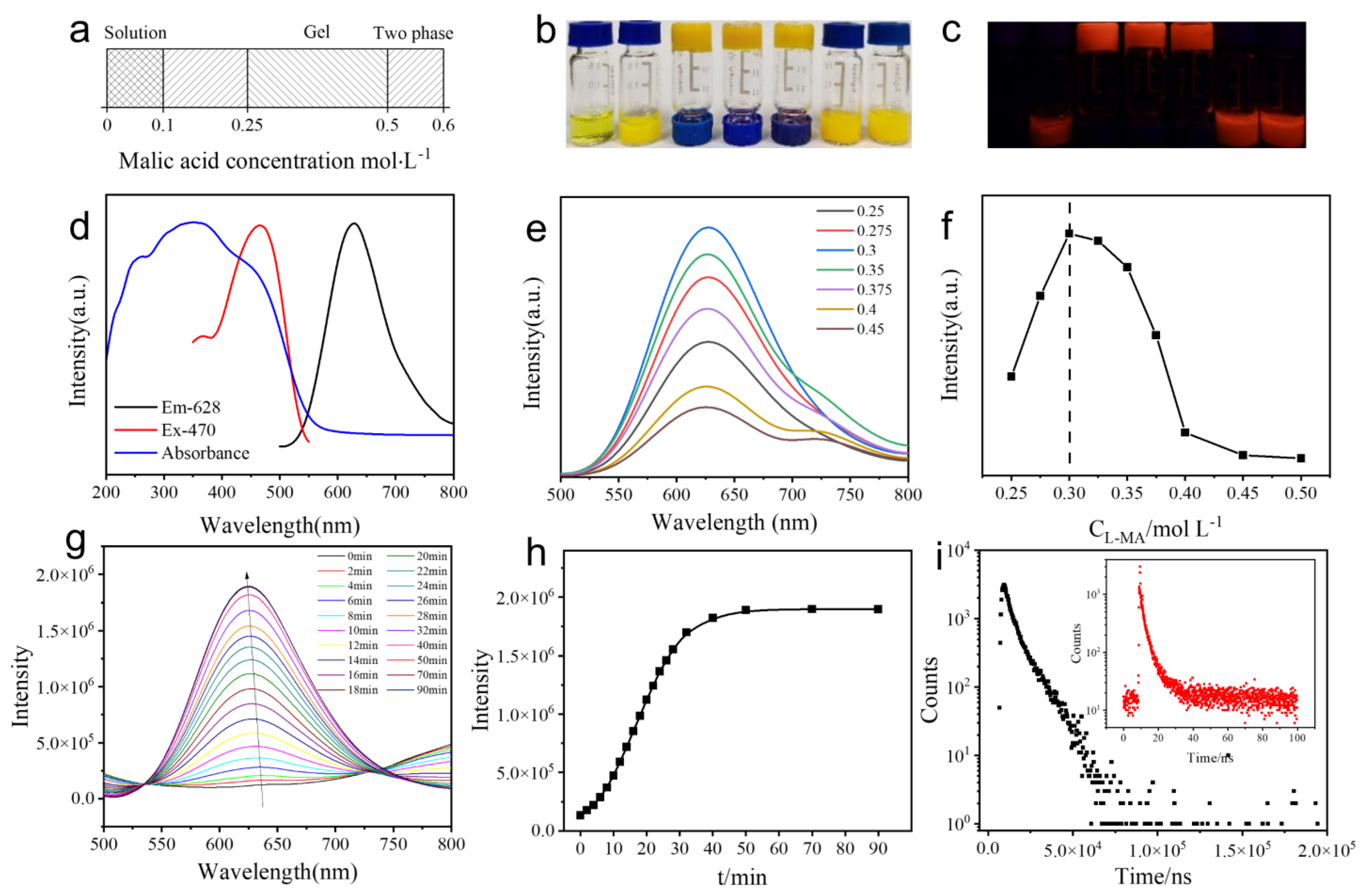
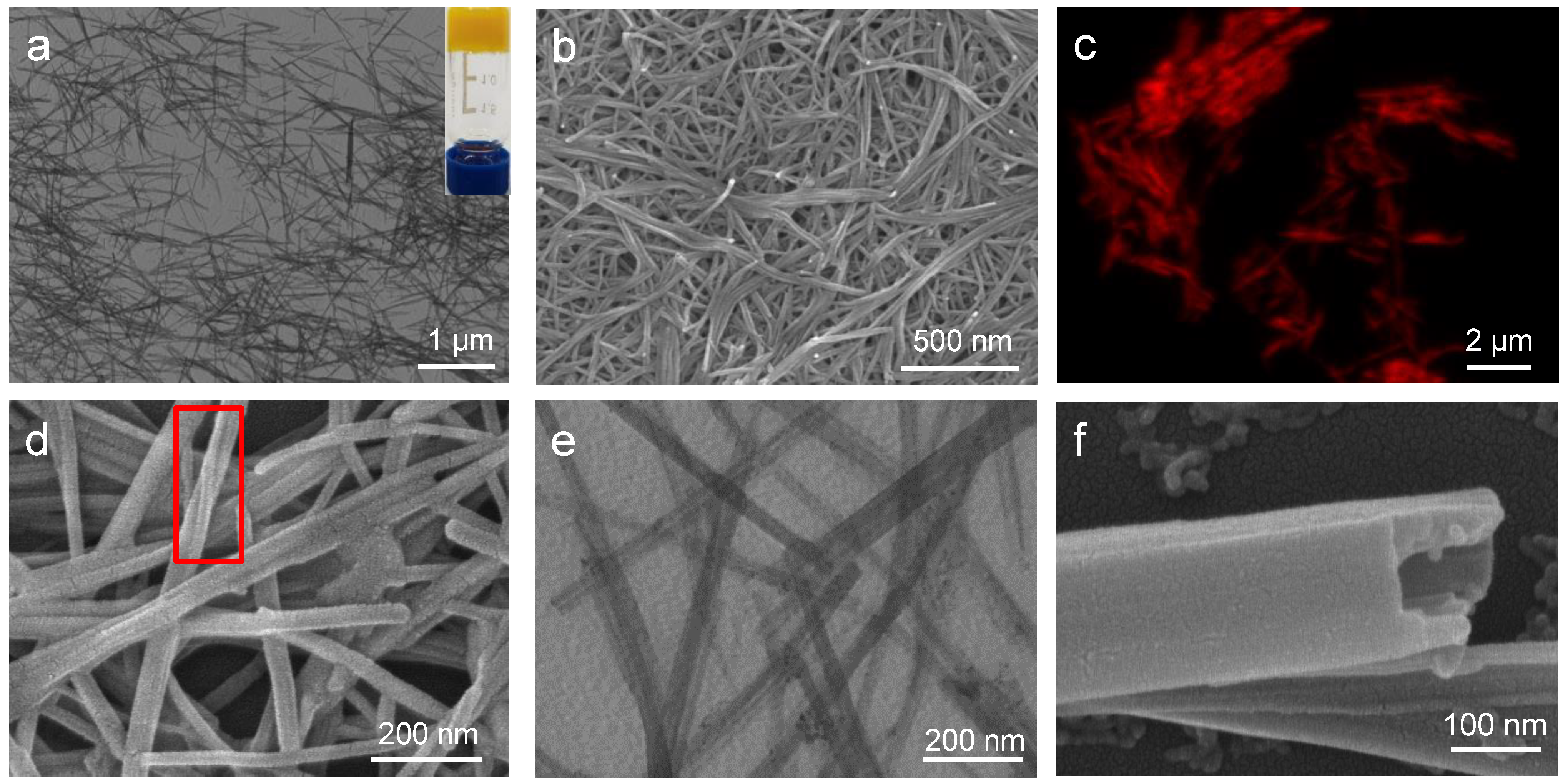
2.1. Self-Assembly of Ag9-NCs/L–MA Hydrogels
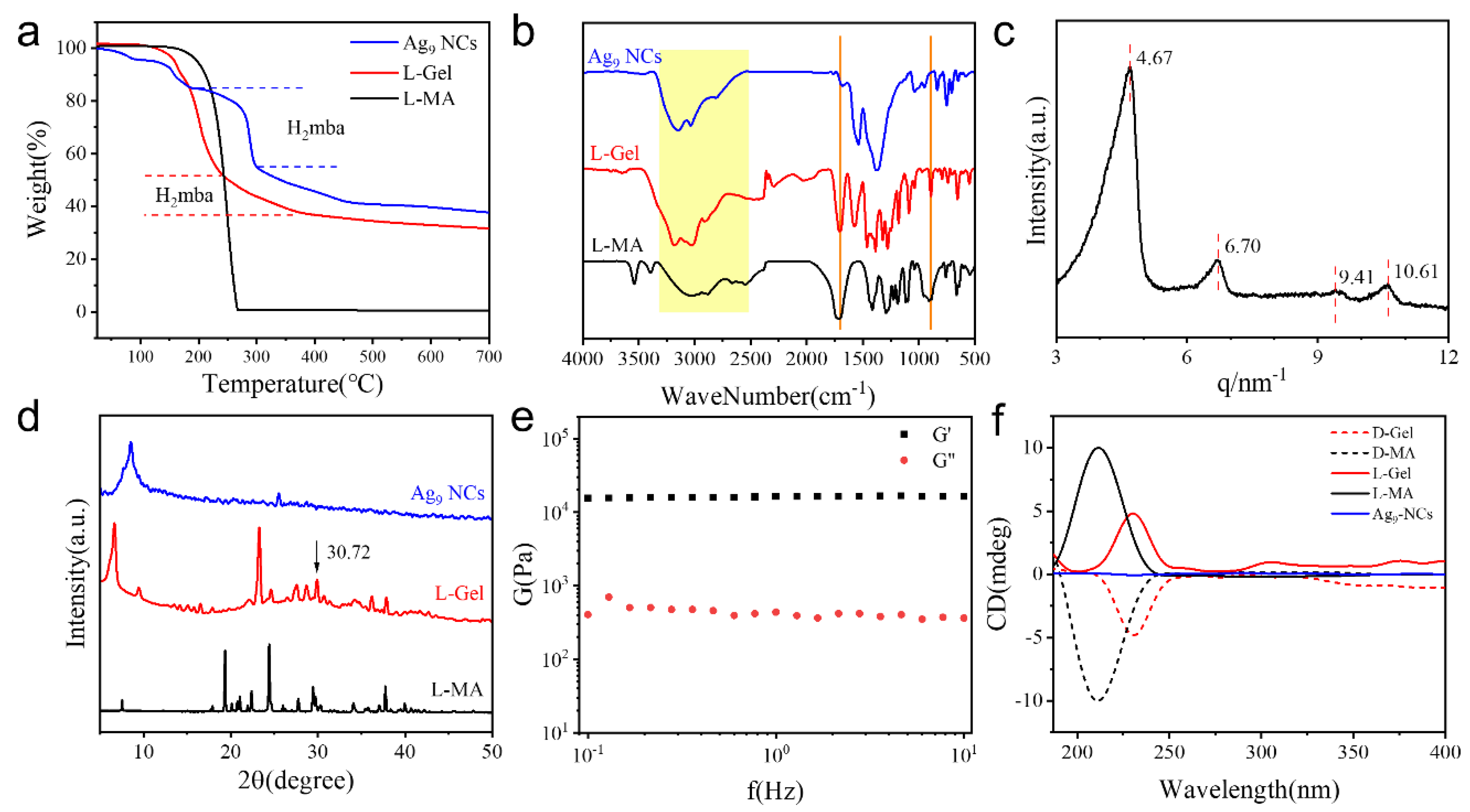
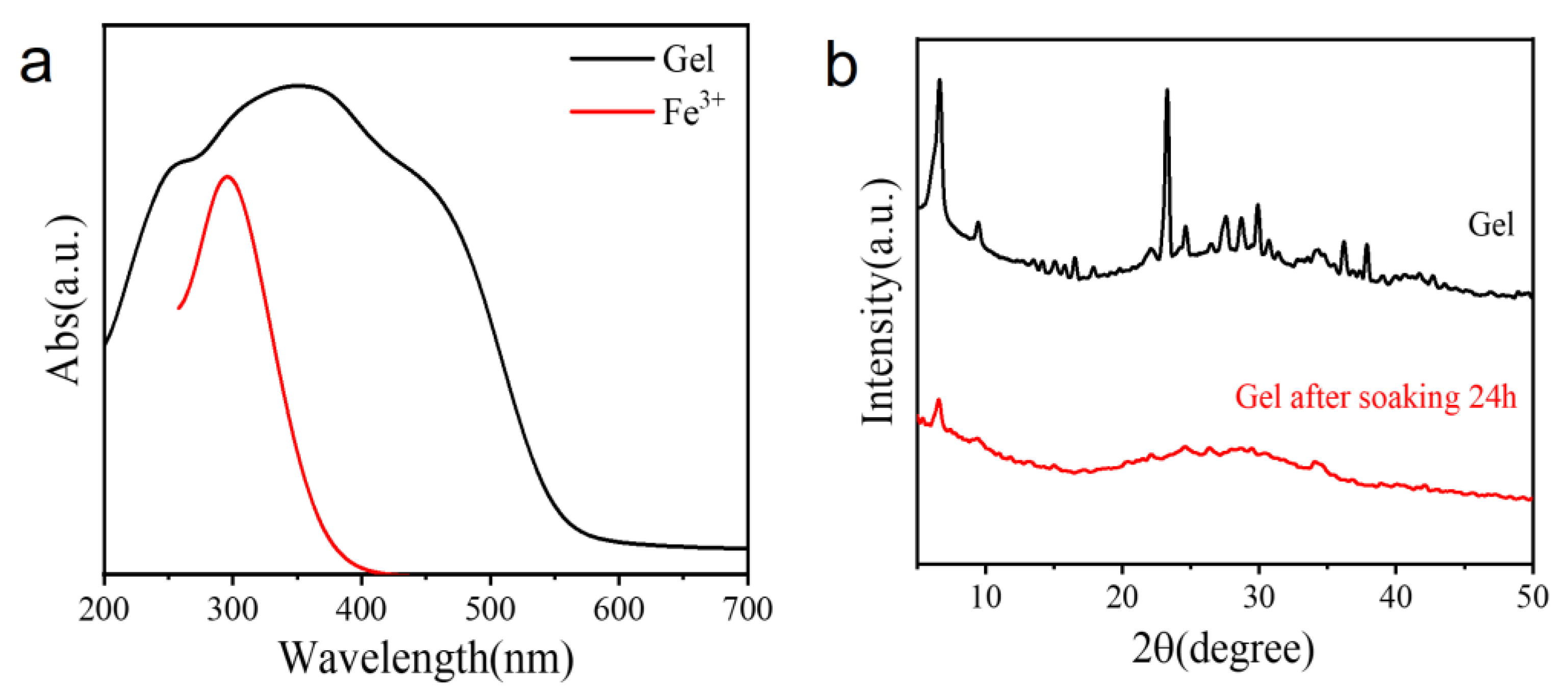
2.2. Structural Analysis of the Hydrogel
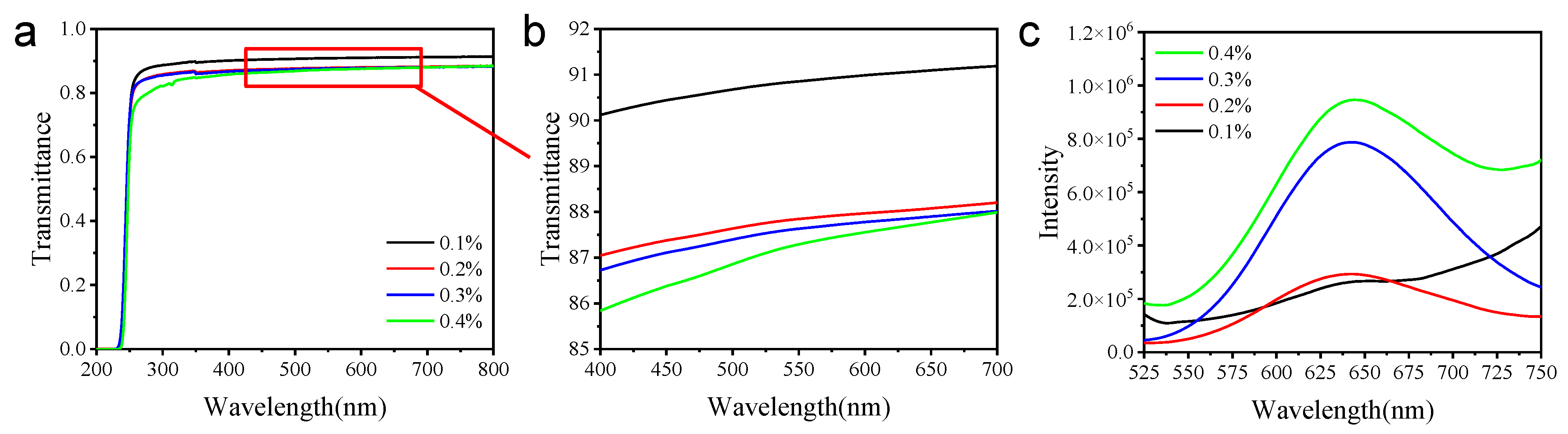
2.3. Ag9-NCs/L–MA Xerogel/PMMA Film for Sensing
3. Conclusions
4. Experiment Section
4.1. Materials
4.2. Synthesis of Ag9-NCs/L–MA Hybrid Nanostructures
4.3. Fabrication of Ag9-NCs/L–MA/PMMA Composite Thin Film
4.4. Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Jin, R. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Cuaran-Acosta, D.; Londono-Larrea, P.; Zaballos-Garcia, E.; Perez-Prieto, J. Reversible pH-induced fluorescence colour change of gold nanoclusters based on pH-regulated surface interactions. Chem. Commun. 2019, 55, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xu, T.; Zhang, Y.; Zhang, C.; Shi, L.; Zhang, G.; Shuang, S.; Dong, C. Green synthesis of fluorescent copper nanoclusters for reversible pH-sensors. Sens. Actuators B 2015, 220, 1064–1069. [Google Scholar] [CrossRef]
- Wang, W.; Zhan, L.; Du, Y.Q.; Leng, F.; Chang, Y.; Gao, M.X.; Huang, C.Z. Label-free DNA detection on the basis of fluorescence resonance energy transfer from oligonucleotide-templated silver nanoclusters to multi-walled carbon nanotubes. Anal. Methods 2013, 5, 5555. [Google Scholar] [CrossRef]
- Burratti, L.; De Matteis, F.; Francini, R.; Lim, J.; Scheu, C.; Prosposito, P. Fluorescent Silver Nanoclusters Embedded in Hydrogel Matrix and Its Potential Use in Environmental Monitoring. Appl. Sci. 2021, 11, 3470. [Google Scholar] [CrossRef]
- Yuan, X.; Tay, Y.; Dou, X.; Luo, Z.; Leong, D.T.; Xie, J. Glutathione-protected silver nanoclusters as cysteine-selective fluorometric and colorimetric probe. Anal. Chem. 2013, 85, 1913–1919. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Cheng, X.; Huo, L.; Lu, L. Gold-Nanocluster-Based Fluorescent Sensors for Highly Sensitive and Selective Detection of Cyanide in Water. Adv. Funct. Mater. 2010, 20, 951–956. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Li, R.; Tu, K.; Li, J.; Xie, Z.; Lei, J.; Liu, D.; Qu, D. Self-assembled N-doped carbon with a tube-in-tube nanostructure for lithium-sulfur batteries. J. Colloid. Interface Sci. 2020, 559, 244–253. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Diez, I.; Pusa, M.; Kulmala, S.; Jiang, H.; Walther, A.; Goldmann, A.S.; Muller, A.H.; Ikkala, O.; Ras, R.H. Color tunability and electrochemiluminescence of silver nanoclusters. Angew. Chem. Int. Ed. 2009, 48, 2122–2125. [Google Scholar] [CrossRef]
- Diez, I.; Kanyuk, M.I.; Demchenko, A.P.; Walther, A.; Jiang, H.; Ikkala, O.; Ras, R.H. Blue, green and red emissive silver nanoclusters formed in organic solvents. Nanoscale 2012, 4, 4434–4437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niesen, B.; Rand, B.P. Thin film metal nanocluster light-emitting devices. Adv. Mater. 2014, 26, 1446–1449. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Y.; Zhao, L.; Liu, R.; Gao, F.; Gao, L.; Gao, X. Peptide protected gold clusters: Chemical synthesis and biomedical applications. Nanoscale 2016, 8, 12095–12104. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, C.W.; Yuan, Z.; Chang, H.T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Abbas, M.A.; Kamat, P.V.; Bang, J.H. Thiolated Gold Nanoclusters for Light Energy Conversion. ACS Energy Lett. 2018, 3, 840–854. [Google Scholar] [CrossRef]
- Shiang, Y.-C.; Huang, C.-C.; Chen, W.-Y.; Chen, P.-C.; Chang, H.-T. Fluorescent gold and silver nanoclusters for the analysis of biopolymers and cell imaging. J. Mater. Chem. 2012, 22, 12972–12982. [Google Scholar] [CrossRef]
- Yang, L.; Shang, L.; Nienhaus, G.U. Mechanistic aspects of fluorescent gold nanocluster internalization by live HeLa cells. Nanoscale 2013, 5, 1537–1543. [Google Scholar] [CrossRef]
- Kunwar, P.; Hassinen, J.; Bautista, G.; Ras, R.H.A.; Toivonen, J. Direct Laser Writing of Photostable Fluorescent Silver Nanoclusters in Polymer Films. ACS Nano 2014, 8, 11165–11171. [Google Scholar] [CrossRef] [Green Version]
- Pourreza, N.; Ghomi, M. In Situ synthesized and embedded silver nanoclusters into poly vinyl alcohol-borax hydrogel as a novel dual mode “on and off” fluorescence sensor for Fe (III) and thiosulfate. Talanta 2018, 179, 92–99. [Google Scholar] [CrossRef]
- Chen, B.; Li, C.; Zhang, J.; Kan, J.; Jiang, T.; Zhou, J.; Ma, H. Sensing and imaging of mitochondrial viscosity in living cells using a red fluorescent probe with a long lifetime. Chem. Commun. 2019, 55, 7410–7413. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, J.; Wang, E. Cu nanoclusters with aggregation induced emission enhancement. Small 2013, 9, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, S.; Bi, Y.; Sun, D.; Kong, F.; Yuan, Z.; Xin, X. Development and characterisation of multi-form composite materials based on silver nanoclusters and cellulose nanocrystals. Colloid Surf. A 2020, 603, 125257. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.C. Simultaneous synthesis and assembly of noble metal nanoclusters with variable micellar templates. J. Am. Chem. Soc. 2014, 136, 13805–13817. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Tan, C.; Qi, X.; Liu, Z.; Zhao, F.; Li, H.; Huang, X.; Shi, L.; Zheng, B.; Zhang, X.; Xie, L.; et al. Self-assembled chiral nanofibers from ultrathin low-dimensional nanomaterials. J. Am. Chem. Soc. 2015, 137, 1565–1571. [Google Scholar] [CrossRef]
- Hong, X.; Tan, C.; Liu, J.; Yang, J.; Wu, X.J.; Fan, Z.; Luo, Z.; Chen, J.; Zhang, X.; Chen, B.; et al. AuAg nanosheets assembled from ultrathin AuAg nanowires. J. Am. Chem. Soc. 2015, 137, 1444–1447. [Google Scholar] [CrossRef]
- Xia, Y.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.; Glotzer, S.C.; Kotov, N.A. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat. Nanotechnol. 2011, 6, 580–587. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, Q.; Zang, S.; Xie, J. Directed Self-Assembly of Ultrasmall Metal Nanoclusters. ACS Mater. Lett. 2019, 1, 237–248. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Sun, D.; Xia, C.; Yuan, S.; Sun, P.; Xin, X. pH-Responsive Nanovesicles with Enhanced Emission Co-Assembled by Ag(I) Nanoclusters and Polyethyleneimine as a Superior Sensor for Al(3). ACS. Appl. Mater. Interfaces 2018, 10, 3955–3963. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Gao, Y.; Liu, H.; Li, T.; Zou, H.; Wang, Z.; Zhang, K.; Wang, Y.; Zhang, H.; et al. Assembly-Induced Enhancement of Cu Nanoclusters Luminescence with Mechanochromic Property. J. Am. Chem. Soc. 2015, 137, 12906–12913. [Google Scholar] [CrossRef]
- Chen, W.; Fan, R.; Fan, J.; Liu, H.; Sun, T.; Wang, P.; Yang, Y. Lanthanide Coordination Polymer-Based Composite Films for Selective and Highly Sensitive Detection of Cr2O72− in Aqueous Media. Inorg. Chem. 2019, 58, 15118–15125. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Liu, Y.; Zhang, S.X.-A. Recent advances in film-based fluorescence sensing. Sci. Sin. Chim. 2019, 50, 39–69. [Google Scholar] [CrossRef]
- Bhasin, A.K.K.; Chauhan, P.; Chaudhary, S. A novel sulfur-incorporated naphthoquinone as a selective “turn-on” fluorescence chemical sensor for rapid detection of Ba2+ ion in semi-aqueous medium. Sens. Actuators B 2019, 294, 116–122. [Google Scholar] [CrossRef]
- Song, C.; Liu, Y.; Jiang, X.; Zhang, J.; Dong, C.; Li, J.; Wang, L. Ultrasensitive SERS determination of avian influenza A H7N9 virus via exonuclease III-assisted cycling amplification. Talanta 2019, 205, 120137. [Google Scholar] [CrossRef]
- Aysha, T.S.; El-Sedik, M.S.; Mohamed, M.B.I.; Gaballah, S.T.; Kamel, M.M. Dual functional colorimetric and turn-off fluorescence probe based on pyrrolinone ester hydrazone dye derivative for Cu2+ monitoring and pH change. Dyes Pigment. 2019, 170, 107549. [Google Scholar] [CrossRef]
- Li, J.J.; Qiao, D.; Zhao, J.; Weng, G.J.; Zhu, J.; Zhao, J.W. Ratiometric fluorescence detection of Hg2+ and Fe3+ based on BSA-protected Au/Ag nanoclusters and His-stabilized Au nanoclusters. Methods Appl. Fluoresc. 2019, 7, 045001. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Duan, J.; Hou, J.; Hou, Q.; Yang, Y.; Li, H.; Ai, S. Rapid and ultrasensitive detection of mercury ion (II) by colorimetric and SERS method based on silver nanocrystals. Microchem. J. 2021, 161, 105790. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alqarni, S.; Mohammed, G.I.; Al Sheheri, S.Z.; Alam, M.M.; Ismail, S.H.; Asiri, A.M.; Hussein, M.A.; Rahman, M.M. Selective Hg2+ sensor performance based various carbon-nanofillers into CuO-PMMA nanocomposites. Polym. Adv. Technol. 2020, 31, 1946–1962. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Bi, Y.; Sun, D.; Zhao, T.; Zhao, F.; Wang, W.; Xin, X. Self-Assembly-Driven Aggregation-Induced Emission of Silver Nanoclusters for Light Conversion and Temperature Sensing. ACS Appl. Nano Mater. 2020, 3, 2038–2046. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Sun, D.; Bai, H.; Zhu, Z.; Bi, Y.; Zhao, T.; Xin, X. pH-guided self-assembly of silver nanoclusters with aggregation-induced emission for rewritable fluorescent platform and white light emitting diode application. J. Colloid Interface Sci. 2020, 567, 235–242. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, P.; Zhang, N.; Zhou, S.; Xin, X. Self-assembly of silver nanoclusters and phthalic acid into hollow tubes as a superior sensor for Fe3+. J. Mol. Liq. 2021, 323, 115032. [Google Scholar] [CrossRef]
- Barreiro, E.; Casas, J.S.; Couce, M.D.; Laguna, A.; López-de-Luzuriaga, J.M.; Monge, M.; Sánchez, A.; Sordo, J.; Vázquez López, E.M. A novel hexanuclear silver(i) cluster containing a regular Ag6 ring with short Ag–Ag distances and an argentophilic interaction. Dalton Trans. 2013, 42, 5916. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, J. Metal-Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2020, 59, 9922–9927. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, C.; Wang, Z.; Zhang, N.; Feng, N.; Wang, W.; Xin, X. Luminescent Hydrogel Based on Silver Nanocluster/Malic Acid and Its Composite Film for Highly Sensitive Detection of Fe3+. Gels 2021, 7, 192. https://doi.org/10.3390/gels7040192
Liu X, Li C, Wang Z, Zhang N, Feng N, Wang W, Xin X. Luminescent Hydrogel Based on Silver Nanocluster/Malic Acid and Its Composite Film for Highly Sensitive Detection of Fe3+. Gels. 2021; 7(4):192. https://doi.org/10.3390/gels7040192
Chicago/Turabian StyleLiu, Xiangkai, Chunhui Li, Zhi Wang, Na Zhang, Ning Feng, Wenjuan Wang, and Xia Xin. 2021. "Luminescent Hydrogel Based on Silver Nanocluster/Malic Acid and Its Composite Film for Highly Sensitive Detection of Fe3+" Gels 7, no. 4: 192. https://doi.org/10.3390/gels7040192
APA StyleLiu, X., Li, C., Wang, Z., Zhang, N., Feng, N., Wang, W., & Xin, X. (2021). Luminescent Hydrogel Based on Silver Nanocluster/Malic Acid and Its Composite Film for Highly Sensitive Detection of Fe3+. Gels, 7(4), 192. https://doi.org/10.3390/gels7040192







