Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
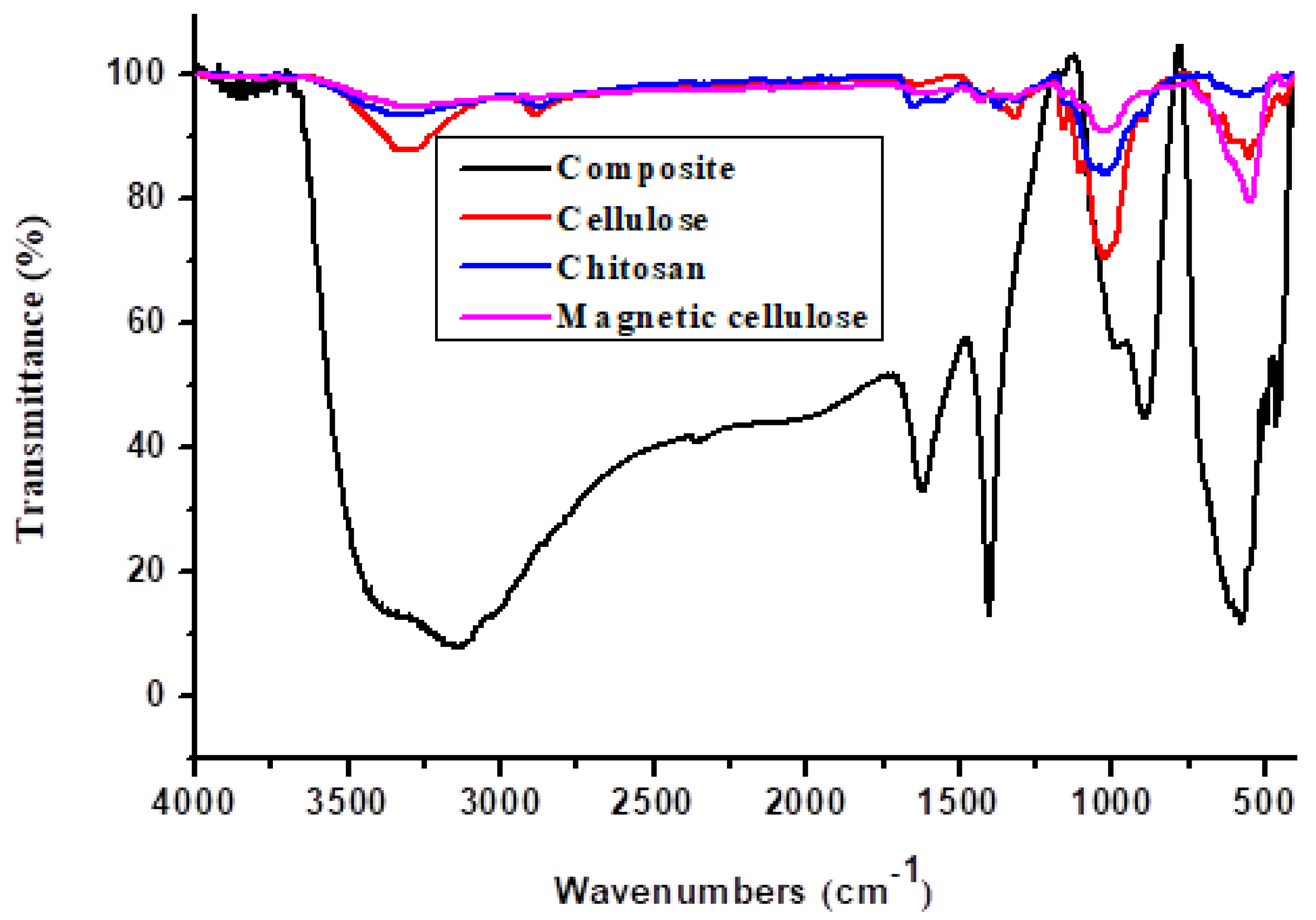
2.1.1. Fourier Transform Infrared Spectroscopy
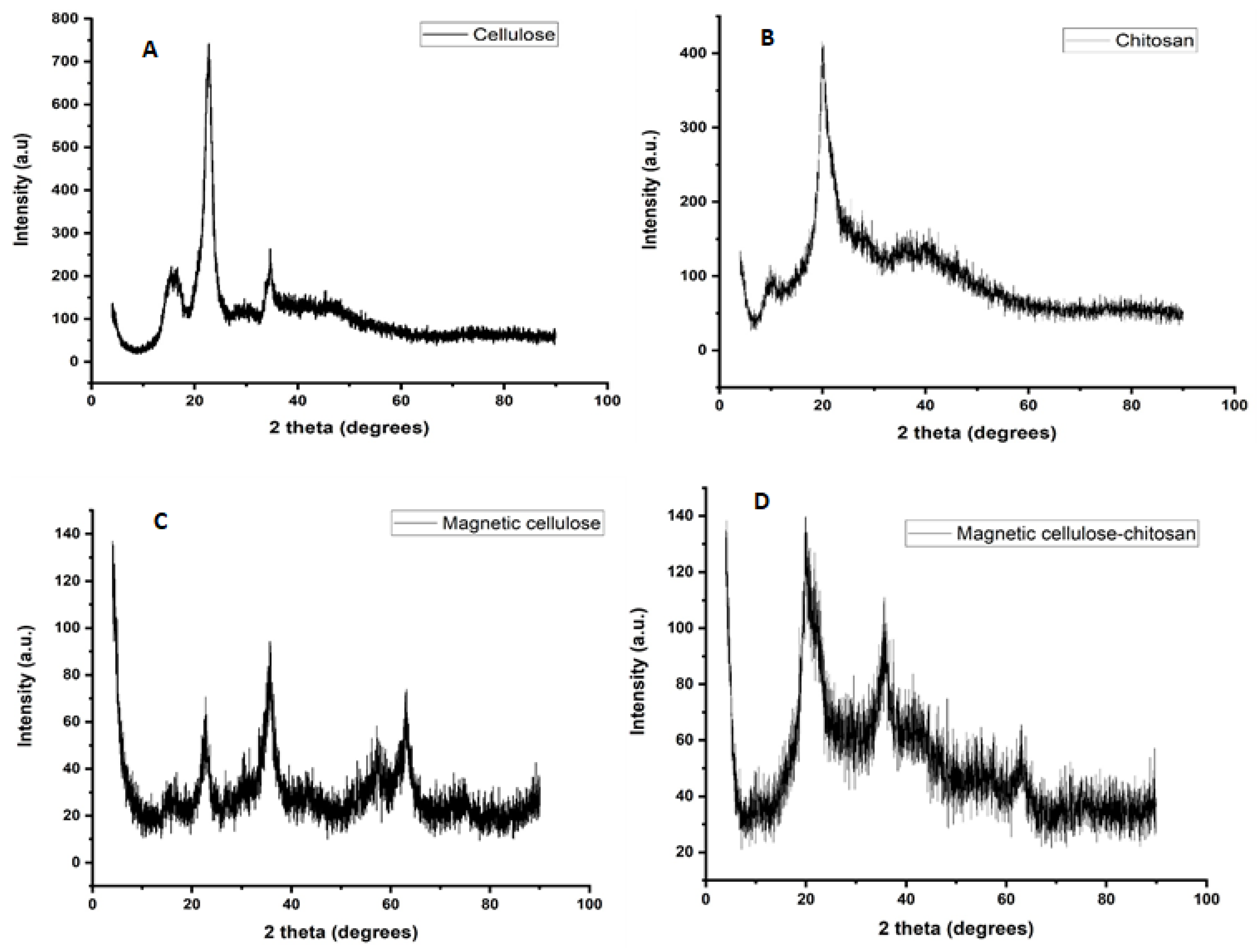
2.1.2. X-ray Diffraction Spectroscopy
2.1.3. Transmission Electron Microscopy
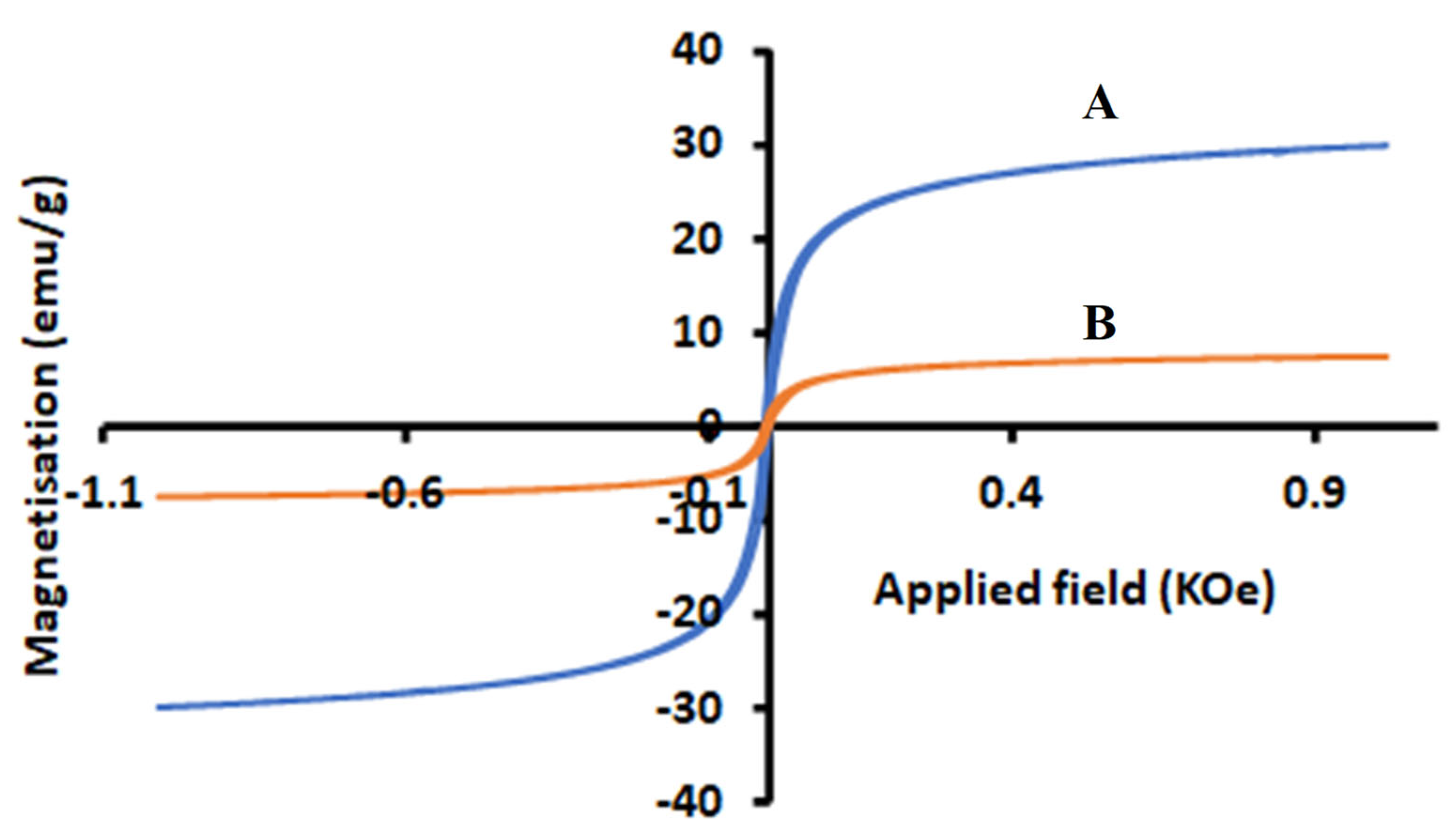
2.1.4. Magnetization Analysis
2.1.5. N2 Adsorption-Desorption Analysis
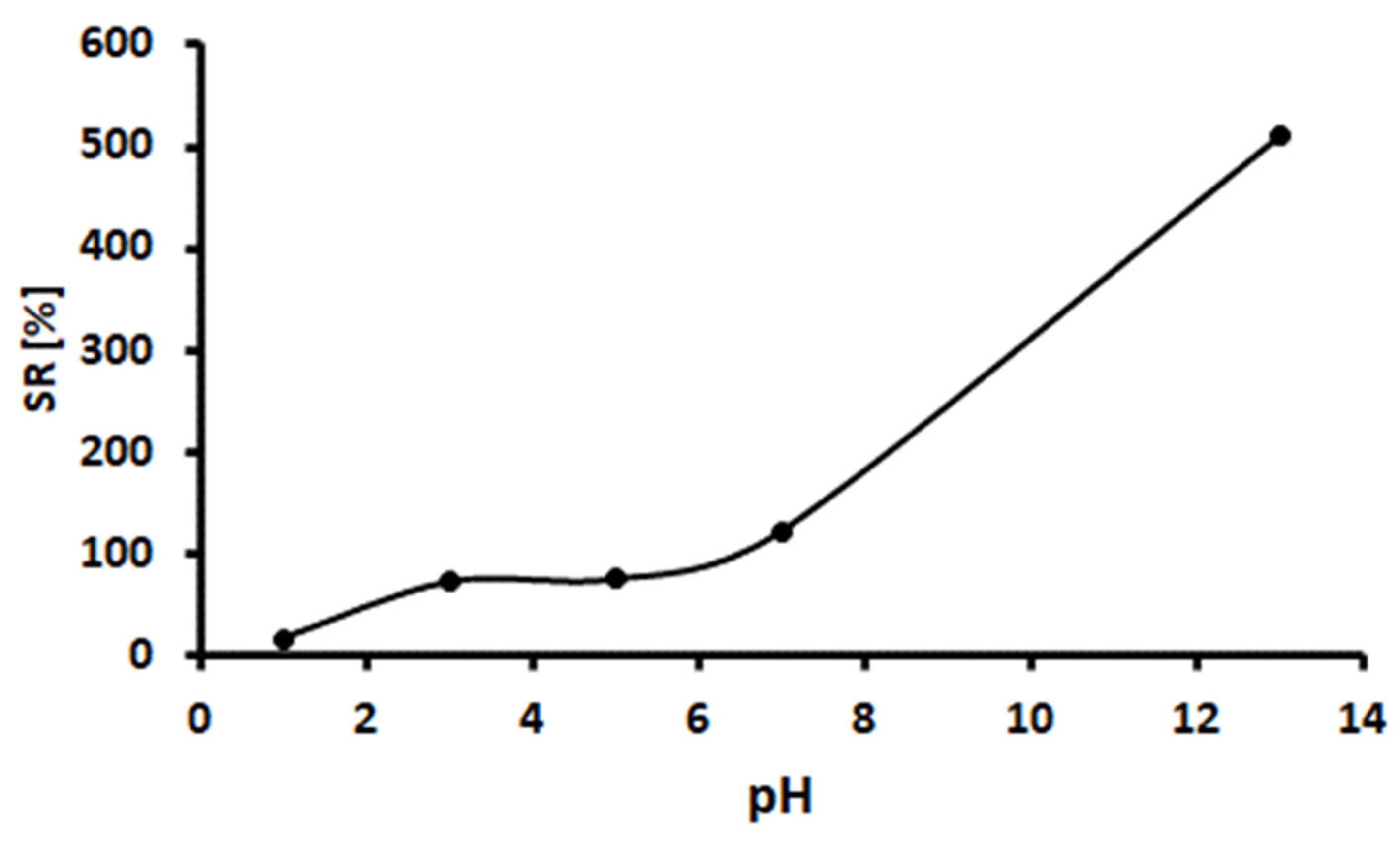
2.2. Percentage Swelling Ratio of the Hydrogel
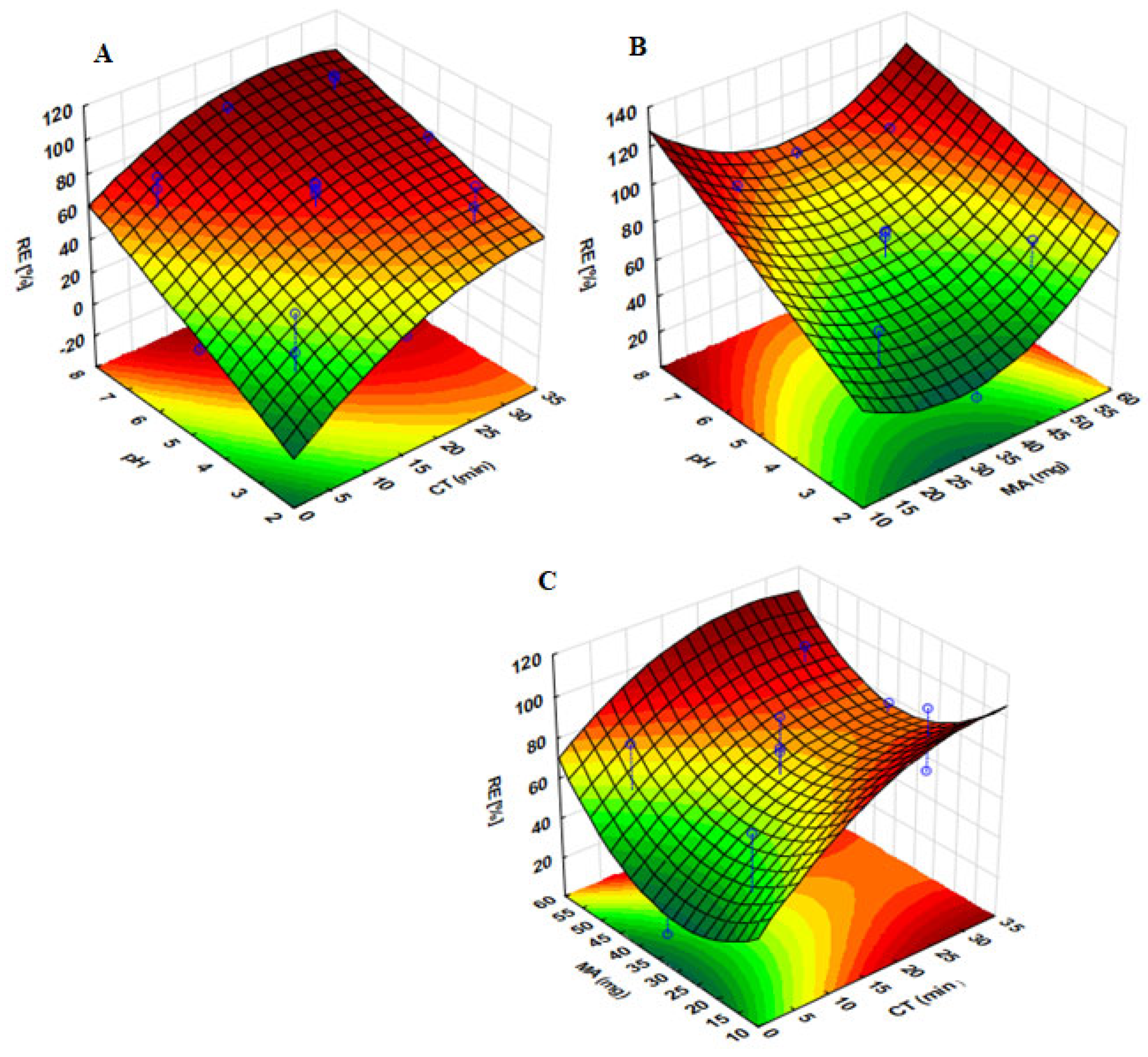
2.3. Optimization of the Adsorption Process
2.3.1. Response Surface Methodology
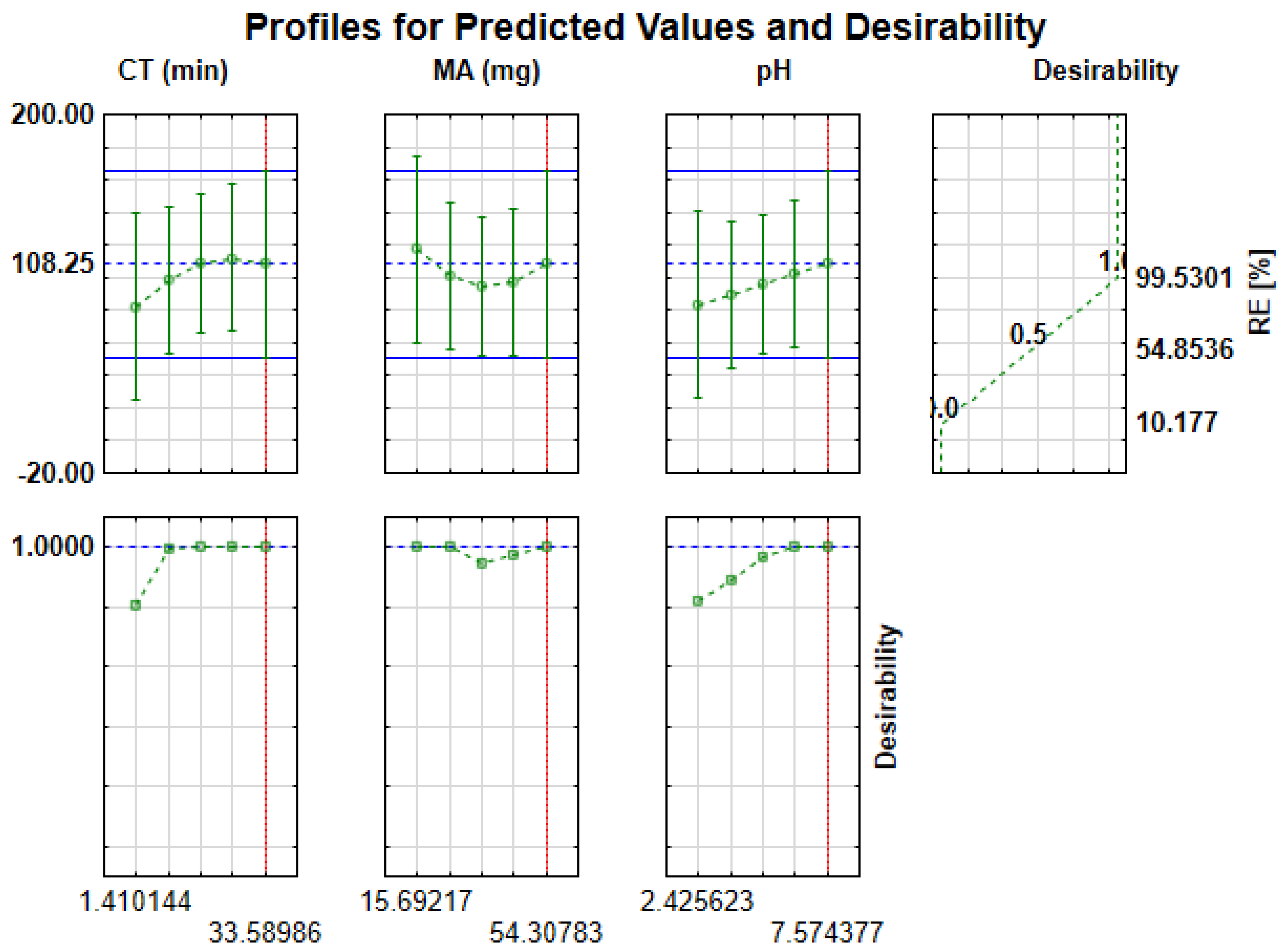
2.3.2. Desirability Function
2.4. Equilibrium Isotherms
2.5. Adsorption Kinetics
2.6. Thermodynamics Studies
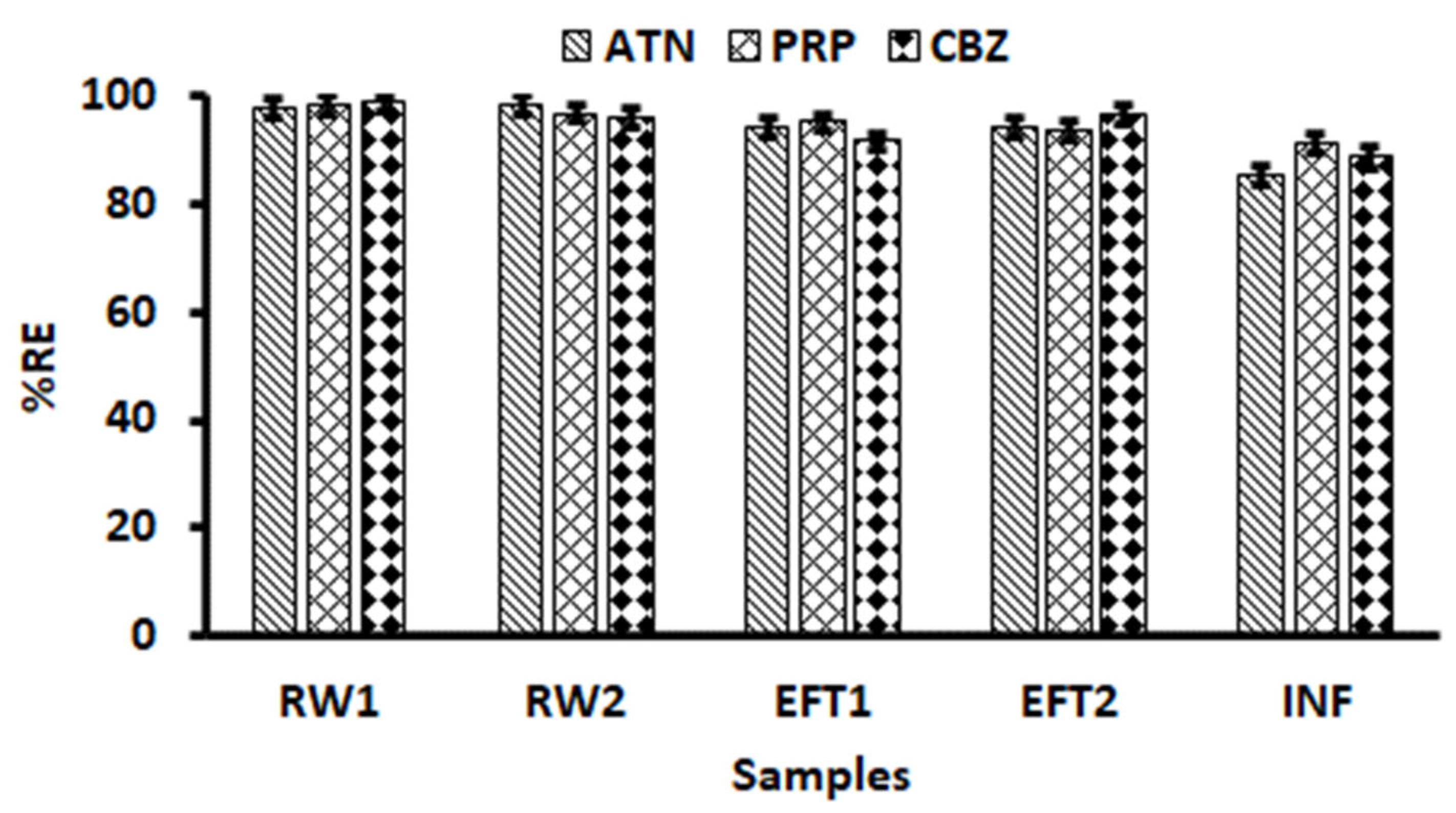
2.7. Application to Real Samples
2.7.1. Occurrence of Atenolol, Propranolol, and Carbamazepine in Water Samples
2.7.2. Removal Efficiency of Selected Pharmaceuticals
2.8. Regeneration Studies
2.9. Comparison with Previous Studies
3. Experimental
3.1. Material and Reagents
3.2. Instrumentation
3.3. Preparation of the Nanocomposite
3.3.1. Synthesis of the Magnetic Cellulose
3.3.2. Synthesis of the Magnetic Cellulose-Chitosan Hydrogel Nanocomposite
3.4. Ultrasound Assisted Batch Adsorption Studies
3.5. Adsorption Isotherms, Kinetics and Thermodynamic Experiments
3.6. Adsorption Data Analysis
3.6.1. Adsorption Isotherms
3.6.2. Adsorption Kinetic Models
3.6.3. Thermodynamics Studies
3.7. Swelling Test
3.8. Real Water Samples
3.9. Reusability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Lorenzo, T.; Castaño-Sánchez, A.; Di Marzio, W.D.; García-Doncel, P.; Nozal Martínez, L.; Galassi, D.M.P.; Iepure, S. The role of freshwater copepods in the environmental risk assessment of caffeine and propranolol mixtures in the surface water bodies of Spain. Chemosphere 2019, 220, 227–236. [Google Scholar] [CrossRef]
- Karaman, R.; Dajani, K.; Hallak, H. Computer-assisted design for atenolol prodrugs for the use in aqueous formulations. J. Mol. Model. 2012, 18, 1523–1540. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, P.; Reddy, M.N.; Ramakrishna, S.; Diwan, P.V. Comparative in vivo evaluation of propranolol hydrochloride after oral and transdermal administration in rabbits. Eur. J. Pharm. Biopharm. 2003, 56, 81–85. [Google Scholar] [CrossRef]
- Rajendran, K.; Sen, S. Adsorptive removal of carbamazepine using biosynthesized hematite nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 9, 122–127. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Rocha, L.S.; Gil, M.V.; Otero, M.; Silva, N.J.O.; Esteves, V.I.; Calisto, V. In situ functionalization of a cellulosic-based activated carbon with magnetic iron oxides for the removal of carbamazepine from wastewater. Environ. Sci. Pollut. Res. 2021, 28, 18314–18327. [Google Scholar] [CrossRef]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. Adsorption of Congo Red dye by native amine and carboxyl modified biomass of Funalia trogii: Isotherms, kinetics and thermodynamics mechanisms. Korean J. Chem. Eng. 2018, 35, 1303–1311. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Kunduzcu, G.; Arica, M.Y. Preparation and characterization of strong cation exchange terpolymer resin as effective adsorbent for removal of disperse dyes. Polym. Eng. Sci. 2020, 60, 192–201. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Chen, D.; Sun, B. New tissue engineering material copolymers of derivatives of cellulose and lactide: Their synthesis and characterization. Mater. Sci. Eng. C 2000, 11, 57–60. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- O’Toole, S.; Metcalfe, C. Synthetic Musks in Fish from Urbanized Areas of the Lower Great Lakes, Canada. J. Great Lakes Res. 2008, 32, 361–369. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M. Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv. 2015, 5, 64106–64118. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym.-Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Ma, L.; Xiang, C.; Li, L. TiO2-doped chitosan microspheres supported on cellulose acetate fibers for adsorption and photocatalytic degradation of methyl orange. Polymers 2019, 11, 1293. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Fu, Y.Q.; Zong, E.M.; Jiang, S.T.; Li, J.B.; Zhu, J.Q.; Zhu, Y.Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- OU, A.; BO, I. Chitosan Hydrogels and their Glutaraldehyde-Crosslinked Counterparts as Potential Drug Release and Tissue Engineering Systems—Synthesis, Characterization, Swelling Kinetics and Mechanism. J. Phys. Chem. Biophys. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sheng, C.; Zhou, Y.; Lu, J.; Zhang, X.; Xue, G. Preparation and characterization of chitosan based hydrogels chemical cross-linked by oxidized cellulose nanowhiskers. Polym. Compos. 2019, 40, 2432–2440. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, P.; Yu, F. Enhanced adsorption of sulfonamides by a novel carboxymethyl cellulose and chitosan-based composite with sulfonated graphene oxide. Bioresour. Technol. 2021, 320, 124373. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous magnetic cellulose-chitosan composite microspheres: Preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, B.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X. Biocompatible magnetic cellulose-chitosan hybrid gel microspheres reconstituted from ionic liquids for enzyme immobilization. J. Mater. Chem. 2012, 22, 15085–15091. [Google Scholar] [CrossRef]
- Karami, S.; Zeynizadeh, B. Reduction of 4-nitrophenol by a disused adsorbent: EDA-functionalized magnetic cellulose nanocomposite after the removal of Cu2+. Carbohydr. Polym. 2019, 211, 298–307. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Ma, L.; Zhou, H.; Yu, Y.; Guo, T.; Zhang, Y.; Huang, H. Green pH/magnetic sensitive hydrogels based on pineapple peel cellulose and polyvinyl alcohol: Synthesis, characterization and naringin prolonged release. Carbohydr. Polym. 2019, 209, 51–61. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Y.; Xue, Z.; Yin, W.; Liang, X.; Li, M.; Chang, J. Modified nanoporous magnetic cellulose–chitosan microspheres for efficient removal of Pb(II) and methylene blue from aqueous solution. Cellulose 2017, 24, 4793–4806. [Google Scholar] [CrossRef]
- Lei, Y.; Su, H.; Tian, F. A Novel Nitrogen Enriched Hydrochar Adsorbents Derived from Salix Biomass for Cr (VI) Adsorption. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon-HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Bazrafshan, A.A.; Ghaedi, A.M. Trace determination of safranin O dye using ultrasound assisted dispersive solid-phase micro extraction: Artificial neural network-genetic algorithm and response surface methodology. Ultrason. Sonochem. 2016, 33, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archin, S.; Sharifi, S.H.; Asadpour, G. Optimization and modeling of simultaneous ultrasound-assisted adsorption of binary dyes using activated carbon from tobacco residues: Response surface methodology. J. Clean. Prod. 2019, 239, 118136. [Google Scholar] [CrossRef]
- Nie, W.; Li, Y.; Chen, L.; Zhao, Z.; Zuo, X.; Wang, D.; Zhao, L.; Feng, X. Interaction between multi-walled carbon nanotubes and propranolol. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- To, M.H.; Hadi, P.; Hui, C.W.; Lin, C.S.K.; McKay, G. Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Abu Bakar, N.F.; Nik Him, N.R.; Lau, W.J. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Dong, S.; Jing, X.; Cao, Y.; Xia, E.; Gao, S.; Mao, L. Non-covalent assembled laccase-graphene composite: Property, stability and performance in beta-blocker removal. Environ. Pollut. 2019, 252, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Schwaab, M.; Steffani, E.; Barbosa-Coutinho, E.; Severo Júnior, J.B. Critical analysis of adsorption/diffusion modelling as a function of time square root. Chem. Eng. Sci. 2017, 173, 179–186. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Equilibrium and kinetic modelling of adsorption of Rhodamine B on MoS2. Mater. Res. Bull. 2019, 111, 238–244. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Wang, T.; Chen, F.; Li, X.; Hou, H.; Zhou, M. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: Adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019, 285, 62–74. [Google Scholar] [CrossRef]
- Mhuka, V.; Dube, S.; Nindi, M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-OrbitrapTM MS. Emerg. Contam. 2020, 6, 250–258. [Google Scholar] [CrossRef]
- Zong, E.; Huang, G.; Liu, X.; Lei, W.; Jiang, S.; Ma, Z.; Wang, J.; Song, P. A lignin-based nano-adsorbent for superfast and highly selective removal of phosphate. J. Mater. Chem. A 2018, 6, 9971–9983. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Su, T.; Lin, L.; Gong, Q. Honeycomb-like hydrogel adsorbents derived from salecan polysaccharide for wastewater treatment. Cellulose 2019, 26, 8759–8773. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Microporous Mesoporous Mater. 2016, 232, 273–280. [Google Scholar] [CrossRef]
- Mhammed Alzayd, A.A.; Atyaa, A.I.; Radhy, N.D.; Jasim Al-Hayder, L.S. A new adsorption material based GO/PVP/AAc composite hydrogel characterization, study kinetic and thermodynamic to removal Atenolol drug from wast water. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 062023. [Google Scholar] [CrossRef]
- Del Mar Orta, M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of propranolol onto montmorillonite: Kinetic, isotherm and pH studies. Appl. Clay Sci. 2019, 173, 107–114. [Google Scholar] [CrossRef]
- Zhao, Y.; Cho, C.W.; Wang, D.; Choi, J.W.; Lin, S.; Yun, Y.S. Simultaneous scavenging of persistent pharmaceuticals with different charges by activated carbon fiber from aqueous environments. Chemosphere 2020, 247, 125909. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, G.; Chen, K.; Lu, J.; Lei, J.; Pu, S. Adsorptive removal of bisphenol A, chloroxylenol, and carbamazepine from water using a novel β-cyclodextrin polymer. Ecotoxicol. Environ. Saf. 2019, 170, 278–285. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, Y.; Zhang, X.; Lu, C. Facile synthesis of magnetic nanocomposites of cellulose@ ultrasmall iron oxide nanoparticles for water treatment. RSC Adv. 2014, 4, 22632–22641. [Google Scholar] [CrossRef]
- Mashile, G.P.; Mpupa, A.; Nqombolo, A.; Dimpe, K.M.; Nomngongo, P.N. Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J. Water Process Eng. 2020, 33, 101011. [Google Scholar] [CrossRef]
- Sharififard, H.; Nabavinia, M.; Soleimani, M. Evaluation of adsorption efficiency of activated carbon/chitosan composite for removal of Cr (VI) and Cd (II) from single and bi-solute dilute solution. Adv. Environ. Technol. 2017, 2, 215–227. [Google Scholar]
- Mashile, P.P.; Mpupa, A.; Nomngongo, P.N. Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: Kinetics and isotherms. Toxicon 2018, 145, 25–31. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Characterisation of water-soluble protein powder and optimisation of process parameters for the removal of sulphonamides from wastewater. Environ. Sci. Pollut. Res. 2019, 26, 21450–21462. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural Polymers and the Hydrogels Prepared from Them; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128164211. [Google Scholar]
- Karzar Jeddi, M.; Mahkam, M. Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef]
- Gao, X.; He, C.; Xiao, C.; Zhuang, X.; Chen, X. Biodegradable pH-responsive polyacrylic acid derivative hydrogels with tunable swelling behavior for oral delivery of insulin. Polymer 2013, 54, 1786–1793. [Google Scholar] [CrossRef]
- Ali, W.; Gebert, B.; Hennecke, T.; Graf, K.; Ulbricht, M.; Gutmann, J.S. Design of Thermally Responsive Polymeric Hydrogels for Brackish Water Desalination: Effect of Architecture on Swelling, Deswelling, and Salt Rejection. ACS Appl. Mater. Interfaces 2015, 7, 15696–15706. [Google Scholar] [CrossRef]
- Akhgari, A.; Farahmand, F.; Afrasiabi Garekani, H.; Sadeghi, F.; Vandamme, T.F. Permeability and swelling studies on free films containing inulin in combination with different polymethacrylates aimed for colonic drug delivery. Eur. J. Pharm. Sci. 2006, 28, 307–314. [Google Scholar] [CrossRef]

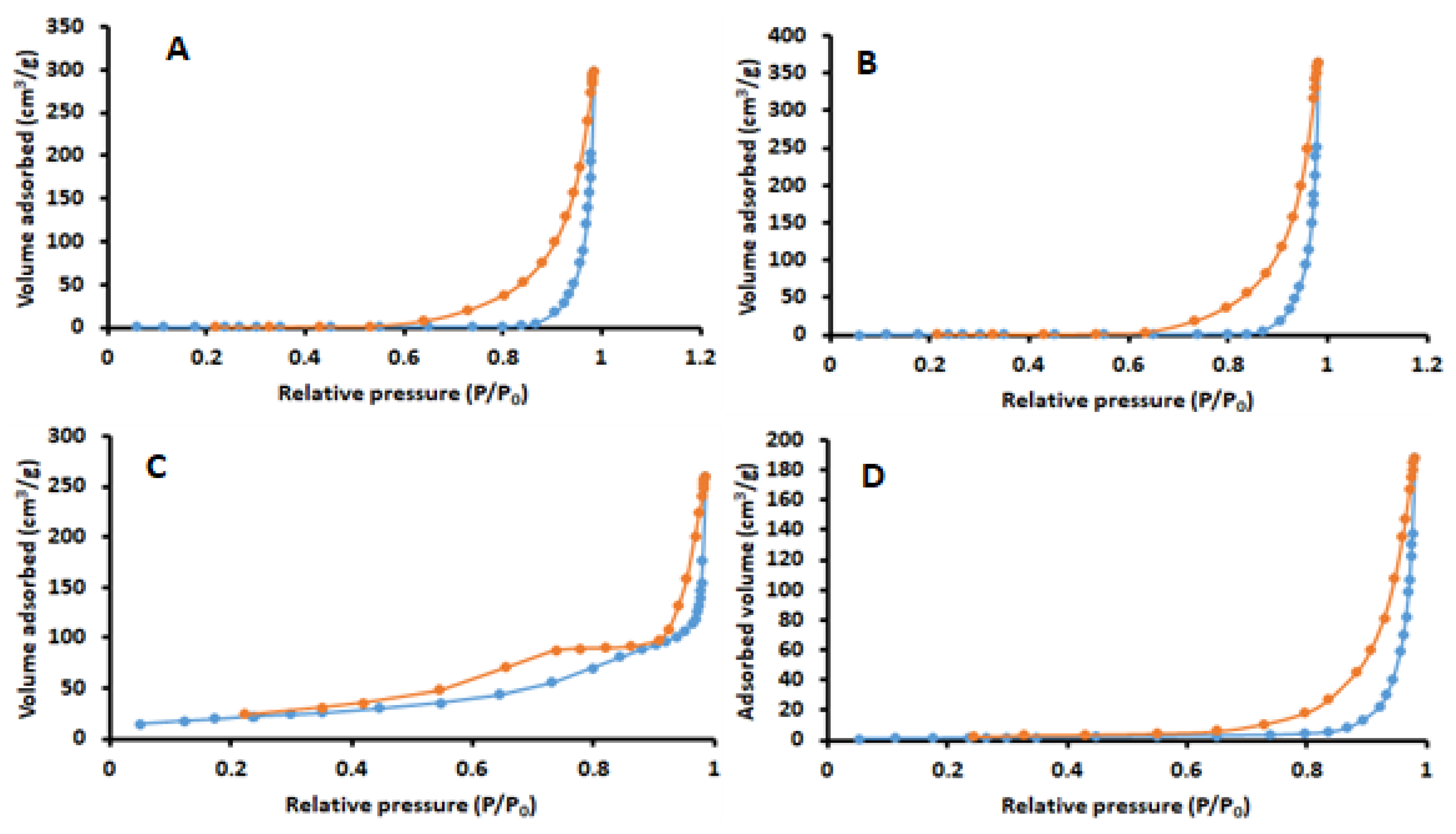


| Surface Properties | Cellulose | Chitosan | Magnetic Cellulose | Nanocomposite |
|---|---|---|---|---|
| SBET (m2 g−1) | 1.53 | 1.58 | 75.3 | 112 |
| Total pore volume (cm3 g−1) | 0.46 | 0.36 | 0.39 | 0.59 |
| Average pore size (nm) | 21.2 | 12.8 | 20.7 | 24.1 |
| Isotherms | Parameters | Atenolol | Propranolol Hydrochloride | Carbamazepine |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 341 | 313 | 291 |
| kL (min −1) | 0.08 | 0.30 | 0.13 | |
| R2 | 0.9939 | 0.9951 | 0.9942 | |
| Freundlich | KF | 42.2 | 114 | 73.0 |
| N | 1.7 | 3.8 | 2.9 | |
| R2 | 0.9208 | 0.9836 | 0.9877 | |
| Sips | KS (L mg−1) | 0.07 | 0.34 | 0.27 |
| qmS (mg g−1) | 345 | 310 | 294 | |
| nS | 1.0 | 0.95 | 1.3 | |
| R2 | 0.9956 | 0.9928 | 0.9907 | |
| Redlich–Peterson | β | 1.2 | 0.95 | 0.98 |
| KR-P (L g−1) | 23.0 | 40.5 | 10.3 | |
| αR-P | 1.25 | 1.97 | 1.56 | |
| R2 | 0.9925 | 0.9936 | 0.9917 |
| Kinetics | Parameters | Atenolol | Propranolol Hydrochloride | Carbamazepine |
|---|---|---|---|---|
| Experimental | qe (mg g−1) | 340 | 308 | 291 |
| Pseudo-first order | qe (mg g−1) | 172 | 183 | 119 |
| k1 | 0.0899 | 0.0854 | 0.0929 | |
| R2 | 0.8812 | 0.8720 | 0.8733 | |
| Pseudo-second order | qe (mg g−1) | 341 | 309 | 286 |
| k2 | 0.00078 | 0.0032 | 0.0013 | |
| R2 | 0.9984 | 0.9970 | 0.9994 |
| Parameters | Atenolol | Propranolol Hydrochloride | Carbamazepine |
|---|---|---|---|
| Kid1 (g mg−1 min−0.5) | 41.6 | 33.4 | 31.7 |
| C1 | 114 | 108 | 128 |
| R2 | 0.9917 | 0.9983 | 0.9988 |
| Kid2 (g mg−1 min−0.5) | 8.80 | 20.2 | 11.3 |
| C2 | 284 | 184 | 220 |
| R2 | 0.7669 | 0.8286 | 0.9077 |
| Kid3 (g mg−1 min−0.5) | 0.295 | 0.301 | 0.056 |
| C3 | 336 | 306 | 289 |
| R2 | 0.9875 | 0.9877 | 0.6812 |
| Analytes | Temp (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|
| Atenolol | 298 | −4.28 | 61.9 | 21.3 |
| 303 | −4.40 | |||
| 308 | −4.48 | |||
| 313 | −4.61 | |||
| Propranolol | 298 | −4.08 | 77.3 | 22.3 |
| 303 | −4.20 | |||
| 308 | −4.31 | |||
| 313 | −4.41 | |||
| Carbamazepine | 298 | −3.82 | 84.7 | 31.4 |
| 303 | −3.96 | |||
| 308 | −4.11 | |||
| 313 | −4.30 |
| Samples | Atenolol | Propranolol | Carbamazepine |
|---|---|---|---|
| River water upstream | 105 ± 2 | ND | 103 ± 4 |
| River downstream | 116 ± 3 | 83.4 ± 2 | 167 ± 3 |
| Effluent (outflow) | 457 ± 4 | 116 ± 3 | 406 ± 6 |
| Effluent before chlorination and UV treatment | 1033 ± 11 | 176 ± 2 | 537 ± 7 |
| Influent | 1455 ± 15 | 201 ± 3 | 894 ± 10 |
| Analytes | Adsorbent | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| Propranolol | Smectite clay mineral montmorillonite | 161 | [55] |
| Carbamazepine and Propranolol | Activated carbon fiber | (0.300 ± 0.014, 0.277 ± 0.021 | [56] |
| Atenolol | Granular activated carbon | 1.2 | [57] |
| Atenolol and Carbamazepine | Activated palm kernel shell | 0.184 and 0.170 | [43] |
| Carbamazepine | Hematite nanoparticles | 2.89 | [4] |
| Carbamazepine | β-cyclodextrin polymer | 136.4 | [58] |
| Atenolol | Magnetic polymer clay composite | 15.6 | [53] |
| Atenolol | GO/PVP/AAc composite hydrogel | 0.0107 | [54] |
| Carbamazepine, Propranolol and Atenolol | Magnetic cellulose-chitosan nanocomposite | 291, 313 and 341 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashile, P.P.; Nomngongo, P.N. Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies. Gels 2021, 7, 190. https://doi.org/10.3390/gels7040190
Mashile PP, Nomngongo PN. Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies. Gels. 2021; 7(4):190. https://doi.org/10.3390/gels7040190
Chicago/Turabian StyleMashile, Phodiso Prudence, and Philiswa Nosizo Nomngongo. 2021. "Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies" Gels 7, no. 4: 190. https://doi.org/10.3390/gels7040190
APA StyleMashile, P. P., & Nomngongo, P. N. (2021). Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies. Gels, 7(4), 190. https://doi.org/10.3390/gels7040190








