Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers
Abstract
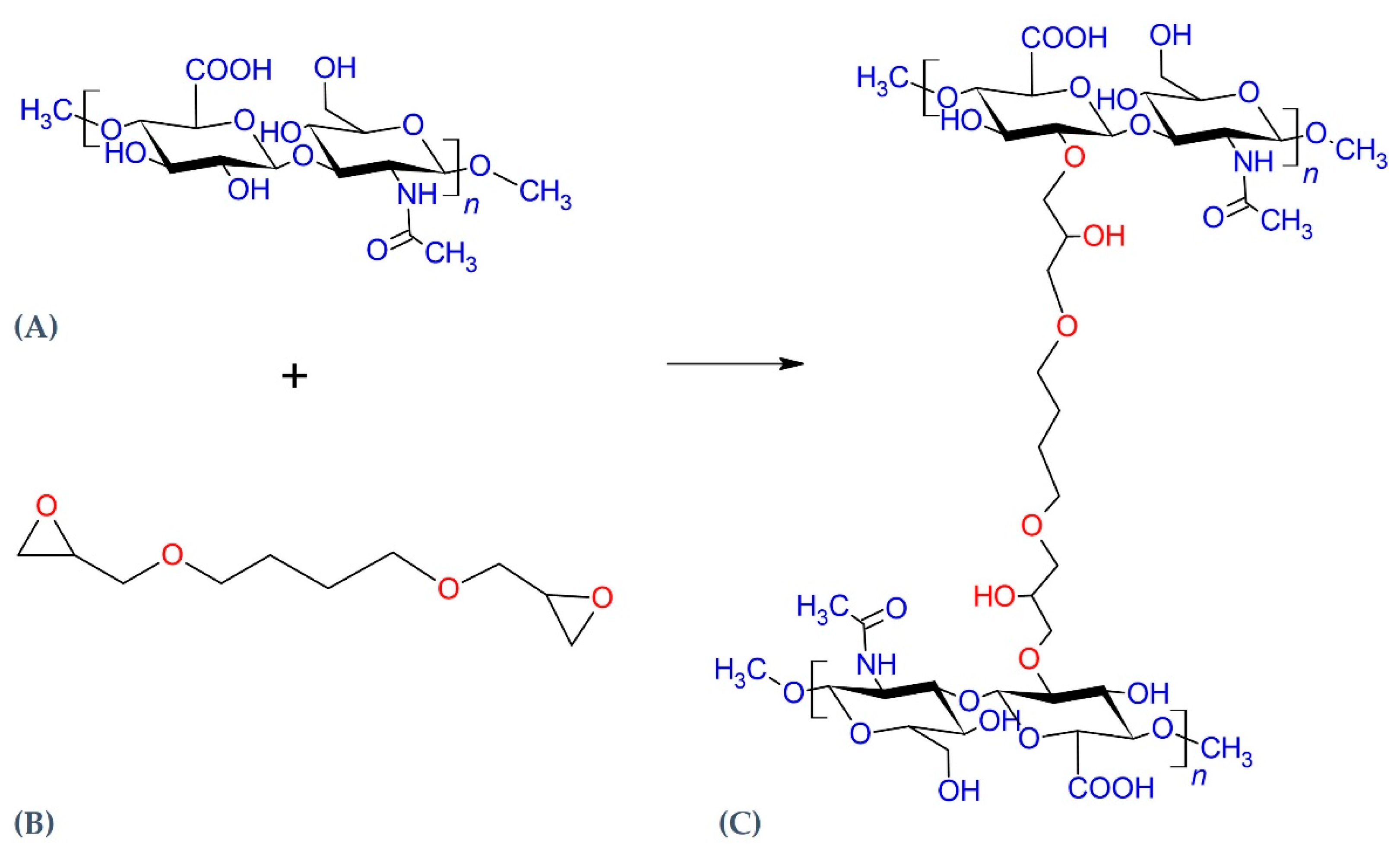
:1. Introduction
2. Results and Discussion
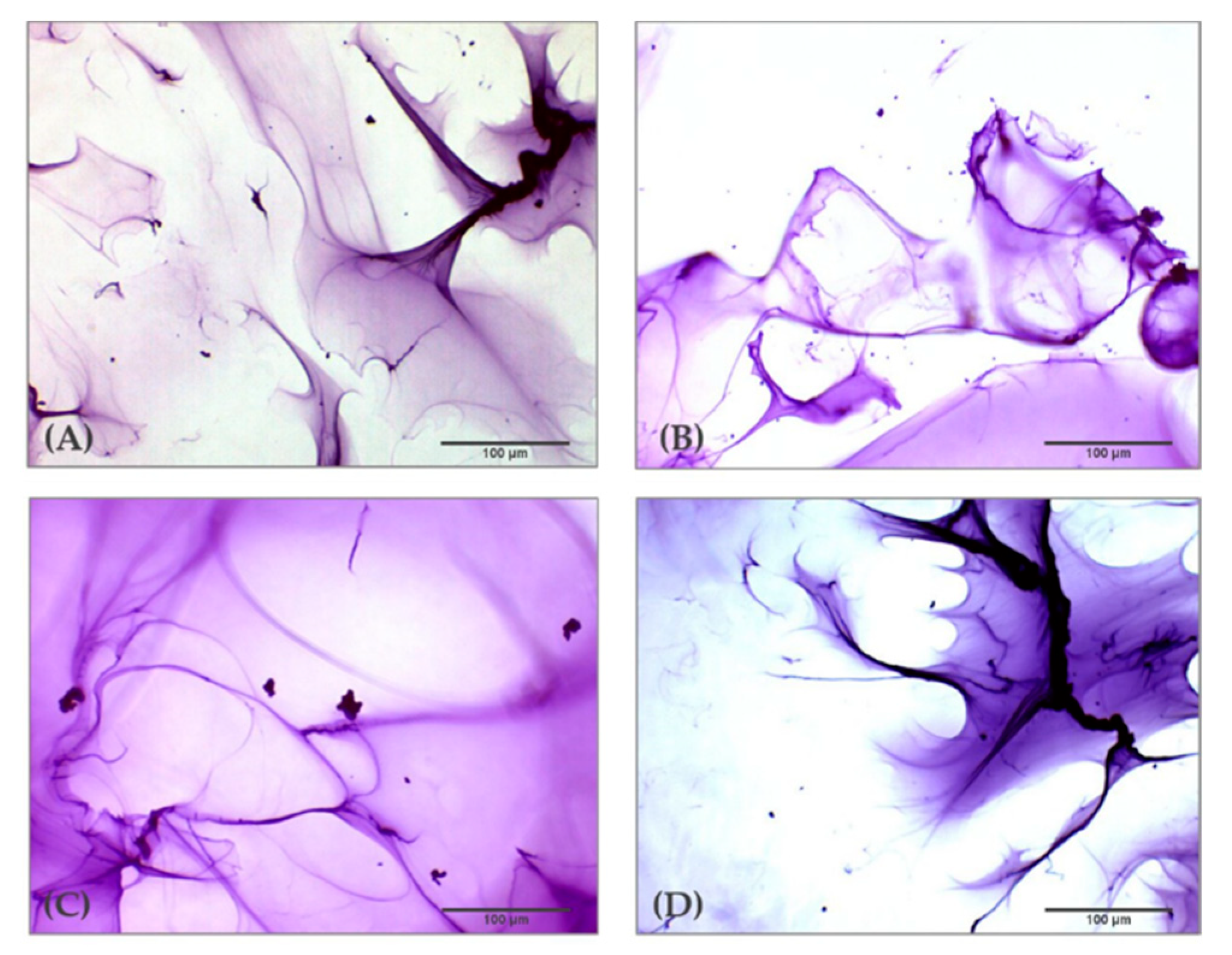
2.1. Optical Microscopic Examination
2.2. Amplitude Sweet Test
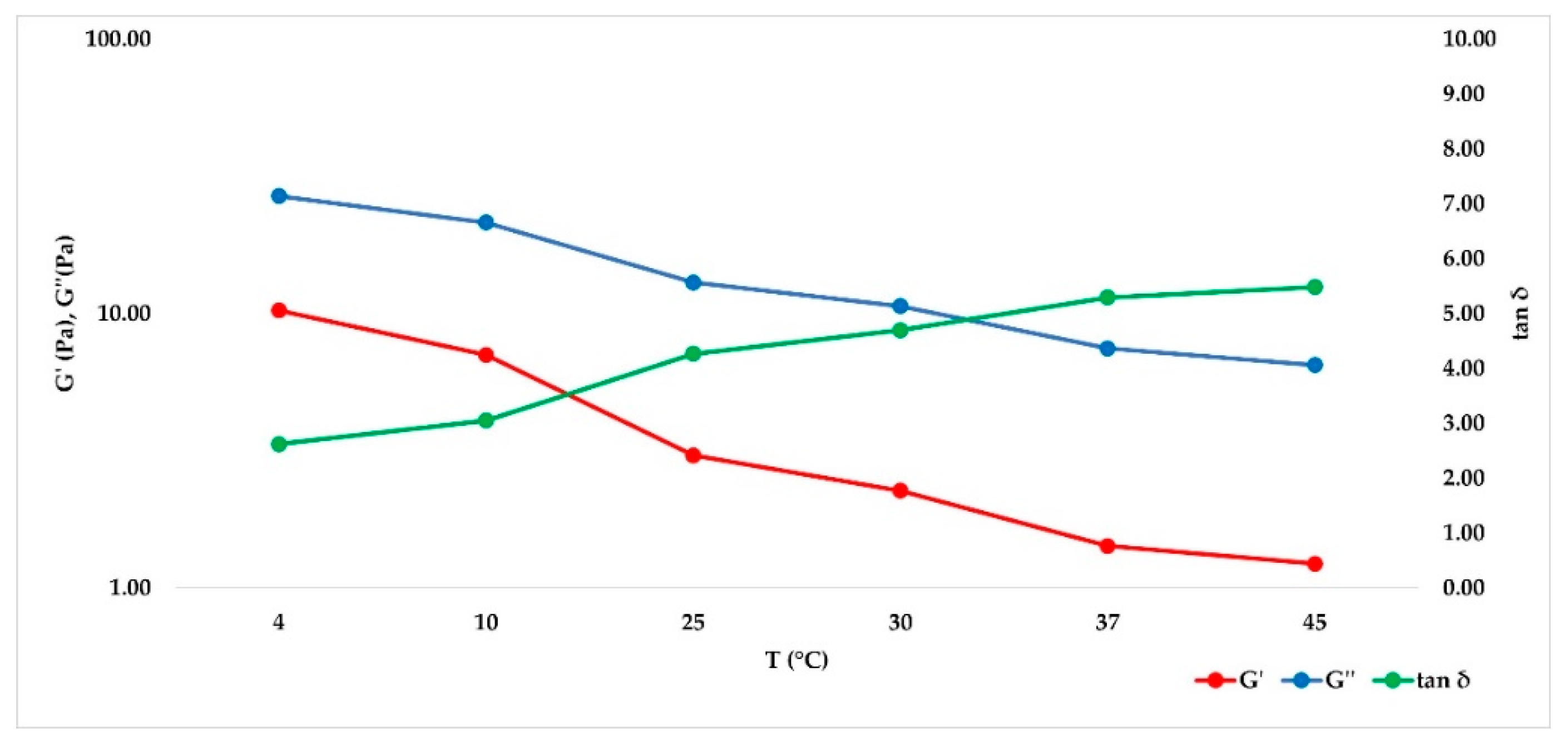
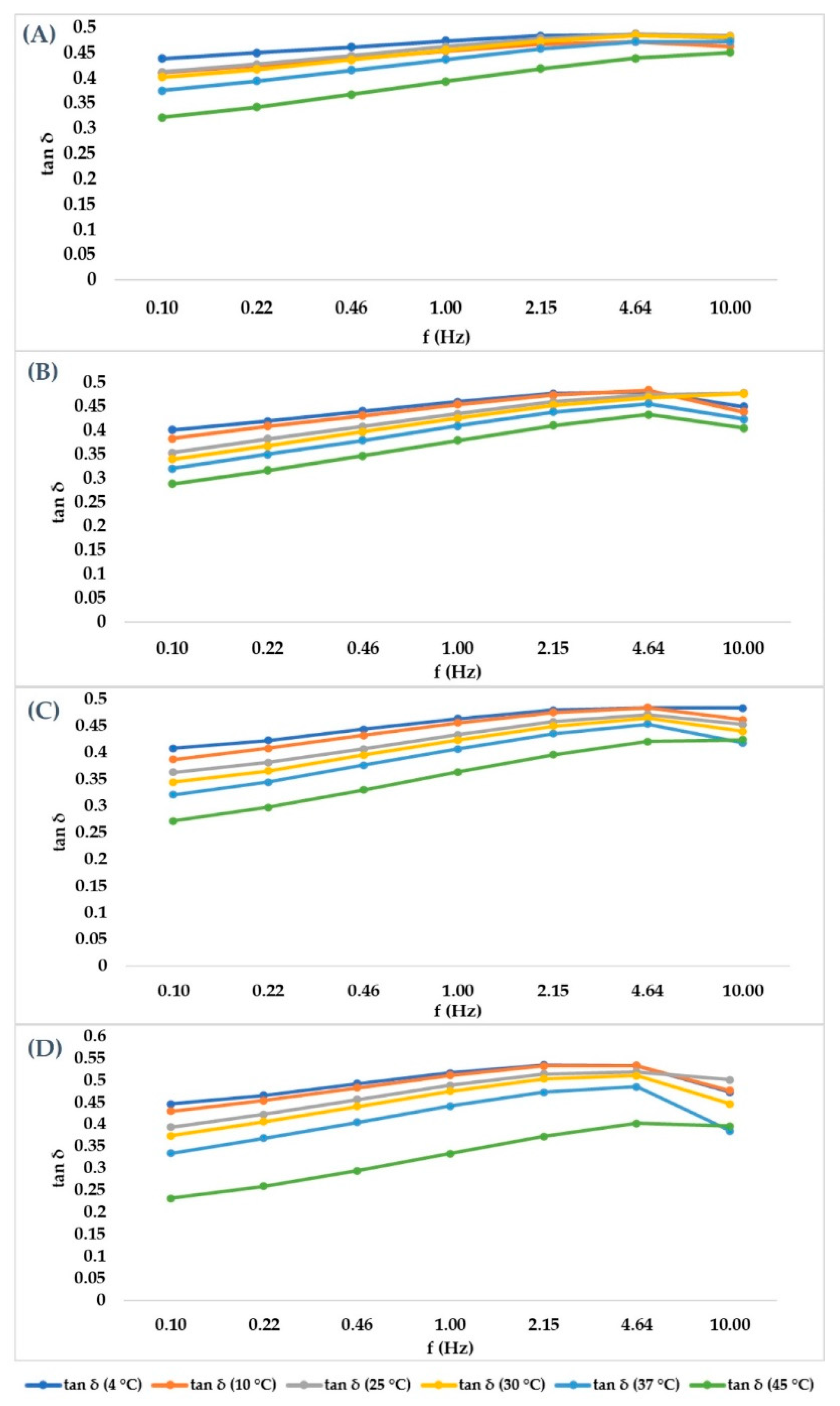
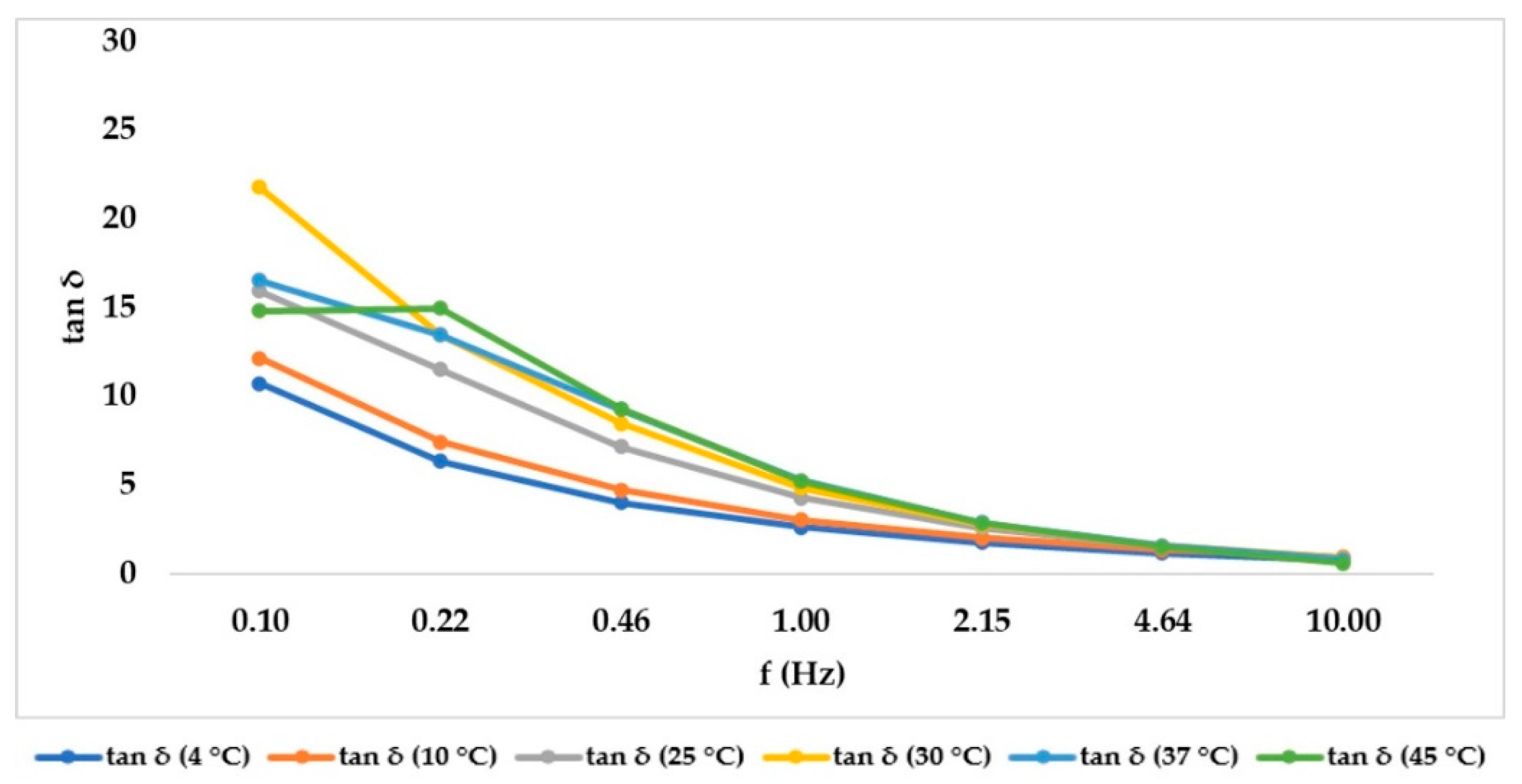
2.3. Frequency Sweep Test in Ramp Temperature Modality
3. Materials and Methods
3.1. Hyaluronic Acid Fillers and Study Design
3.2. Optical Microscopic Examination
3.3. Amplitude Sweep Test for Linear Viscoelastic Region (LVER) Determination
3.4. Frequency Sweep Test with Increasing Temperature
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397. [Google Scholar] [CrossRef] [Green Version]
- Tomihata, K.; Ikada, Y. Preparation of cross-linked hyaluronic acid films of low water content. Biomaterials 1997, 18, 189–195. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Raei, M.; Hushmandi, K.; Zarrabi, A.; Voelcker, N.H.; Aref, A.R.; et al. Hyaluronic acid-based nanoplatforms for Doxorubicin: A review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr. Polym. 2021, 272, 118491. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Kamiński, K.; Kaczor-Kamińska, M.; Szafraniec-Szczęsny, J.; Kmak, A.; Kassassir, H.; Watała, C.; Wróbel, M.; Zapotoczny, S. Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity. Nanomaterials 2021, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Shiedlin, A.; Bigelow, R.; Christopher, W.; Arbabi, S.; Yang, L.; Maier, R.V. Evaluation of hyaluronan from different sources: Streptococcus zooepidermicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 2004, 5, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Nord, L.I.; Ohrlund, A.; Larkner, H. Gel Properties of Hyaluronic Acid Dermal Fillers. Dermatol. Surg. 2012, 38, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Gavard Molliard, S.; Albert, S.; Mondon, K. Key importance of compression properties in the biophysical characteristics of hyaluronic acid soft-tissue fillers. J. Mech. Behav. Biomed. Mater. 2016, 61, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Deepa, K.B. Rheology of hydrogels. In Rheology of Polymer Blends and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 193–204. [Google Scholar]
- Peppas, N.A.; Slaughter, B.V.; Kanzelberger, M.A. Hydrogels. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 385–395. [Google Scholar]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced Concepts in Rheology for the Evaluation of Hyaluronic Acid-Based Soft Tissue Fillers. Dermatol. Surg. 2021, 47, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.; Liew, S.; Bernardin, A. Basics of dermal filler rheology. Dermatol. Surg. 2015, 41, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Henning Winter, H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 2000, 30, 367. [Google Scholar] [CrossRef]
- Gavard Molliard, S.; Bon Bétemps, J.; Hadjab, B.; Topchian, D.; Micheels, P.; Salomon, D. Key rheological properties of hyaluronic acid fillers: From tissue integration to product degradation. Plast. Aesthetic Res. 2018, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Santoro, S.; Russo, L.; Argenzio, V.; Borzacchiello, A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J. Appl. Biomater. Biomech. 2011, 9, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, H.C.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Mondon, K.; Dadras, M. Influence of the macro- and/or microstructure of cross-linked hyaluronic acid hydrogels on the release of two model drugs. J. Glycobiol. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Capillo, M.C.; Grimaldi, G.; Alonci, G.; Protasoni, M.; Rauso, R.; Mocchi, R. Toward Physicochemical and Rheological Characterization of Different Injectable Hyaluronic Acid Dermal Fillers Cross-Linked with Polyethylene Glycol Diglycidyl Ether. Polymers 2021, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, Z.P.; Öhrlund, A.; Edsman, K. Factors Affecting the Rheological Measurement of Hyaluronic Acid Gel Fillers. J. Drugs Dermatol. 2017, 16, 876–882. [Google Scholar] [PubMed]



| Product | HA Content (mg/mL) | Cross-Linker |
|---|---|---|
| HA hydrogel 22 mg/mL | 22 | BDDE |
| HA hydrogel 24 mg/mL | 24 | BDDE |
| HA hydrogel 26 mg/mL | 26 | BDDE |
| HA hydrogel 28 mg/mL | 28 | BDDE |
| HA not cross-linked 18 mg/mL | 18 | Not cross-linked |
| Product | G’ (Pa) | G’’ (Pa) | G* (Pa) | tan δ | η* (Pa s) |
|---|---|---|---|---|---|
| 22 mg/mL | 56.67 ± 1.98 | 26.03 ± 1.97 | 62.37 ± 2.62 | 0.46 ± 0.02 | 9.93 ± 0.42 |
| 24 mg/mL | 97.27 ± 8.22 | 42.16 ± 2.99 | 106.02 ± 8.74 | 0.43 ± 0.01 | 16.87 ± 1.39 |
| 26 mg/mL | 93.22 ± 4.91 | 41.65 ± 2.11 | 102.16 ± 4.15 | 0.45 ± 0.04 | 16.25 ± 0.66 |
| 28 mg/mL | 120.57 ± 2.59 | 53.19 ± 0.10 | 132.60 ± 1.81 | 0.44 ± 0.01 | 21.10 ± 0.29 |
| Product | G’ (Pa) | G’’ (Pa) | G* (Pa) | tan δ | η* (Pa s) |
|---|---|---|---|---|---|
| 22 mg/mL | 56.99 ± 6.08 | 21.89 ± 1.77 | 61.05 ± 6.24 | 0.39 ± 0.02 | 9.72 ± 0.99 |
| 24 mg/mL | 92.85 ± 1.51 | 37.01 ± 3.39 | 100.00 ± 0.92 | 0.40 ± 0.03 | 15.91 ± 0.15 |
| 26 mg/mL | 95.26 ± 5.13 | 39.35 ± 0.94 | 103.04 ± 4.91 | 0.41 ± 0.02 | 16.40 ± 0.79 |
| 28 mg/mL | 121.73 ± 5.59 | 52.86 ± 1.83 | 132.70 ± 5.88 | 0.43 ± 0.36 | 21.13 ± 0.93 |
| Product | G’ (Pa) | G’’ (Pa) | tan δ |
|---|---|---|---|
| 22 mg/mL | 46.39 ± 3.53 | 22.72 ± 6.91 | 0.49 ± 3.39 |
| 24 mg/mL | 92.17 ± 6.12 | 39.96 ± 5.62 | 0.43 ± 4.73 |
| 26 mg/mL | 100.75 ± 4.87 | 43.77 ± 7.13 | 0.43 ± 3.07 |
| 28 mg/mL | 160.30 ± 8.62 | 73.98 ± 8.75 | 0.46 ± 4.92 |
| Not cross-linked | 3.04 ± 6.71 | 12.99 ± 10.22 | 4.27 ± 5.16 |
| Product | G’ (Pa) | G’’ (Pa) | tan δ |
|---|---|---|---|
| 22 mg/mL | 45.03 ± 5.77 | 19.90 ± 7.22 | 0.44 ± 4.46 |
| 24 mg/mL | 82.13 ± 8.58 | 33.31 ± 5.79 | 0.41 ± 5.99 |
| 26 mg/mL | 89.13 ± 6.78 | 34.49 ± 8.41 | 0.41 ± 2.92 |
| 28 mg/mL | 145.20 ± 7.32 | 63.42 ± 7.14 | 0.44 ± 5.60 |
| Not cross-linked | 1.57 ± 6.08 | 6.73 ± 1.98 | 5.02 ± 7.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbinati, N.; Sommatis, S.; Maccario, C.; Capillo, M.C.; Grimaldi, G.; Alonci, G.; Rauso, R.; Guida, S.; Mocchi, R. Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers. Gels 2021, 7, 139. https://doi.org/10.3390/gels7030139
Zerbinati N, Sommatis S, Maccario C, Capillo MC, Grimaldi G, Alonci G, Rauso R, Guida S, Mocchi R. Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers. Gels. 2021; 7(3):139. https://doi.org/10.3390/gels7030139
Chicago/Turabian StyleZerbinati, Nicola, Sabrina Sommatis, Cristina Maccario, Maria Chiara Capillo, Giulia Grimaldi, Giuseppe Alonci, Raffaele Rauso, Stefania Guida, and Roberto Mocchi. 2021. "Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers" Gels 7, no. 3: 139. https://doi.org/10.3390/gels7030139
APA StyleZerbinati, N., Sommatis, S., Maccario, C., Capillo, M. C., Grimaldi, G., Alonci, G., Rauso, R., Guida, S., & Mocchi, R. (2021). Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers. Gels, 7(3), 139. https://doi.org/10.3390/gels7030139







