Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation
Abstract
1. Introduction
2. Results and Discussion
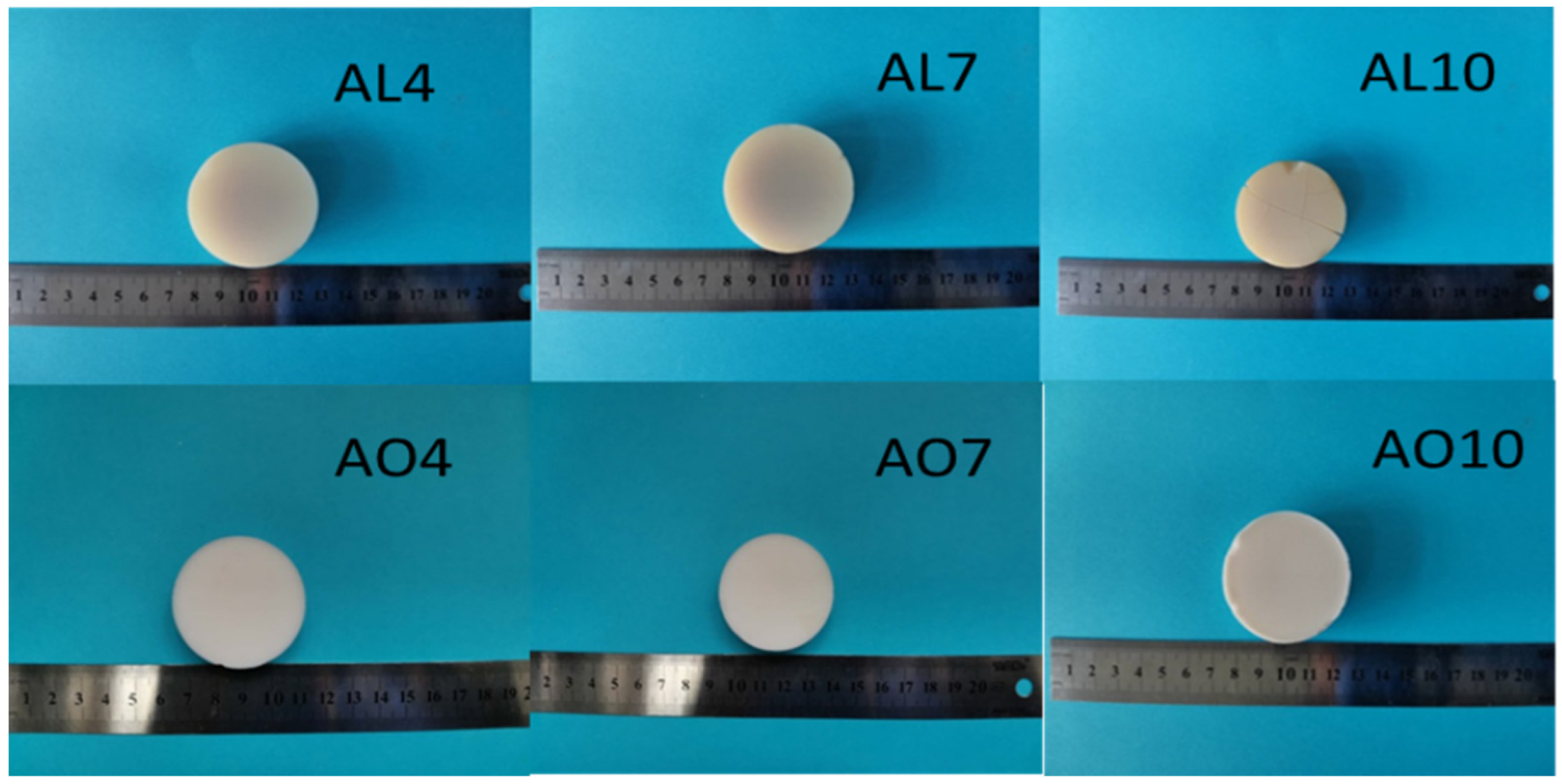
2.1. Macroscopic Properties of As-Prepared Samples
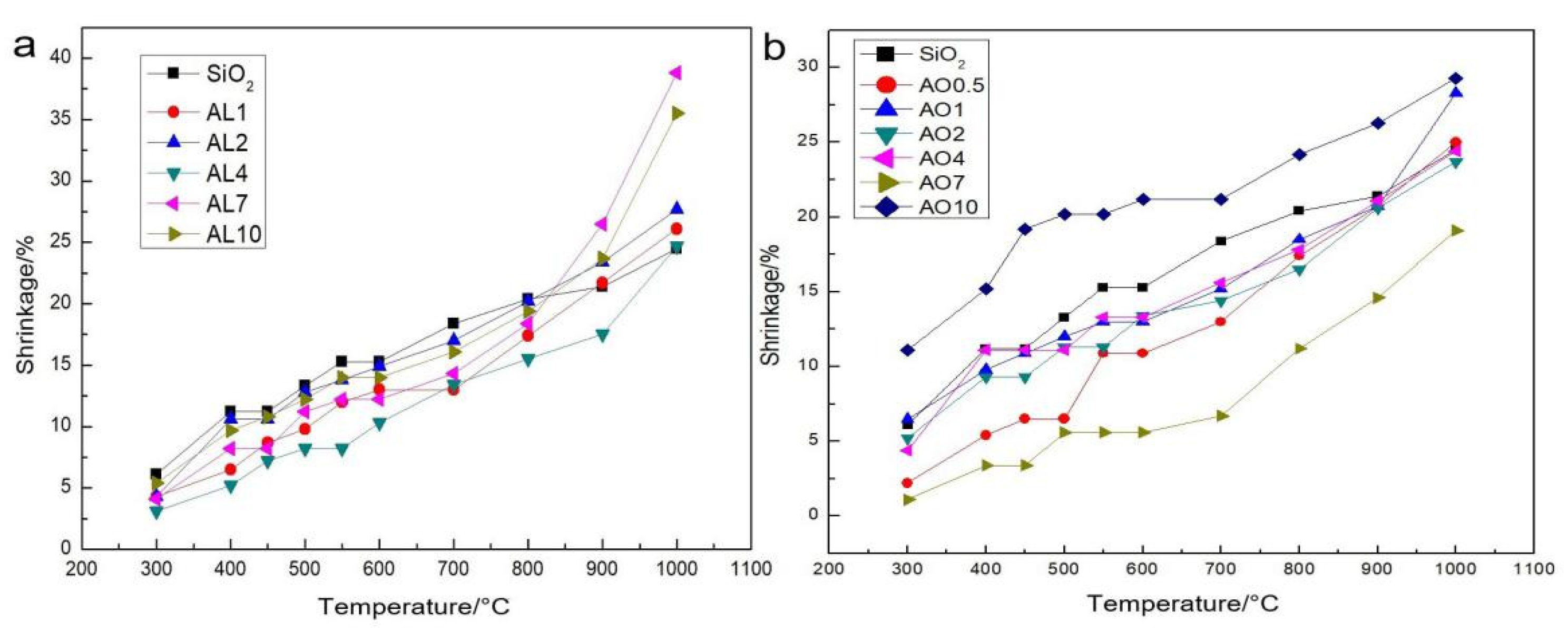
2.2. Shrinkage after Different Heating Temperatures
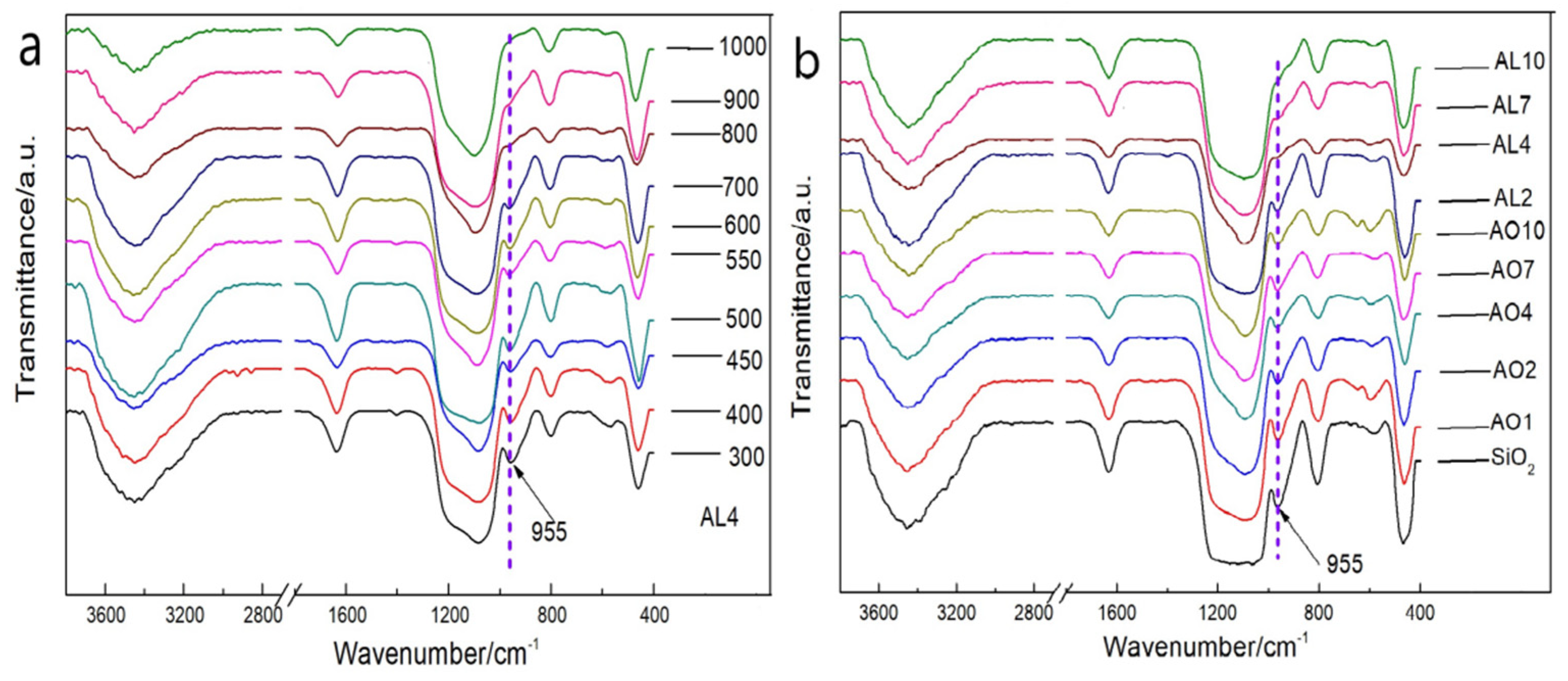
2.3. Variation of Chemical Composition at Different Temperatures
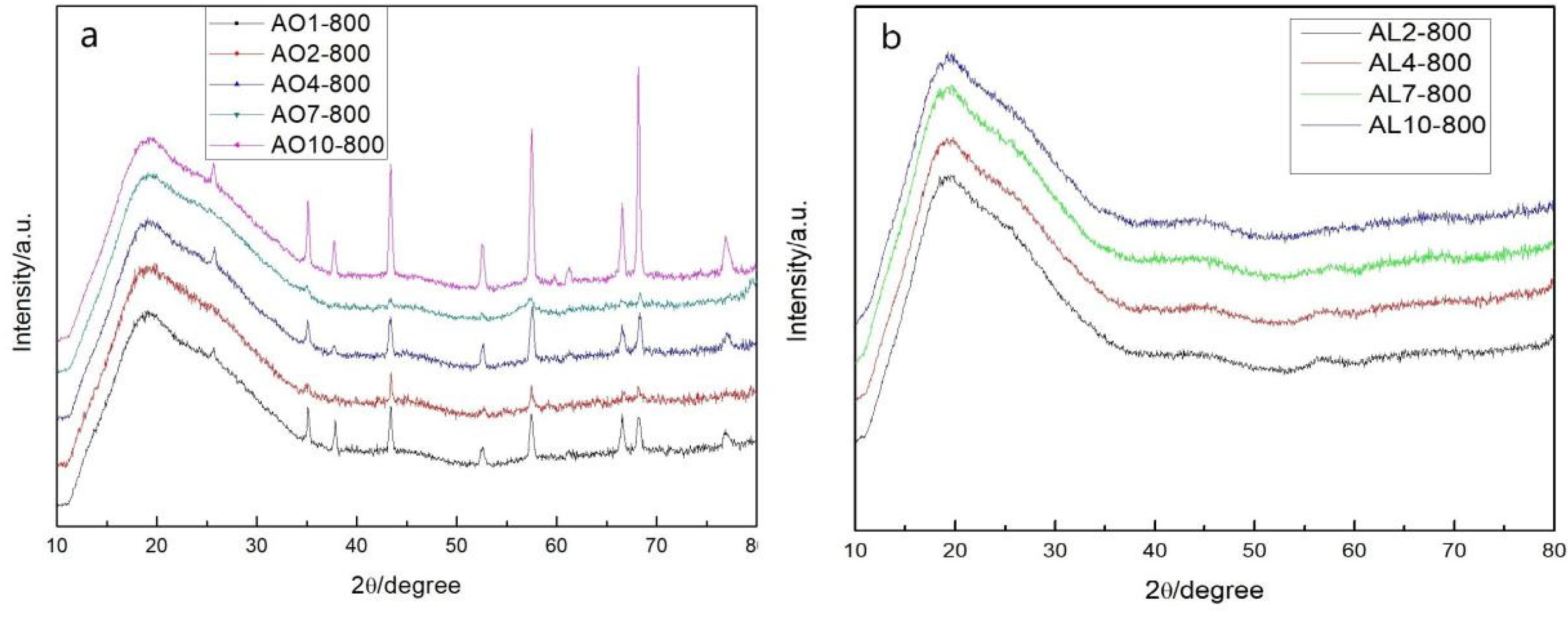
2.4. Crystal Phase of Aerogels after Heat Treatment at 800 °C
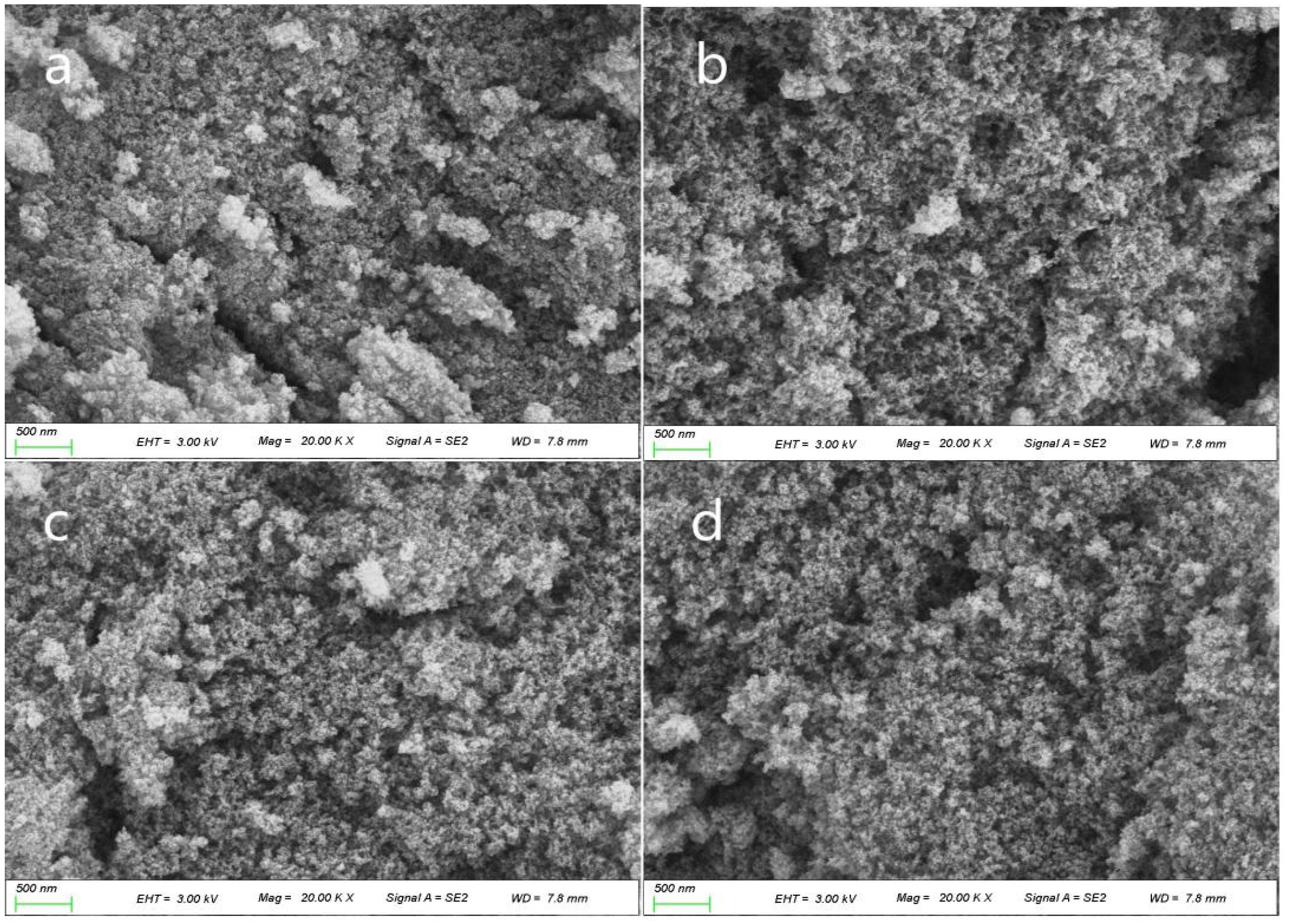
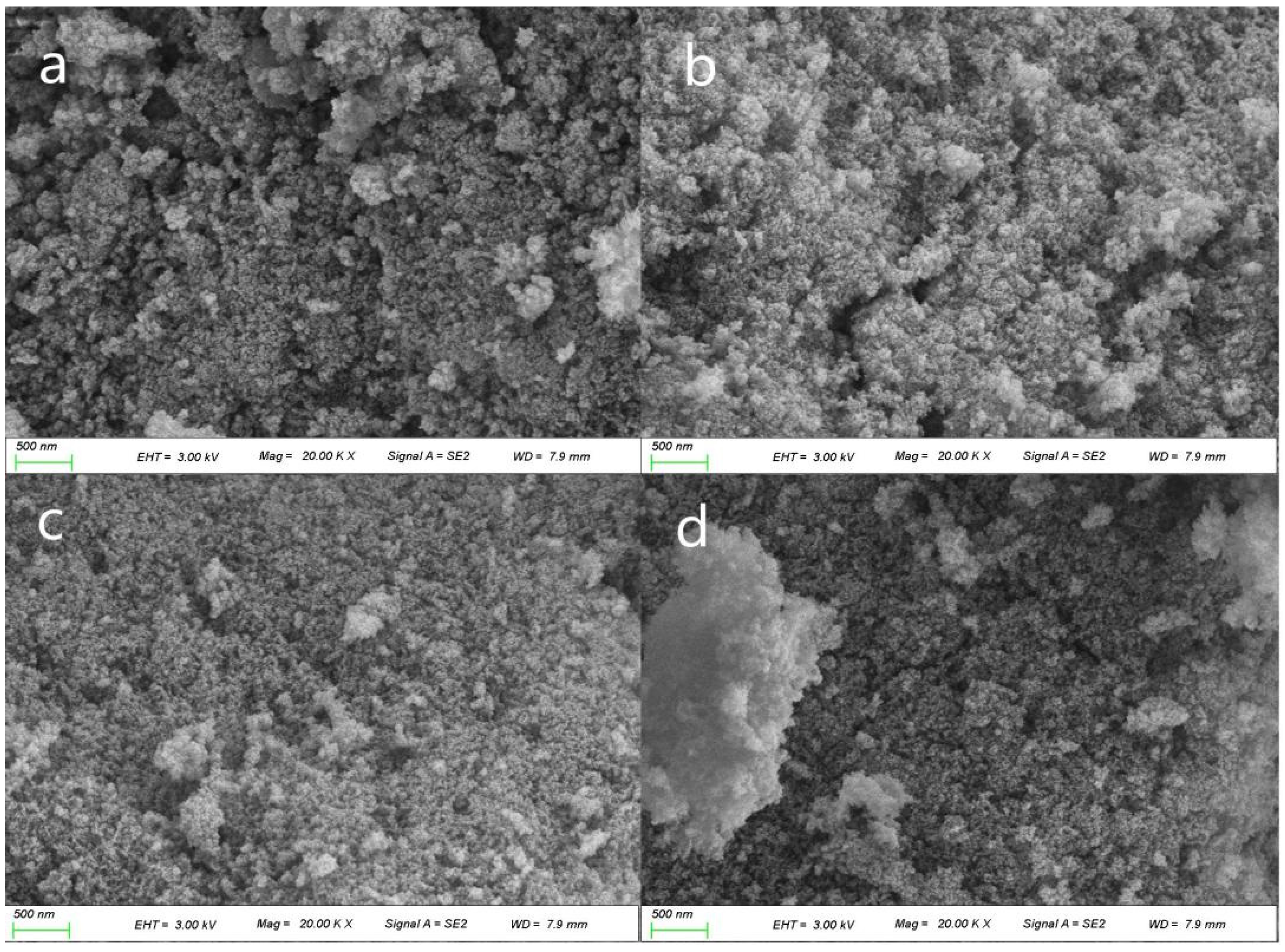
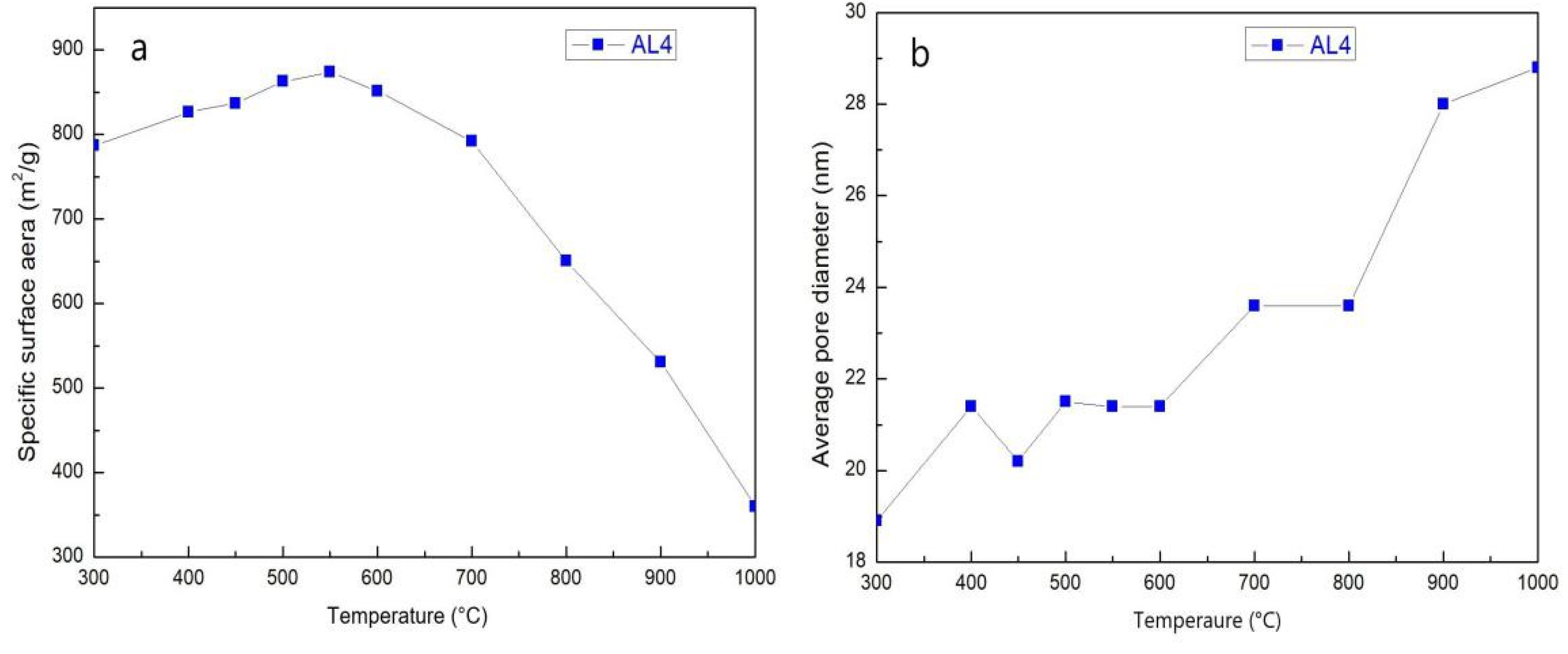
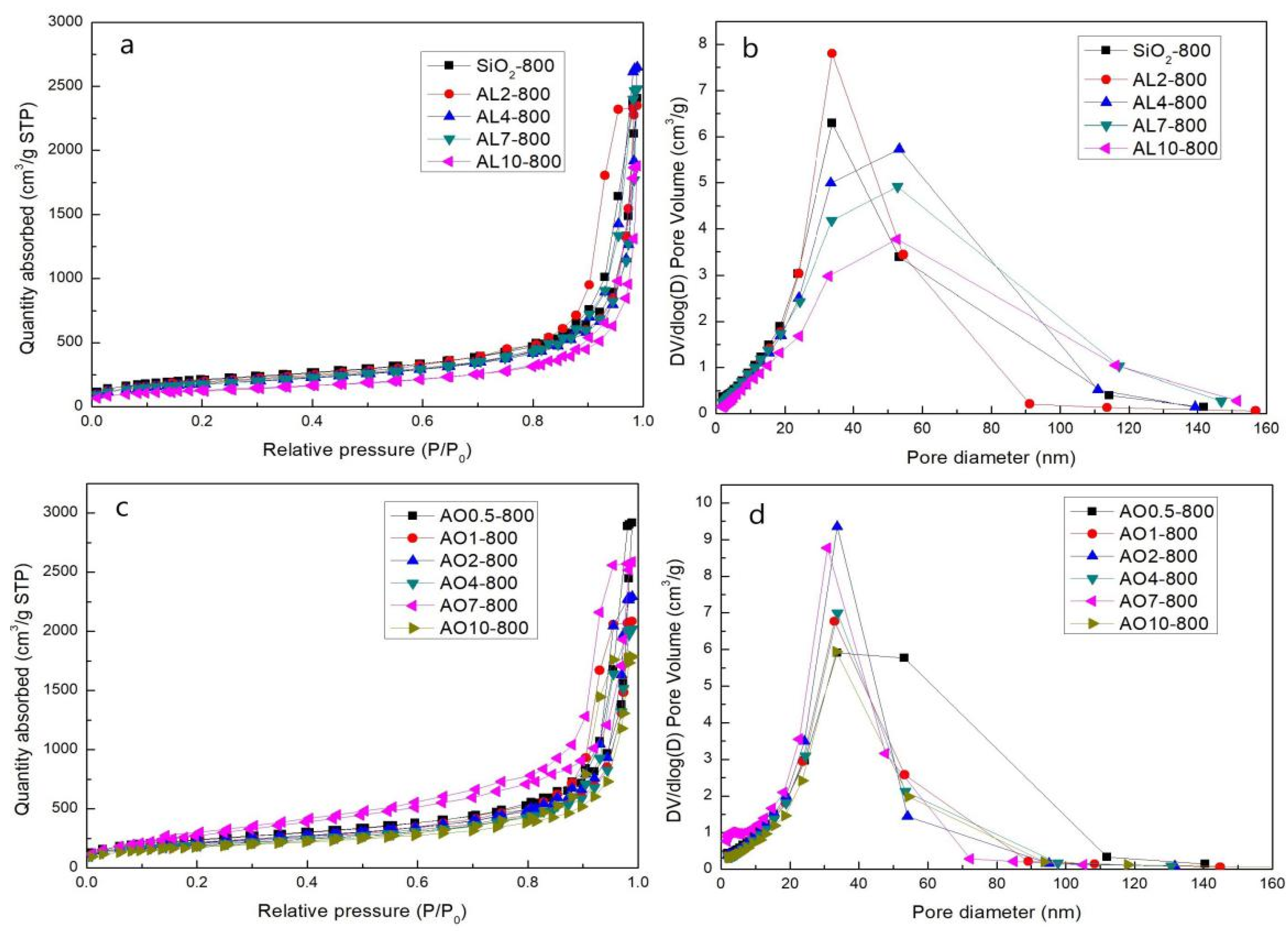
2.5. SSA and Pore Size Distribution at Different Temperatures
2.6. Analysis of Sol-Gel Process and Thermal Stability
- (a) Hydrolysis.
- (b) Condensation.
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
4.2. Synthesis
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Despetis, F.; Calas-Etienne, S.; Etienne, P. Slow crack growth in silica aerogels: A review. J. Sol.-Gel Sci. Technol. 2019, 90, 20–27. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, C.; Zhang, H.; Peng, S.; Wei, X.; Hu, Y. Regeneration of mesoporous silica aerogel for hydrocarbon adsorption and recovery. Mar. Pollut. Bulletin. 2017, 122, 129–138. [Google Scholar] [CrossRef]
- Parale, V.G.; Lee, K.Y.; Park, H.H. Flexible and transparent silica aerogels: An overview. J. Korean Ceram. Soc. 2017, 54, 184–199. [Google Scholar] [CrossRef]
- He, Y.; Xie, T. A review of heat transfer models of nanoporous silica aerogel insulation material. Chin. Sci. Bull. 2015, 60, 137–163. [Google Scholar]
- Pisal, A.A.; Rao, A.V. Comparative studies on the physical properties of TEOS, TMOS and Na2SiO3 based silica aerogels by ambient pressure drying method. J. Porous Mater. 2016, 23, 1547–1556. [Google Scholar] [CrossRef]
- Reim, M.; Korner, W.; Manara, J.; Korder, S.; Arduini-Schuster, M.; Ebert, H.P.; Fricke, J. Silica aerogel granulate material for thermal insulation and daylighting. Sol. Energy 2005, 79, 131–139. [Google Scholar] [CrossRef]
- Cao, S.L.; Yao, N.; Yeung, K.L. Synthesis of freestanding silica and titania-silica aerogels with ordered and disordered mesopores. J. Sol.-Gel Sci. Technol. 2008, 46, 323–333. [Google Scholar] [CrossRef]
- Kaya, G.G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Hung, W.-C.; Horng, R.S.; Shia, R.-E. Investigation of thermal insulation performance of glass/carbon fiber-reinforced silica aerogel composites. J. Sol.-Gel Sci. Technol. 2021, 97, 414–421. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhong, T.; Wu, Z.; Zhan, X.; Ye, J. Thermal insulation and hydrophobization of wood impregnated with silica aerogel powder. J. Wood Sci. 2020, 66, 1–11. [Google Scholar] [CrossRef]
- Yay, B.; Gizli, N. A review on silica aerogels for CO2 capture applications. Pamukkale Univ. J. Eng. Sci. Pamukkale Univ. Muhendis. Bilimleri Derg. 2019, 25, 907–913. [Google Scholar] [CrossRef]
- Jia, H.J.; Liu, S.; Mao, Z.Y.; Wang, D.J. Preparation and properties of the Al2O3-SiO2 aerogel/alumina framework composite. Ceram. Int. 2021, 47, 1466–1471. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Li, L.; Cai, H.; Feng, J. A facile method to fabricate monolithic alumina-silica aerogels with high surface areas and good mechanical properties. J. Eur. Ceram. Soc. 2020, 40, 2480–2488. [Google Scholar] [CrossRef]
- Posada, L.F.; Carroll, M.K.; Anderson, A.M.; Bruno, B.A. Inclusion of Ceria in alumina- and silica-based aerogels for catalytic applications. J. Supercrit. Fluids 2019, 152, 104536. [Google Scholar] [CrossRef]
- Zou, W.B.; Wang, X.D.; Wu, Y.; Zou, L.P.; Zu, G.Q.; Chen, D.; Shen, J. Opacifier embedded and fiber reinforced alumina-based aerogel composites for ultra-high temperature thermal insulation. Ceram. Int. 2019, 45, 644–650. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Wu, Y.; Zu, G.Q.; Zou, L.P.; Zhang, R.; Yao, X.; Shen, J. Highly thermally stable alumina-based aerogels modified by partially hydrolyzed aluminum tri-sec-butoxide. J. Sol-Gel Sci. Technol. 2017, 84, 507–514. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Shen, X.; Cui, S.; Wang, L. Novel Al2O3-SiO2 composite aerogels with high specific surface area at elevated temperatures with different alumina/silica molar ratios prepared by a non-alkoxide sol-gel method. RSC Adv. 2016, 6, 5611–5620. [Google Scholar] [CrossRef]
- Zhang, R.B.; Ye, C.S.; Wang, B.L. Novel Al2O3-SiO2 aerogel/porous zirconia composite with ultra-low thermal conductivity. J. Porous Mater. 2018, 25, 171–178. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Zou, L.P.; Zou, W.B.; Guan, D.Y.; Wu, Y.; Zhang, Y.W. Highly thermally stable zirconia/silica composite aerogels prepared by supercritical deposition. Microporous Mesoporous Mat. 2017, 238, 90–96. [Google Scholar] [CrossRef]
- Long, J.W.; Chervin, C.N.; Balow, R.B.; Jeon, S.; Miller, J.B.; Helms, M.E.; Owrutsky, J.C.; Rolison, D.R.; Fears, K.P. Zirconia-based aerogels for sorption and degradation of dimethyl methylphosphonate. Ind. Eng. Chem. Res. 2020, 59, 19584–19592. [Google Scholar] [CrossRef]
- Gao, H.B.; Zhang, Z.Y.; Shi, Z.Y.; Zhang, J.Z.; Zhi, M.J.; Hong, Z.L. Synthesis of high-temperature resistant monolithic zirconia-based aerogel via facile water glass assisted sol-gel method. J. Sol.-Gel Sci. Technol. 2018, 85, 567–573. [Google Scholar] [CrossRef]
- Yu, Y.X.; Zhu, M.W. Preparation and characterization of highly spherical silica-titania aerogel beads with high surface area. Cailiao Gongcheng-J. Mater. Eng. 2017, 45, 7–11. [Google Scholar]
- Wang, X.K.; Liu, J.X.; Shi, F.; Liu, S.H.; Feng, X.; Bao, L. Influences of heat-treatment on the microstructure and properties of silica-titania composite aerogels. J. Porous Mater. 2014, 21, 293–301. [Google Scholar] [CrossRef]
- An, L.; Petit, D.; Di Luigi, M.; Sheng, A.; Huang, Y.; Hu, Y.; Li, Z.; Ren, S. Reflective paint consisting of mesoporous silica aerogel and titania nanoparticles for thermal management. ACS Appl. Nano Mater. 2021, 4, 6357–6363. [Google Scholar] [CrossRef]
- Yoda, S.; Otake, K.; Takebayashi, Y.; Sugeta, T.; Sato, T. Effects of supercritical impregnation conditions on the properties of silica-titania aerogels. J. Non-Cryst. Solids 2001, 285, 8–12. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Zou, L.P.; Wang, W.Q.; Lian, Y.; Zhang, Z.H.; Du, A. Nanoengineering super heat-resistant, strong alumina aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Yang, H.; Tian, S.; Ge, Q.; Huang, S.; Wang, M. Removal of chloride ions from acidic solution with antimony oxides. J. Ind. Eng. Chem. 2021, 93, 170–175. [Google Scholar] [CrossRef]
- Sun, F.; Liu, W.; Yan, K.; Yishuai, E. Removal of chloride ion from desulfurization wastewater by ion-exchange resin. Inorg. Chem. Ind. 2019, 51, 45–48. [Google Scholar]
- Yang, C.; Yang, G.; Shi, Y.; Chen, S. Study on electrosorption removal of chloride ion from reclaimed water. Adv. Mater. Res. 2012, 356–360, 2015–2019. [Google Scholar] [CrossRef]
- Sun, J. Selective sodium removal from lithium chloride brine with novel inorganic ion exchanger. Bull. Chem. Soc. Ethiop. 2013, 27, 241–248. [Google Scholar] [CrossRef][Green Version]
- Wu, X.D.; Ding, J.; Kong, Y.; Sun, Z.; Shao, G.F.; Li, B.Y.; Wu, J.; Zhong, Y.; Shen, X.D.; Cui, S. Synthesis of a novel three-dimensional Na2SO4@SiO2@Al2O3-SiO2 phase change material doped aerogel composite with high thermal resistance and latent heat. Ceram. Int. 2018, 44, 21855–21865. [Google Scholar] [CrossRef]
- Chen, H.; Sui, X.Y.; Zhou, C.L.; Wang, C.H.; Liu, F.T. Preparation and characterization of monolithic Al2O3-SiO2 aerogel. J. Ceram. Soc. Jpn. 2016, 124, 442–447. [Google Scholar] [CrossRef]
- Lei, Y.F.; Chen, X.H.; Song, H.H.; Hu, Z.J.; Cao, B. Improvement of thermal insulation performance of silica aerogels by Al2O3 powders doping. Ceram. Int. 2017, 43, 10799–10804. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z. Synthesis and thermal behavior of high-surface-area monolithic alumina aerogels. Rare Met. Mat. Eng. 2016, 45, 522–529. [Google Scholar]
- Tang, X.B.; Sun, A.H.; Chu, C.Y.; Yu, M.L.; Ma, S.; Cheng, Y.C.; Guo, J.J.; Xu, G.J. A novel silica nanowire-silica composite aerogels dried at ambient pressure. Mater. Des. 2017, 115, 415–421. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wei, X.; Ni, X.; Zhang, Z.; Wang, J.; Liu, G. Preparation and characterization of monolithic alumina aerogels. J. Non-Cryst. Solids 2011, 357, 2903–2906. [Google Scholar] [CrossRef]
- Horiuchi, T.; Osaki, T.; Sugiyama, T.; Masuda, H.; Horio, M.; Suzuki, K.; Mori, T.; Sago, T. High-surface-area alumina aerogel at elevated-temperatures. J. Chem. Soc. -Faraday Trans. 1994, 90, 2573–2578. [Google Scholar] [CrossRef]


| Sample | Density (mg/cm3) | Thermal Conductivity (W/m·K) | Sample | Density (mg/cm3) | Thermal Conductivity (W/m∙K) |
|---|---|---|---|---|---|
| AO0.5 | 151 | 0.0383 ± 0.0019 | SiO2 | 132 | 0.0286 ± 0.0014 |
| AO1 | 149 | 0.0374 ± 0.0020 | AL1 | 134 | 0.0283 ± 0.0016 |
| AO2 | 155 | 0.0340 ± 0.0017 | AL2 | 140 | 0.0296 ± 0.0015 |
| AO4 | 161 | 0.0360 ± 0.0019 | AL4 | 114 | 0.0269 ± 0.0014 |
| AO7 | 170 | 0.0357 ± 0.0018 | AL7 | 102 | 0.0284 ± 0.0014 |
| AO10 | 144 | 0.0304 ± 0.0015 | AL10 | 103 | 0.0292 ± 0.0015 |
| Sample | Density (mg/cm3) | Thermal Conductivity (W/m∙K) | Sample | Density (mg/cm3) | Thermal Conductivity (W/m∙K) |
|---|---|---|---|---|---|
| AO1 | 273 | 0.058 ± 0.003 | AL1 | 334 | 0.057 ± 0.003 |
| AO2 | 277 | 0.054 ± 0.003 | AL2 | 299 | 0.056 ± 0.003 |
| AO4 | 315 | 0.056 ± 0.003 | AL4 | 234 | 0.050 ± 0.003 |
| AO7 | 280 | 0.055 ± 0.003 | AL7 | 409 | 0.067 ± 0.003 |
| AO10 | 314 | 0.056 ± 0.003 | AL10 | 331 | 0.062 ± 0.003 |
| Sample | SSA (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| AL4-300 | 787 ± 3 | 18.9 | 3.5 |
| AL4-400 | 827 ± 3 | 21.4 | 4.1 |
| AL4-450 | 837 ± 3 | 20.2 | 3.9 |
| AL4-500 | 862.8 ± 1.9 | 21.5 | 4.6 |
| AL4-550 | 873.5 ± 1.3 | 21.4 | 4.8 |
| AL4-600 | 851.5 ± 1.3 | 21.4 | 4.8 |
| AL4-700 | 791.8 ± 1.2 | 23.6 | 5.0 |
| AL4-800 | 650.3 ± 1.0 | 23.6 | 4.1 |
| AL4-900 | 530.6 ± 0.8 | 28.0 | 4.2 |
| AL4-1000 | 360.2 ± 0.6 | 28.8 | 2.9 |
| Sample | SSA(m2/g) | Pore Dimeter (nm) | Pore Volumes (cm3/g) |
|---|---|---|---|
| AL2 | 683.9 ± 1.1 | 20.5 | 3.7 |
| AL4 | 650.3 ± 1.0 | 23.6 | 4.1 |
| AL7 | 652.5 ± 0.9 | 21.8 | 3.9 |
| AL10 | 471.9 ± 0.7 | 22.2 | 2.9 |
| AO0.5 | 871.9 ± 1.7 | 20.9 | 4.5 |
| AO1 | 743.4 ± 1.7 | 17.8 | 3.3 |
| AO2 | 786.0 ± 1.3 | 18.0 | 3.6 |
| AO4 | 706.3 ± 1.3 | 17.9 | 3.2 |
| AO7 | 1056 ± 12 | 12.2 | 4.0 |
| AO10 | 633.0 ± 1.4 | 17.9 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, X.; Liu, L.; Zhang, Z.; Shen, J. Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation. Gels 2021, 7, 122. https://doi.org/10.3390/gels7030122
Wu Y, Wang X, Liu L, Zhang Z, Shen J. Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation. Gels. 2021; 7(3):122. https://doi.org/10.3390/gels7030122
Chicago/Turabian StyleWu, Yu, Xiaodong Wang, Lin Liu, Ze Zhang, and Jun Shen. 2021. "Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation" Gels 7, no. 3: 122. https://doi.org/10.3390/gels7030122
APA StyleWu, Y., Wang, X., Liu, L., Zhang, Z., & Shen, J. (2021). Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation. Gels, 7(3), 122. https://doi.org/10.3390/gels7030122








