Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties
Abstract
1. Introduction
2. Results and Discussion
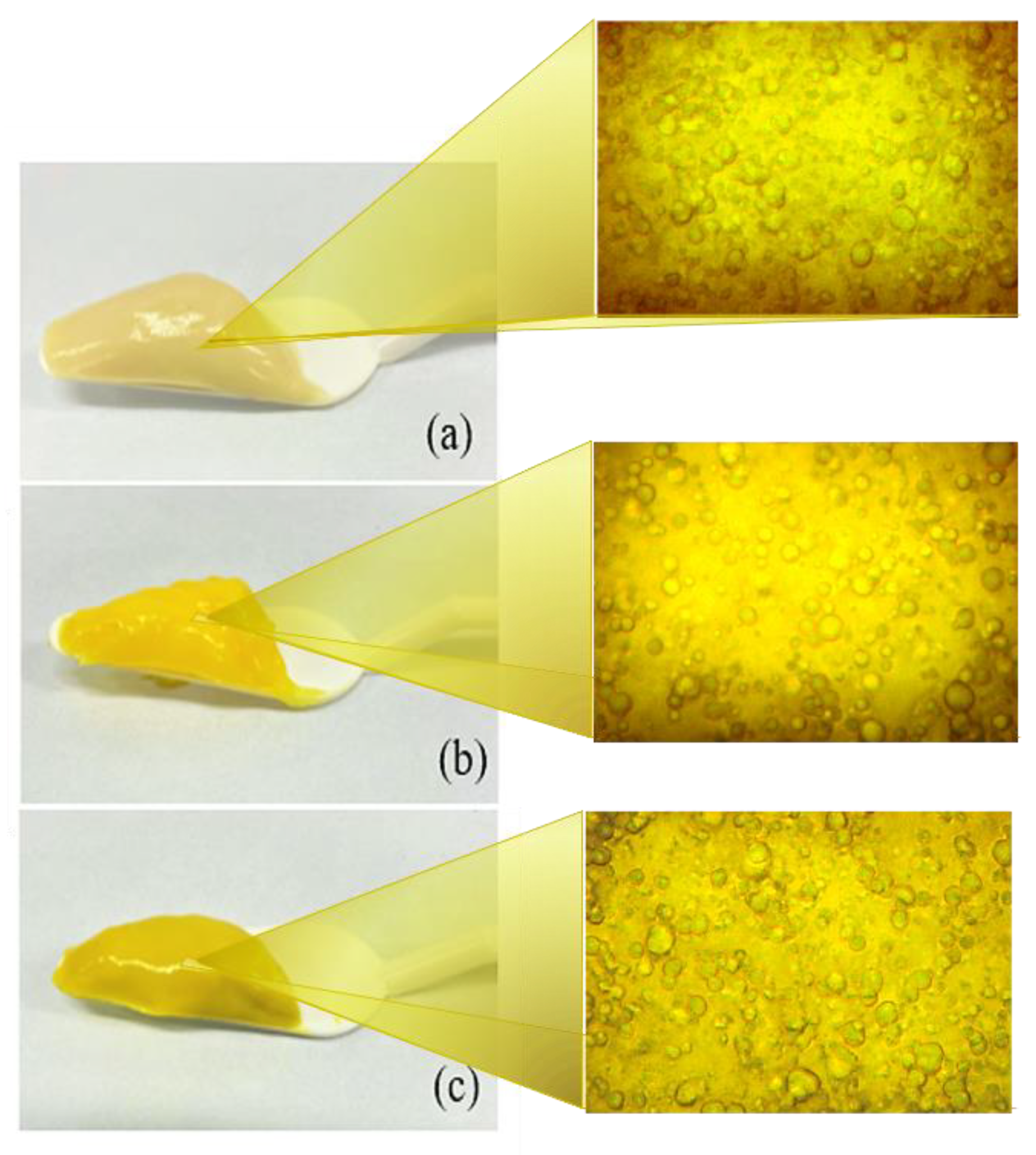
2.1. Preparation and Microarchitecture of Emulgel Systems
2.2. Physicochemical Stability Study
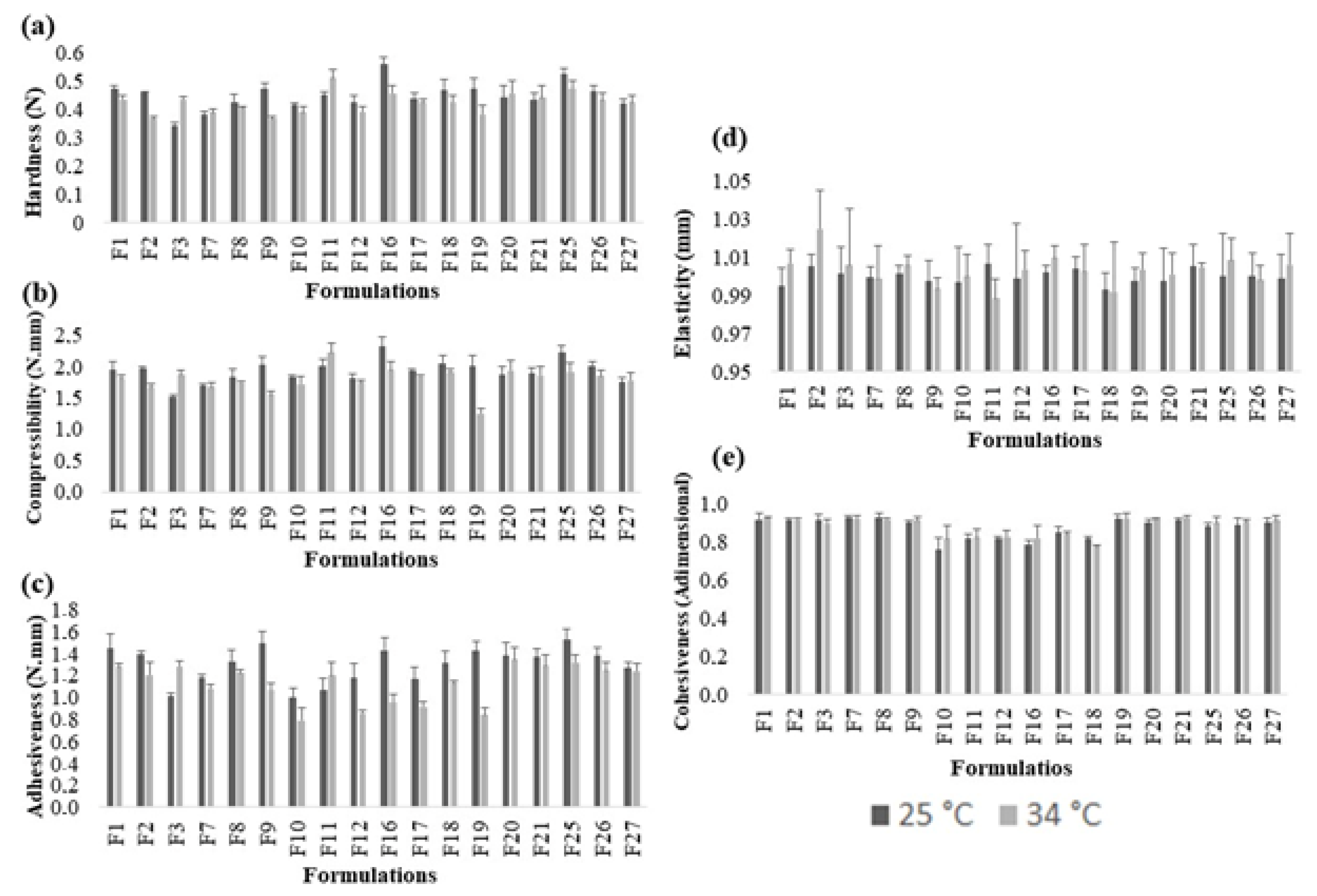
2.3. Texture Profile Analysis (TPA)
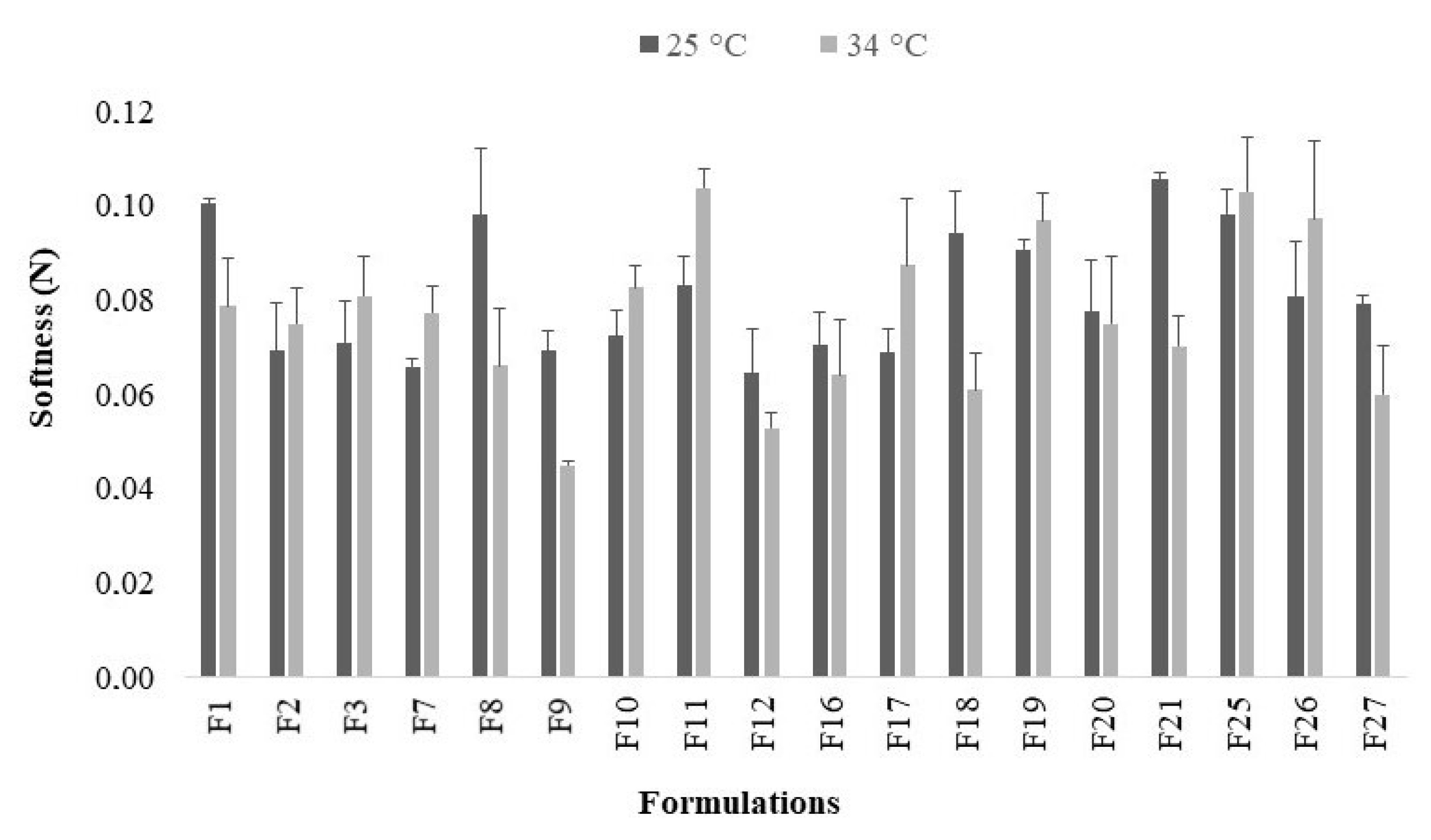
2.4. Softness
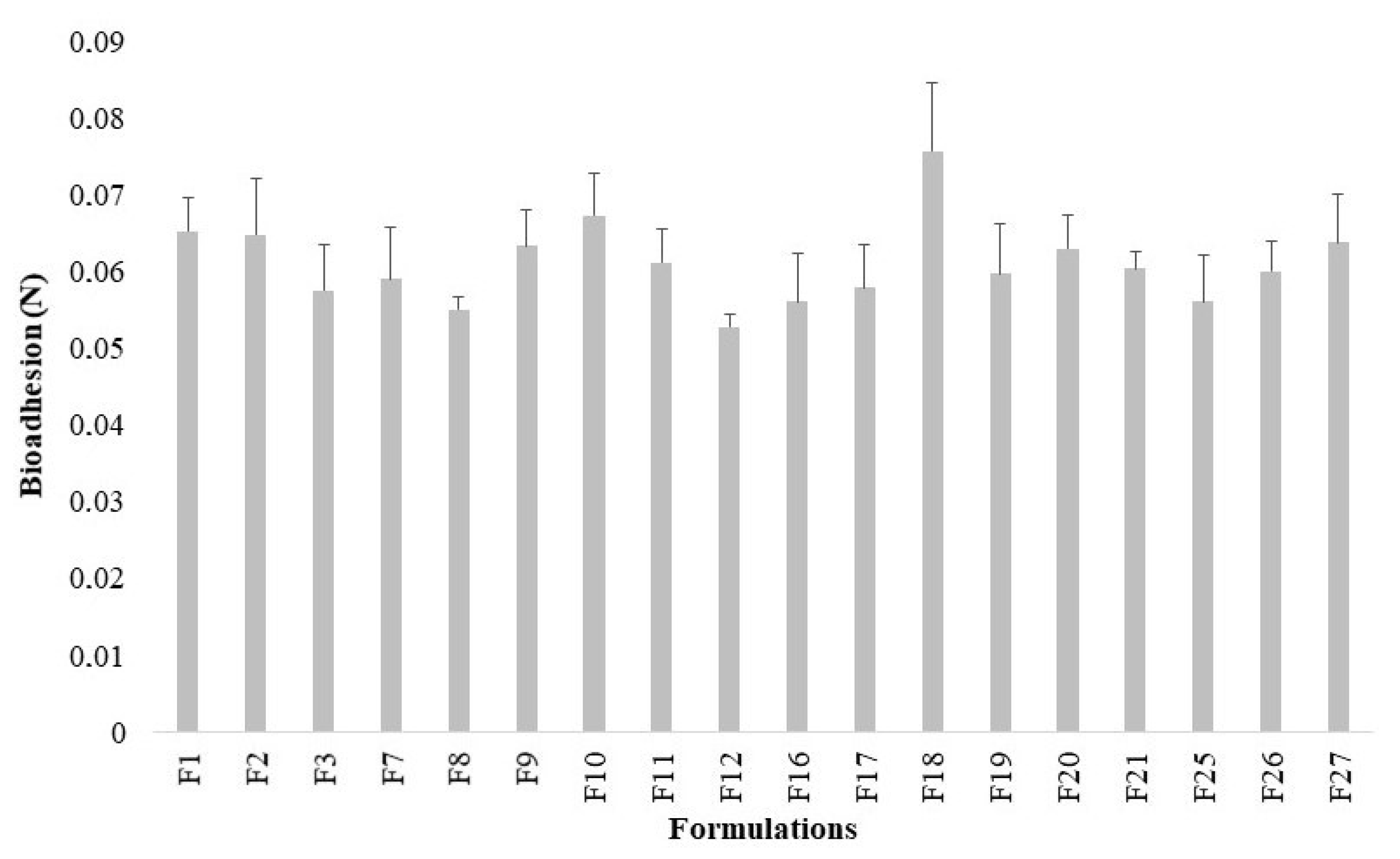
2.5. Evaluation of Bioadhesive Properties
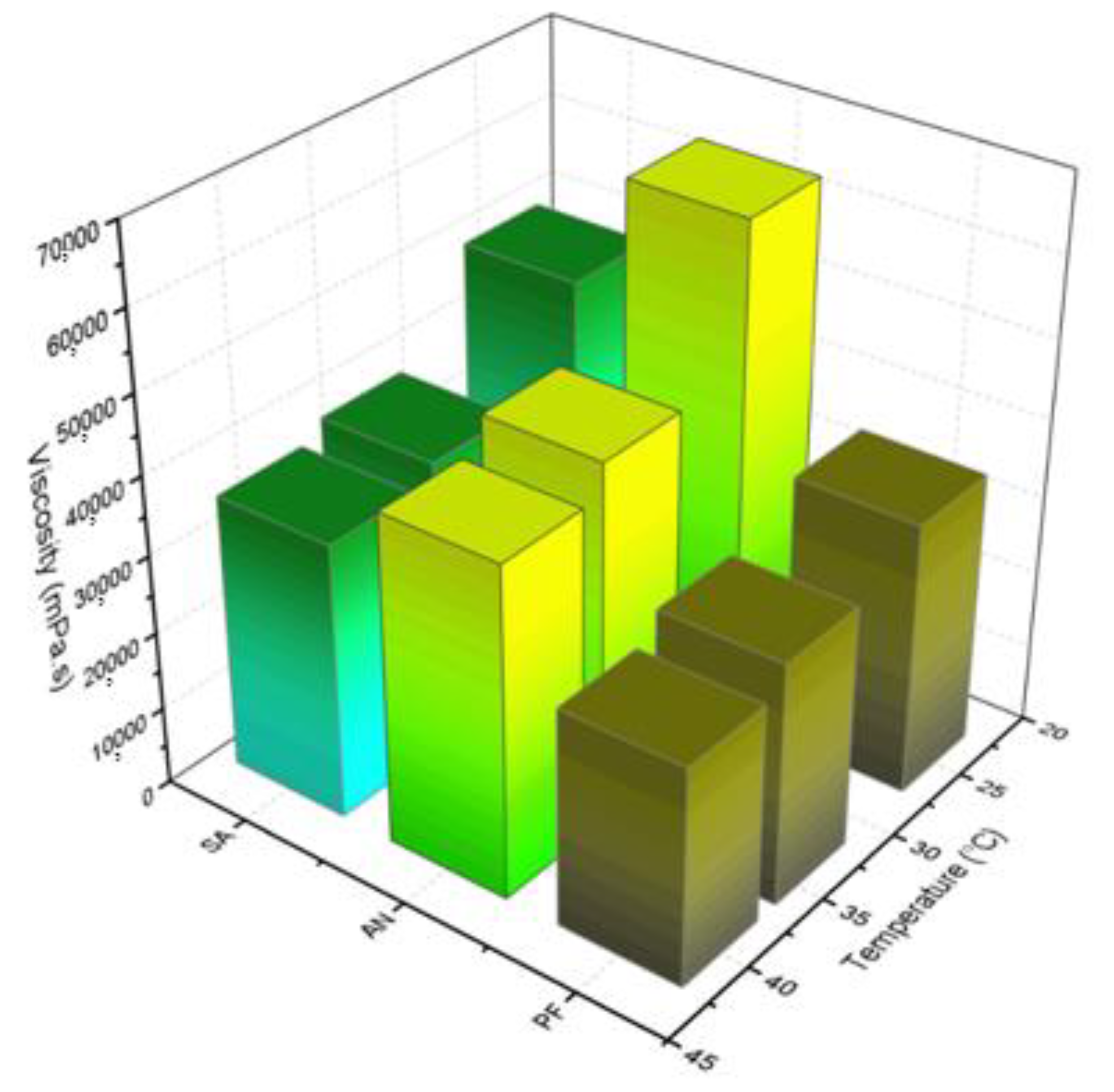
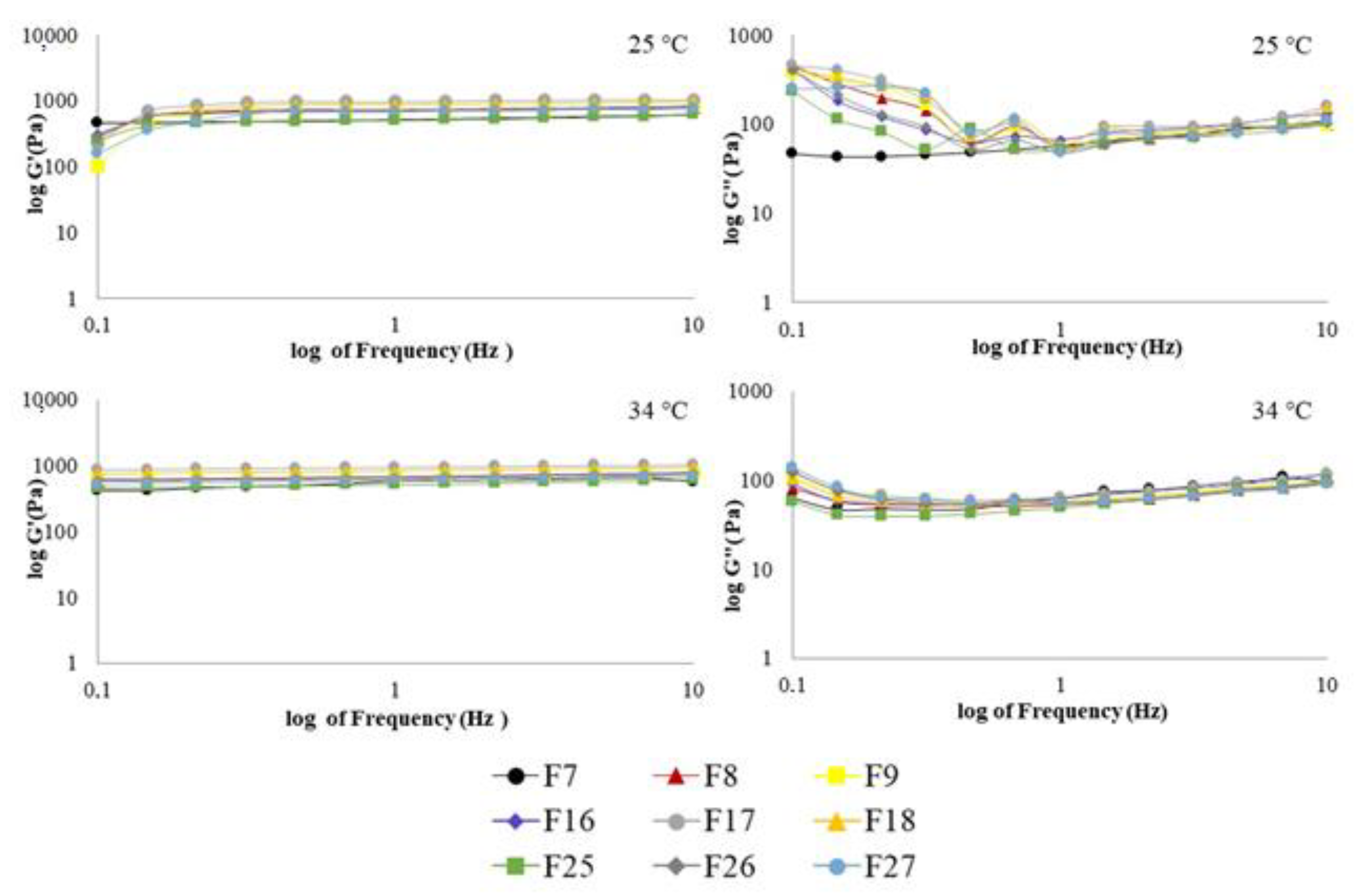
2.6. Rheological Analysis
2.6.1. Continuous Shear
2.6.2. Viscoelastic Measurements
2.7. Physicochemical Properties Correlation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Emulgel Systems
4.3. Physicochemical Stability Study
4.4. Viscosity Study
4.5. Texture Profile Analyses (TPA)
4.6. Determination of Softness
4.7. Ex Vivo Evaluation of Bioadhesive Strength
4.8. Rheometry
4.8.1. Continuous Shear (Flow)
4.8.2. Oscillatory Rheometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.B.; Ferreira, S.B.D.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; Moço, T.D.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Rheological, mucoadhesive and textural properties of thermoresponsive polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2016, 55, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.D.S.; da Silva, J.B.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Linear correlation between rheological, mechanical and mucoadhesive properties of polycarbophil polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 68, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef]
- Uddin, A.; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.; Giri, T.K.; Tripathi, D. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Hughes, N.E.; Marangoni, A.G.; Wright, A.J.; Rogers, M.; Rush, J.W. Potential food applications of edible oil organogels. Trends Food Sci. Technol. 2009, 20, 470–480. [Google Scholar] [CrossRef]
- de Francisco, L.M.B.; Rosseto, H.C.; Toledo, L.D.A.S.D.; dos Santos, R.S.; Ferreira, S.B.D.S.; Bruschi, M.L. Organogel composed of poloxamer 188 and passion fruit oil: Sol-gel transition, rheology, and mechanical properties. J. Mol. Liq. 2019, 289, 111170. [Google Scholar] [CrossRef]
- Singla, V.; Saini, S.; Joshi, B.; Rana, A.C. Emulgel: A new platform for topical drug delivery. Int. J. Pharma Bio Sci. 2012, 3, 485–498. [Google Scholar]
- Vintiloiu, A.; Leroux, J.-C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Akomeah, F.K.; Martin, G.P.; Brown, M.B. Variability in Human Skin Permeability In Vitro: Comparing Penetrants with Different Physicochemical Properties. J. Pharm. Sci. 2007, 96, 824–834. [Google Scholar] [CrossRef]
- Wagner, H.; Kostka, K.-H.; Lehr, C.-M.; Schaefer, U.F. Interrelation of permeation and penetration parameters obtained from in vitro experiments with human skin and skin equivalents. J. Control. Release 2001, 75, 283–295. [Google Scholar] [CrossRef]
- Goebel, K.; Sato, M.E.O.; De Souza, D.F.; Murakami, F.S.; Andreazza, I.F. In vitro release of diclofenac diethylamine from gels: Evaluation of generic semisolid drug products in Brazil. Braz. J. Pharm. Sci. 2013, 49, 211–219. [Google Scholar] [CrossRef][Green Version]
- Tamburic, S.; Craig, D.Q.M. Rheological Evaluation of Polyacrylic Acid Hydrogels. Pharm. Pharmacol. Commun. 1995, 1, 107–109. [Google Scholar] [CrossRef]
- Gómez-Carracedo, A.; Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Concheiro, A. Glass transitions and viscoelastic properties of Carbopol® and Noveon® compacts. Int. J. Pharm. 2004, 274, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Chawla, V.; Saraf, S. Rheological studies on solid lipid nanoparticle based carbopol gels of aceclofenac. Colloids Surf. B Biointerfaces 2012, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Suljaković, S.; Skalko-Basnet, N.; Kristl, A. Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. Eur. J. Pharm. Biopharm. 2014, 87, 40–46. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2012, 39, 1750–1757. [Google Scholar] [CrossRef]
- Suhail, M.; Wu, P.-C.; Minhas, M.U. Using Carbomer-Based Hydrogels for Control the Release Rate of Diclofenac Sodium: Preparation and In Vitro Evaluation. Pharmaceuticals 2020, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Hosmani, A.H. Carbopol and Its Pharmaceutical Significance: A Review. Pharmainfo.net-Pharmaceutical Information for You. 2006. Available online: www.pharmainfo.net/exclusive/reviews/carbopol_and_its_pharmaceutical_significance:a_review (accessed on 9 April 2021).
- Pereira, R.R.D.A.; Godoy, J.S.R.; Svidzinski, T.I.S.; Bruschi, M.L. Preparation and Characterization of Mucoadhesive Thermoresponsive Systems Containing Propolis for the Treatment of Vulvovaginal Candidiasis. J. Pharm. Sci. 2013, 102, 1222–1234. [Google Scholar] [CrossRef]
- Mendonça, A.P.; Ferraz, I.D.K. Óleo de andiroba: Processo tradicional da extração, uso e aspectos sociais no estado do Amazonas, Brasil. Acta Amaz. 2007, 37, 353–364. [Google Scholar] [CrossRef]
- Cela, E.V.S.S.; da Rocha, M.d.B.; Gomes, T.M.; Chia, C.Y.; Alves, C.F. Clinical evaluation of the effectiveness of andiroba oil in burns caused by hair removal with intense pulsed light: A prospective, comparative and double-blind study. Surg. Cosmet. Dermatol. 2012, 4, 248–251. [Google Scholar]
- Souza, V.M.; Junior, D.A. Ativos Dermatologicos: Dermocosméticos e Nutracêuticos, 9th ed.; RBE Editor: São Paulo, Brazil, 2016. [Google Scholar]
- Delfine, F.d.T. Microencapsulação de Óleo da Semente de Maracujá Através da Secagem por Atomização. 2016. Available online: http://www.repositorio.unicamp.br/handle/REPOSIP/305405 (accessed on 19 April 2021).
- Morais, G.G. Development and Evaluation of the Stability of O/W Emulsion with Liquid Crystals Added of Xantine to Treatment of the Gynoid Hydrolipodystrophy (Cellulite); Faculdade de Ciências Farmacêuticas de Ribeirão Preto: Ribeirão Preto, Brazil, 2006. [Google Scholar] [CrossRef]
- Cordeiro, L.B.A. Desenvolvimento Farmacotécnico e Estudo de Estabilidade de Emulsões à Base de Óleo de Semente de Maracujá Para Prevenção de Feridas; Universidade Federal Fluminense: Niterói, Brazil, 2017. [Google Scholar]
- Rosani, L. Desenvolvimento e Estudo da Estabilidade de Nanoemulsões do Tipo óleo em Água com Óleos Vegetais; Universidade Federal de São Carlos: São Carlos, Brazil, 2011. [Google Scholar]
- dos Santos Marques, A.P. Óleo de Amêndoa do Barueiro (Dipteryxalata Vog.): Obtenção, Caracterização e Uso em Emulsões. 2014. Available online: https://ppgcal.iq.ufrj.br/wp-content/uploads/2017/06/Adriana_Marques.pdf (accessed on 19 April 2021).
- Dos Santos, R.S.; Rosseto, H.C.; Da Silva, J.B.; Vecchi, C.F.; Caetano, W.; Bruschi, M.L. The effect of carbomer 934P and different vegetable oils on physical stability, mechanical and rheological properties of emulsion-based systems containing propolis. J. Mol. Liq. 2020, 307, 112969. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Moustafa, N.; Asran, A.E.M.A.A.; Mowafy, L. Cypermethrin-induced histopathological, ultrastructural and biochemical changes in liver of albino rats: The protective role of propolis and curcumin. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 160–173. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Thabet, H.Z.; Abdel-Hafez, A.M. Toxicity of Monosodium Glutamate on Male Rat Reproductive System and Effect of Curcumin and Propolis Co-Administeration. Egypt. J. Forensic Sci. Appl. Toxicol. 2017, 17, 129–146. [Google Scholar] [CrossRef][Green Version]
- Bruschi, M.L.; Klein, T.; Lopes, R.S.; Franco, S.L.; Gremião, M.P.D. Contribuição ao protocolo de controle de qualidade da própolis e de seus extratos. Rev. Cienc. Farm. 2002, 23, 289–306. [Google Scholar]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Burdock, G. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Cabral, I.S.R.; Oldoni, T.; De Alencar, S.M.; Rosalen, P.L.; Ikegaki, M. The correlation between the phenolic composition and biological activities of two varieties of Brazilian propolis (G6 and G12). Braz. J. Pharm. Sci. 2012, 48, 557–564. [Google Scholar] [CrossRef]
- De Francisco, L.M.B.; Cerquetani, J.A.; Bruschi, M.L. Development and characterization of gelatin and ethylcellulose microparticles designed as platforms to delivery fluoride. Drug Dev. Ind. Pharm. 2013, 39, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrazek, A.; Mahmoud, S.; ElMoneim, A.A. The comparison between curcumin and propolis against sepsis-induced oxidative stress, inflammation, and apoptosis in kidney of adult male rat. Futur. J. Pharm. Sci. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.; de Freitas, O.; Lara, E.H. Semisolid Systems Containing Propolis for the Treatment of Periodontal Disease: In Vitro Release Kinetics, Syringeability, Rheological, Textural, and Mucoadhesive Properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ahmad, S.; Mallick, N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef]
- Sueth-Santiago, V.; Mendes-Silva, G.P.; Decoté-Ricardo, D.; De Lima, M.E.F. Curcumin, the golden powder from turmeric: Insights into chemical and biological activities. Quím. Nova 2015, 38, 538–552. [Google Scholar] [CrossRef]
- Goel, A.; Aggarwal, B.B. Curcumin, the Golden Spice from Indian Saffron, Is a Chemosensitizer and Radiosensitizer for Tumors and Chemoprotector and Radioprotector for Normal Organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Ji, H.-F.; Shen, L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol. Sci. 2014, 35, 265–266. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Jun, H. Diffusion studies of methotrexate in Carbopol and Poloxamer gels. Int. J. Pharm. 1998, 160, 1–9. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Lobo, J.M.S. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Bhattacharjee, K. Importance of Surface Energy in Nanoemulsion. In Nanoemulsions: Properties, Fabrications and Applications; IntechOpen Limited: London, UK, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Khadem, B.; Sheibat-Othman, N. Modeling droplets swelling and escape in double emulsions using population balance equations. Chem. Eng. J. 2020, 382, 122824. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.; Moraes, I.C.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, S.S.; da Conceição, L.R.V.; Costa, C.; Zamian, J.R.; Filho, G.N.D.R. Oxidative stability of biodiesels produced from vegetable oils having different degrees of unsaturation. Energy Convers. Manag. 2013, 74, 293–298. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterization and investigation of interactions between formulations components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.; Brown, A.F.; Coulter, A.W.; McClelland, C.; Irwin, C.R. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J. Control. Release 2000, 67, 357–368. [Google Scholar] [CrossRef]
- Da Silva, J.B.; Ferreira, S.B.D.S.; Reis, A.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Junqueira, M.V.; Borghi-Pangoni, F.B.; Ferreira, S.B.D.S.; Bruschi, M.L. Evaluation of the methylene blue addition in binary polymeric systems composed by poloxamer 407 and Carbopol 934P using quality by design: Rheological, textural, and mucoadhesive analysis. Drug Dev. Ind. Pharm. 2016, 42, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; da Silva, J.B.; Junqueira, M.V.; Borghi-Pangoni, F.B.; Gomes, R.G.; Bruschi, M.L. The importance of the relationship between mechanical analyses and rheometry of mucoadhesive thermoresponsive polymeric materials for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 74, 142–153. [Google Scholar] [CrossRef]
- Demiröz, F.N.T.; Acartürk, F.; Erdoğan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef]
- Brasil, Farmacopeia Brasileira. 2019. Available online: http://portal.anvisa.gov.br (accessed on 20 April 2021).
- FDA. Guidance for Industry: Q1A(R2) Stability Testing of New Drug Substances and Products; U.S. Department of Health and Human Services, Food and Drug Administration: Washinghton, DC, USA, 2003; pp. 1–22. [Google Scholar]
- Callens, C.; Ceulemans, J.; Ludwig, A.; Foreman, P.; Remon, J. Rheological study on mucoadhesivity of some nasal powder formulations. Eur. J. Pharm. Biopharm. 2003, 55, 323–328. [Google Scholar] [CrossRef]
- De Francisco, L.; Pinto, D.; Rosseto, H.; Toledo, L.; dos Santos, R.S.; Tobaldini-Valério, F.; Svidzinski, T.; Bruschi, M.; Sarmento, B.; Oliveira, B.; et al. Evaluation of radical scavenging activity, intestinal cell viability and antifungal activity of Brazilian propolis by-product. Food Res. Int. 2018, 105, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, H.C.; Toledo, L.D.A.S.D.; de Francisco, L.M.B.; Esposito, E.; Lim, Y.; Valacchi, G.; Cortesi, R.; Bruschi, M.L. Nanostructured lipid systems modified with waste material of propolis for wound healing: Design, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Tunin, L.M.; Borghi, F.B.; Nogueira, A.C.; Higachi, L.; Barbosa, D.S.; Baesso, M.L.; Hernandes, L.; Diniz, A.; Truiti, M.D.C.T. Employing photoacoustic spectroscopy in the evaluation of the skin permeation profile of emulsion containing antioxidant phenolic-rich extract of Melochia arenosa. Pharm. Biol. 2015, 54, 1–7. [Google Scholar] [CrossRef]
- Madsen, F. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- De Francisco, L.M.B.; Pinto, D.; Rosseto, H.C.; De Toledo, L.de.A.S.; Dos Santos, R.S.; Costa, P.; Rodrigues, F.; Oliveira, M.B.P.P.; Sarmento, B.; Bruschi, M.L. Correction: Development of a microparticulate system containing Brazilian propolis by-product and gelatine for ascorbic acid delivery: Evaluation of intestinal cell viability and radical scavenging activity. Food Funct. 2018, 9, 4194–4206. [Google Scholar] [CrossRef]
- Toledo, L.D.A.S.D.; Bavato, M.I.; Rosseto, H.C.; Cortesi, R.; Bruschi, M.L. Pharmaceutical films made from the waste material from the preparation of propolis extracts: Development and characterization. Braz. J. Pharm. Sci. 2015, 51, 847–859. [Google Scholar] [CrossRef]
- Da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- ANVISA-Brazilian Health Regulatory Agency. Cosmetic Stability Guide; ANVISA: Brasilia, Brazil, 2004. [Google Scholar]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Borghi-Pangoni, F.B.; Junqueira, M.V.; Ferreira, S.B.D.S.; Silva, L.L.; Rabello, B.R.; Caetano, W.; Diniz, A.; Bruschi, M.L. Screening and In Vitro Evaluation of Mucoadhesive Thermoresponsive System Containing Methylene Blue for Local Photodynamic Therapy of Colorectal Cancer. Pharm. Res. 2015, 33, 776–791. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Lara, E.H.G.; Martins, C.H.G.; Vinholis, A.H.C.; Casemiro, L.A.; Panzeri, H.; Gremião, M.P.D. Preparation and Antimicrobial Activityof Gelatin Microparticles Containing Propolis Against Oral Pathogens. Drug Dev. Ind. Pharm. 2006, 32, 229–238. [Google Scholar] [CrossRef]
- Yadav, D.N.; Chhikara, N.; Anand, T.; Sharma, M.; Singh, A.K. Rheological quality of pearl millet porridge as affected by grits size. J. Food Sci. Technol. 2014, 51, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Catalán, A.; Palou, L.; Taberner, V.; Grimal, A.; Argente-Sanchis, M.; Pérez-Gago, M. Hydroxypropyl Methylcellulose-Based Edible Coatings Formulated with Antifungal Food Additives to Reduce Alternaria Black Spot and Maintain Postharvest Quality of Cold-Stored ‘Rojo Brillante’ Persimmons. Agronomy 2021, 11, 757. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological Characterization of Topical Carbomer Gels Neutralized to Different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Hemphil, T.; Campos, W.; Pilehvari, A. Yield-power law model more accurately predicts mud rheology. Oil Gas J. 1993, 91, 45–50. [Google Scholar]
- Folzer, E.; Gonzalez, D.; Singh, R.S.; Derendorf, H. Comparison of skin permeability for three diclofenac topical formulations: An in vitro study. Die Pharm. 2014, 69, 27–31. [Google Scholar]
- Ferrari, M.; Oliveira, M.S.C.; Nakano, A.K.; Rocha-Filho, P.A. Determinação do fator de proteção solar (FPS) in vitro e in vivo de emulsões com óleo de andiroba (Carapa guianensis). Rev. Bras. Farm. 2007, 17, 626–630. [Google Scholar] [CrossRef]


| Formulations | K (Pa.s) | n (Dimensionless) | ||
|---|---|---|---|---|
| 25 °C | 34 °C | 25 °C | 34 °C | |
| F1 | 68.45 ± 0.35 | 67.65 ± 0.29 | 0.35 ± 0.00 | 0.34 ± 0.00 |
| F2 | 83.06 ± 6.39 | 82.06 ± 6.74 | 0.33 ± 0.00 | 0.33 ± 0.00 |
| F3 | 60.78 ± 0.96 | 60.78 ± 0.96 | 0.34 ± 0.00 | 0.34 ± 0.00 |
| F7 | 71.39 ± 0.19 | 71.39 ± 0.19 | 0.33 ± 0.00 | 0.33 ± 0.00 |
| F8 | 75.17 ± 4.22 | 70.22 ± 1.06 | 0.33 ± 0.00 | 0.33 ± 0.00 |
| F9 | 86.72 ± 7.06 | 69.71 ± 0.99 | 0.28 ± 0.02 | 0.33 ± 0.00 |
| F10 | 191.65 ± 1.25 | 191.65 ± 1.25 | 0.16 ± 0.04 | 0.16 ± 0.04 |
| F11 | 67.84 ± 1.06 | 67.84 ± 1.06 | 0.35 ± 0.00 | 0.35 ± 0.00 |
| F12 | 71.55 ± 4.89 | 61.27 ± 2.50 | 0.36 ± 0.01 | 0.35 ± 0.04 |
| F16 | 107.07 ± 2.53 | 110.67 ± 4.51 | 0.29 ± 0.00 | 0.28 ± 0.00 |
| F17 | 138.77 ± 6.29 | 128.00 ± 7.10 | 0.25 ± 0.00 | 0.25 ± 0.00 |
| F18 | 103.00 ± 2.08 | 77.72 ± 6.05 | 0.28 ± 0.00 | 0.31 ± 0.01 |
| F19 | 71.37 ± 1.44 | 74.53 ± 0.17 | 0.34 ± 0.00 | 0.32 ± 0.00 |
| F20 | 85.64 ± 6.31 | 80.71 ± 1.89 | 0.32 ± 0.01 | 0.32 ± 0.00 |
| F21 | 66.76 ± 2.46 | 62.64 ± 2.73 | 0.34 ± 0.00 | 0.35 ± 0.00 |
| F25 | 66.27 ± 1.67 | 65.78 ± 0.54 | 0.34 ± 0.01 | 0.33 ± 0.00 |
| F26 | 75.47 ± 2.22 | 75.85 ± 6.78 | 0.33 ± 0.00 | 0.32 ± 0.01 |
| F27 | 73.35 ± 2.14 | 54.65 ± 0.77 | 0.33 ± 0.00 | 0.35 ± 0.00 |
| Correlation Parameter | R2-Value |
|---|---|
| Droplet size/Hardness | 0.0570 |
| Droplet size/Compressibility | 0.0932 |
| Droplet size/Tan δ | 0.2954 |
| Droplet size/Hysteresis area | 0.0170 |
| Hardness/Compressibility | 0.9617 |
| K */Hardness | 0.0017 |
| K */Compressibility | 0.0102 |
| Independent Variables | Levels | ||
|---|---|---|---|
| Low (−1) | Central (0) | High (+1) | |
| X1 = Polymer type | C934P | C974P | PC |
| X2 = Oil type | PF | SA | AN |
| X3 = Bioctive agent | PE | CUR | PE+CUR |
| Standard run (formulations) | X1 | X2 | X3 |
| F1 | −1 | −1 | −1 |
| F2 | 0 | −1 | −1 |
| F3 | +1 | −1 | −1 |
| F4 | −1 | 0 | −1 |
| F5 | 0 | 0 | −1 |
| F6 | +1 | 0 | −1 |
| F7 | −1 | +1 | −1 |
| F8 | 0 | +1 | −1 |
| F9 | +1 | +1 | −1 |
| F10 | −1 | −1 | 0 |
| F11 | 0 | −1 | 0 |
| F12 | +1 | −1 | 0 |
| F13 | −1 | 0 | 0 |
| F14 | 0 | 0 | 0 |
| F15 | +1 | 0 | 0 |
| F16 | −1 | +1 | 0 |
| F17 | 0 | +1 | 0 |
| F18 | +1 | +1 | 0 |
| F19 | −1 | −1 | +1 |
| F20 | 0 | −1 | +1 |
| F21 | +1 | −1 | +1 |
| F22 | −1 | 0 | +1 |
| F23 | 0 | 0 | +1 |
| F24 | +1 | 0 | +1 |
| F25 | −1 | +1 | +1 |
| F26 | 0 | +1 | +1 |
| F27 | +1 | +1 | +1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said dos Santos, R.; Bassi da Silva, J.; Rosseto, H.C.; Vecchi, C.F.; Campanholi, K.d.S.S.; Caetano, W.; Bruschi, M.L. Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties. Gels 2021, 7, 120. https://doi.org/10.3390/gels7030120
Said dos Santos R, Bassi da Silva J, Rosseto HC, Vecchi CF, Campanholi KdSS, Caetano W, Bruschi ML. Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties. Gels. 2021; 7(3):120. https://doi.org/10.3390/gels7030120
Chicago/Turabian StyleSaid dos Santos, Rafaela, Jéssica Bassi da Silva, Hélen Cássia Rosseto, Camila Felix Vecchi, Katieli da Silva Souza Campanholi, Wilker Caetano, and Marcos Luciano Bruschi. 2021. "Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties" Gels 7, no. 3: 120. https://doi.org/10.3390/gels7030120
APA StyleSaid dos Santos, R., Bassi da Silva, J., Rosseto, H. C., Vecchi, C. F., Campanholi, K. d. S. S., Caetano, W., & Bruschi, M. L. (2021). Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties. Gels, 7(3), 120. https://doi.org/10.3390/gels7030120






