Tissue Adhesion-Anisotropic Polyrotaxane Hydrogels Bilayered with Collagen
Abstract
:1. Introduction
2. Results and Discussion
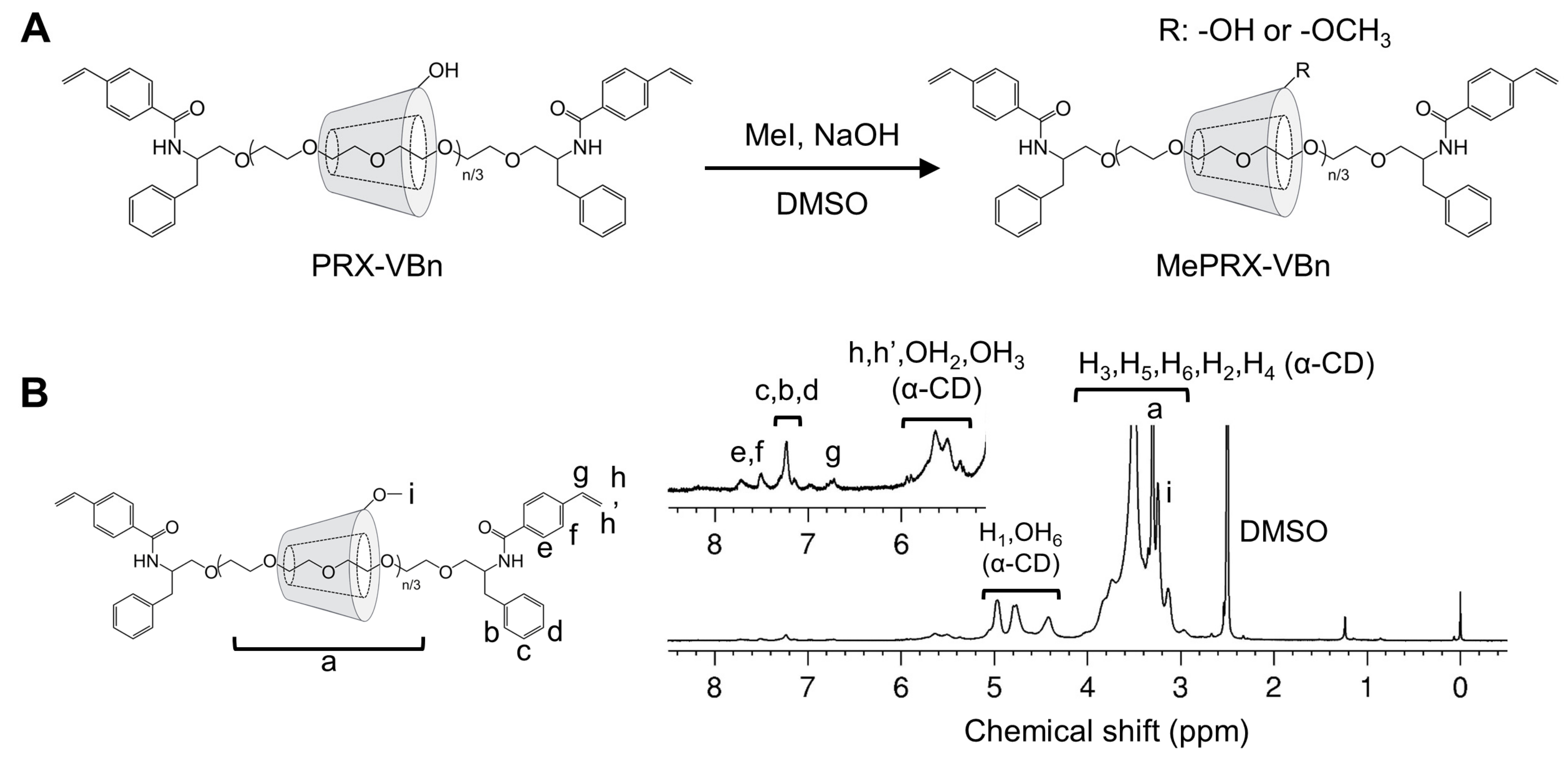
2.1. Characterization of Methylated Polyrotaxanes Capped with 4-vinylbenzyl Groups (MePRX-VBn)
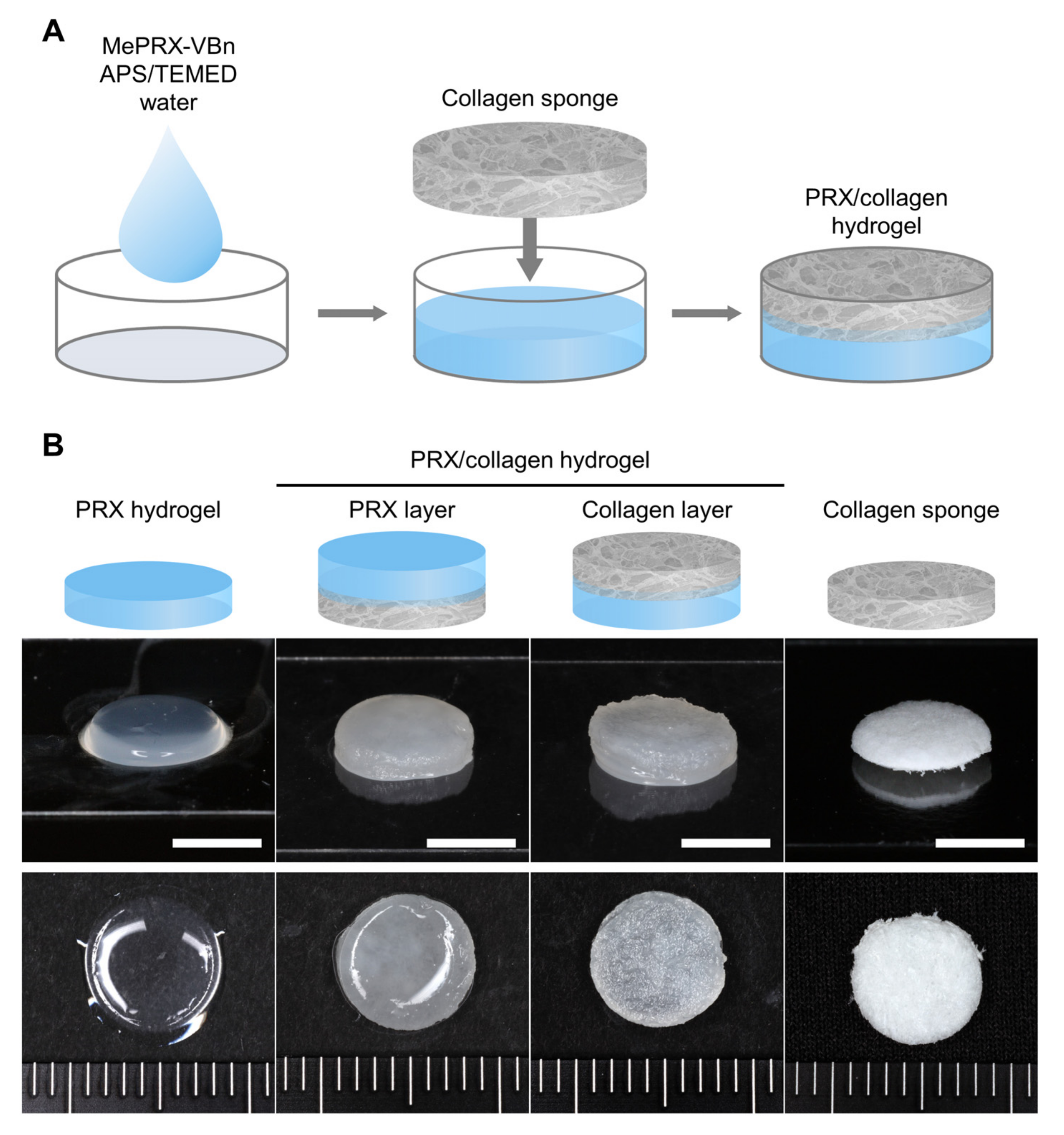
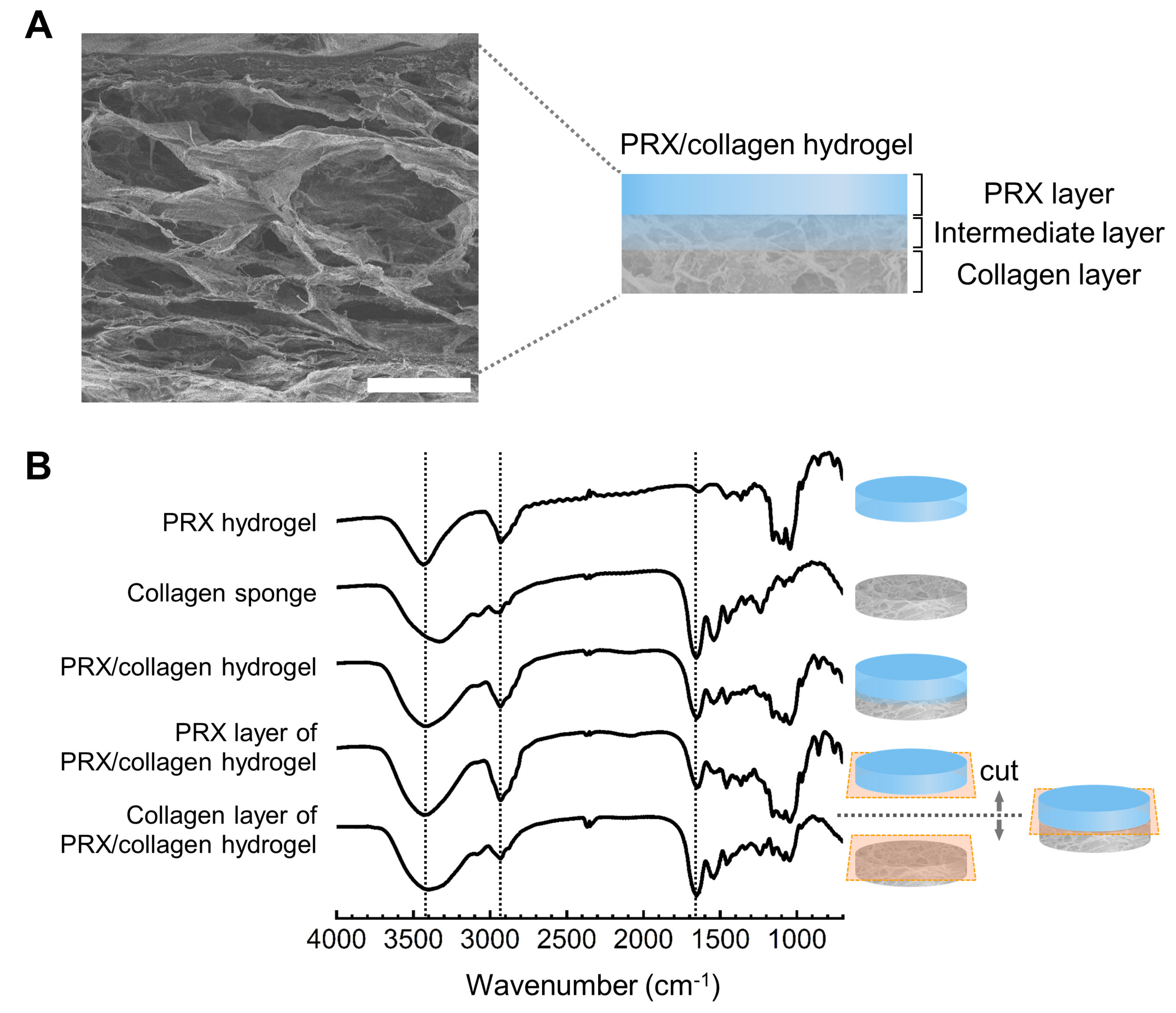
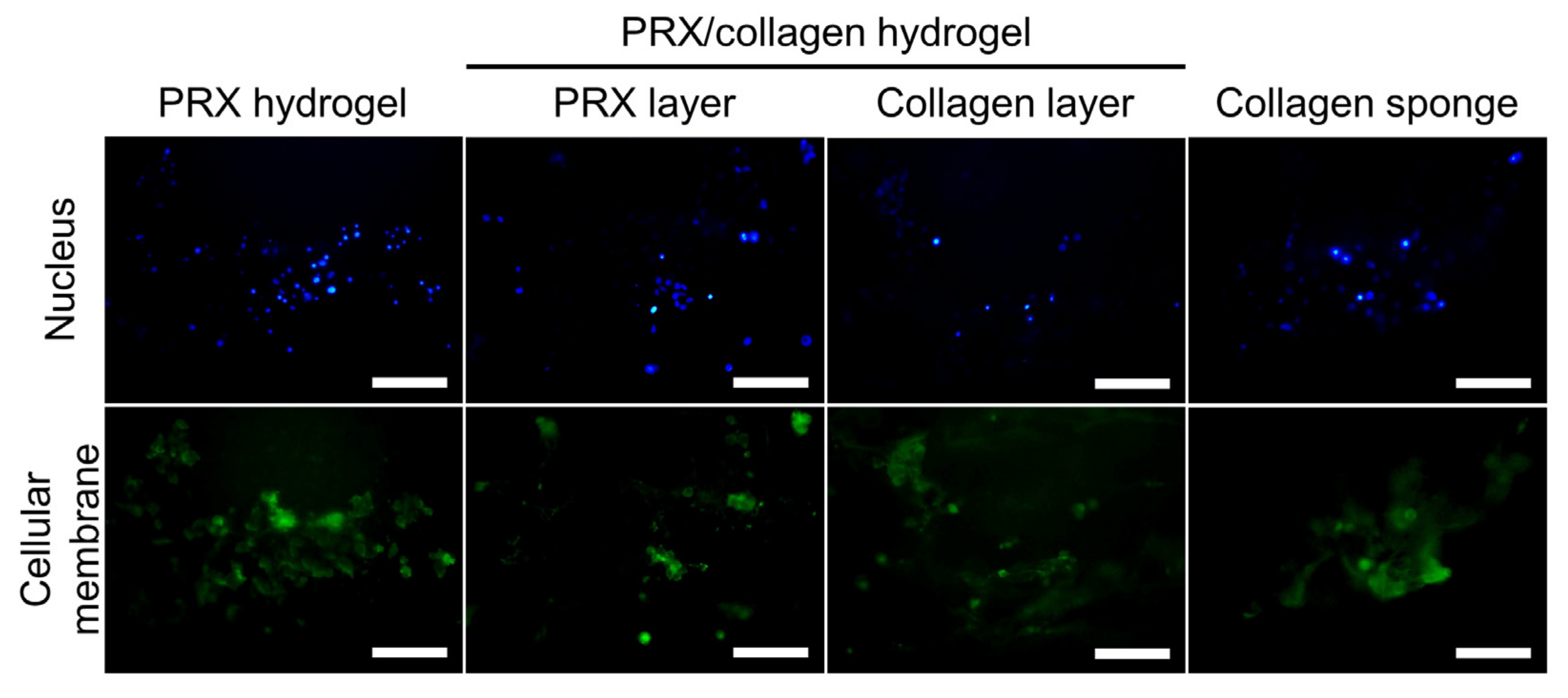
2.2. Fabrication and Characterization of PRX Hydrogels and PRX/Collagen Hydrogels
2.3. Subcutaneous Implantation of the PRX/Collagen Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Methylated PRX-VBn
4.3. Characterization of Polyrotaxane
4.4. Fabrication of PRX Hydrogel and PRX/Collagen Hydrogel
4.5. FT-IR Analysis
4.6. SEM Analysis
4.7. Culturing of Fibroblasts on PRX Hydrogel and PRX/Collagen Hydrogel
4.8. In Vivo Subcutaneous Implantation of PRX Hydrogel and PRX/Collagen Hydrogel
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, K.; Ito, K. Structure and dynamics of polyrotaxane and slide-ring materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Ooya, T.; Eguchi, M.; Yui, N. Supramolecular Design for Multivalent Interaction: Maltose Mobility along Polyrotaxane Enhanced Binding with Concanavalin A. J. Am. Chem. Soc. 2003, 125, 13016–13017. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Ito, K. Novel Cross-Linking Concept of Polymer Network: Synthesis, Structure, and Properties of Slide-Ring Gels with Freely Movable Junctions. Polym. J. 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Rajendan, A.K.; Arisaka, Y.; Yui, N.; Iseki, S. Polyrotaxanes as emerging biomaterials for tissue engineering applications: A brief review. Inflamm. Regener. 2020, 40, 27. [Google Scholar] [CrossRef]
- Loethen, S.; Kim, J.M.; Thompson, D.H. Biomedical Applications of Cyclodextrin Based Polyrotaxanes. Polym. Rev. 2007, 47, 383–418. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef]
- Arisaka, Y.; Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Seo, J.H.; Kakinoki, S.; Yamaoka, T.; Yui, N. Directing Stem Cell Differentiation by Changing the Molecular Mobility of Supramolecular Surfaces. Adv. Healthc. Mater. 2015, 4, 215–222. [Google Scholar] [CrossRef]
- Arisaka, Y.; Tonegawa, A.; Tamura, A.; Yui, N. Terminally cross-linking polyrotaxane hydrogels applicable for cellular microenvironments. J. Appl. Polym. Sci. 2021, 138, 49706. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef] [PubMed]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Li, Y.C.E.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue adhesives: From research to clinical translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef]
- Rathi, S.; Saka, R.; Domb, A.J.; Khan, W. Protein-based bioadhesives and bioglues. Polym. Adv. Technol. 2019, 30, 217–234. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharmaceut. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Lin, Z.; Rios, H.F.; Cochran, D.L. Emerging Regenerative Approaches for Periodontal Reconstruction: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S134–S152. [Google Scholar] [CrossRef] [Green Version]
- Araki, J.; Ito, K. Polyrotaxane derivatives. I. Preparation of modified polyrotaxanes with nonionic functional groups and their solubility in organic solvents. J. Polym. Sci. A Polym. Chem. 2006, 44, 6312–6323. [Google Scholar] [CrossRef]
- Kidowaki, M.; Zhao, C.; Kataoka, T.; Ito, K. Thermoreversible sol–gel transition of an aqueous solution of polyrotaxane composed of highly methylated α-cyclodextrin and polyethylene glycol. Chem. Commun. 2006, 4102–4103. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Dogenski, M.; Gurikov, P.; Baudron, V.; Oliveira, J.V.; Smirnova, I.; Ferreira, S.R.S. Starch-Based Aerogels Obtained via Solvent-Induced Gelation. Gels 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kakinoki, S.; Inoue, Y.; Yamaoka, T.; Ishihara, K.; Yui, N. Designing dynamic surfaces for regulation of biological responses. Soft Matter. 2012, 8, 5477–5485. [Google Scholar] [CrossRef]
- Sekiya-Aoyama, R.; Arisaka, Y.; Hakariya, M.; Masuda, H.; Iwata, T.; Yoda, T.; Yui, N. Dual effect of molecular mobility and functional groups of polyrotaxane surfaces on the fate of mesenchymal stem cells. Biomater. Sci. 2021, 9, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Tonegawa, A.; Arisaka, Y.; Yui, N. Versatile synthesis of end-reactive polyrotaxanes applicable to fabrication of supramolecular biomaterials. Beilstein J. Org. Chem. 2016, 12, 2883–2892. [Google Scholar] [CrossRef] [Green Version]


| Code a | Mn of PEG Axle | Number of Threaded α-CDs on PRX | Mn,NMR of PRX | Number of Methyl Groups on PRX |
|---|---|---|---|---|
| PRX-VBn | 5000 | 22.3 | 27,200 | 0 |
| Me30PRX-VBn | 5000 | 22.3 | 27,600 | 30.0 |
| Me112PRX-VBn | 5000 | 22.3 | 28,800 | 112.3 |
| Me322PRX-VBn | 5000 | 22.3 | 31,700 | 322.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakariya, M.; Arisaka, Y.; Masuda, H.; Yoda, T.; Tamura, A.; Iwata, T.; Yui, N. Tissue Adhesion-Anisotropic Polyrotaxane Hydrogels Bilayered with Collagen. Gels 2021, 7, 168. https://doi.org/10.3390/gels7040168
Hakariya M, Arisaka Y, Masuda H, Yoda T, Tamura A, Iwata T, Yui N. Tissue Adhesion-Anisotropic Polyrotaxane Hydrogels Bilayered with Collagen. Gels. 2021; 7(4):168. https://doi.org/10.3390/gels7040168
Chicago/Turabian StyleHakariya, Masahiro, Yoshinori Arisaka, Hiroki Masuda, Tetsuya Yoda, Atsushi Tamura, Takanori Iwata, and Nobuhiko Yui. 2021. "Tissue Adhesion-Anisotropic Polyrotaxane Hydrogels Bilayered with Collagen" Gels 7, no. 4: 168. https://doi.org/10.3390/gels7040168
APA StyleHakariya, M., Arisaka, Y., Masuda, H., Yoda, T., Tamura, A., Iwata, T., & Yui, N. (2021). Tissue Adhesion-Anisotropic Polyrotaxane Hydrogels Bilayered with Collagen. Gels, 7(4), 168. https://doi.org/10.3390/gels7040168







