Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles and Surfactants
Abstract
:1. Introduction
2. Results and Discussion
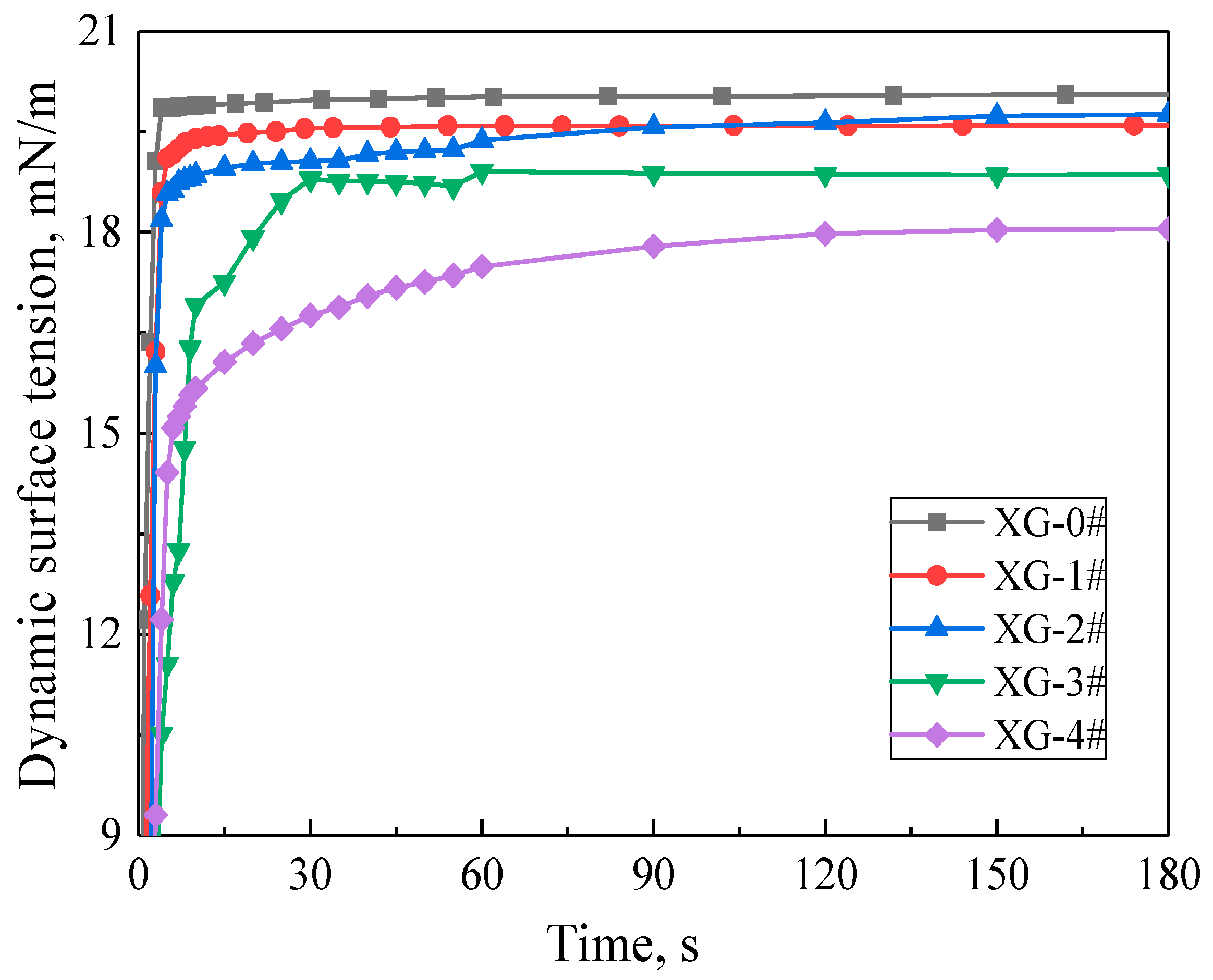
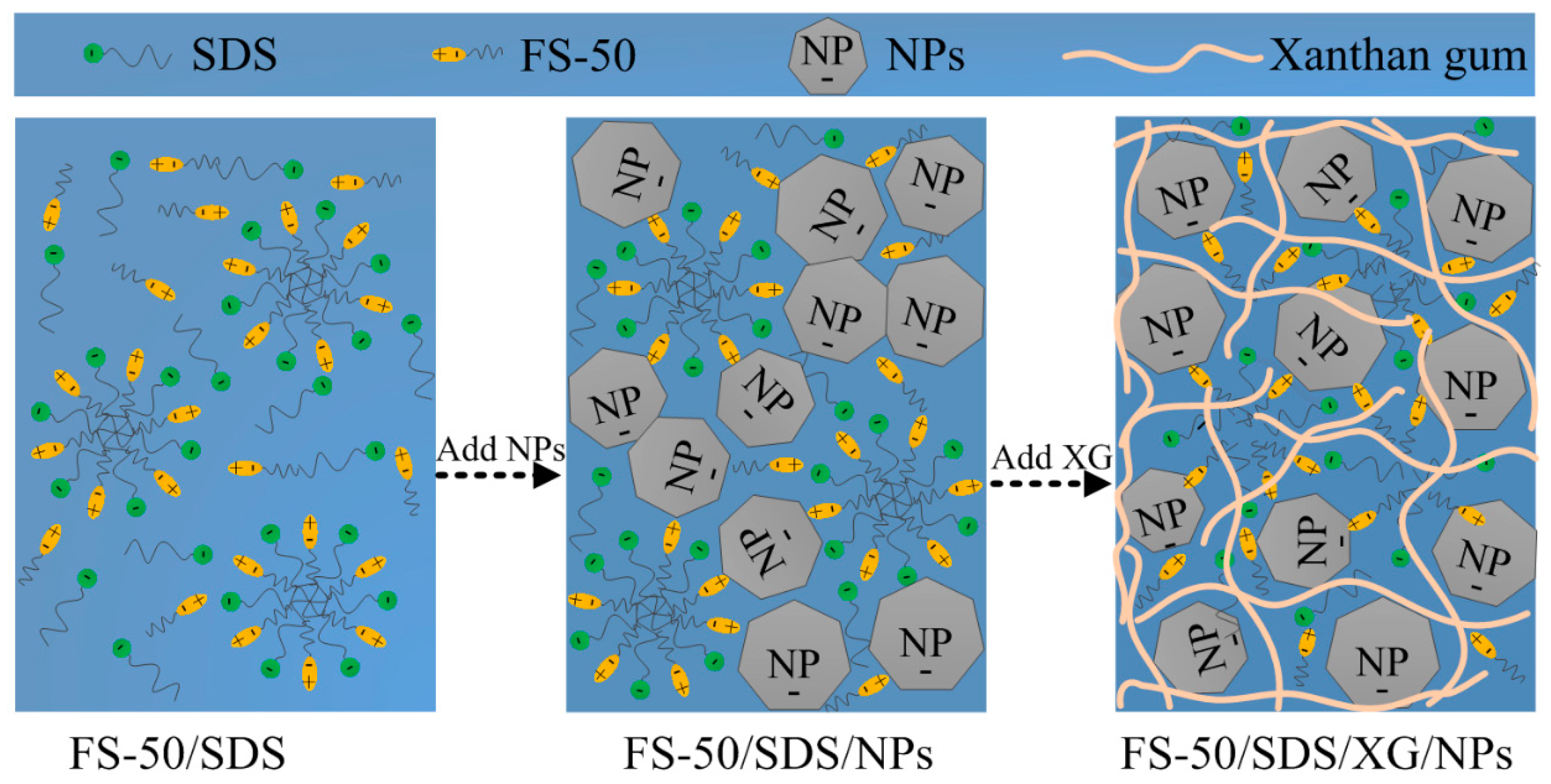
2.1. Properties of Foam Dispersions
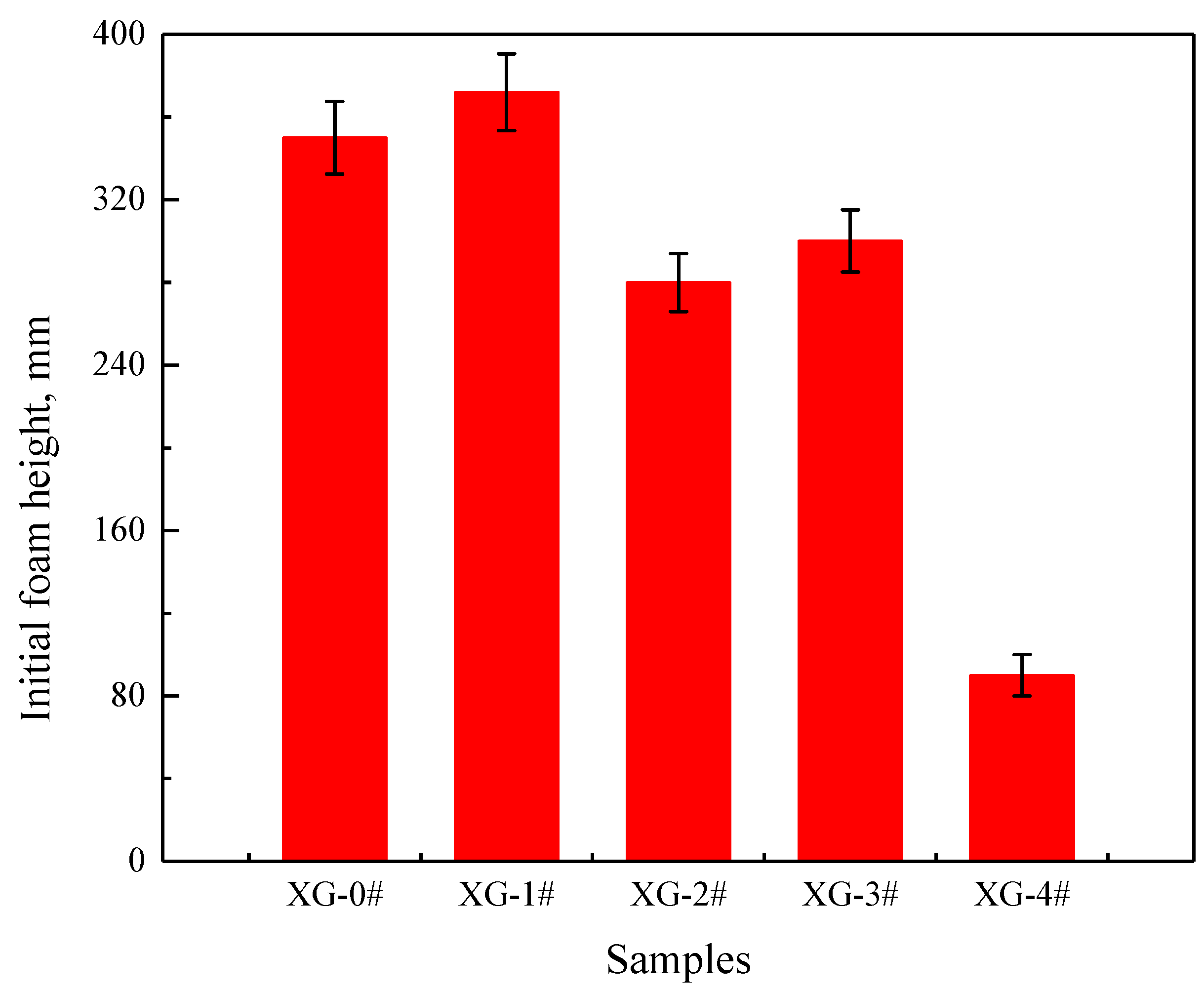
2.2. Foaming Ability
2.3. Foam Thermal Stability
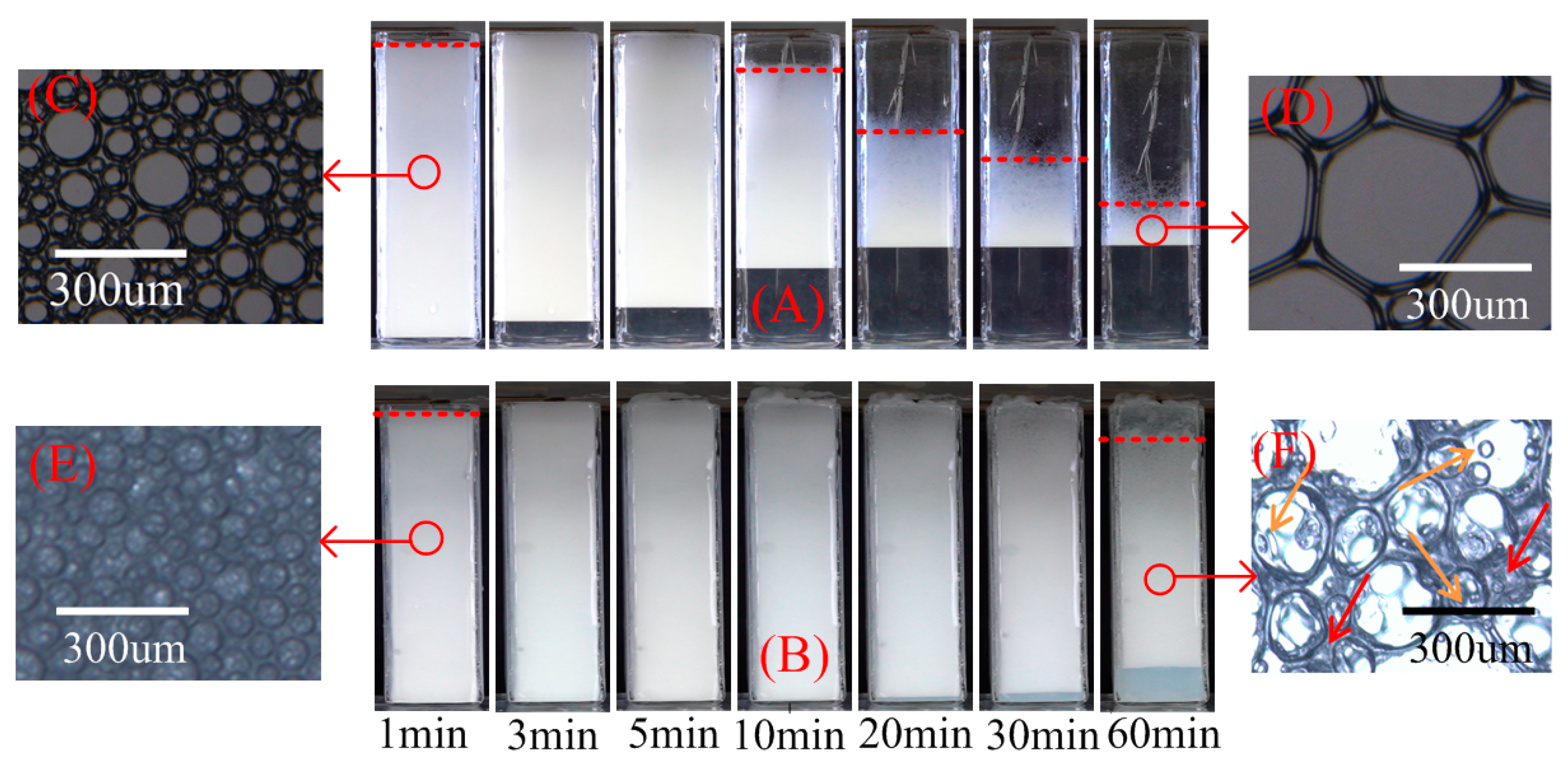
2.3.1. Foam Drainage and Decay Process
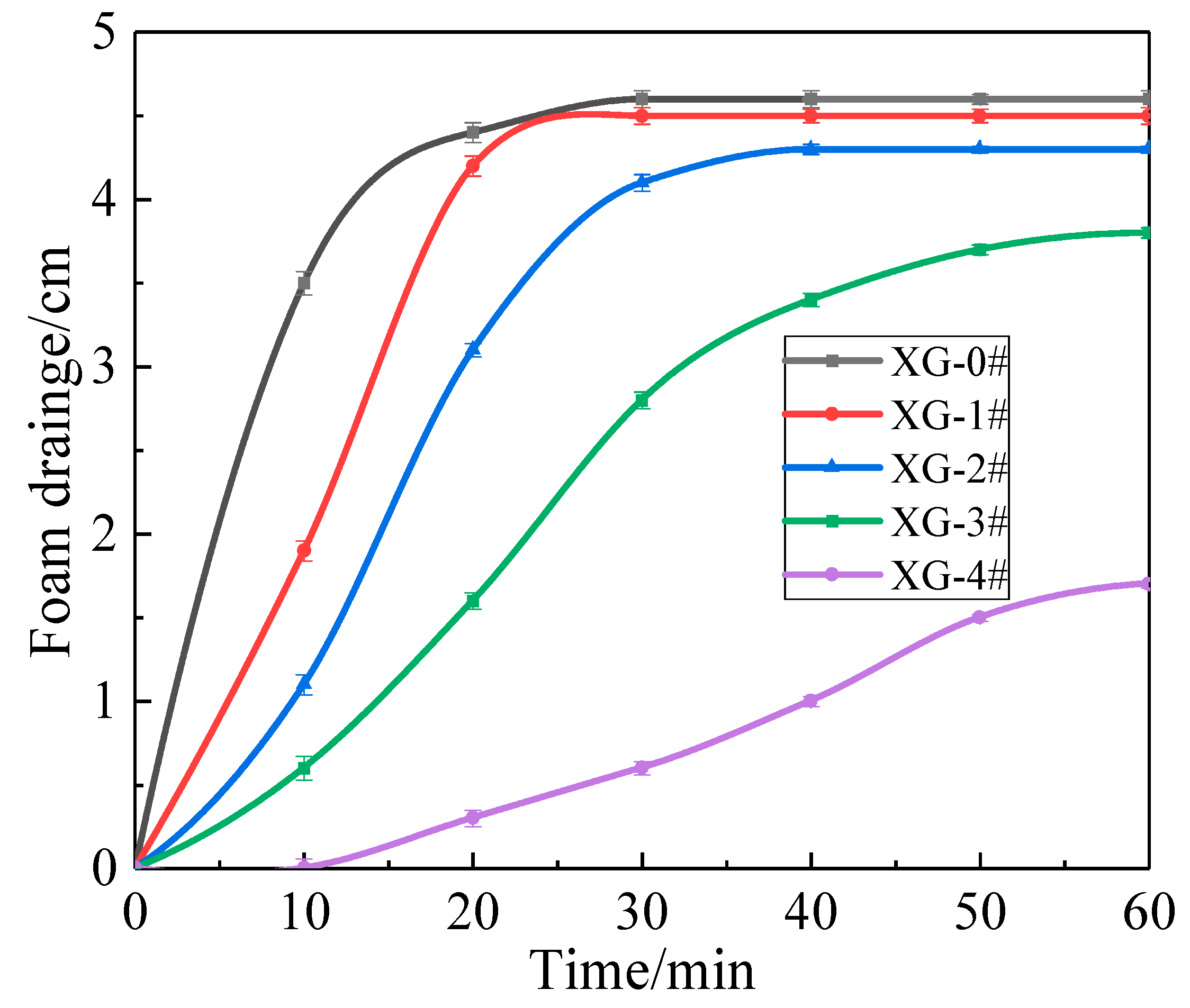
2.3.2. Variation in Foam Thickness and Drainage Height Versus Time
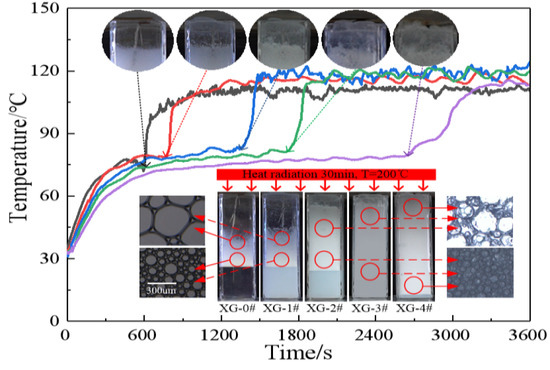
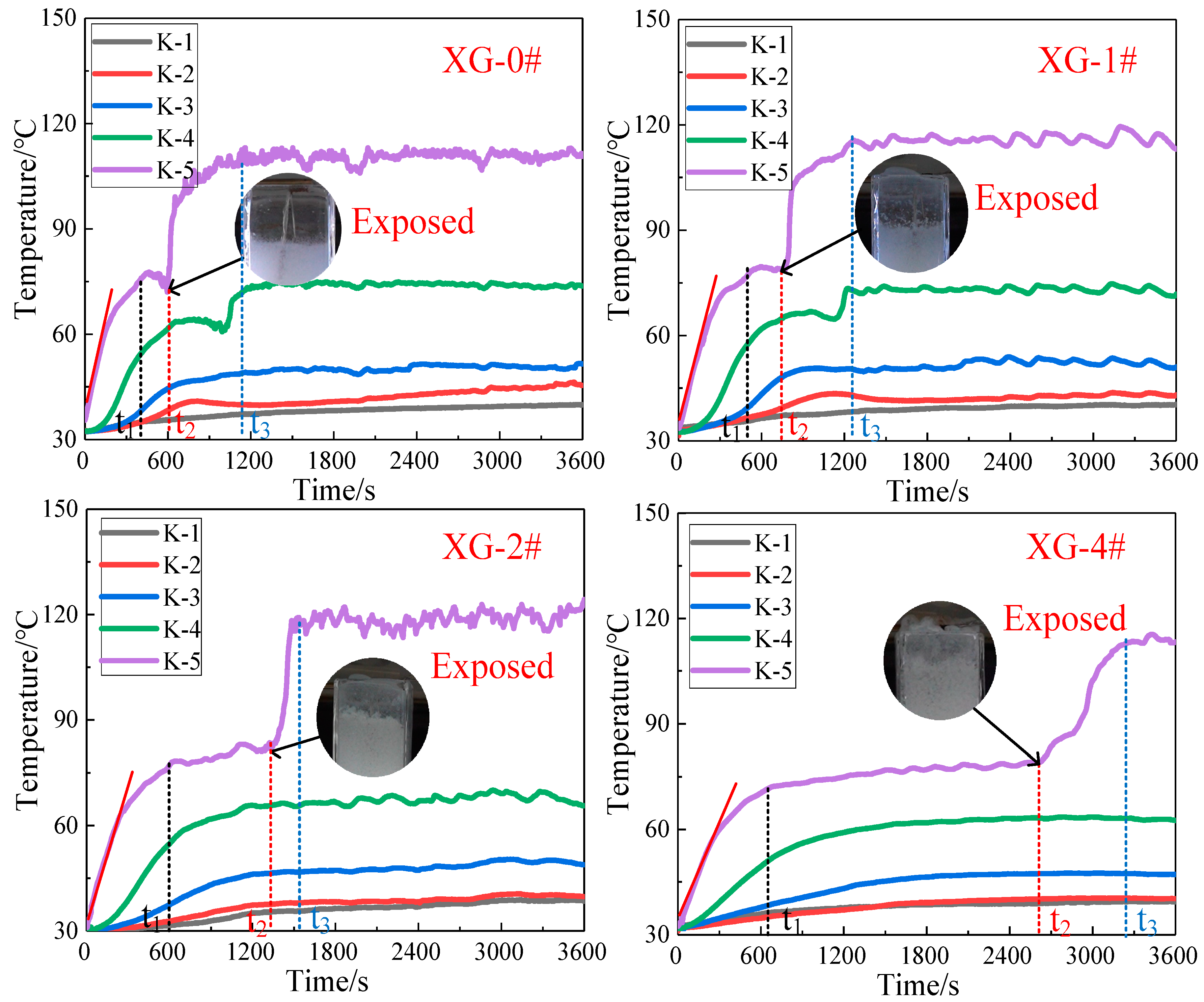
2.3.3. Temperature Distribution of the Foam Layer
3. Conclusions
4. Experimental
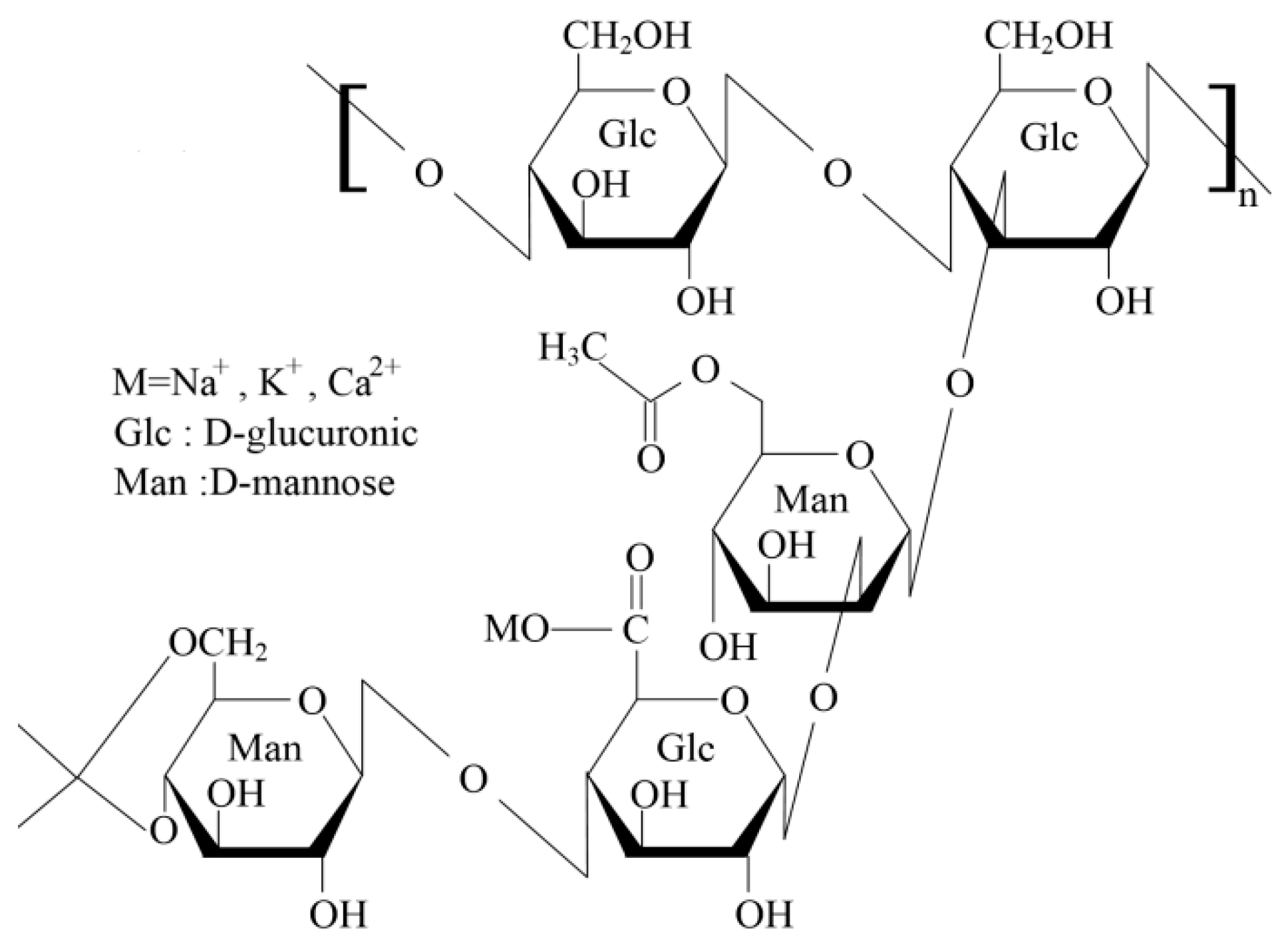
4.1. Materials
4.2. Preparation of Foam Dispersions
4.3. Characterization of Foam Dispersions
4.4. Testing Device for Foam Thermal Stability
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, A.J.; Littlejohn, K.A.; Hooley, P.; Cox, P.W. Formation and Stability of Food Foams and Aerated Emulsions: Hydro-phobins as Novel Functional Ingredients. Curr. Opin. Colloid Interface Sci. 2013, 18, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.; Okuda, T.; Kurose, K.; Tsai, T.-Y.; Nakai, S.; Nishijima, W.; Okada, M. Surface ozonation of polyvinyl chloride for its separation from waste plastic mixture by froth floatation. J. Mater. Cycles Waste Manag. 2010, 12, 326–331. [Google Scholar] [CrossRef]
- Tran, D.N.; Whitby, C.P.; Fornasiero, D.; Ralston, J. Foamability of aqueous suspensions of fine graphite and quartz particles with a triblock copolymer. J. Colloid Interface Sci. 2010, 348, 460–468. [Google Scholar] [CrossRef]
- Polanish, E. Foam Applicators to Apply Cosmetics or Nail Polish. U.S. Patent 2013. [Google Scholar]
- Sheng, Y.; Xue, M.; Zhang, S.; Wang, Y.; Zhai, X.; Zhao, Y.; Ma, L.; Liu, X. Role of nanoparticles in the performance of foam stabilized by a mixture of hydrocarbon and fluorocarbon surfactants. Chem. Eng. Sci. 2020, 228, 115977. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Li, C. Fluorinated and fluorine-free firefighting foams spread on heptane surface. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Ye, H.; Yang, L.L.; Peng, B. Nanoparticles as Foam Stabilizer: Mechanism, Control Parameters and Ap-plication in Foam Flooding for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2021, 202, 108561–108576. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic effect of mixed surfactant systems on foam behavior and surface tension. J. Surfactants Detergents 2013, 16, 621–630. [Google Scholar] [CrossRef]
- Kishi, T.; Arai, M. Study on the generation of perfluorooctane sulfonate from the aqueous film-forming foam. J. Hazard. Mater. 2008, 159, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef]
- Rodriguez-Freire, L.; Abad-Fernández, N.; Sierra-Alvarez, R.; Hoppe-Jones, C.; Peng, H.; Giesy, J.P.; Snyder, S.; Keswani, M. Sonochemical degradation of perfluorinated chemicals in aqueous film-forming foams. J. Hazard. Mater. 2016, 317, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.L.; Snow, A.W.; Hinnant, K.M.; Ananth, R. Modulation of fluorocarbon surfactant diffusion with diethylene glycol butyl ether for improved foam characteristics and fire suppression. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123660. [Google Scholar] [CrossRef]
- Ananth, R.; Snow, A.W.; Hinnant, K.M.; Giles, S.L.; Farley, J.P. Synergisms between siloxane-polyoxyethylene and alkyl polyglycoside surfactants in foam stability and pool fire extinction. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123686. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Conroy, M.W.; Ananth, R. Influence of fuel on foam degradation for fluorinated and fluorine-free foams. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 1–17. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Sun, X.; Lu, S.; Li, C. Experimental Study on Effect of Foam Stabilizers on Aqueous Film-Forming Foam. Fire Technol. 2017, 54, 211–228. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Verma, A.; Chauhan, G.; Baruah, P.P.; Ojha, K. Morphology, Rheology, and Kinetics of Nanosilica Stabilized Gelled Foam Fluid for Hydraulic Fracturing Application. Ind. Eng. Chem. Res. 2018, 57, 13449–13462. [Google Scholar] [CrossRef]
- Sheng, Y.; Lu, S.; Xu, M.; Wu, X.; Li, C. Effect of Xanthan Gum on the Performance of Aqueous Film-Forming Foam. J. Dispers. Sci. Technol. 2016, 37, 1664–1670. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, N.; Miao, X.; Zong, R.; Lu, S. Formation of Stable Aqueous Foams on the Ethanol Layer: Synergistic Stabiliza-tion of Fluorosurfactant and Polymers. Colloids Surf. A 2020, 591, 124545–124561. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Lee, S.; Lim, J. Surface modification of calcium carbonate nanoparticles by fluorosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 213–223. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z. Highly stable foam stabilized by alumina nanoparticles for EOR: Effects of sodium cumenesul-fonate and electrolyte concentrations. Energy Fuels 2017, 31, 9016–9025. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Binks, B.P.; Kirkland, M.; Rodrigues, J.A. Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures. Soft Matter 2008, 4, 2373–2382. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Íñiguez-Palomares, R.; Rio, E.; Langevin, D. On the long-term stability of foams stabilised by mixtures of nano-particles and oppositely charged short chain surfactants. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Wang, J.; Li, S.; Li, B.; Jiang, L.; Wang, H.; Lu, Q.; Zhang, C.; Liu, W. Aqueous foam stabilized by partially hydrophobic nanoparticles in the presence of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 54–64. [Google Scholar] [CrossRef]
- Tang, F.-Q.; Xiao, Z.; Tang, J.-A.; Jiang, L. The effect of SiO2 particles upon stabilization of foam. J. Colloid Interface Sci. 1989, 131, 498–502. [Google Scholar] [CrossRef]
- Dickinson, E.; Ettelaie, R.; Kostakis, T.; Murray, B.S. Factors Controlling the Formation and Stability of Air Bubbles Stabi-lized by Partially Hydrophobic Silica Nanoparticles. Langmuir ACS J. Surf. Colloids 2004, 20, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Schulze, H.J. Colloidal Science of Flotation; CRC Press: Boca Raton, FL, USA, 2003; Volume 118, pp. 39–45. [Google Scholar]
- Singh, R.; Mohanty, K.K. Synergy between Nanoparticles and Surfactants in Stabilizing Foams for Oil Recovery. Energy Fuels 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z.; Miao, Q.; Deng, Z.; Zhu, Y. Foams Stabilized by In Situ-Modified Nanoparticles and Anionic Surfactants for Enhanced Oil Recovery. Energy Fuels 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Binks, B.; Bernard, P. Colloidal Particles at a Range of Fluid-Fluid Interfaces. Langmuir 2017, 33, 6947–6963. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, A.R.; Kam, S.I. A New Foam Rheology Model for Shale-Gas Foam Fracturing Applications. SPE Canadian unconventional resources conference. OnePetro 2012, 2012, 162709. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Impact of Natural Surfactant (Reetha), Polymer (Xanthan Gum), and Silica Nanoparticles To Enhance Heavy Crude Oil Recovery. Energy Fuels 2019, 33, 4225–4236. [Google Scholar] [CrossRef]
- Clavijo, J.V.; Moncayo-Riascos, I.; Husein, M.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Theoretical and Experimental Approach for Understanding the Interactions Among SiO2 Nanoparticles, CaCO3, and Xanthan Gum Components of Water-Based Mud. Energy Fuels 2021, 35, 4803–4814. [Google Scholar] [CrossRef]
- Bhattacharyya, A.B.; Monroy, F.; Langevin, D.; Argillier, J.F. Surface Rheology and Foam Stability of Mixed Surfac-tant-Polyelectrolyte Solutions. Langmuir 2000, 16, 8727–8732. [Google Scholar] [CrossRef]
- Abro, S.H.; Moria, H.A.; Al-Khazaal, A.Z.; Omer, U. Synthesis of Carbon Foam Using Xanthan-Gum Additive and Charac-terization for its Heat Absorbing and Retarding Properties. Mater. Sci. Forum 2020, 1013, 14–18. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Al-Amodi, A.O.H. Study of Polymer-Surfactant Interactions for Chemical Enhanced Oil Recovery in Carbonate Reservoirs. Ph.D. Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Eastern Province, Saudi Arabia, 2013; pp. 31–33. [Google Scholar]
- Sheng, Y.; Wu, X.; Lu, S.; Li, C. Experimental Study on Foam Properties of Mixed Systems of Silicone and Hydrocarbon Sur-factants. J. Surfactants Deterg. 2016, 19, 823–831. [Google Scholar] [CrossRef]
- Wang, J.; Xue, G.; Tian, B.; Li, S.; Chen, K.; Wang, D.; Li, Z. Interaction between Surfactants and SiO2 Nanoparticles in Multi-phase Foam and Its Plugging Ability. Energy Fuels 2017, 31, 408–417. [Google Scholar] [CrossRef]
- Tang, B.; Wu, Z.; Chen, W.H. Effect of nano-silica on foam and thermal stability of a foam extinguishing agent. Nano-Mater. Energy 2017, 6, 67–73. [Google Scholar]
- Zhou, R.; Lang, X.; Zhang, X.; Tao, B.; He, L. Thermal stability and insulation characteristics of three-phase fire-fighting foam exposed to radiant heating. Process. Saf. Environ. Prot. 2021, 146, 360–368. [Google Scholar] [CrossRef]
- Jiang, N.; Sheng, Y.; Li, C.; Lu, S. Surface activity, foam properties and aggregation behavior of mixtures of short-chain fluorocarbon and hydrocarbon surfactants. J. Mol. Liq. 2018, 268, 249–255. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Zhao, Y.; Wang, Q.; Ma, L.; Liu, X. Molecular interaction and foaming property of the mixtures of hydrocarbon, fluorocarbon and silicone surfactants. J. Mol. Liq. 2019, 296, 111836. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, X.; Sheng, Y.; Zong, R.; Li, C.; Lu, S. Role of salts in performance of foam stabilized with sodium dodecyl sulfate. Chem. Eng. Sci. 2020, 216, 115474. [Google Scholar] [CrossRef]
- Mitrinova, Z.; Tcholakova, S.; Denkov, N.; Ananthapadmanabhan, K. Role of interactions between cationic polymers and surfactants for foam properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 378–391. [Google Scholar] [CrossRef]
- Gaillard, T.; Roché, M.; Honorez, C.; Jumeau, M.; Balan, A.; Jedrzejczyk, C.; Drenckhan, W. Controlled foam generation using cyclic diphasic flows through a constriction. Int. J. Multiph. Flow 2017, 96, 173–187. [Google Scholar] [CrossRef]



| Samples | SDS (mM) | FS-50 (wt%) | NaCl (mM) | XG (wt%) | NPs (wt%) |
|---|---|---|---|---|---|
| XG-0# | 16 | 0.25 | 1 | 0 | 0 |
| XG-1# | 16 | 0.25 | 1 | 0 | 5 |
| XG-2# | 16 | 0.25 | 1 | 0.01 | 5 |
| XG-3# | 16 | 0.25 | 1 | 0.03 | 5 |
| XG-4# | 16 | 0.25 | 1 | 0.05 | 5 |
| Sample | EST (mN/m) | Conductivity (uS/ms) | Viscosity (mPa‧s) |
|---|---|---|---|
| XG-0# | 20.041 | 211 | 1.22 |
| XG-1# | 19.601 | 202 | 2.32 |
| XG-2# | 19.744 | 206 | 53.44 |
| XG-3# | 18.867 | 203.3 | 370.33 |
| XG-4# | 18.094 | 196.9 | 870.58 |
| Sample | t1 (s) | t2 (s) | t3 (s) | Tt1 (°C) | Tt3 (°C) | K (0–t1) |
|---|---|---|---|---|---|---|
| XG-0# | 445 | 595 | 1150 | 78.3 | 118.5 | 0.140 |
| XG-1# | 518 | 771 | 1270 | 78.5 | 118.4 | 0.119 |
| XG-2# | 660 | 1340 | 1557 | 78.3 | 118 | 0.111 |
| XG-3# | 816 | 1710 | 2040 | 76.3 | 117.1 | 0.106 |
| XG-4# | 805 | 2675 | 3270 | 72.8 | 116.9 | 0.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Yan, C.; Li, Y.; Peng, Y.; Ma, L.; Wang, Q. Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles and Surfactants. Gels 2021, 7, 179. https://doi.org/10.3390/gels7040179
Sheng Y, Yan C, Li Y, Peng Y, Ma L, Wang Q. Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles and Surfactants. Gels. 2021; 7(4):179. https://doi.org/10.3390/gels7040179
Chicago/Turabian StyleSheng, Youjie, Canbin Yan, Yang Li, Yunchuan Peng, Li Ma, and Qiuhong Wang. 2021. "Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles and Surfactants" Gels 7, no. 4: 179. https://doi.org/10.3390/gels7040179
APA StyleSheng, Y., Yan, C., Li, Y., Peng, Y., Ma, L., & Wang, Q. (2021). Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles and Surfactants. Gels, 7(4), 179. https://doi.org/10.3390/gels7040179