The Effect of Different Additives on the Hydration and Gelation Properties of Composite Dental Gypsum
Abstract
1. Introduction
2. Results
2.1. Effect of Accelerant on the Gelation Characteristics of Dental Gypsum
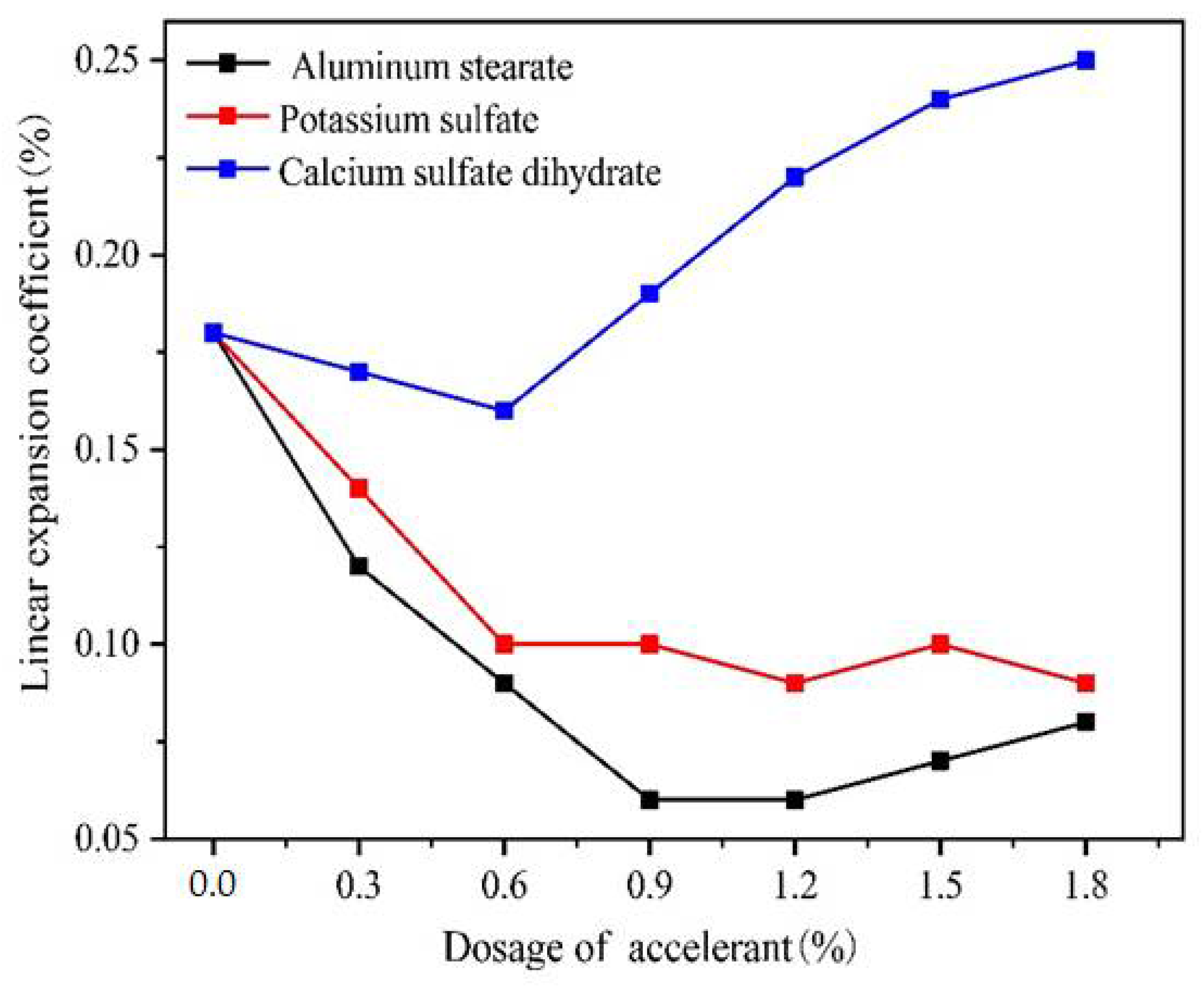
2.2. Linear Expansion Coefficient
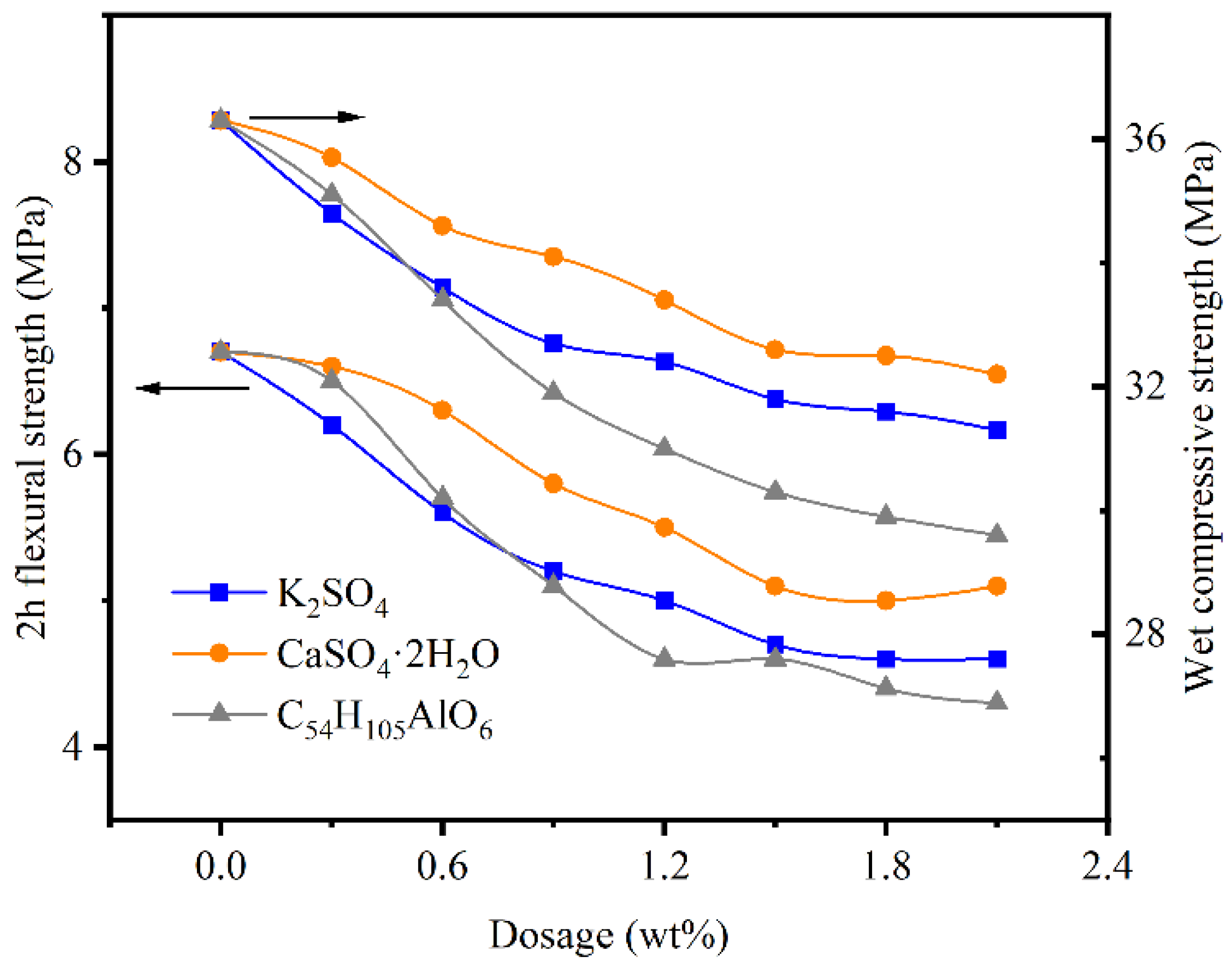
2.3. Mechanical Properties
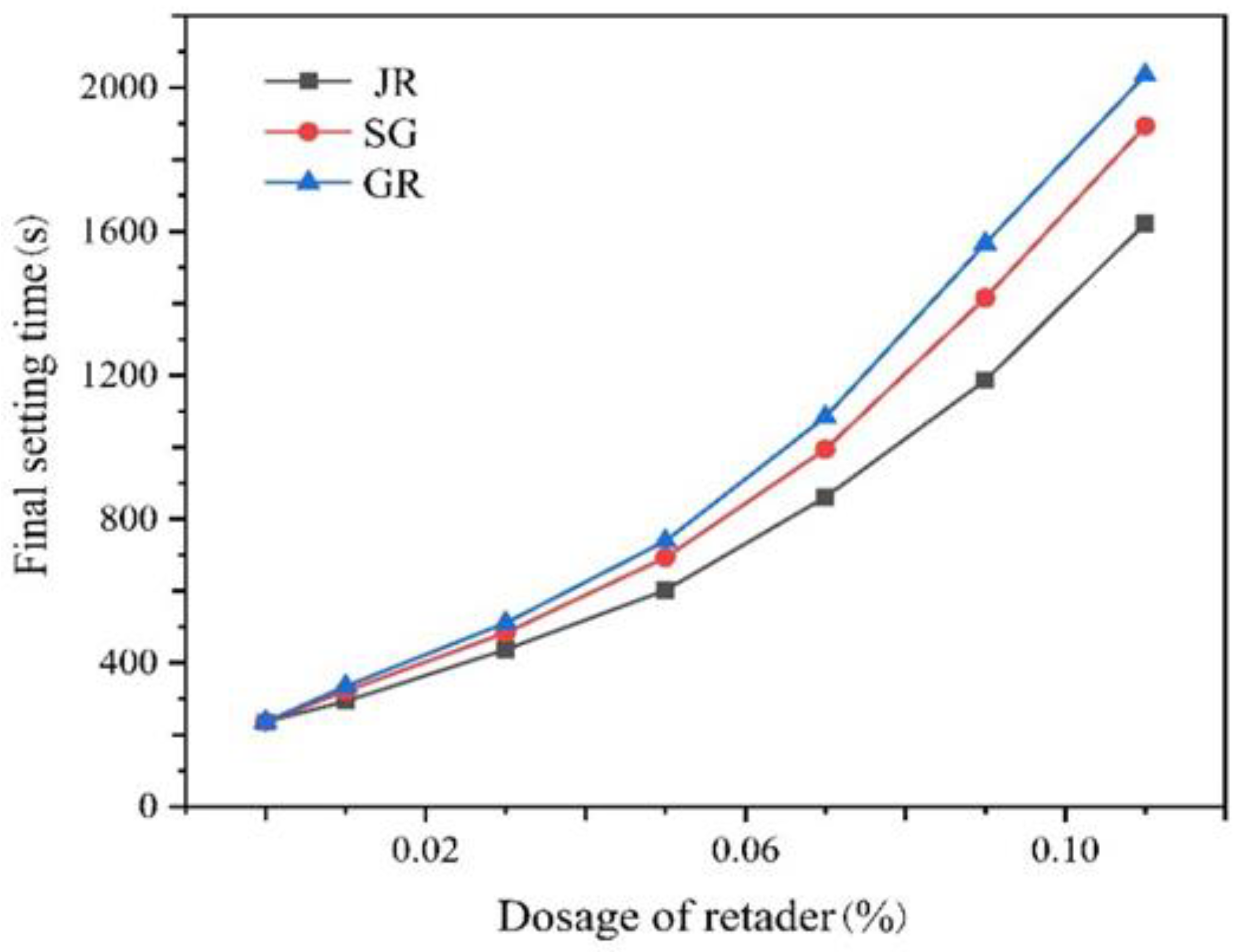
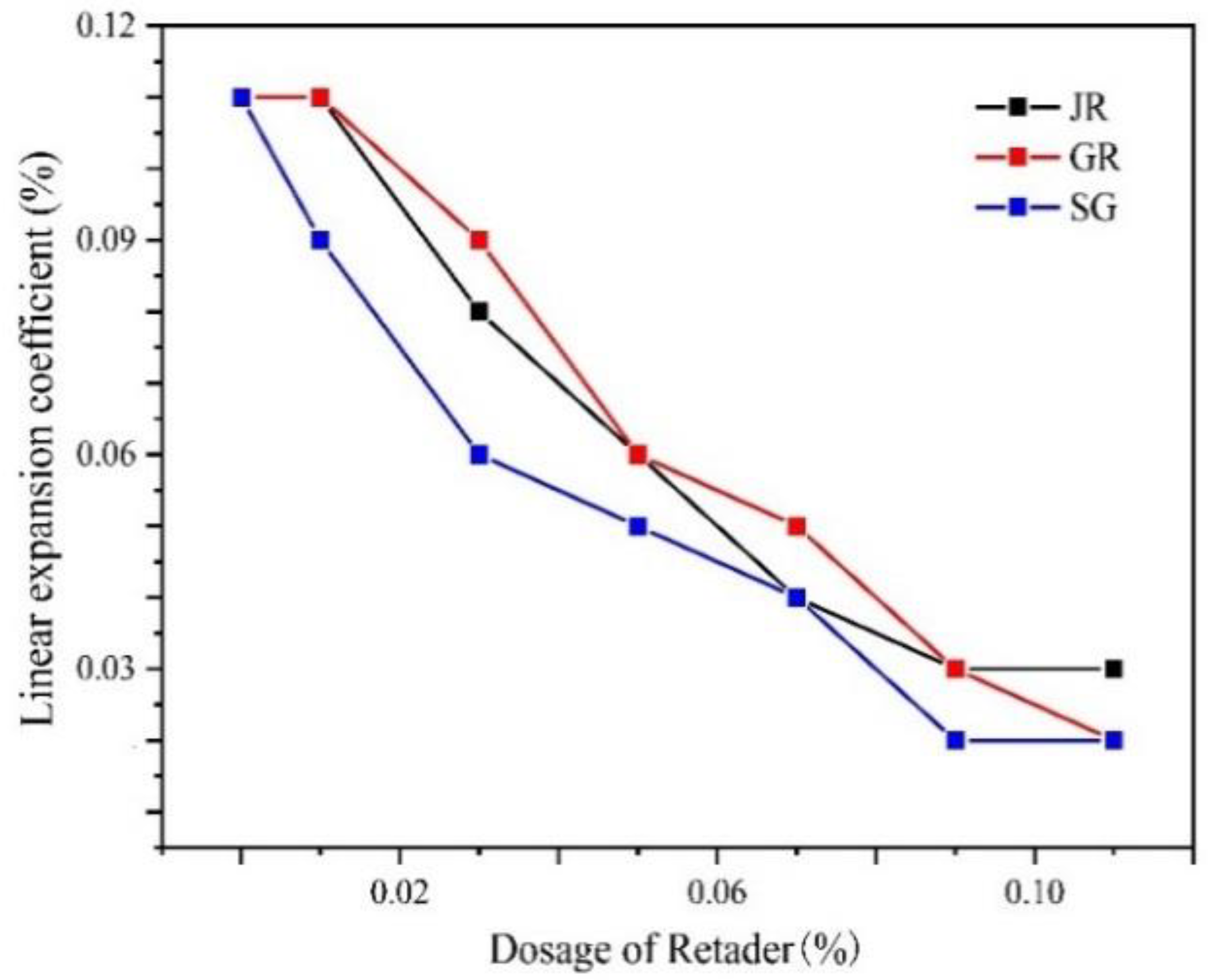
2.4. Effect of the Retarder on Linear Expansion Coefficient
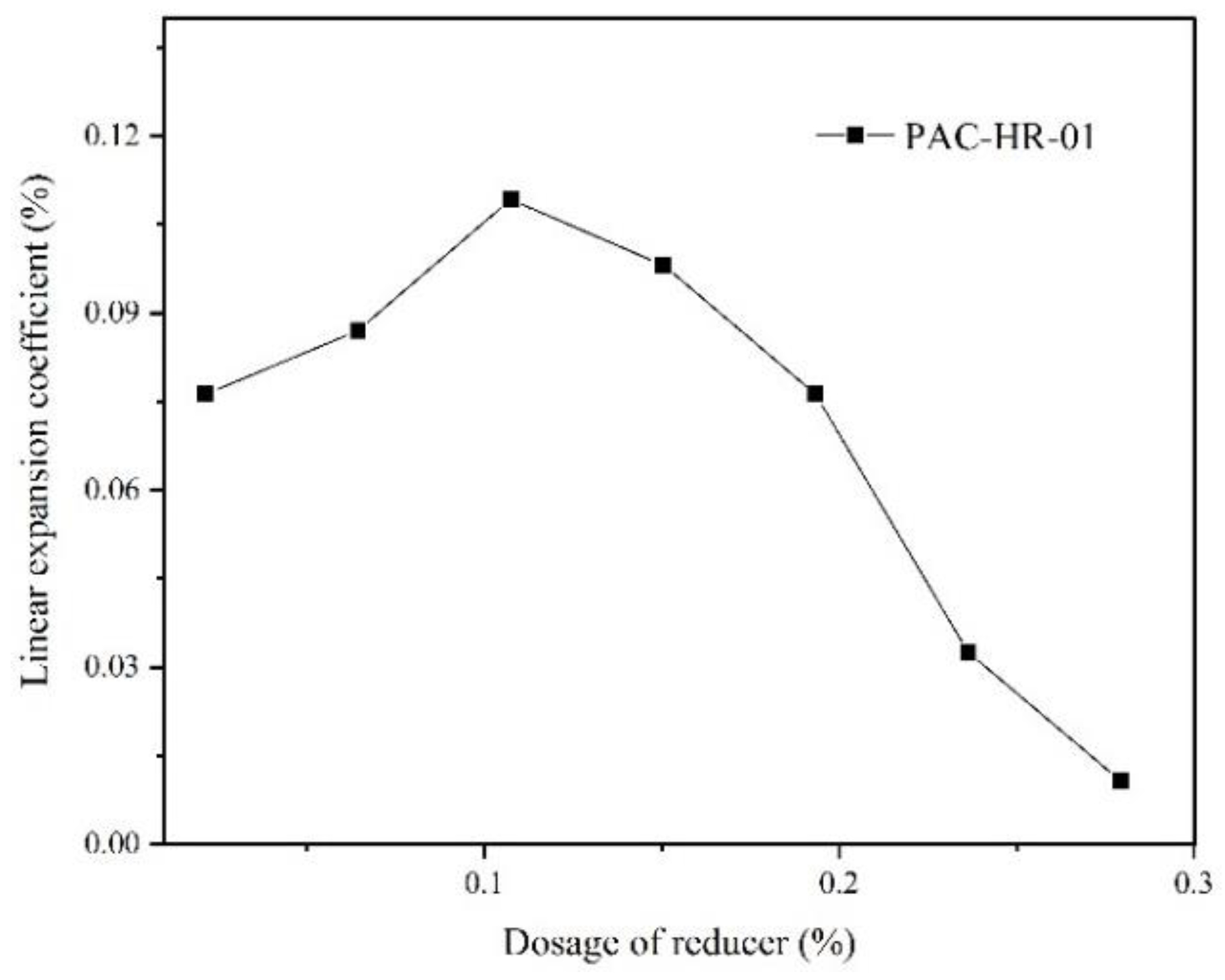
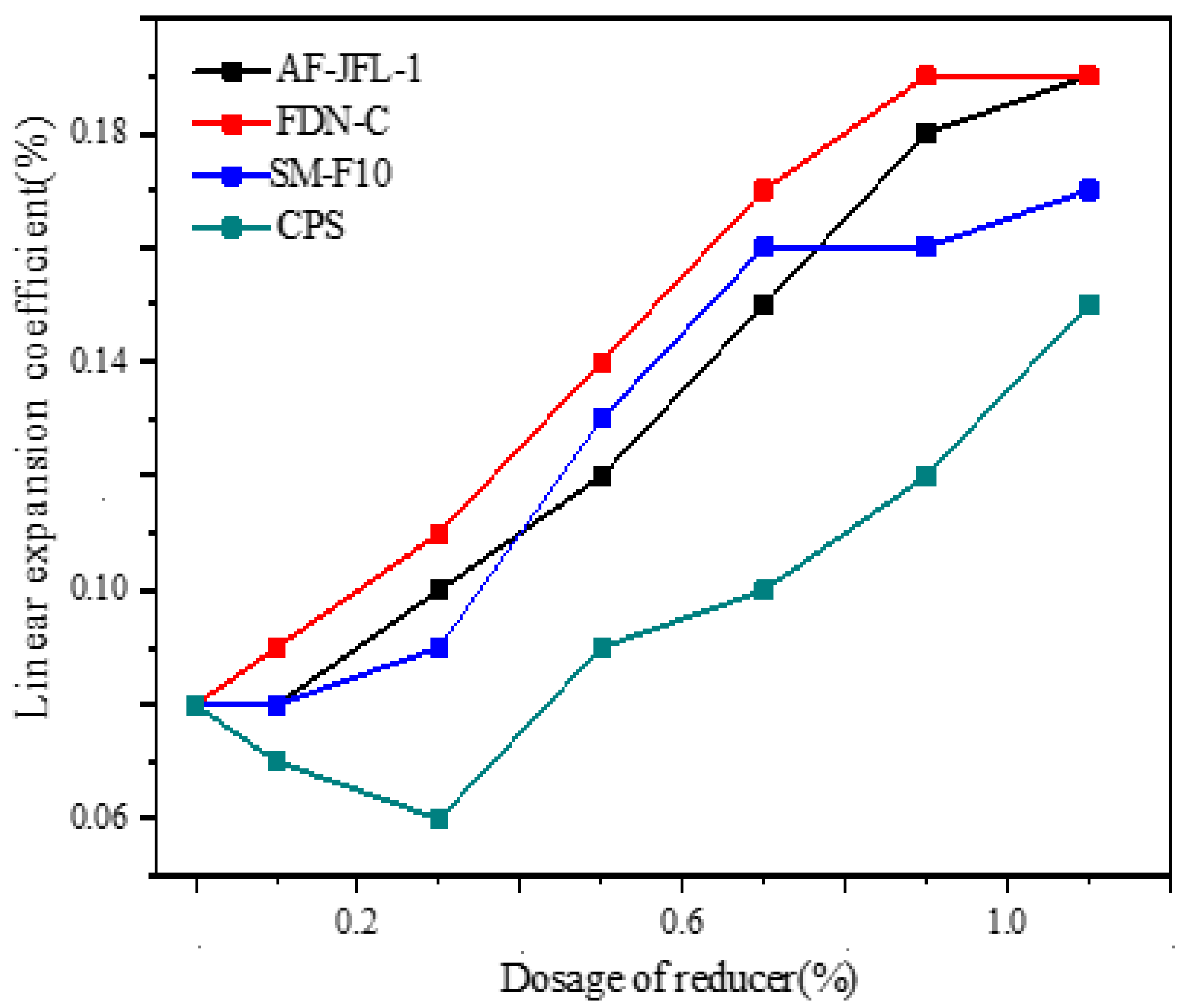
2.5. Effect of Water Reducer on Linear Expansion Coefficient
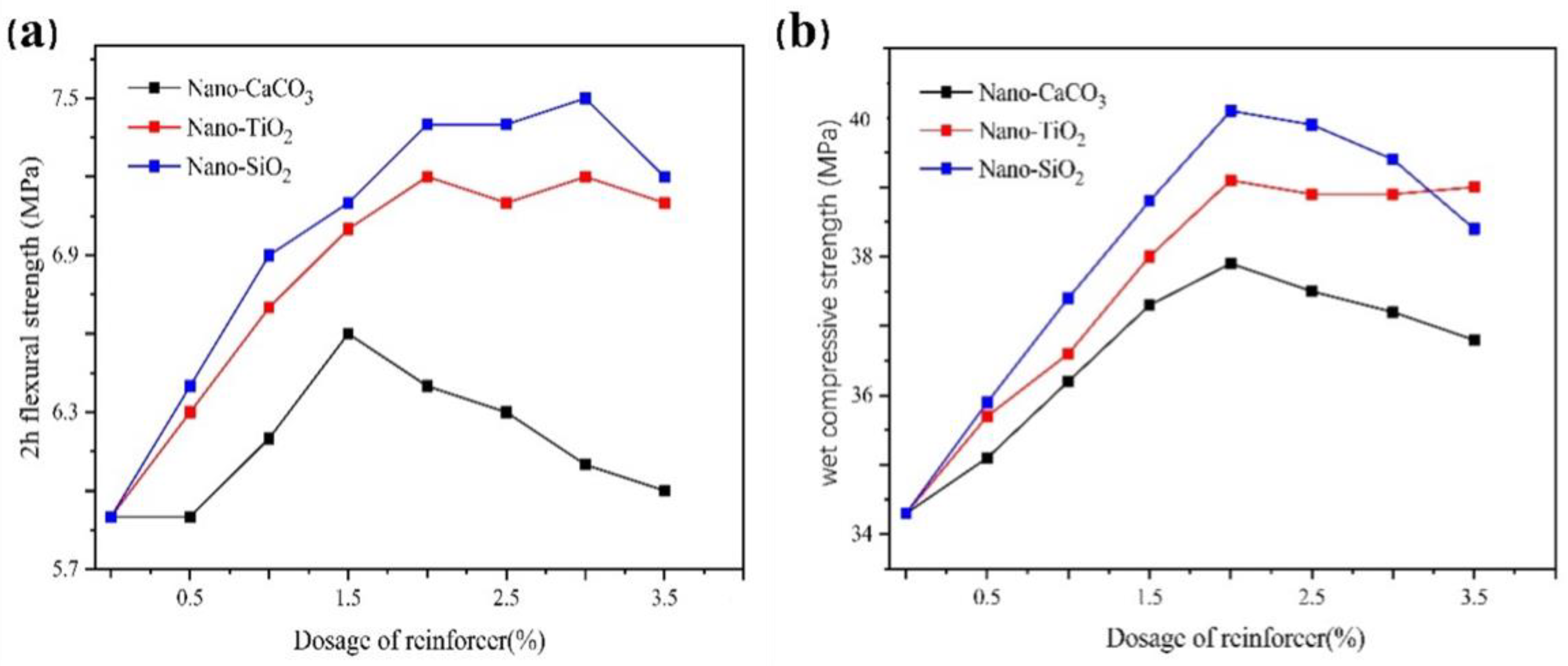
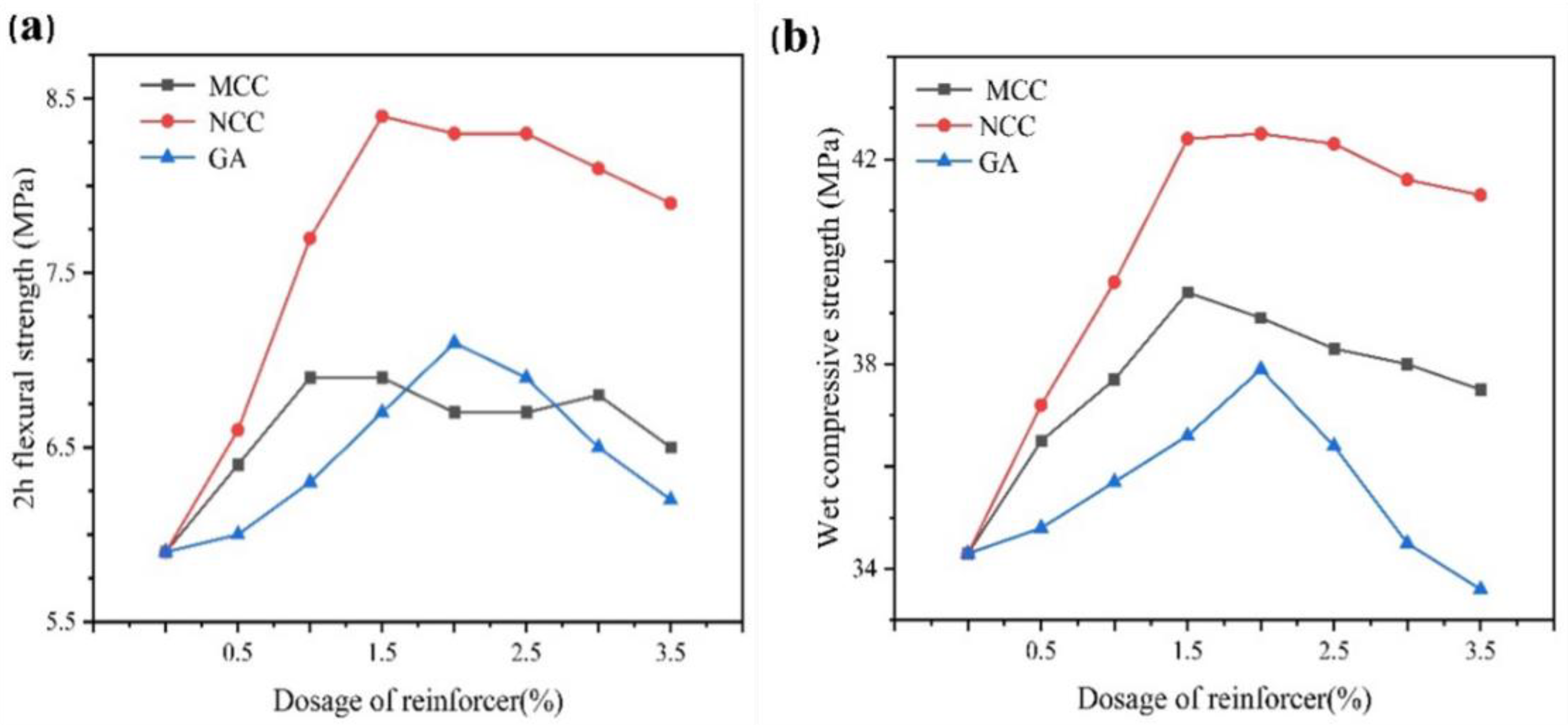
2.6. Effect of Reinforcer on the Gelation Characteristics of Dental Gypsum
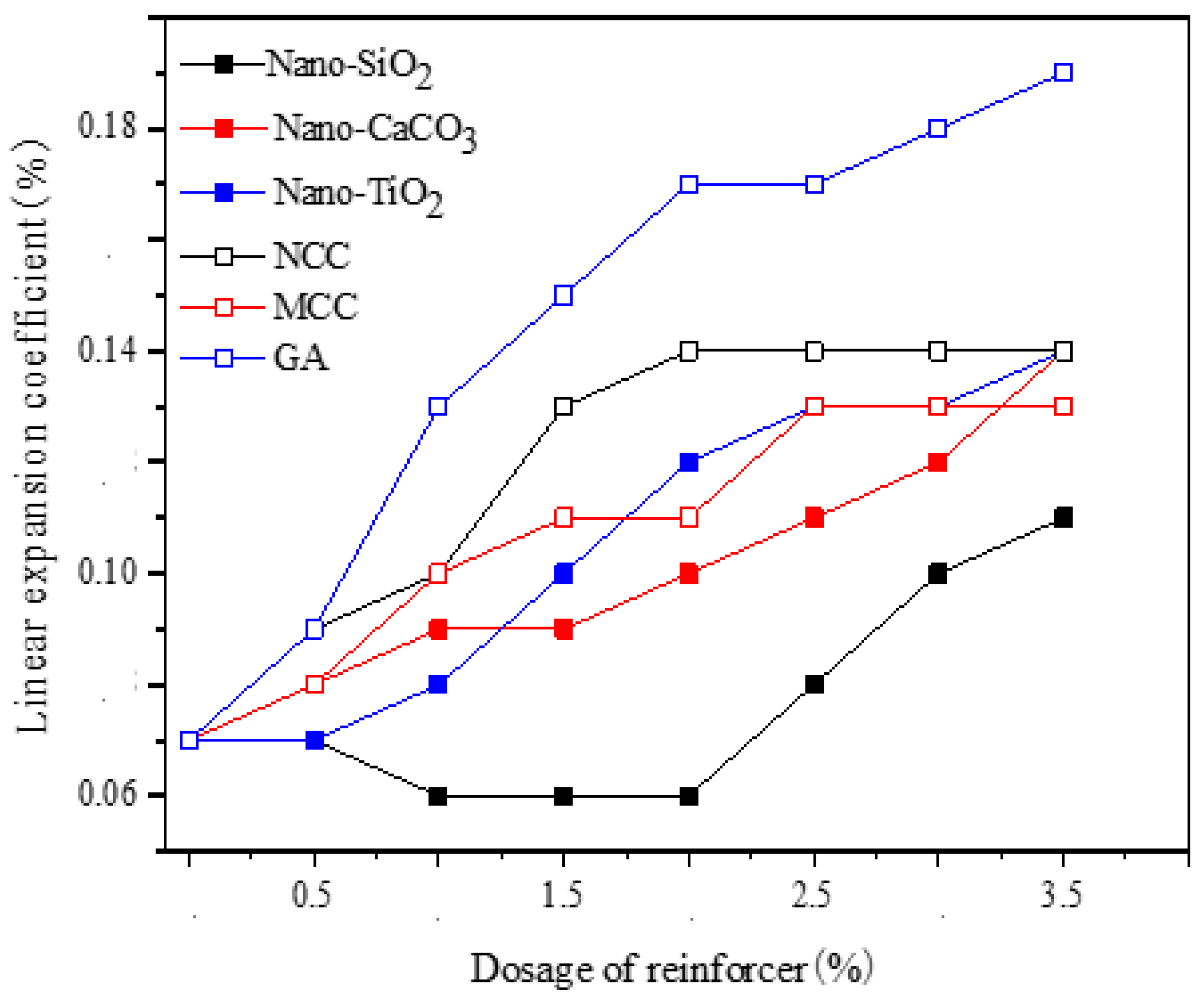
2.6.1. Linear Expansion Coefficient
2.6.2. Mechanical Properties
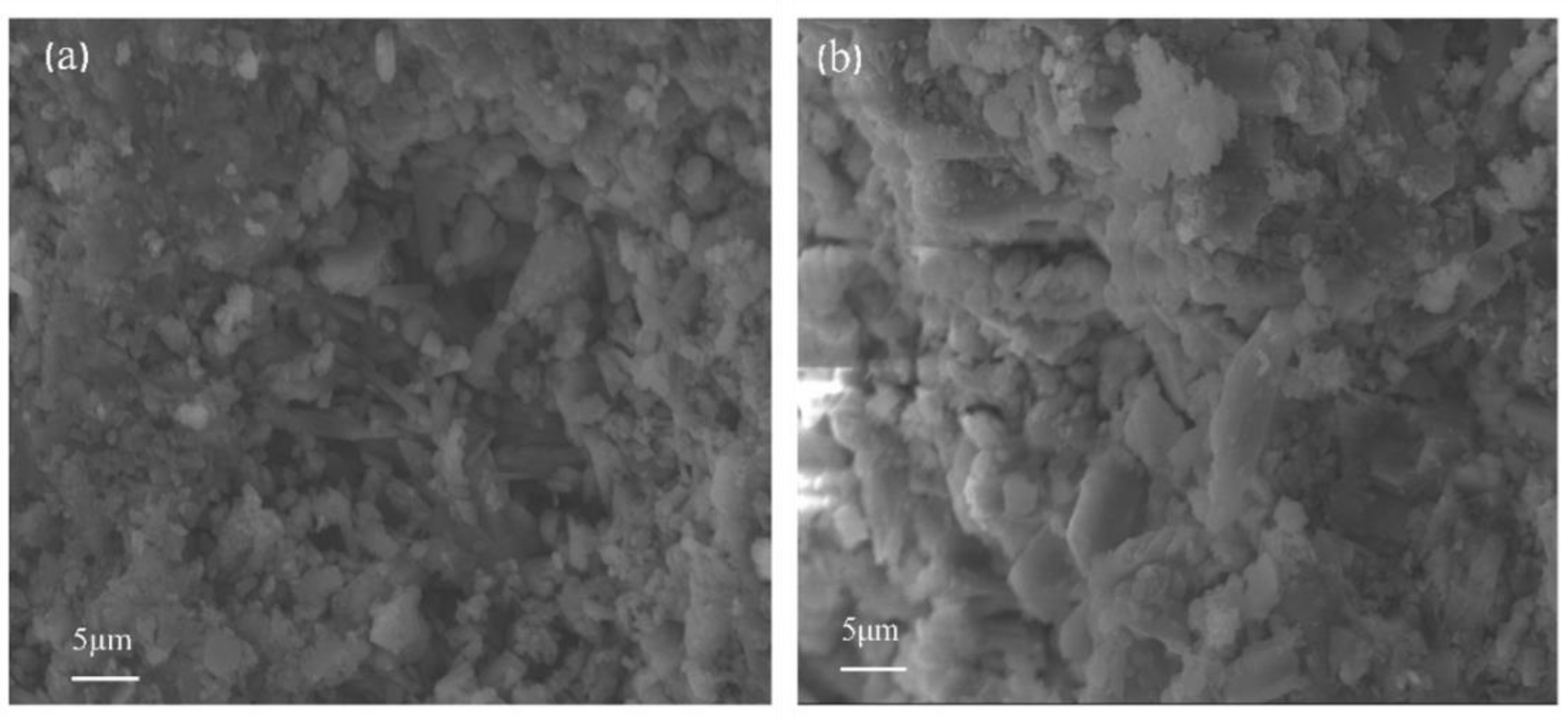
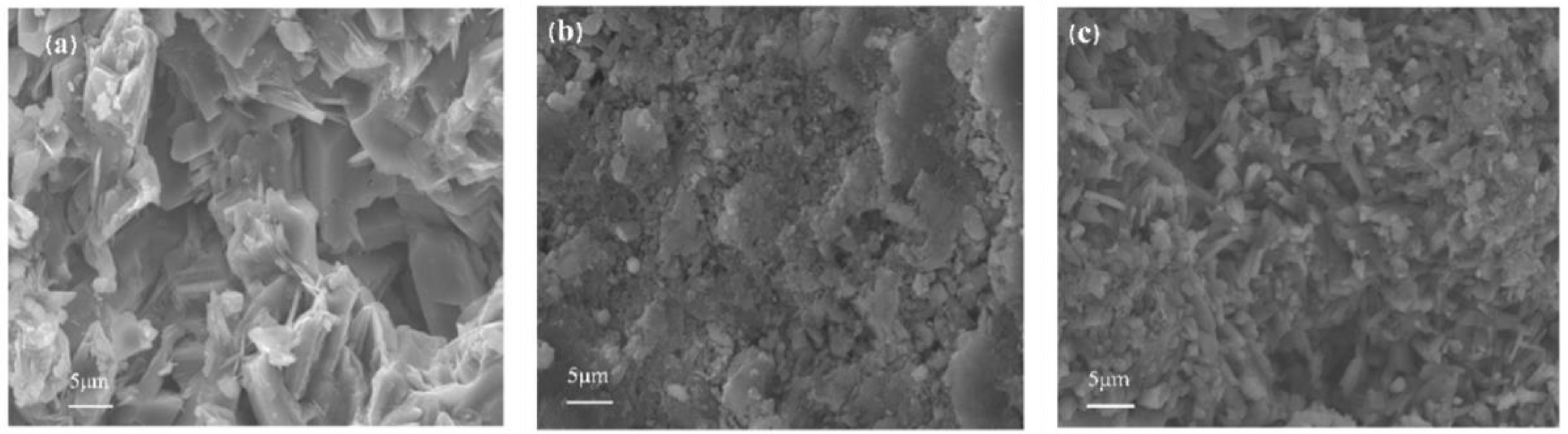
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Instruments
5.3. Methods
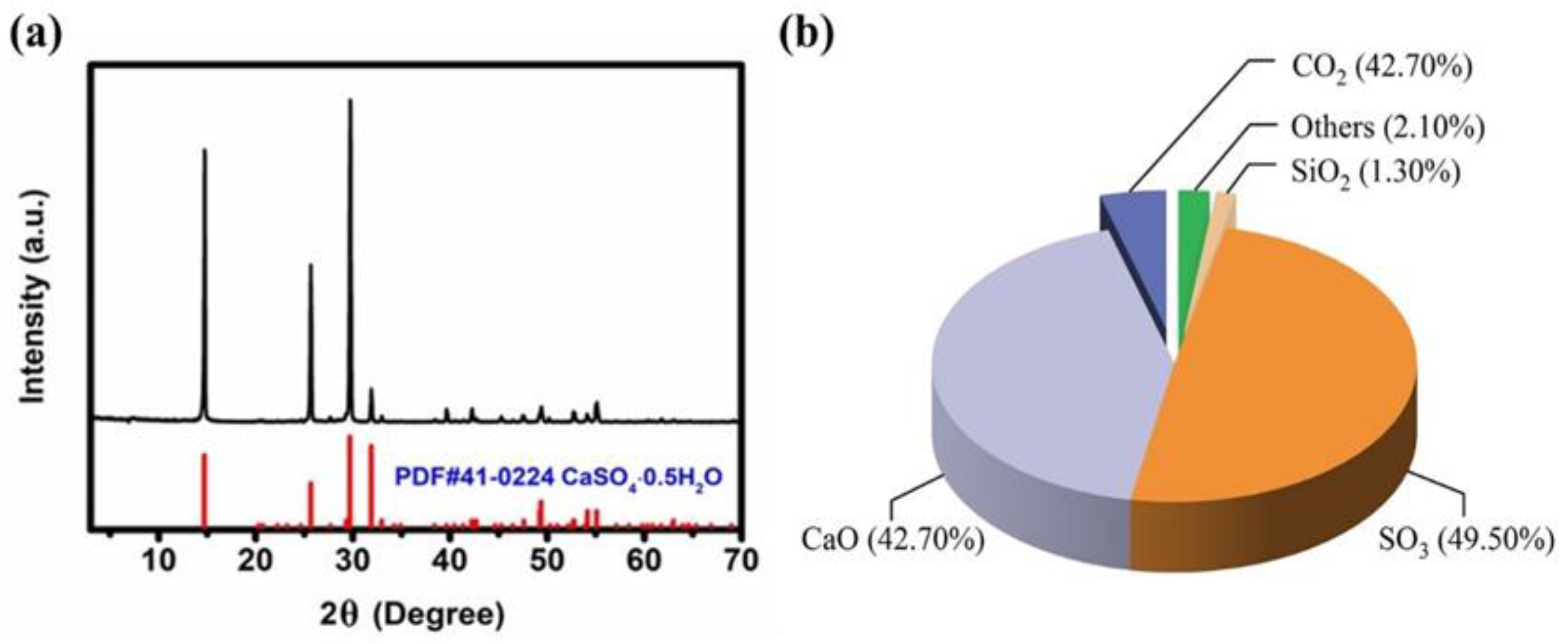
5.3.1. Preparation of Low-Expansion Gypsum Powder for Dentistry
5.3.2. Mixing and Molding
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nejatian, T.; Firouzmanesh, P.; Yaqin, A.U. Dental gypsum and investments. Adv. Dent. Biomater. 2019, 3, 37–39. [Google Scholar]
- Abdelaziz, K.M.; Combe, E.C.; Hodges, J.S. The effect of disinfectants on the properties of dental gypsum, part 2: Surface properties. J. Prosthodont. 2002, 11, 234–240. [Google Scholar] [CrossRef]
- Oancea, L.; Bilinschi, L.G.; Burlibasa, M.; Petre, A.; Sandu, M.; Costela, S. Effects of disinfectant solutions incorporated in dental stone on setting expansion, compression and flexural strength of dental models. Rom. Biotechnol. Lett. 2020, 25, 2095–2102. [Google Scholar] [CrossRef]
- Rudolph, H.; Salmen, H.; Moldan, M.; Kuhn, K.; Sichwardt, V.; Wöstmann, B. Accuracy of intraoral and extraoral digital data acquisition for dental restorations. J. Appl. Oral Sci. 2008, 24, 85–94. [Google Scholar] [CrossRef]
- Michalakis, K.X.; Stratos, A.; Hirayama, H.; Pissiotis, A.L.; Touloumi, F. Delayed setting and hygroscopic linear expansion of three gypsum products used for cast articulation. J. Prosthet. Dent. 2009, 102, 313–318. [Google Scholar] [CrossRef]
- Silva, M.A.B.; Vitti, R.P.; Consani, S.; Sinhoreti, M.A.C.; Mesquita, M.F.; Consani, R.L.X. Linear dimensional change, compressive strength and detail reproduction in type IV dental stone dried at room temperature and in a microwave oven. J. Appl. Oral Sci. 2012, 20, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.; Ferracane, J.; Powers, J.M. Craig’s Restorative Dental Materials. Br. Dent. J. 2019, 226, 293–314. [Google Scholar]
- Hashedi, A.A.; Laurenti, M.; Mezour, M.A.; Basiri, T.; Touazine, H.; Jahazi, M. Advanced inorganic nanocomposite for decontaminating titanium dental implants. J. Biomed. Mater. 2019, 107, 761–772. [Google Scholar] [CrossRef]
- American Dental Association. The dentist, the forensic pathologist, and the identification of human remains. J. Am. Dent. Assoc. 1972, 85, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Duke, P.; Moore, B.K.; Haug, S.P.; Andres, C.J. Study of the physical properties of type IV gypsum, resin-containing, and epoxy die materials. J. Prosthet. Dent. 2000, 83, 466–473. [Google Scholar] [CrossRef]
- Mahler, D.B.; Asgarzadeh, K. The Volumetric Contraction of Dental Gypsum Materials on Setting. J. Dent. Res. 1953, 32, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, E.P.; Corbin, F. Investigation on the expansion of dental stone. J. Dent. Res. 1969, 48, 206–210. [Google Scholar] [CrossRef]
- Asaoka, K.; Bae, J.Y.; Lee, H.H. Porosity of dental gypsum-bonded investments in setting and heating process. Dent. Mater. J. 2012, 31, 120–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lodovici, E.; Meira, J.B.C.; Filho, L.E.; Ballester, R.Y. Expansion of high flow mixtures of gypsum-bonded in investments in contact with absorbent liners. Dent. Mater. 2005, 21, 573–579. [Google Scholar] [CrossRef]
- Heshmati, R.H.; Nagy, W.W.; Wirth, G.G.; Dhuru, V.B. Delayed linear expansion of improved dental stone. J. Prosthet. Dent. 2002, 88, 26–31. [Google Scholar] [CrossRef]
- Carvalho, M.A.; Calil, C.C.; Savastano, H.; Tubino, R.; Carvalho, M.T. Microstructure and Mechanical Properties of Gypsum Composites Reinforced with Recycled Cellulose Pulp. Am. J. Mater. 2008, 11, 391–397. [Google Scholar] [CrossRef]
- Michalakis, K.X.; Asar, N.V.; Kapsampeli, V.; Trikka, P.M.; Pissiotis, A.L.; Hirayama, H. Delayed linear dimensional changes of five high strength gypsum products used for the fabrication of definitive casts. J. Prosthet. Dent. 2012, 108, 189–195. [Google Scholar] [CrossRef]
- Kumar, S.R.; Patnaik, A.; Bhat, I.K. Optimum selection of nano- and microsized filler for the best combination of physical, mechanical, and wear properties of dental composites. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2016, 232, 416–428. [Google Scholar] [CrossRef]
- He, L.H.; Vuuren, L.J.; Planitz, N.; Swain, M. A micro-mechanical evaluation of the effects of die hardener on die stone. Dent. Mater. J. 2010, 29, 433–437. [Google Scholar] [CrossRef]
- Kalahasti, D.; Hegde, V.; Kosaraju, K.; Baliga, X.; Reddy, N.K.; Sujatha, B.K. Evaluation of Efficacy of Microwave Irradiation in Disinfecting Dental Gypsum Casts: An Ex Vivo Study. J. Indian Prosthodont. Soc. 2014, 14, 381–392. [Google Scholar] [CrossRef]
- Paula, P.R.; Lucas, M.G.; Spolidorio, D.M.P. Antimicrobial activity of disinfectant agents incorporated into type IV dental stone. Gerodontology 2012, 29, 267–274. [Google Scholar]
- Dalmay, P.; Smith, A.; Chotard, T.; Sahay, T.P.; Gloaguen, V.; Krausz, P. Properties of cellulosic fibre reinforced plaster: Influence of hemp or flax fibres on the properties of set gypsum. J. Mater. Sci. 2010, 45, 793–803. [Google Scholar] [CrossRef]
- Yan, M.; Takahashi, H.; Nishimura, F. Dimensional accuracy and surface property of titanium casting using gypsum-bonded alumina investment. Dent. Mater. J. 2004, 23, 539–544. [Google Scholar] [CrossRef]
- Lewry, A.J.; Williamson, J. The setting of gypsum plaster.3. The effect of additives and impurities. J. Mater. Sci. 1994, 29, 6085–6090. [Google Scholar] [CrossRef]
- Touraj, N.; Zohaib, K.; Muhammad, Z.; Shariq, N.; Sana, Z.; Masoud, M. Dental biocomposites. Biomater. Oral Dent. Tissue Eng. 2017, 5, 65–84. [Google Scholar]
- Romanec, C.; Rosu, S.; Macovei, G.; Scutariu, M.M.; Dragomir, B.; Olteanu, N.D. Morphofunctional Features in Angle Second Class Malocclusion on Dental Gypsum Models. Mater. Plast. 2018, 55, 686–690. [Google Scholar] [CrossRef]
- Choi, J.W.; Ahn, J.J.; Son, K.; Huh, J.B. Three-Dimensional Evaluation on Accuracy of Conventional and Milled Gypsum Models and 3D Printed Photopolymer Models. Materials 2019, 12, 3499. [Google Scholar] [CrossRef]
- Prombonas, A.E.; Paralika, M.A.; Sotiriou, M.P.; Vlissidis, D.S. The peak-amplitude method of vibration analysis for nondestructively studying the structural integrity of dental gypsum. J. Biomed. Mater. Res. 2002, 63, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Miyaji, H.; Imamura, T.; Kaga, N.; Yokoyama, A.; Yoshida, Y. Submicro-patterning of curable dental materials by molding methods: A screening trail. Dig. J. Nanomater. Biostruct. 2017, 12, 281–292. [Google Scholar]
| Run | A (SG) % | B (CPS) % | C (Nano-SiO2) % | D (C54H105AlO6) % | Expansion Value % |
|---|---|---|---|---|---|
| 1 | 1 (0.01) | 1 (0.2) | 1 (1.0) | 1 (0.3) | 0.131 |
| 2 | 1 (0.01) | 2 (0.3) | 2 (1.5) | 2 (0.6) | 0.078 |
| 3 | 1 (0.01) | 3 (0.4) | 3 (2.0) | 3 (0.9) | 0.064 |
| 4 | 2 (0.02) | 1 (0.2) | 2 (1.5) | 3 (0.9) | 0.082 |
| 5 | 2 (0.02) | 2 (0.3) | 3 (2.0) | 1 (0.3) | 0.114 |
| 6 | 2 (0.02) | 3 (0.4) | 1 (1.0) | 2(0.6) | 0.102 |
| 7 | 3 (0.03) | 1 (0.2) | 3 (2.0) | 2 (0.6) | 0.076 |
| 8 | 3 (0.03) | 2 (0.3) | 1 (1.0) | 3 (0.9) | 0.058 |
| 9 | 3 (0.03) | 3 (0.4) | 2 (1.5) | 1 (0.3) | 0.125 |
| K1 | 0.273 | 0.289 | 0.291 | 0.370 | |
| K2 | 0.298 | 0.250 | 0.285 | 0.256 | |
| K3 | 0.259 | 0.291 | 0.254 | 0.204 | |
| k1 | 0.091 | 0.096 | 0.097 | 0.123 | |
| k2 | 0.099 | 0.083 | 0.095 | 0.085 | |
| k3 | 0.086 | 0.097 | 0.085 | 0.068 | |
| ΔR | 0.013 | 0.014 | 0.012 | 0.055 |
| Sample | CK | Heraeus | Dentona CAD/CAM | Bowin KKK | Formula Sample | |
|---|---|---|---|---|---|---|
| Time interval (h) | 2 | 0.26 | 0.26 | 0.13 | 0.22 | 0.06 |
| 6 | 0.26 | 0.26 | 0.15 | 0.24 | 0.06 | |
| 12 | 0.27 | 0.27 | 0.15 | 0.24 | 0.07 | |
| 24 | 0.27 | 0.27 | 0.18 | 0.25 | 0.07 | |
| 36 | 0.29 | 0.28 | 0.18 | 0.26 | 0.07 | |
| 48 | 0.30 | 0.28 | 0.19 | 0.27 | 0.07 | |
| 60 | 0.31 | 0.28 | 0.20 | 0.27 | 0.07 | |
| 72 | 0.31 | 0.28 | 0.20 | 0.27 | 0.07 | |
| growth rate(%) | 19.23 | 7.69 | 53.85 | 22.73 | 16.67 | |
| Tested Parameters | Properties |
|---|---|
| Standard consistency water demand (%) | 27 |
| Initial setting time (s) | 634 |
| Final setting time (s) | 930 |
| Linear expansion coefficient (%) | 0.26 |
| 2 h flexural strength (MPa) | 6.70 |
| Wet compressive strength (MPa) | 36.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Xie, Q.; Evelina, A.; Long, W.; Ma, C.; Zhou, F.; Cha, R. The Effect of Different Additives on the Hydration and Gelation Properties of Composite Dental Gypsum. Gels 2021, 7, 117. https://doi.org/10.3390/gels7030117
Ma L, Xie Q, Evelina A, Long W, Ma C, Zhou F, Cha R. The Effect of Different Additives on the Hydration and Gelation Properties of Composite Dental Gypsum. Gels. 2021; 7(3):117. https://doi.org/10.3390/gels7030117
Chicago/Turabian StyleMa, Liang, Qianting Xie, Amutenya Evelina, Wenjun Long, Cunfa Ma, Fengshan Zhou, and Ruitao Cha. 2021. "The Effect of Different Additives on the Hydration and Gelation Properties of Composite Dental Gypsum" Gels 7, no. 3: 117. https://doi.org/10.3390/gels7030117
APA StyleMa, L., Xie, Q., Evelina, A., Long, W., Ma, C., Zhou, F., & Cha, R. (2021). The Effect of Different Additives on the Hydration and Gelation Properties of Composite Dental Gypsum. Gels, 7(3), 117. https://doi.org/10.3390/gels7030117






