Novel Fabrication of Zein-Soluble Soybean Polysaccharide Nanocomposites Induced by Multifrequency Ultrasound, and Their Roles on Microstructure, Rheological Properties and Stability of Pickering Emulsions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Ultrasonic Modes on Storage Stability of HIPEs Stabilized by Zein Composite Nanoparticles
2.2. Physicochemical Properties of Zein Composite Nanoparticles
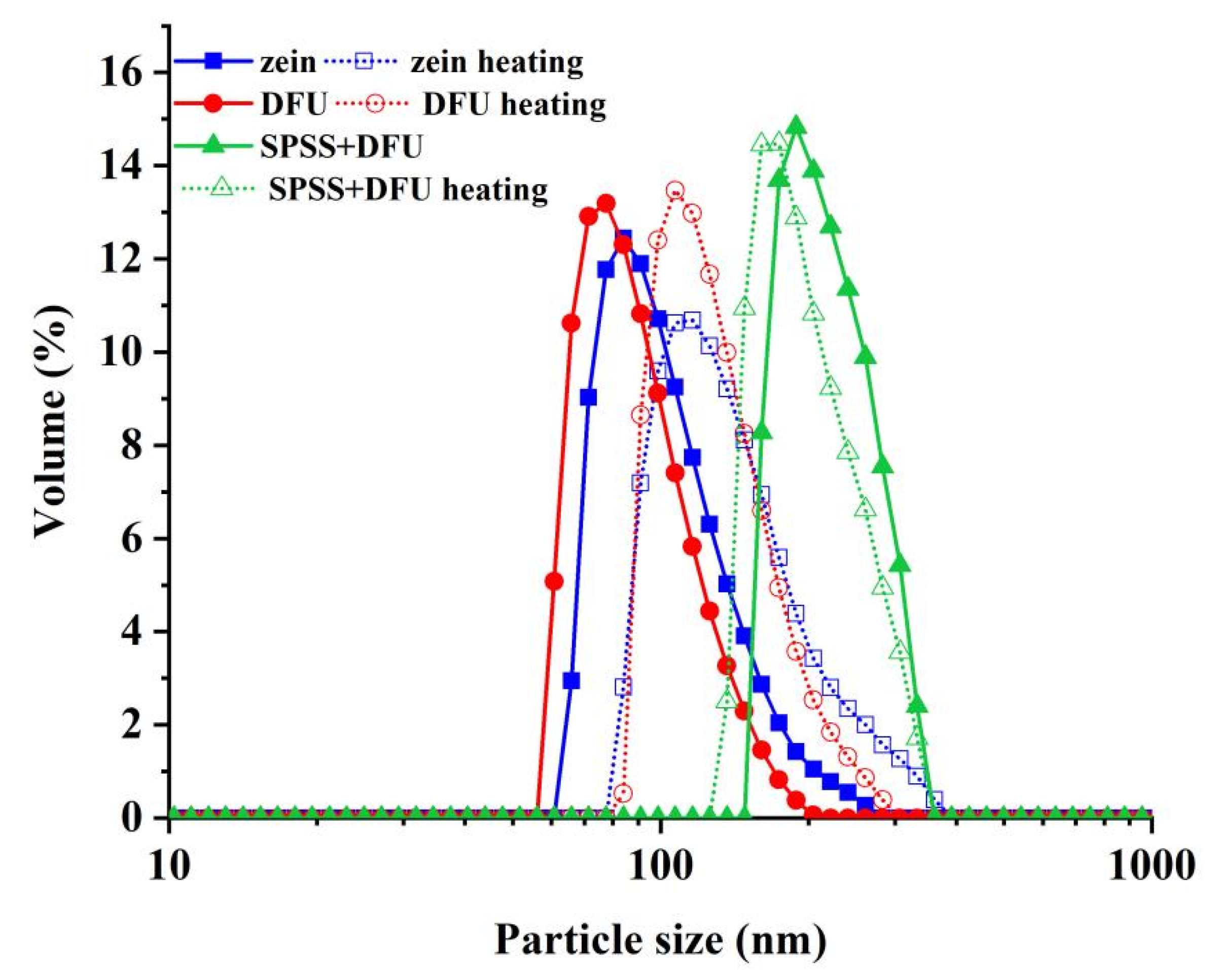
2.2.1. Zeta-Potential, PDI and Particle Size Distribution of Nanoparticles
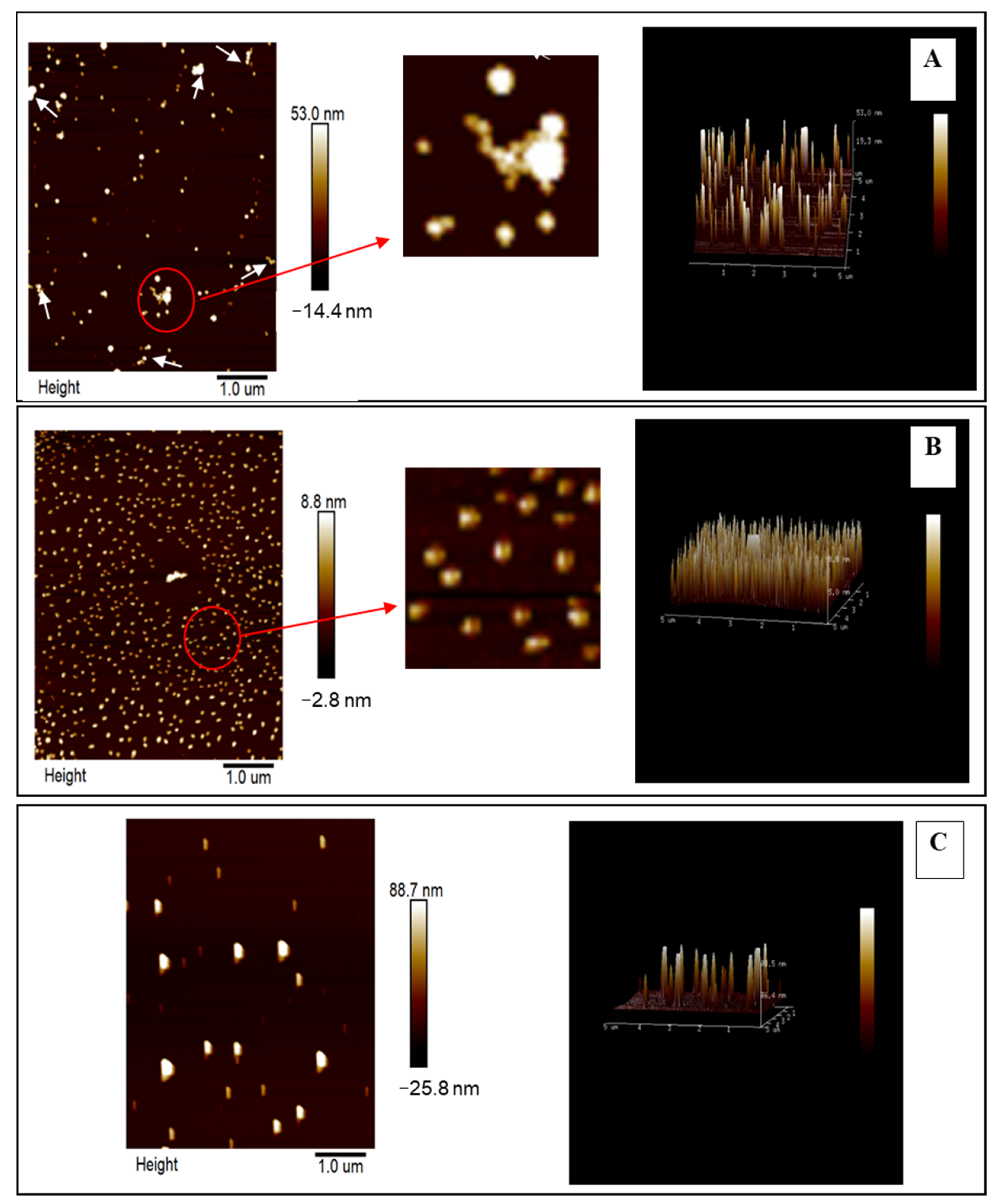
2.2.2. Atomic Force Microscopy (AFM)
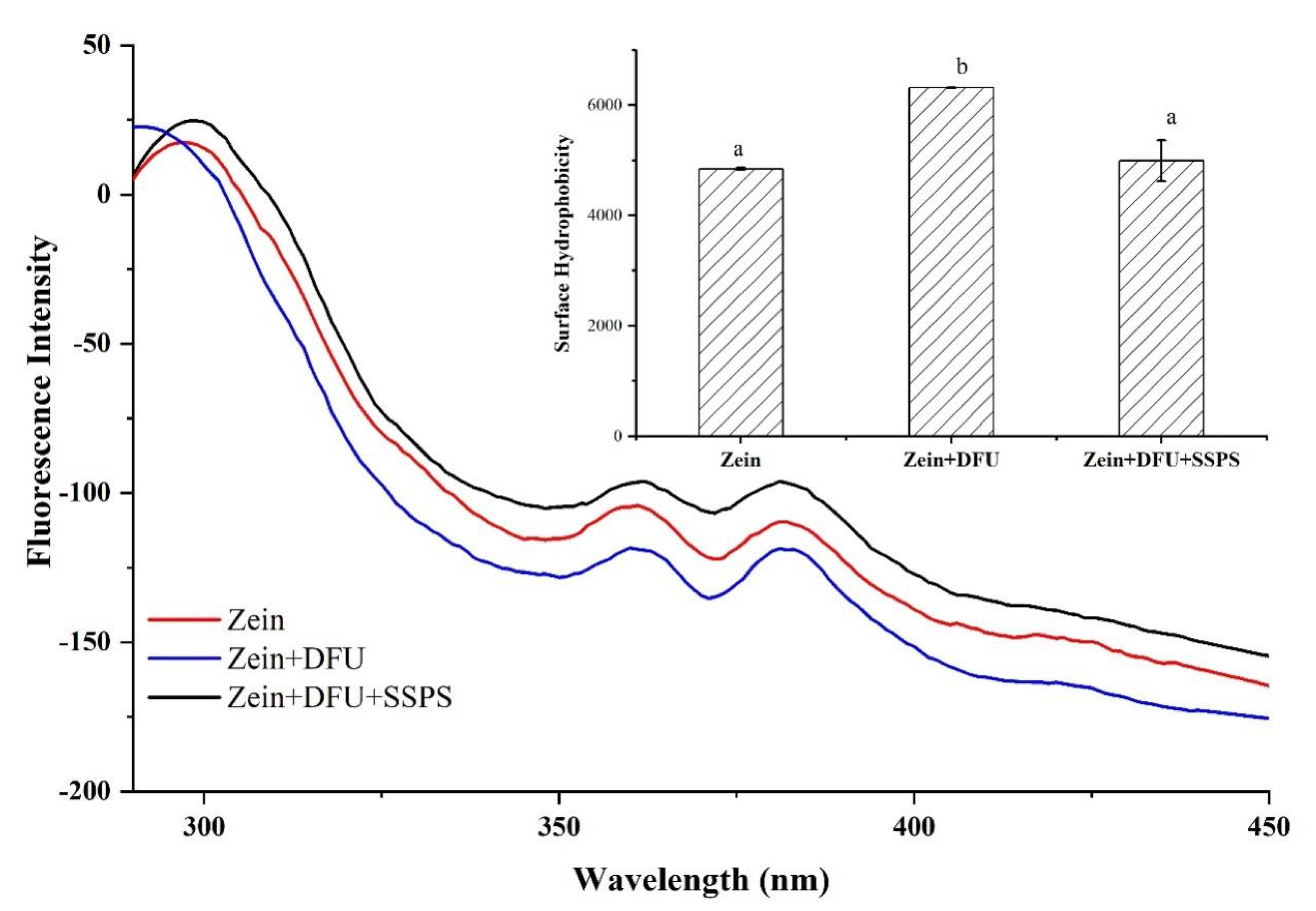
2.2.3. Fluorescence Spectroscopy and Surface Hydrophobicity
2.2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.5. Circular Dichroism (CD) Spectroscopy Analysis
2.2.6. Thermal Stability
2.3. Rheological Properties, Microstructure and Stability of HIPEs
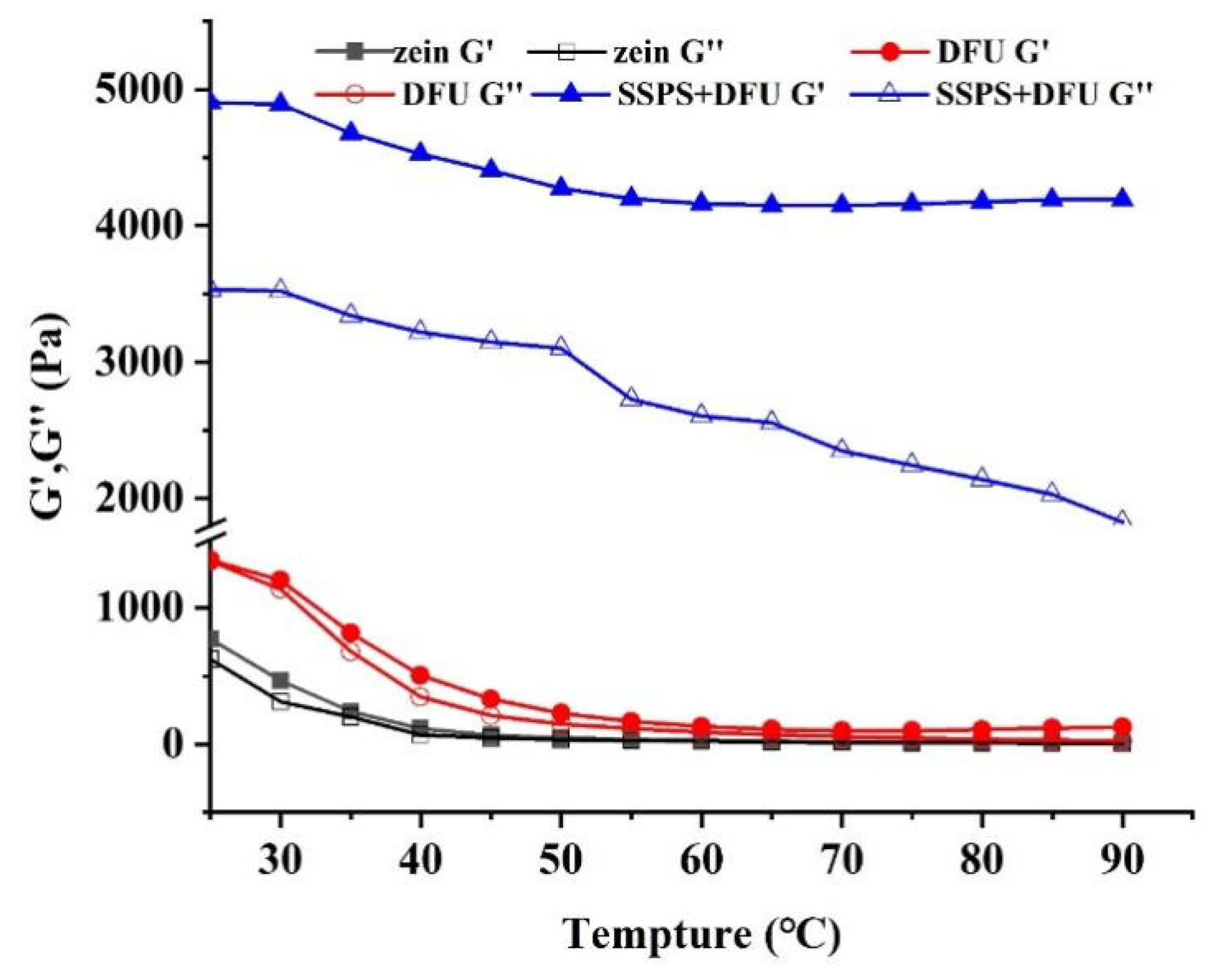
2.3.1. Rheological Properties of Emulsions Stabilized by Zein-SSPS Composite Nanoparticles
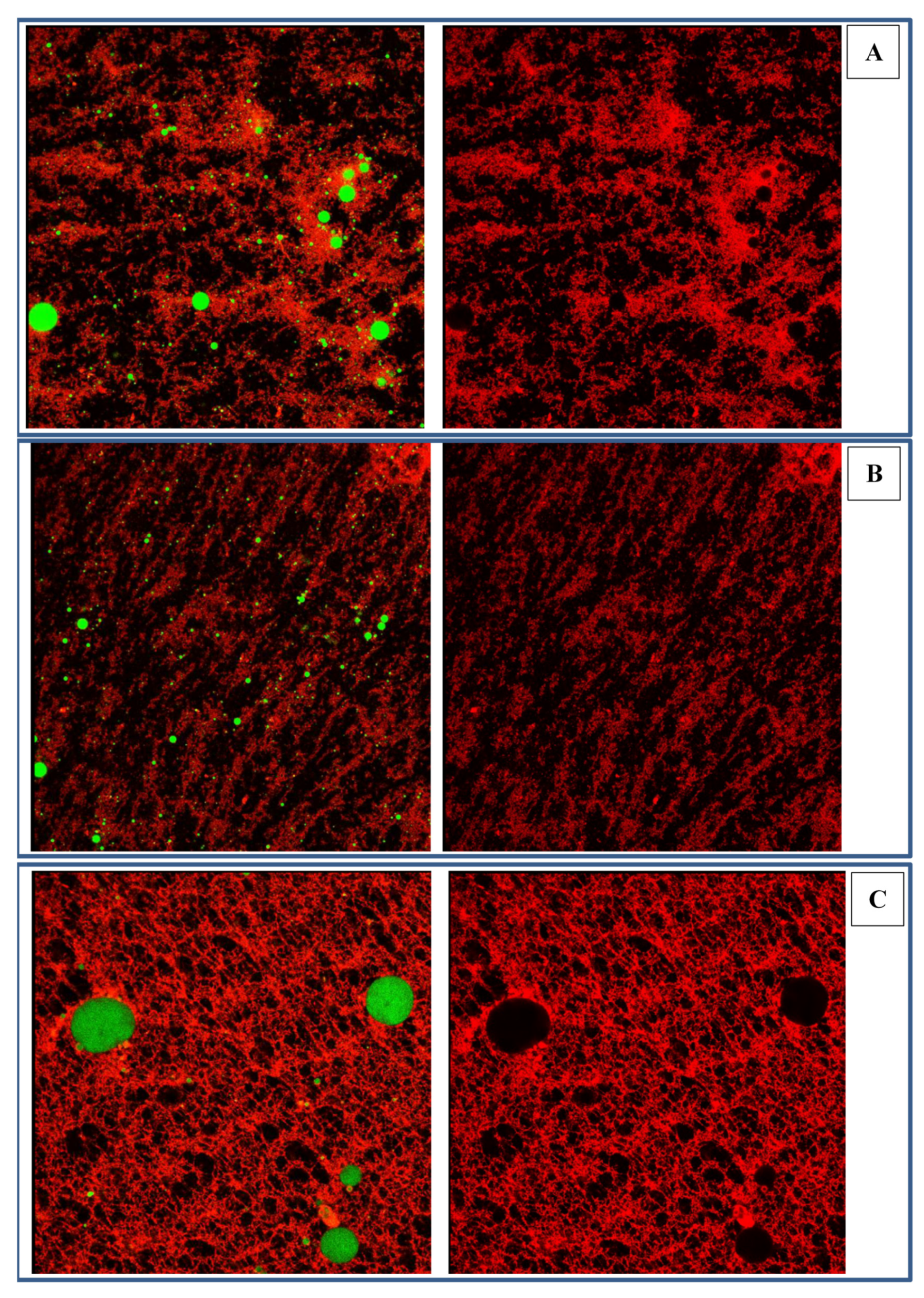
2.3.2. Microstructure of Emulsions Stabilized by Zein-SSPS Composite Nanoparticles
2.3.3. Stability of Zein-SSPS Stabilized HIPEs Based on Water Migration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Zein-SSPS Colloidal Particles with Different Model Frequency Ultrasound
4.3. Preparation of High Internal Phase Pickering Emulsion (HIPE)
4.4. Characteristics of Zein-SSPS Complexes
4.4.1. Determination of Particle Size, Polydispersity Index (PDI) and Zeta-Potential
4.4.2. Atomic Force Microscopy (AFM)
4.4.3. Fluorescence Spectroscopy and Surface Hydrophobicity
4.4.4. Fourier Transform Infrared (FTIR) Spectroscopy
4.4.5. Circular Dichroism (CD) Spectroscopy
4.4.6. Thermal Stability
4.5. Properties of HIPEs
4.5.1. Rheological Properties
4.5.2. Analysis of Water Migration
4.5.3. CLSM
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Lei, Y.; Rong, J.; Zhang, X.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Fang, Z.; et al. Physico-chemical properties of reduced-fat biscuits prepared using O/W cellulose-based Pickering emulsion. LWT 2021, 3, 111745. [Google Scholar] [CrossRef]
- Zhao, Q.; Zaaboul, F.; Liu, Y.; Li, J. Recent advances on protein-based Pickering high internal phase emulsions (Pickering HIPEs): Fabrication, characterization, and applications. Compr. Rev. Food. Sci. Food. Saf. 2020, 19, 1934–1968. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yang, S.; Wei, Y.; Sun, C.; McClements, D.J.; Mao, L.; Gao, Y. Development of stable high internal phase emulsions by pickering stabilization: Utilization of zein-propylene glycol alginate-rhamnolipid complex particles as colloidal emulsifiers. Food Chem. 2018, 275, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-N.; Zhu, J.-J.; Xi, Y.-K.; Yin, S.-W.; Ngai, T.; Yang, X.-Q. Protein-Based Pickering High Internal Phase Emulsions as Nutraceutical Vehicles of and the Template for Advanced Materials: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 9719–9726. [Google Scholar] [CrossRef]
- Maithya, O.M.; Li, X.; Feng, X.L.; Sui, X.F.; Wang, B.J. Microencapsulated phase change material via Pickering emulsion stabi-lized by graphene oxide for photothermal conversion. J. Mater. Sci. 2020, 55, 7731–7742. [Google Scholar] [CrossRef]
- Prabhu, R.; Jeevananda, T.; Mohan, N. Spectral and thermal studies on polyaniline–titanium dioxide nanocomposites by inverted emulsion techniques. Mater. Today Proc. 2019, 27, 2164–2168. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of alkaline deamidation on the structure, surface hydrophobicity, and emulsifying properties of the Z19 alpha-zein. J. Agric. Food Chem. 2007, 55, 439–445. [Google Scholar] [CrossRef]
- Fadoju, O.M.; Osinowo, O.A.; Ogunsuyi, O.; Oyeyemi, I.T.; Alabi, O.; Alimba, C.G.; Bakare, A.A. Interaction of titanium dioxide and zinc oxide nanoparticles induced cytogenotoxicity in Allium cepa. Nucleus 2020, 63, 159–166. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. LWT 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering Emulsion Gels Prepared by Hydrogen-Bonded Zein/Tannic Acid Complex Colloidal Particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef]
- Zhu, C.-P.; Zhang, H.-H.; Huang, G.-Q.; Xiao, J.-X. Whey protein isolate—low methoxyl pectin coacervates as a high internal phase Pickering emulsion stabilizer. J. Dispers. Sci. Technol. 2020, 42, 1009–1020. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, Y. Surface modification of zein colloidal particles with sodium caseinate to stabilize oil-in-water pickering emulsion. Food Hydrocoll. 2016, 56, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2018, 279, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, M.N.; Goli, S.A.H.; Doost, A.S.; Dewettinck, K.; Van der Meeren, P. Bioparticles of flaxseed protein and mucilage enhance the physical and oxidative stability of flaxseed oil emulsions as a potential natural alternative for synthetic surfactants. Colloids Surf. B Biointerfaces 2019, 184, 110489. [Google Scholar] [CrossRef] [PubMed]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Pickering stabilization of thymol through green emulsification using soluble fraction of almond gum—Whey protein isolate nano-complexes. Food Hydrocoll. 2018, 88, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Doost, A.S.; Nasrabadi, M.N.; Goli, S.A.H.; Van Troys, M.; Dubruel, P.; De Neve, N.; Van der Meeren, P. Maillard conjugation of whey protein isolate with water-soluble fraction of almond gum or flaxseed mucilage by dry heat treatment. Food Res. Int. 2020, 128, 108779. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of men-haden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Ding, X.; Yao, P. Soy Protein/Soy Polysaccharide Complex Nanogels: Folic Acid Loading, Protection, and Controlled Delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Chivero, P.; Gohtani, S.; Ikeda, S.; Nakamura, A. The structure of soy soluble polysaccharide in aqueous solution. Food Hydrocoll. 2013, 35, 279–286. [Google Scholar] [CrossRef]
- Bernela, M.; Kaur, P.; Chopra, M.; Thakur, R. Synthesis, characterization of nisin loaded alginate–chitosan–pluronic composite nanoparticles and evaluation against microbes. LWT Food Sci. Technol. 2014, 59, 1093–1099. [Google Scholar] [CrossRef]
- Tran, T.; Rousseau, D. Stabilization of acidic soy protein-based dispersions and emulsions by soy soluble polysaccharides. Food Hydrocoll. 2012, 30, 382–392. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2018, 87, 342–351. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Li, S.; Zhou, B. Development of zein/soluble soybean polysaccharide nanoparticle: Stabilized pickering emul-sions. J. Food Sci. 2021, 86, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Kwon, Y.-J. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. Food Sci. Biotechnol. 2013, 23, 107–113. [Google Scholar] [CrossRef]
- Du, J.; Dai, H.; Wang, H.; Yu, Y.; Zhu, H.; Fu, Y.; Ma, L.; Peng, L.; Li, L.; Wang, Q.; et al. Preparation of high thermal stability gelatin emulsion and its application in 3D printing. Food Hydrocoll. 2020, 113, 106536. [Google Scholar] [CrossRef]
- Ren, X.; Wei, X.; Ma, H.; Zhou, H.; Guo, J.; Mao, S.; Hu, A. Effects of a Dual-Frequency Frequency-Sweeping Ultrasound Treatment on the Properties and Structure of the Zein Protein. Cereal Chem. J. 2015, 92, 193–197. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Fang, R.; Lei, C.; Li, Y.; Li, B.; Pei, Y.; Luo, X.; Liu, S. Application of Nanocellulose as particle stabilizer in food Pickering emulsion: Scope, Merits and challenges. Trends Food Sci. Technol. 2021, 110, 573–583. [Google Scholar] [CrossRef]
- Morales, R.; Martínez, K.D.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhang, B.C.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for en-capsulation of alpha-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- An, H.; Yang, H.; Liu, Z.; Zhang, Z. Effects of heating modes and sources on nanostructure of gelatinized starch molecules using atomic force microscopy. LWT 2008, 41, 1466–1471. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2019, 101, 105503. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Hu, Z.; Cai, J.; Qin, X. Maillard-Reacted Whey Protein Isolates and Epigallocatechin Gallate Complex Enhance the Thermal Stability of the Pickering Emulsion Delivery of Curcumin. J. Agric. Food Chem. 2019, 67, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef]
- Peng, W.; Kong, X.; Chen, Y.; Zhang, C.; Yang, Y.; Hua, Y. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll. 2016, 52, 301–310. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Xing, L.; Zhang, W. Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends Food Sci. Technol. 2021, 110, 493–512. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, Q. Development of Zein Nanoparticles Coated with Carboxymethyl Chitosan for Encapsulation and Controlled Release of Vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef]
- Akbari, A.; Wu, J. Cruciferin nanoparticles: Preparation, characterization and their potential application in delivery of bioactive compounds. Food Hydrocoll. 2016, 54, 107–118. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; He, X.; Liu, F.; Yuan, F.; Gao, Y. Effect of heat treatment on physical, structural, thermal and morphological characteristics of zein in ethanol-water solution. Food Hydrocoll. 2016, 58, 11–19. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Wang, Y.; Yang, X.; Sun, C.; Mao, L.; Gao, Y. Zein-hyaluronic acid binary complex as a delivery vehicle of quercetagetin: Fabrication, structural characterization, physicochemical stability and in vitro release property. Food Chem. 2018, 276, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, Q. Glycation of whey protein to provide steric hindrance against thermal aggregation. J. Agric. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, H.; Muhoza, B.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Whey protein isolate-dextran conjugates: Decisive role of glycation time dependent conjugation degree in size control and stability improvement of colloidal nanoparticles. LWT 2021, 148, 111766. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2020, 107, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.-W.; Guo, J.; Yin, S.-W.; Yang, X.-Q. Wheat gluten based percolating emulsion gels as simple strategy for structuring liquid oil. Food Hydrocoll. 2016, 61, 747–755. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural char-acteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, R.; Li, X.-L.; Liu, W.-J.; Cheng, J.-S.; Wang, W. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Tashiro, Y.; Matsukawa, S.; Ogawa, H. Comparison of horse mackerel and tilapia surimi gel based on rheological and 1H NMR relaxation properties. Fish. Sci. 2005, 71, 655–661. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liu, C.; Liu, C. Application of LF-NMR to the characterization of camellia oil-loaded pickering emulsion fabricated by soy protein isolate. Food Hydrocoll. 2021, 112, 106329. [Google Scholar] [CrossRef]
- Tokifuji, A.; Matsushima, Y.; Hachisuka, K.; Yoshioka, K. Texture, sensory and swallowing characteristics of high-pressure-heat-treated pork meat gel as a dysphagia diet. Meat Sci. 2013, 93, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Hu, Y.-Q.; Yin, S.-W.; Yang, X.-Q.; Lai, F.-R.; Wang, S.-Q. Fabrication and Characterization of Antioxidant Pickering Emulsions Stabilized by Zein/Chitosan Complex Particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dai, L.; Gao, Y. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhou, H.; Guo, T.; Zhu, H.; Wang, H.; Zhang, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces 2020, 196, 111294. [Google Scholar] [CrossRef] [PubMed]
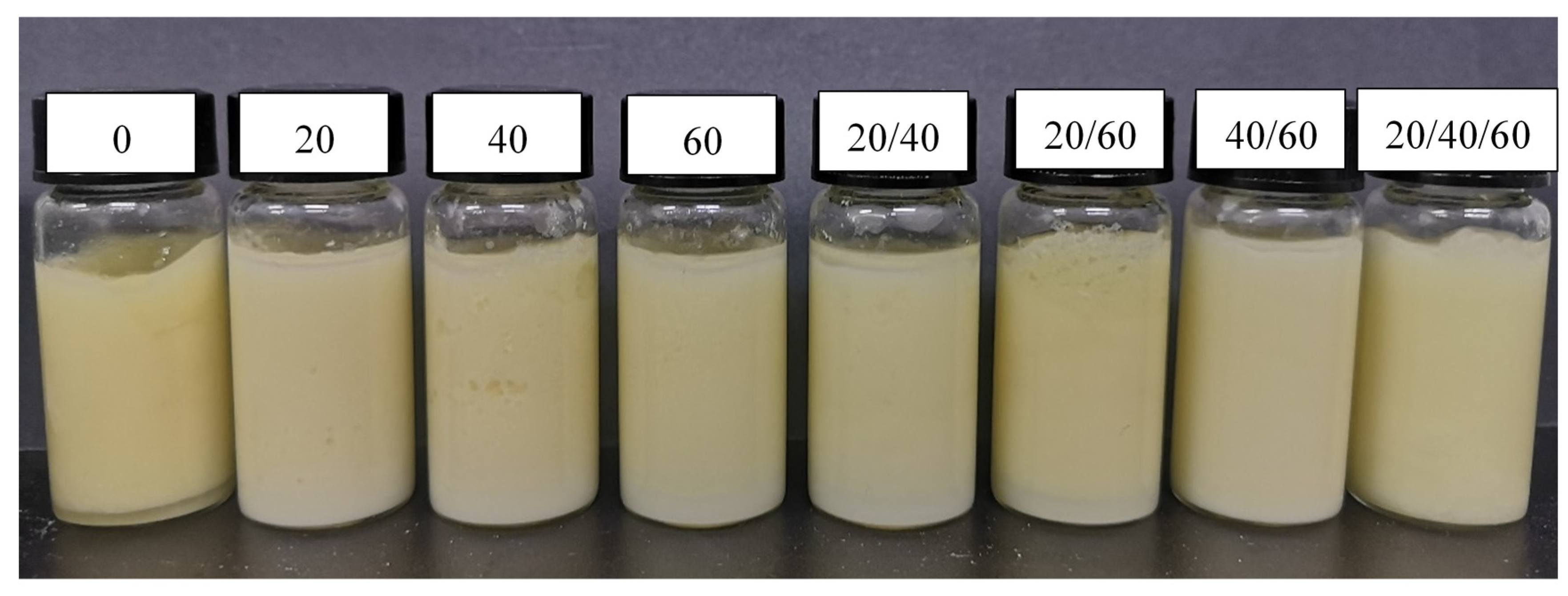


| Samples | Ultrasound Modes | Ultrasonic Frequency (Hz) |
|---|---|---|
| 20 | SFU | 20 |
| 40 | 40 | |
| 60 | 60 | |
| 20/40 | DFU | 20/40 |
| 20/60 | 20/60 | |
| 40/60 | 40/60 | |
| 24/40/60 | MFU | 20/40/60 |
| Samples | Zeta-Potential | PDI (%) | Hydrodynamic Radius |
|---|---|---|---|
| Zein | 18.48 ± 0.23 b | 15.70 ± 0.95 b | 132.23 ± 0.85 b |
| Zein + DFU | 18.33 ± 1.00 b | 11.30 ± 1.15 a | 114.54 ± 0.23 a |
| Zein + DFU + SPSS | −21.90 ± 0.46 a | 17.47 ± 0.90 b | 256.5 ±4.81 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Xiong, Z.; Shi, T.; Monto, A.R.; Yuan, L.; Gao, R. Novel Fabrication of Zein-Soluble Soybean Polysaccharide Nanocomposites Induced by Multifrequency Ultrasound, and Their Roles on Microstructure, Rheological Properties and Stability of Pickering Emulsions. Gels 2021, 7, 166. https://doi.org/10.3390/gels7040166
Song T, Xiong Z, Shi T, Monto AR, Yuan L, Gao R. Novel Fabrication of Zein-Soluble Soybean Polysaccharide Nanocomposites Induced by Multifrequency Ultrasound, and Their Roles on Microstructure, Rheological Properties and Stability of Pickering Emulsions. Gels. 2021; 7(4):166. https://doi.org/10.3390/gels7040166
Chicago/Turabian StyleSong, Teng, Zhiyu Xiong, Tong Shi, Abdul Razak Monto, Li Yuan, and Ruichang Gao. 2021. "Novel Fabrication of Zein-Soluble Soybean Polysaccharide Nanocomposites Induced by Multifrequency Ultrasound, and Their Roles on Microstructure, Rheological Properties and Stability of Pickering Emulsions" Gels 7, no. 4: 166. https://doi.org/10.3390/gels7040166
APA StyleSong, T., Xiong, Z., Shi, T., Monto, A. R., Yuan, L., & Gao, R. (2021). Novel Fabrication of Zein-Soluble Soybean Polysaccharide Nanocomposites Induced by Multifrequency Ultrasound, and Their Roles on Microstructure, Rheological Properties and Stability of Pickering Emulsions. Gels, 7(4), 166. https://doi.org/10.3390/gels7040166






