Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells
Abstract
1. Introduction
2. Results
2.1. Thermal Analysis
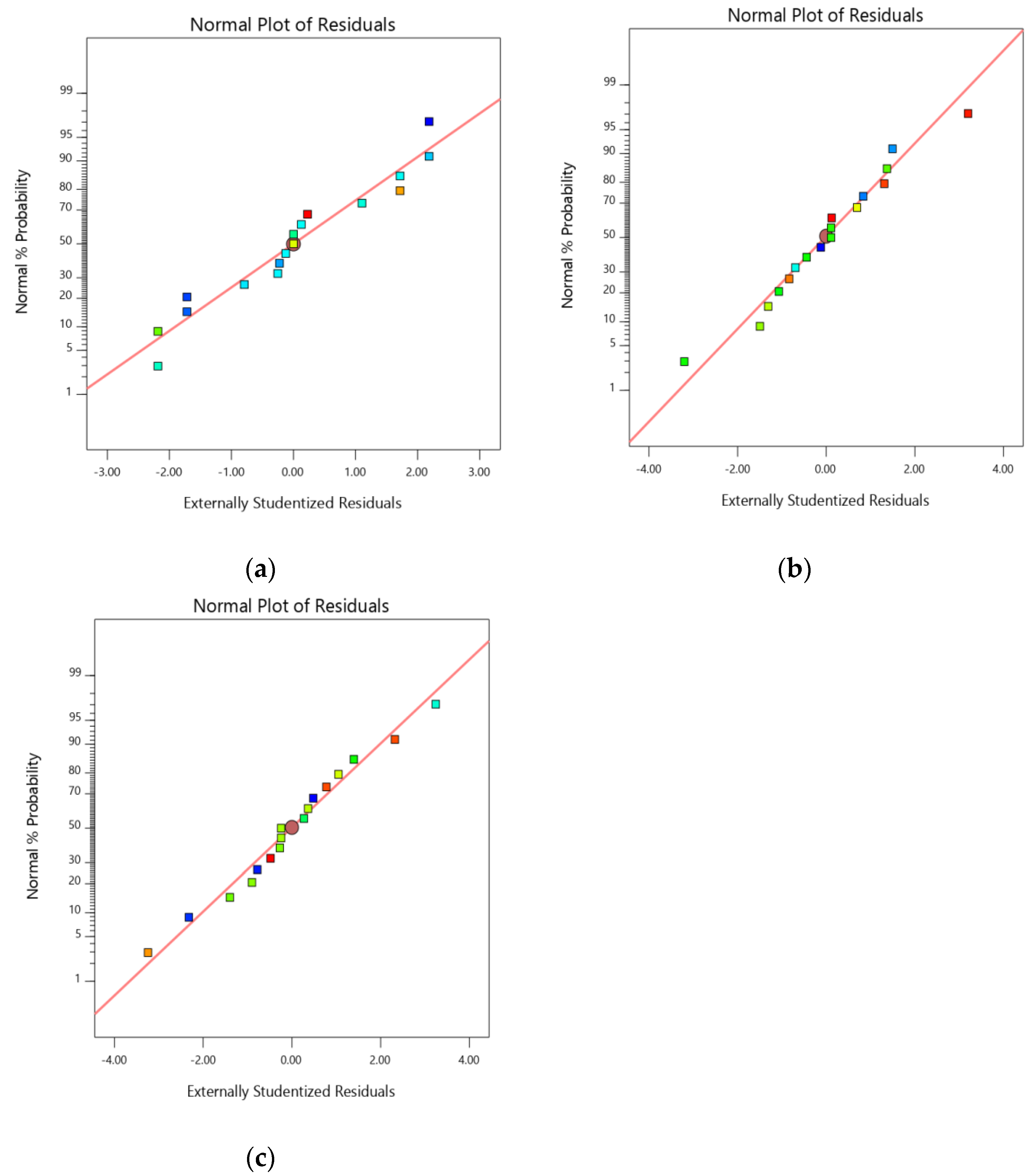
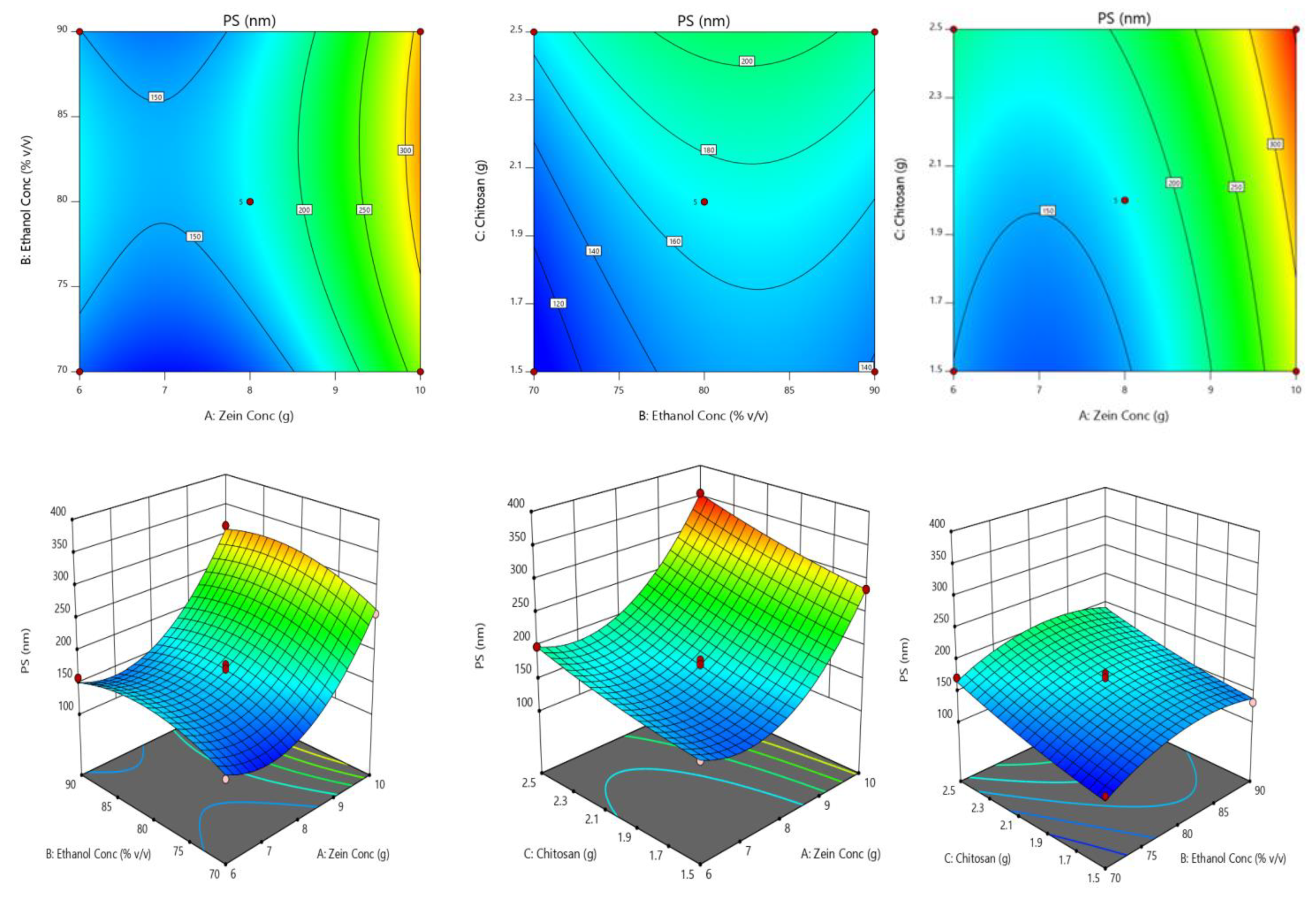
2.2. Optimization of Chitosan-Avanafil Nanocomplex Formulation
2.3. Zeta Potential and PDI
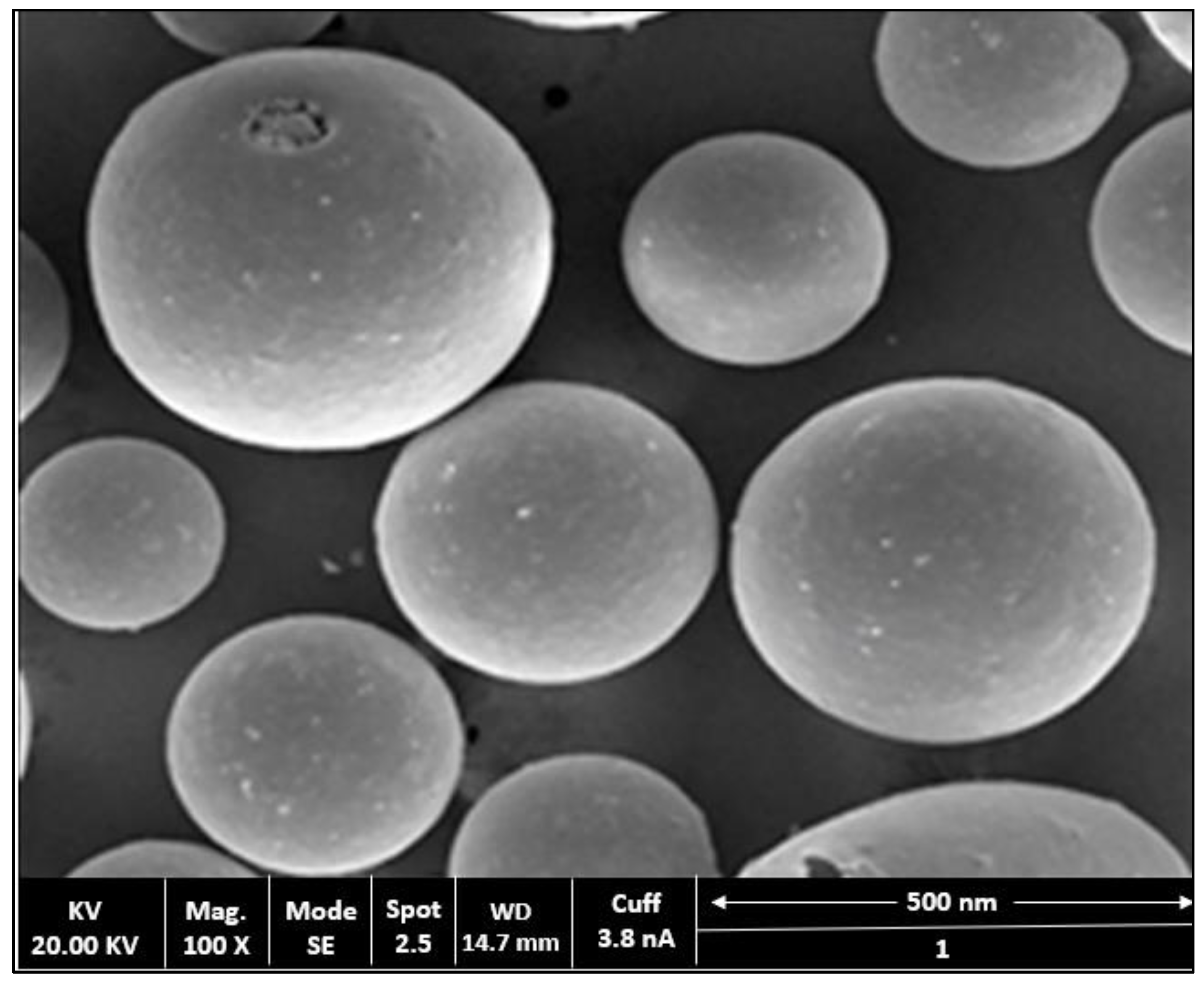
2.4. Surface Morphology Studies
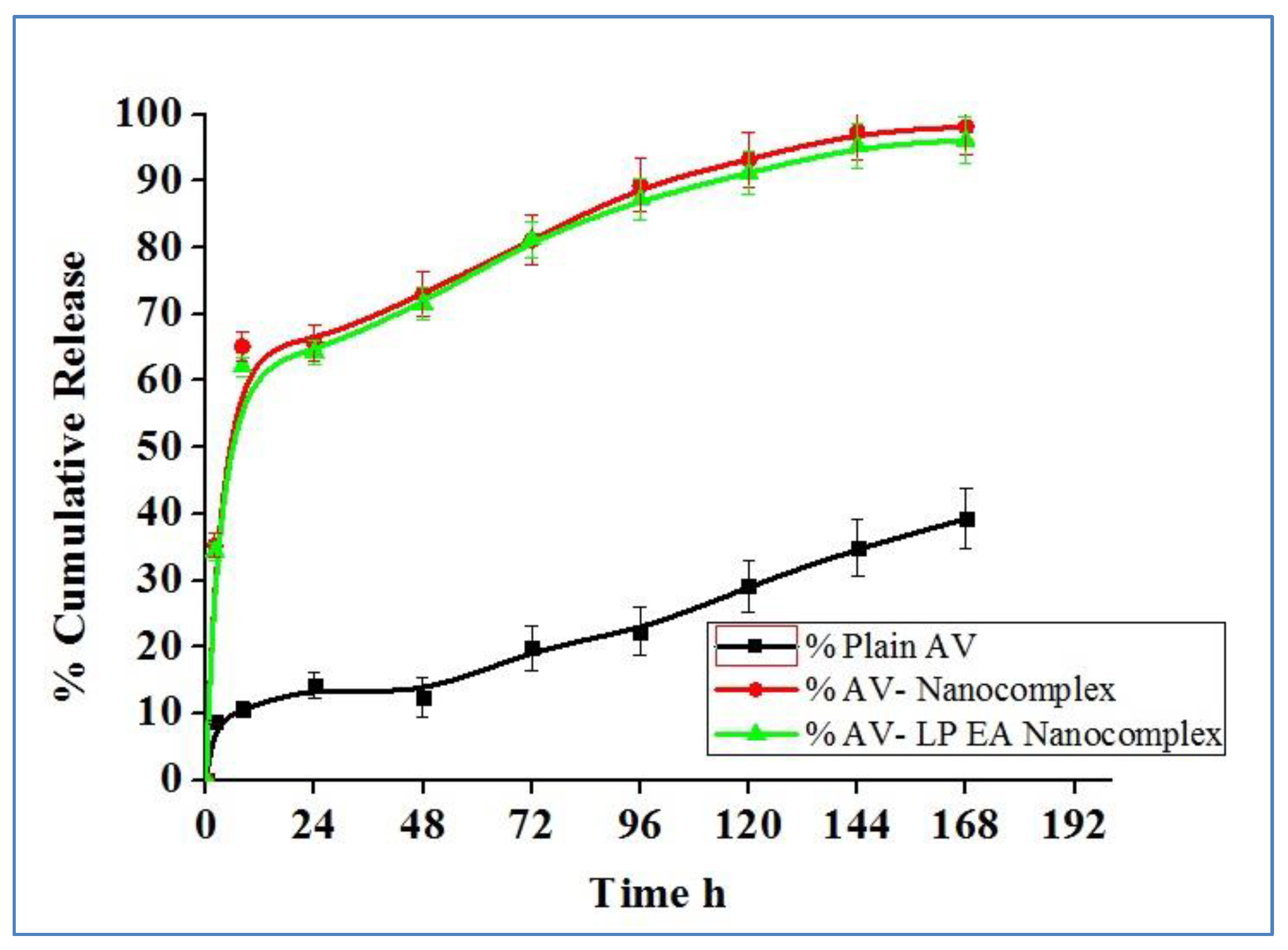
2.5. In-Vitro Release Study
2.6. Measurement of Cell Viability
2.7. Measurement of Intracellular ROS
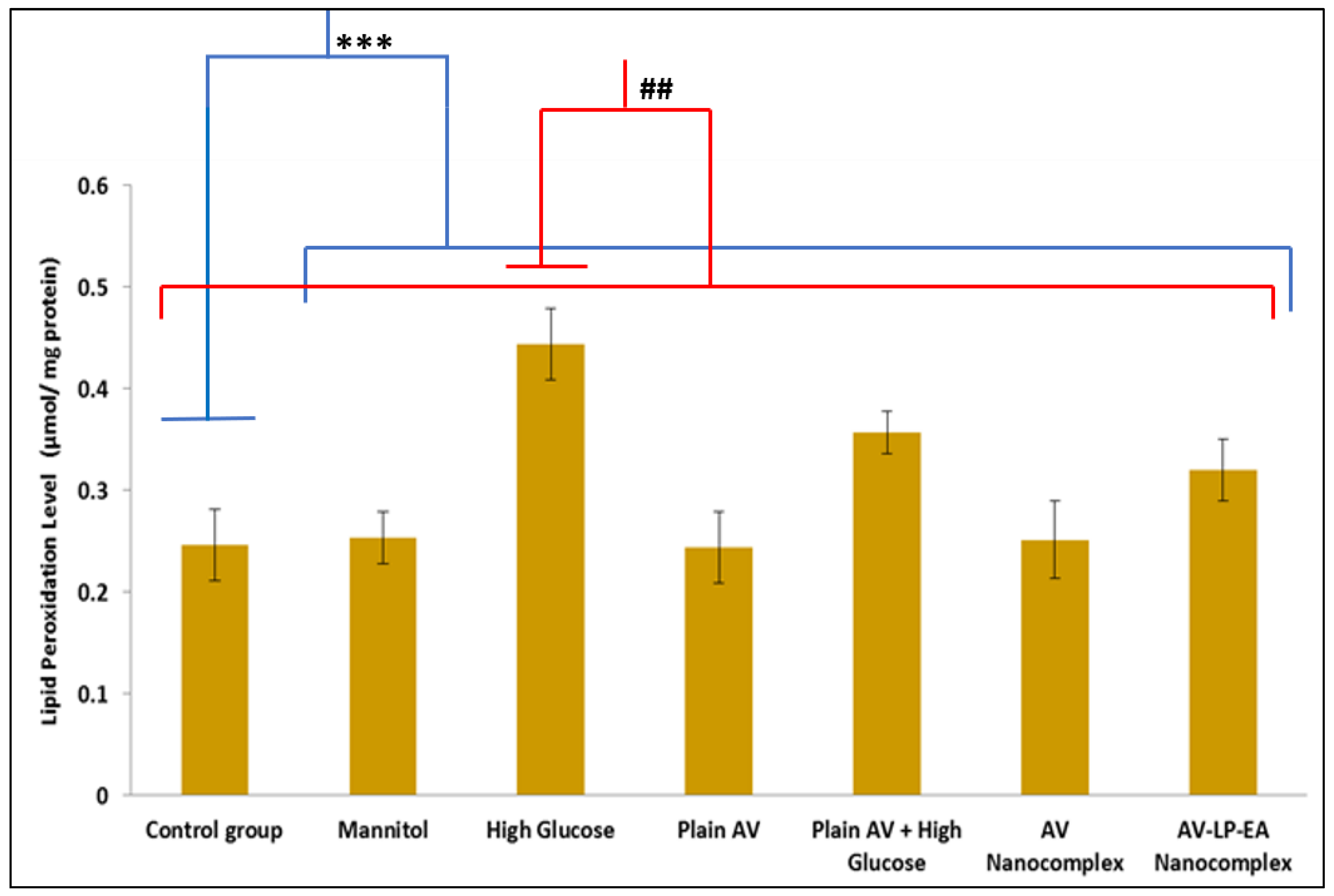
2.8. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Chitosan-Avanafil Nanocomplex
4.2.2. Optimization of Chitosan-Avanafil Nanocomplex Preparation
4.3. Characterization
4.3.1. Particle Size(PS)Determination
4.3.2. Entrapment Efficiency(EE)Determination
4.3.3. Stability Index(SI)
4.3.4. The Rationale of Experimental Design
4.3.5. Polydispersity Index(PDI) and Zeta Potential(mV) Determination
4.3.6. Scanning Electron Microscopy(SEM)
4.3.7. Differential Scanning Calorimetry(DSC) Studies
4.3.8. In-Vitro Drug Release Studies
4.3.9. Cell Culture and Treatment
4.3.10. Cell Viability Measurement
4.3.11. Intracellular ROS Measurement
4.3.12. Lipid Peroxidation Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosny, K.M.; Aldawsari, H.M. Avanafil liposomes as transdermal drug delivery for erectile dysfunction treat-ment: Preparation, characterization, and in vitro, ex vivo and in vivo studies. Trop. J. Pharm. Res. 2015, 14, 559–565. [Google Scholar] [CrossRef][Green Version]
- Kurakula, M.; Ahmad, O.A.A.; Fahmy, U.A.; Ahmed, T.A. Solid lipid nanoparticles for transdermal delivery of avanafil: Optimization, formulation, in-vitro and ex-vivo studies. J. Liposome Res. 2016, 26, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Amar, E.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Montorsi, F.; Vardi, Y.; Wespes, E. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Premature Ejaculation. Eur. Urol. 2010, 57, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, L.; Cao, D.; He, L.; Li, Y.; Wei, Q. Avanafil for the Treatment of men With Erectile Dysfunction: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Men’s Health 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S. Avanafil (Spedra): Increasing the choice of PDE-5 inhibitors. Prescriber 2014, 25, 19–21. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Ahmed, O.A.; Hosny, K.M. Development and evaluation of avanafil self-nanoemulsifying drug delivery system with rapid onset of action and enhanced bioavailability. AAPS PharmSciTech 2015, 16, 53–58. [Google Scholar] [CrossRef]
- Burke, R.M.; Evans, J.D. Avanafil for treatment of erectile dysfunction: Review of its potential. Vasc. Health Risk Manag. 2012, 8, 517–523. [Google Scholar] [CrossRef]
- Fahmy, U.A. Augmentation of fluvastatin cytotoxicity against prostate carcinoma PC3 cell line utilizing alpha lipoic–ellagic acid nanostructured lipid carrier formula. AAPS PharmSciTech 2018, 19, 3454–3461. [Google Scholar] [CrossRef]
- Naguib, G.H.; Al-Hazmi, F.E.; Kurakula, M.; Al-dharrab, A.A.; Hosny, K.M.; Alkhalidi, H.M.; Hamed, M.T.; Hassan, A.H.; Al-mohammadi, A.M.; Alnowaiser, A.M.; et al. Zein Coated Zinc Oxide Nanoparticles: Fabrication and Antimicrobial Evaluation as Dental Aid. Int. J. Pharmacol. 2018, 14, 1051–1059. [Google Scholar] [CrossRef]
- Ohno, Y.; Shimizu, S.; Tokudome, K.; Kunisawa, N.; Sasa, M. New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2015, 134, 104–121. [Google Scholar] [CrossRef]
- Paek, H.J.; Lee, Y.J.; Chung, H.E.; Yoo, N.H.; Lee, J.A.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Prabhakar, M.; Kumar, P.; Deshmukh, R.; Sharma, P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013, 698, 6–18. [Google Scholar] [CrossRef]
- Raposo, C.; Nunes, A.K.; Luna, R.L.; Araújo, S.M.; da Cruz-Höfling, M.A.; Peixoto, C.A. Sildenafil (Viagra) protective effects on neuroinflammation: The role of iNOS/NO system in an inflammatory demyelination model. Mediat. Inflamm. 2013, 321460. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.A.; Nunes, A.K.; Garcia-Osta, A. Phosphodiesterase-5 inhibitors: Action on the signaling pathways of neuroinflammation, neurodegeneration, and cognition. Mediat. Inflamm. 2015, 940207. [Google Scholar] [CrossRef]
- Ota, K.T.; Pierre, V.J.; Ploski, J.E.; Queen, K.; Schafe, G.E. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn. Memory 2008, 15, 792–805. [Google Scholar] [CrossRef]
- Wincott, C.M.; Abera, S.; Vunck, S.A.; Tirko, N.; Choi, Y.; Titcombe, R.F.; Antoine, S.O.; Tukey, D.S.; DeVito, L.M.; Hofmann, F.; et al. cGMP-dependent protein kinase type II knockout mice exhibit working memory impairments, decreased repetitive behavior, and increased anxiety-like traits. Neurobiol. Learn. Mem. 2014, 114, 32–39. [Google Scholar] [CrossRef]
- Liu, S.; Ho, P.C. Formulation optimization of scutellarin-loaded HP-_-CD/chitosan nanoparticles using response surface methodology with Box-Behnken design. Asian J. Pharm. Sci. 2017, 12, 378–385. [Google Scholar] [CrossRef]
- Sarathchandiran, I.; Koumaravelou, K.; Selvasudha, N. Interaction pattern and in vitro, in vivo release behavior of simvastatin-loaded chitosan nanoformulation. Drug Dev. Ind. Pharm. 2019, 45, 1725–1739. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Kurakula, M.; Banjar, Z.M.; Afouna, M.I.; Zidan, A.S. Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J. Pharm. Sci. 2015, 104, 2062–2075. [Google Scholar] [CrossRef]
- Hosny, K.M.; Aldawsari, H.M.; Bahmdan, R.H.; Sindi, A.M.; Kurakula, M.; Alrobaian, M.M.; Aldryhim, A.Y.; Alkhalidi, H.M.; Bahmdan, H.H.; Khallaf, R.A.; et al. Preparation, Optimization, and Evaluation of Hyaluronic Acid-Based Hydrogel Loaded with Miconazole Self-Nanoemulsion for the Treatment of Oral Thrush. AAPS PharmSciTech 2019, 20, 297. [Google Scholar] [CrossRef]
- Alkhalidi, H.M.; Naguib, G.H.; Kurakula, M.; Hamed, M.T.; Attar, M.H.; Almatrook, Z.H.; Aldryhim, A.Y.; Bahmdan, R.H.; Khallaf, R.A.; El Sisi, A.M.; et al. In vitro and preclinical assessment of factorial design based nanoethosomal transdermal film formulation of mefenamic acid to overcome barriers to its use in relieving pain and inflammation. J. Drug Deliv. Sci. Technol. 2018, 48, 450–456. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Statistical modelling and optimization of protease production by an autochthonous Bacillus aryabhattai Ab15-ES: A response surface methodology approach. Biocatal. Agric. Biotechnol. 2020, 24, 101528. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Khosa, A.; Singhvi, G.; Girdhar, V.; Jain, R.; Dubey, S.K. Application of QbD principles in nanocarrier-based drug delivery systems. In Pharmaceutical Quality by Design; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 255–296. [Google Scholar]
- Zhang, H.; Choi, J.P.; Moon, S.K.; Ngo, T.H. A hybrid multi-objective optimization of aerosol jet printing process via response surface methodology. Addit. Manuf. 2020, 33, 101096. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In Situ Gel Loaded with Chitosan-Coated Simvastatin Nanoparticles: Promising Delivery for Effective Anti-Proliferative Activity against Tongue Carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Sun, C.; Dai, L.; Yang, S.; Wei, Y.; Mao, L.; Yuan, F.; Gao, Y. Effect of molecular weight of hyaluronan on zein-based nanoparticles: Fabrication, structural characterization and delivery of curcumin. Carbohydr. Polym. 2018, 201, 599–607. [Google Scholar] [CrossRef]
- Chen, S.; Sun, C.; Wang, Y.; Han, Y.; Dai, L.; Abliz, A.; Gao, Y. Quercetagetin-loaded composite nanoparticles based on zein and hyaluronic acid: Formation, characterization, and physicochemical stability. J. Agric. Food Chem. 2018, 66, 7441–7450. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Hosny, K.M.; Al-Sawahli, M.M.; Fahmy, U.A. Optimization of caseinate-coated simvastatin-zein nanoparticles: Improved bioavailability and modified release characteristics. Drug Des. Devel. Ther. 2015, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Sima, A.; Stevens, M. Diabetic neuropathy and oxidative stress. Diabetes Metab. Res. Rev. 2006, 22, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Zhang, Z.G. PDE5 inhibitors promote recovery of peripheral neuropathy in diabetic mice. Neural Regen. Res. 2017, 12, 218. [Google Scholar] [CrossRef]
- Tancheva, L.P.; Lazarova, M.I.; Alexandrova, A.V.; Dragomanova, S.T.; Nicoletti, F.; Tzvetanova, E.R.; Hodzhev, Y.K.; Kalfin, R.E.; Miteva, S.A.; Mazzon, E.; et al. Neuroprotective Mechanisms of Three Natural Antioxidants on a Rat Model of Parkinson’s Disease: A Comparative Study. Antioxidants 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; McClements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 1, 101–118. [Google Scholar] [CrossRef]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Sathyamoorthy, N.; Magharla, D.; Chintamaneni, P.; Vankayalu, S. Optimization of paclitaxel loaded poly (e-caprolactone) nanoparticles using Box Behnken design. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 362–373. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ahuja, M.; Kaur, H. Thiolated pectin nanoparticles: Preparation, characterization and ex vivo corneal permeation study. Carbohydr. Polym. 2012, 87, 1606–1610. [Google Scholar] [CrossRef]
- Nagasubbareddy, J.; Naveen, R. Etoposide-loaded nanoparticles made from poly-e-caprolactone (PCL): Formulation, characterization, in vitro drug release for controlled drug delivery system. Int. J. Biopharm. 2014, 5, 5–12. [Google Scholar]
- Kumar, V.D.; Verma, P.R.; Singh, S.K. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. Lwt-Food Sci. Technol. 2015, 61, 330–338. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Singh, B.; Kapil, R.; Nandi, M.; Ahuja, N. Developing oral drug delivery systems using formulation by design: Vital precepts, retrospect and prospects. Expert Opin. Drug Deliv. 2011, 8, 1341–1360. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Kurakula, M. Okra-Thioglycolic Acid Conjugate—Synthesis, Characterization, and Evaluation as a Mucoadhesive Polymer. Processes 2020, 8, 316. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gas-troretentive floating tablets: In vitro and in vivo evaluation. Future J. Pharm. Sci. 2017, 3, 140–147. [Google Scholar] [CrossRef]
- Firouzi, S.S.; Sarvestani, N.N.; Bakhtiarian, A.; Khansari, M.G.; Karimi, M.Y.; Ranjbar, A.; Safa, M.; Hosseini, A. Sildenafil protective effects on high glucose-induced neurotoxicity in PC12 cells: The role of oxidative stress, apoptosis, and inflammation pathways in an in vitro cellular model for diabetic neuropathy. Neurol. Res. 2018, 3, 624–636. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harbor Protoc. 2018, 1, pdb-rot095505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wu, H.Y.; Xia, X.K.; Liang, Y.J.; Yan, Y.Y.; She, Z.G.; Lin, Y.C.; Fu, L.W. Anthracenedione derivative 1403P-3 induces apoptosis in KB and KBv200 cells via reactive oxygen species-independent mitochondrial pathway and death receptor pathway. Cancer Biol. Ther. 2007, 1, 1409–1417. [Google Scholar] [CrossRef]



| Run | A:ZeinConcentration (g) | B:EthanolConcentration (% v/v) | C:Chitosan (g) | PS (nm) | EE (%) | SI (%) |
|---|---|---|---|---|---|---|
| 1 | 10 | 80 | 2.5 | 355 | 51 | 98 |
| 2 | 10 | 90 | 2 | 315 | 67 | 95 |
| 3 | 6 | 80 | 2.5 | 199 | 72.5 | 69 |
| 4 | 8 | 80 | 2 | 179 | 70 | 83 |
| 5 | 8 | 80 | 2 | 170 | 67 | 85 |
| 6 | 10 | 80 | 1.5 | 286 | 52 | 92 |
| 7 | 8 | 90 | 1.5 | 132 | 76 | 78 |
| 8 | 6 | 90 | 2 | 158 | 87 | 59 |
| 9 | 8 | 80 | 2 | 172 | 68 | 84 |
| 10 | 8 | 70 | 2.5 | 172 | 57 | 82 |
| 11 | 10 | 70 | 2 | 258 | 46 | 95 |
| 12 | 8 | 80 | 2 | 169 | 68 | 86 |
| 13 | 8 | 70 | 1.5 | 109 | 74 | 74 |
| 14 | 8 | 80 | 2 | 165 | 66 | 84 |
| 15 | 8 | 90 | 2.5 | 185 | 84.5 | 82.5 |
| 16 | 6 | 70 | 2 | 125 | 86 | 58 |
| 17 | 6 | 80 | 1.5 | 148 | 82 | 57 |
| PS | EE | SI | ||||
|---|---|---|---|---|---|---|
| Source | Adjusted R2 | Predicted R2 | Adjusted R2 | Predicted R2 | Adjusted R2 | Predicted R2 |
| Linear | 0.6078 | 0.3678 | 0.7534 | 0.5873 | 0.9167 | 0.8942 |
| 2FI | 0.4954 | -0.4754 | 0.8654 | 0.5955 | 0.8992 | 0.8251 |
| Quadratic | 0.9857 | 0.9199 | 0.9760 | 0.8845 | 0.9822 | 0.9035 |
| Cubic | 0.9944 | --- | 0.9854 | --- | 0.9921 | --- |
| PS | EE | SI | ||||
|---|---|---|---|---|---|---|
| Source | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value |
| Model | 123.31 | <0.0001* | 73.26 | <0.0001* | 98.91 | <0.0001* |
| A-Zein concentration | 628.92 | <0.0001 | 428.91 | <0.0001 | 795.78 | <0.0001 |
| B-Ethanol concentration | 29.28 | 0.0010 | 91.50 | <0.0001 | 1.28 | 0.2947 |
| C-Chitosan concentration | 102.71 | <0.0001 | 12.45 | 0.0096 | 39.44 | 0.0004 |
| AB | 2.12 | 0.1883 | 27.60 | 0.0012 | 0.0848 | 0.7793 |
| AC | 1.19 | 0.3105 | 4.99 | 0.0607 | 3.05 | 0.1241 |
| BC | 0.3688 | 0.5628 | 44.87 | 0.0003 | 1.04 | 0.3420 |
| A2 | 306.54 | <0.0001 | 6.69 | 0.0361 | 21.58 | 0.0024 |
| B2 | 46.12 | 0.0003 | 43.24 | 0.0003 | 20.22 | 0.0028 |
| C2 | 2.05 | 0.1949 | 1.22 | 0.3057 | 3.27 | 0.1136 |
| Residual | ||||||
| Lack of Fit | 4.64 | 0.0863** | 2.51 | 0.1976** | 3.96 | 0.1085** |
| Factors/Independent Variables | Levels | Responses/Dependent Variables | Constraints | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Concentration of Zein (g)-X1 | 6 | 8 | 10 | Particle size (nm) | Minimum |
| Ethanol solution concentration (%v/v)- X2 | 70 | 80 | 90 | Entrapment Efficiency (%) | Maximum |
| Concentration of Chitosan (g)-X3 | 1.5 | 2 | 2.5 | Stability Index (%) | Maximum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurakula, M.; Naveen N., R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels 2021, 7, 96. https://doi.org/10.3390/gels7030096
Kurakula M, Naveen N. R, Patel B, Manne R, Patel DB. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels. 2021; 7(3):96. https://doi.org/10.3390/gels7030096
Chicago/Turabian StyleKurakula, Mallesh, Raghavendra Naveen N., Bhaumik Patel, Ravi Manne, and Devang B. Patel. 2021. "Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells" Gels 7, no. 3: 96. https://doi.org/10.3390/gels7030096
APA StyleKurakula, M., Naveen N., R., Patel, B., Manne, R., & Patel, D. B. (2021). Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels, 7(3), 96. https://doi.org/10.3390/gels7030096






