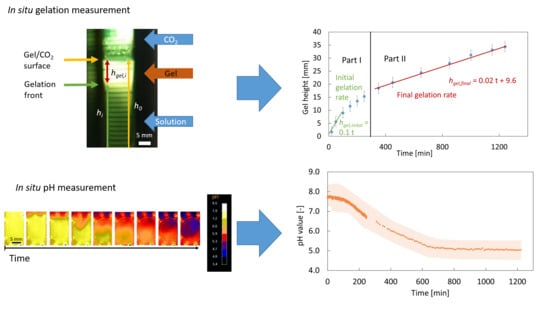
In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems
Abstract
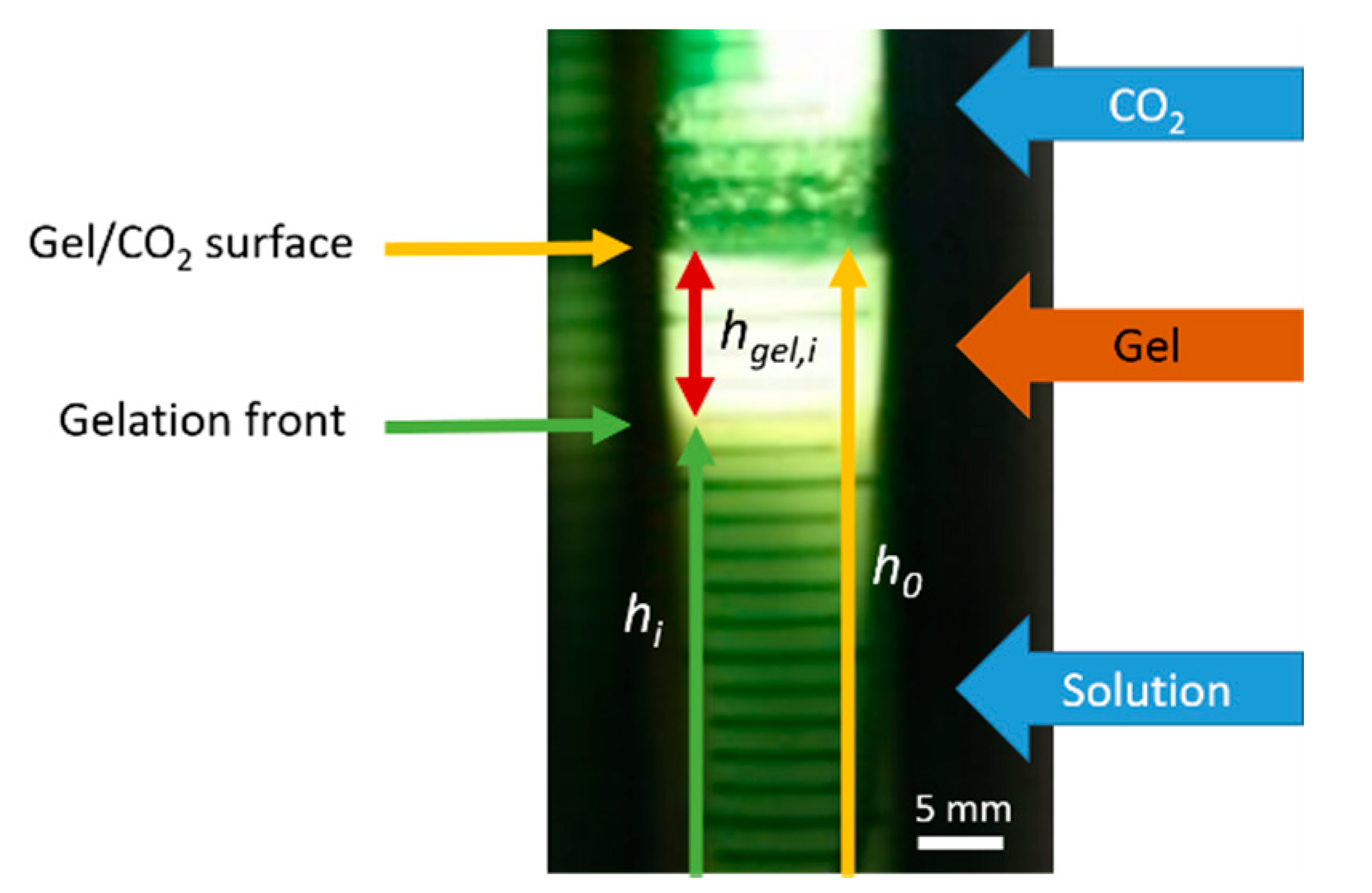
:1. Introduction
- Dissolution of CO2 in the aqueous biopolymer solution;
- Reaction of CO2 with water and formation of carbonic acid;
- Dissociation of the carbonic acid resulting in a pH drop;
- Dissolution of cross-linkers (e.g., CaCO3) due to increased solubility at a reduced pH value;
- Cross-linking of biopolymer chains, which is either induced by reduction of the pH value (step 3) and therefore interaction of biopolymer molecules or by interaction of biopolymer molecules with released cations (step 4).
2. Results and Discussion
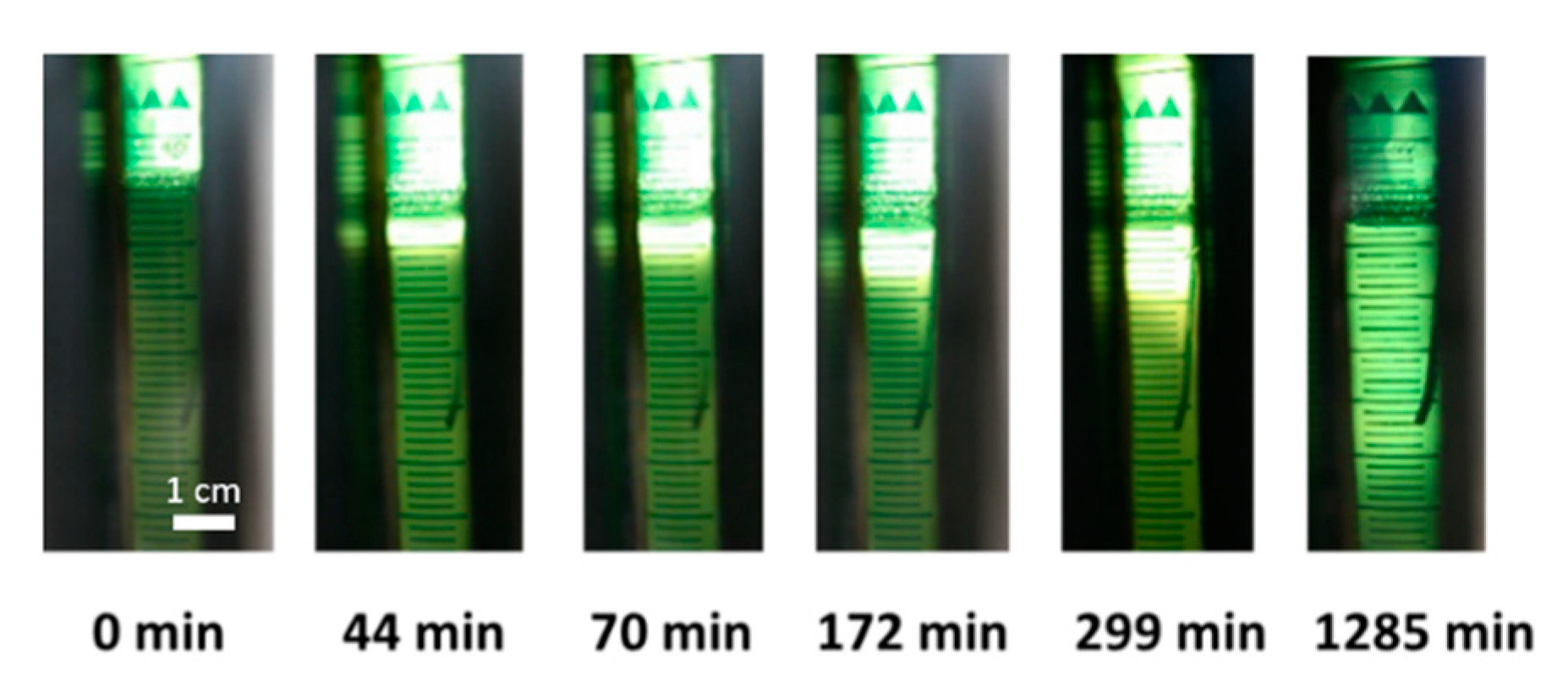
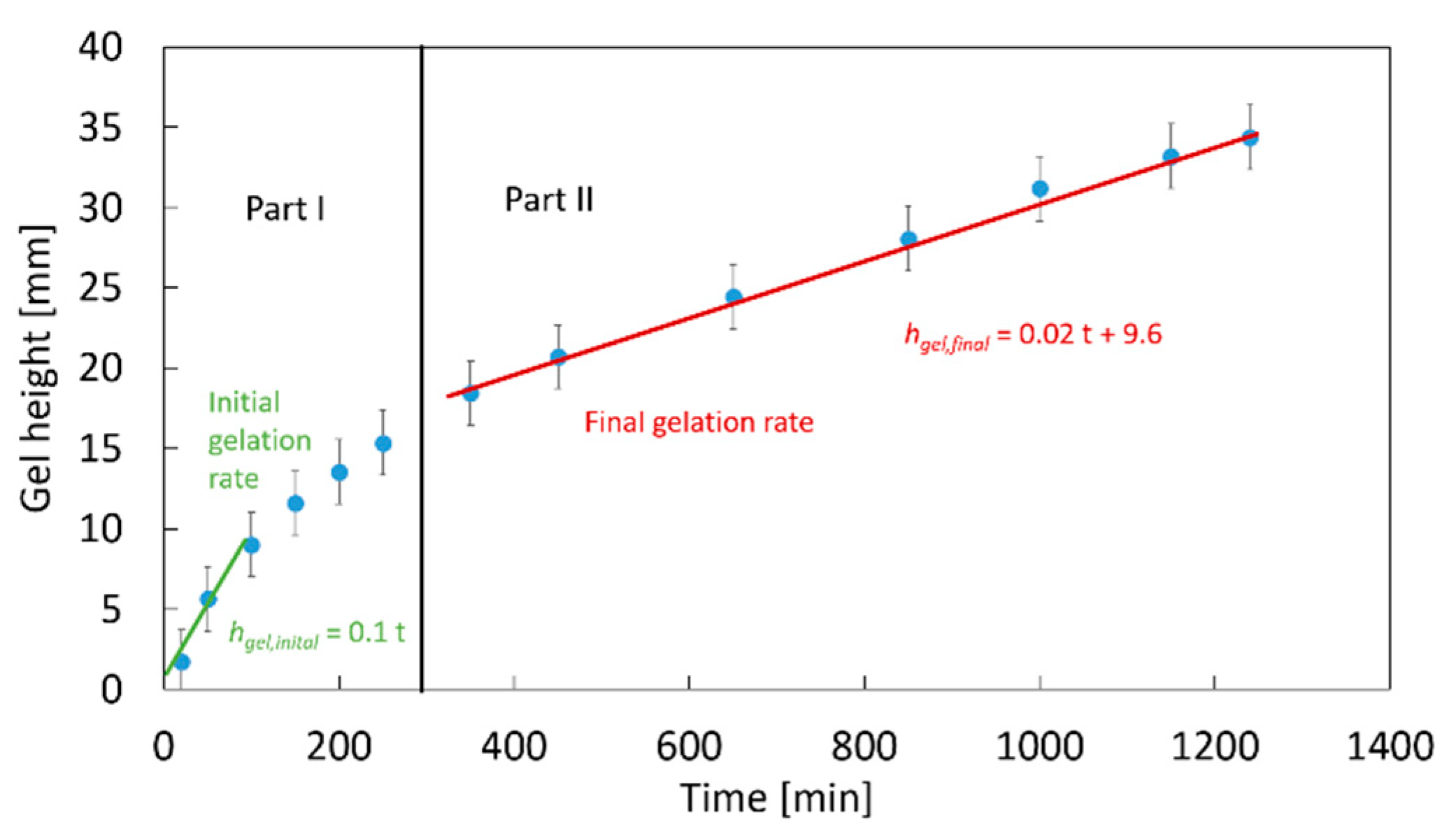
2.1. Optical Measurement of the CaCO3 Dissolution and Gelation
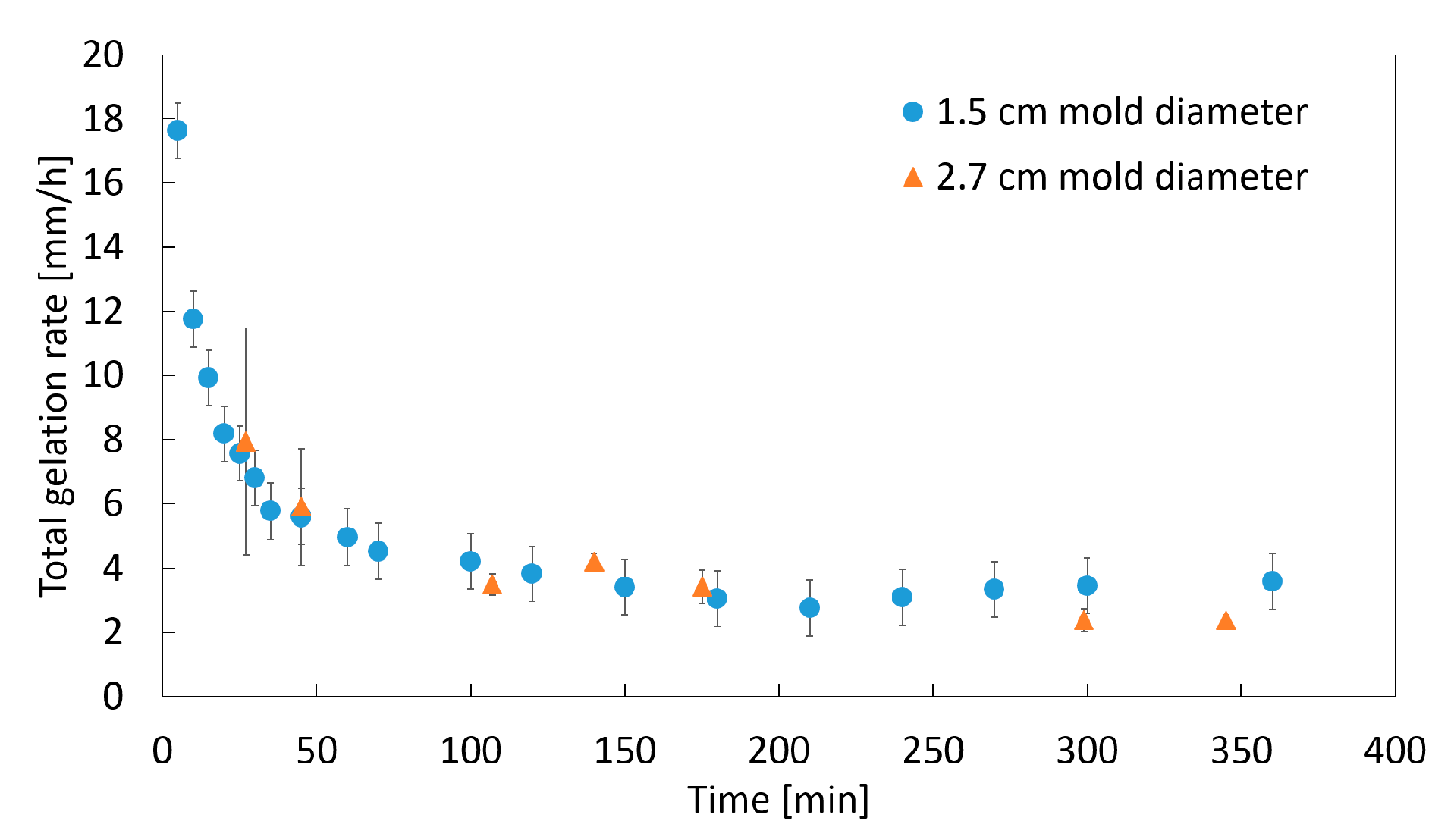
2.1.1. Impact of the Mold Material
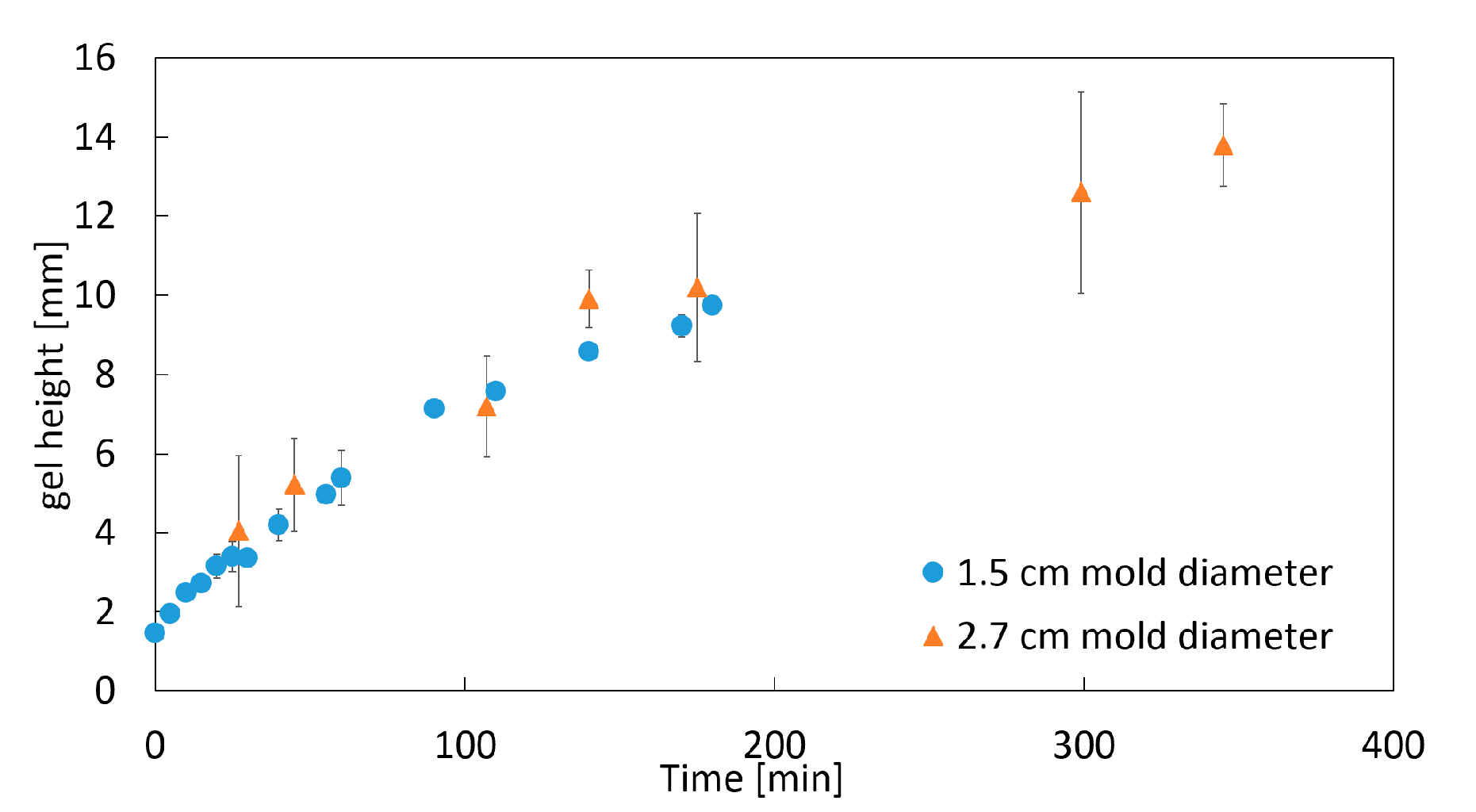
2.1.2. Impact of the Mold Diameter
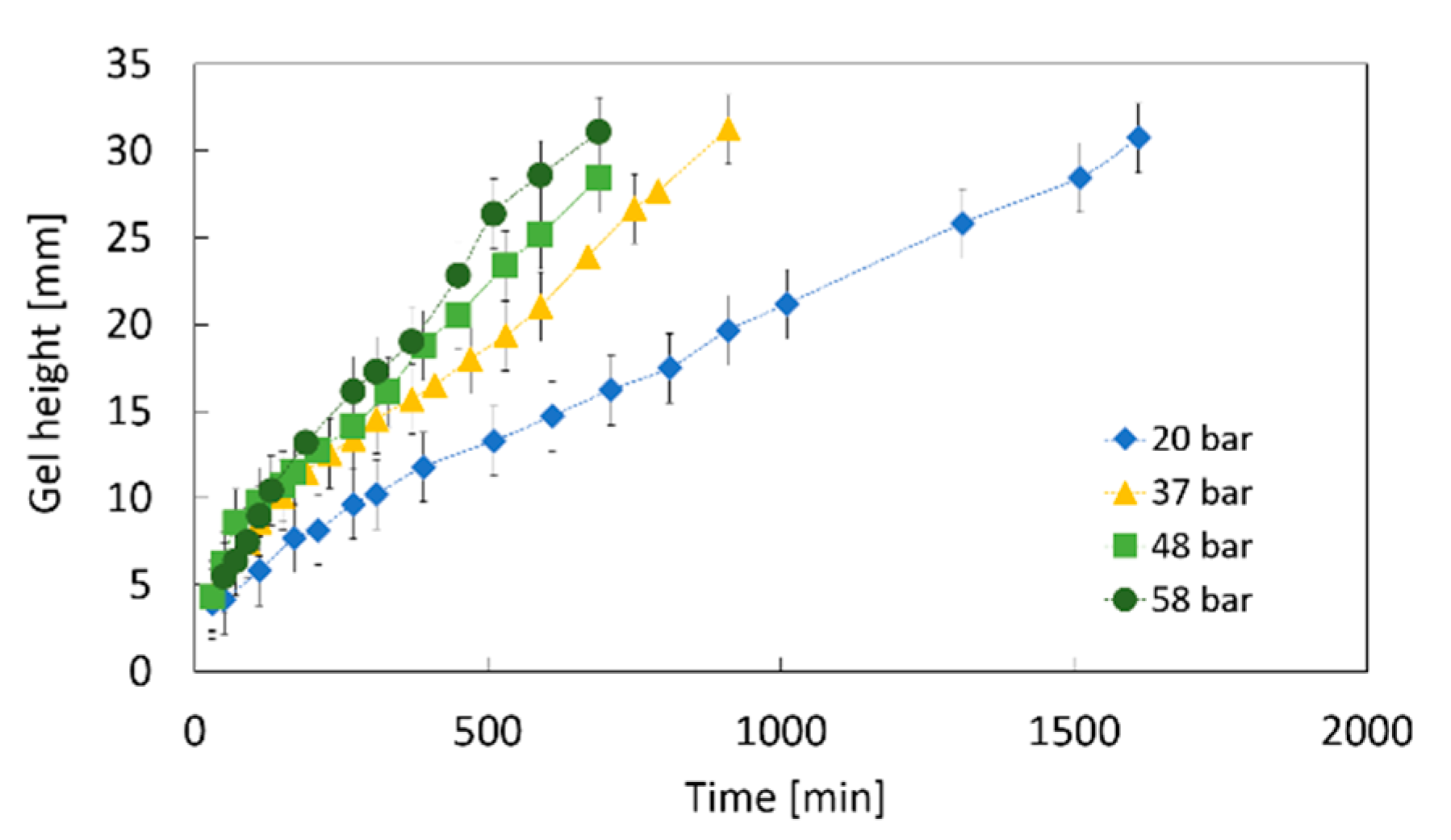
2.1.3. Impact of the CO2 Pressure
2.1.4. Impact of the CaCO3 Concentration
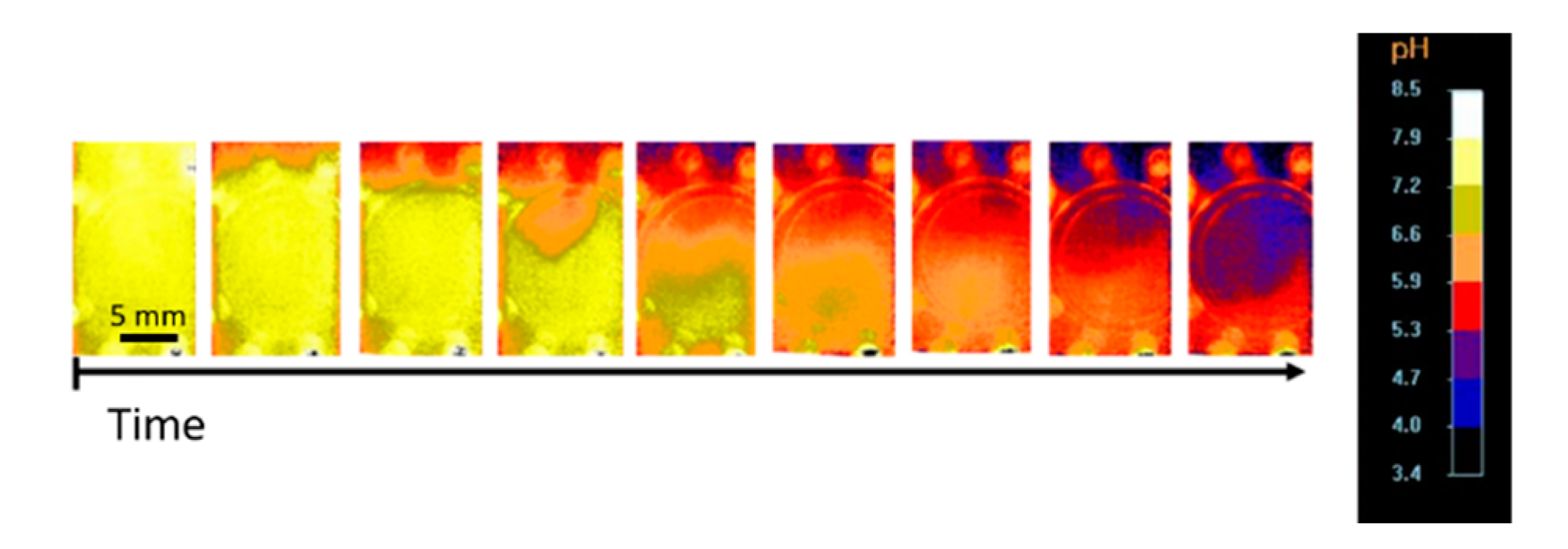
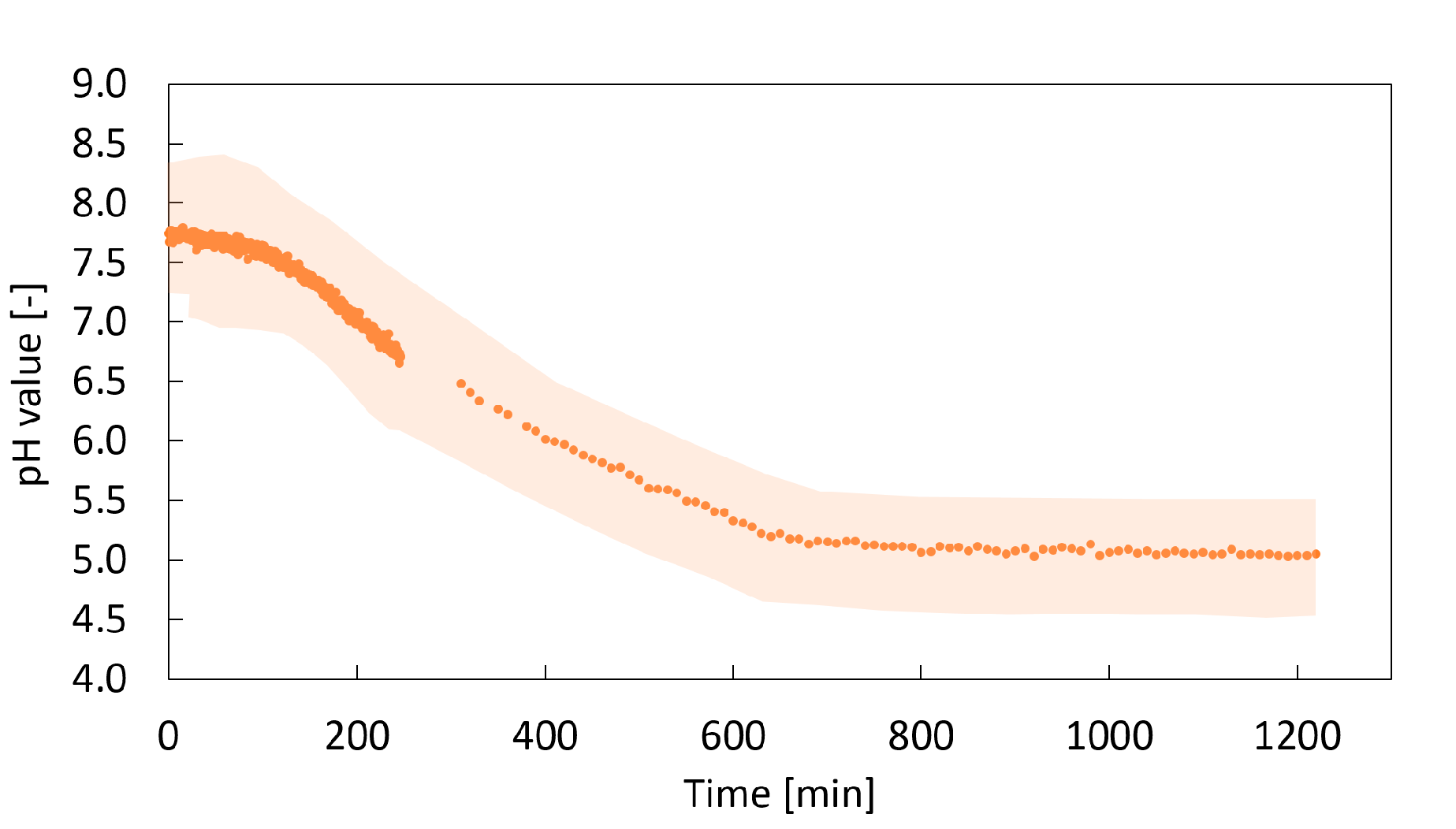
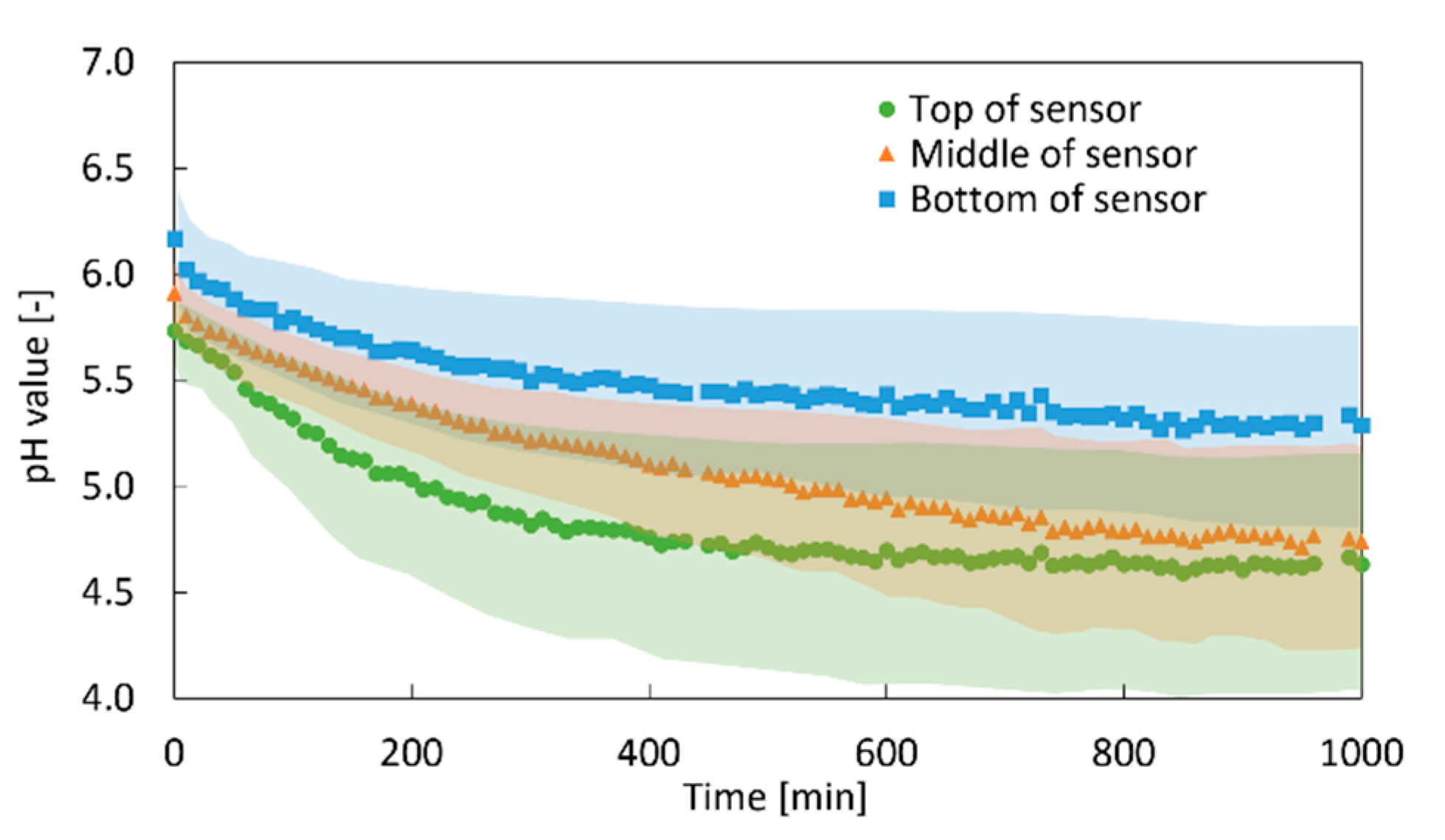
2.2. In Situ pH Measurement
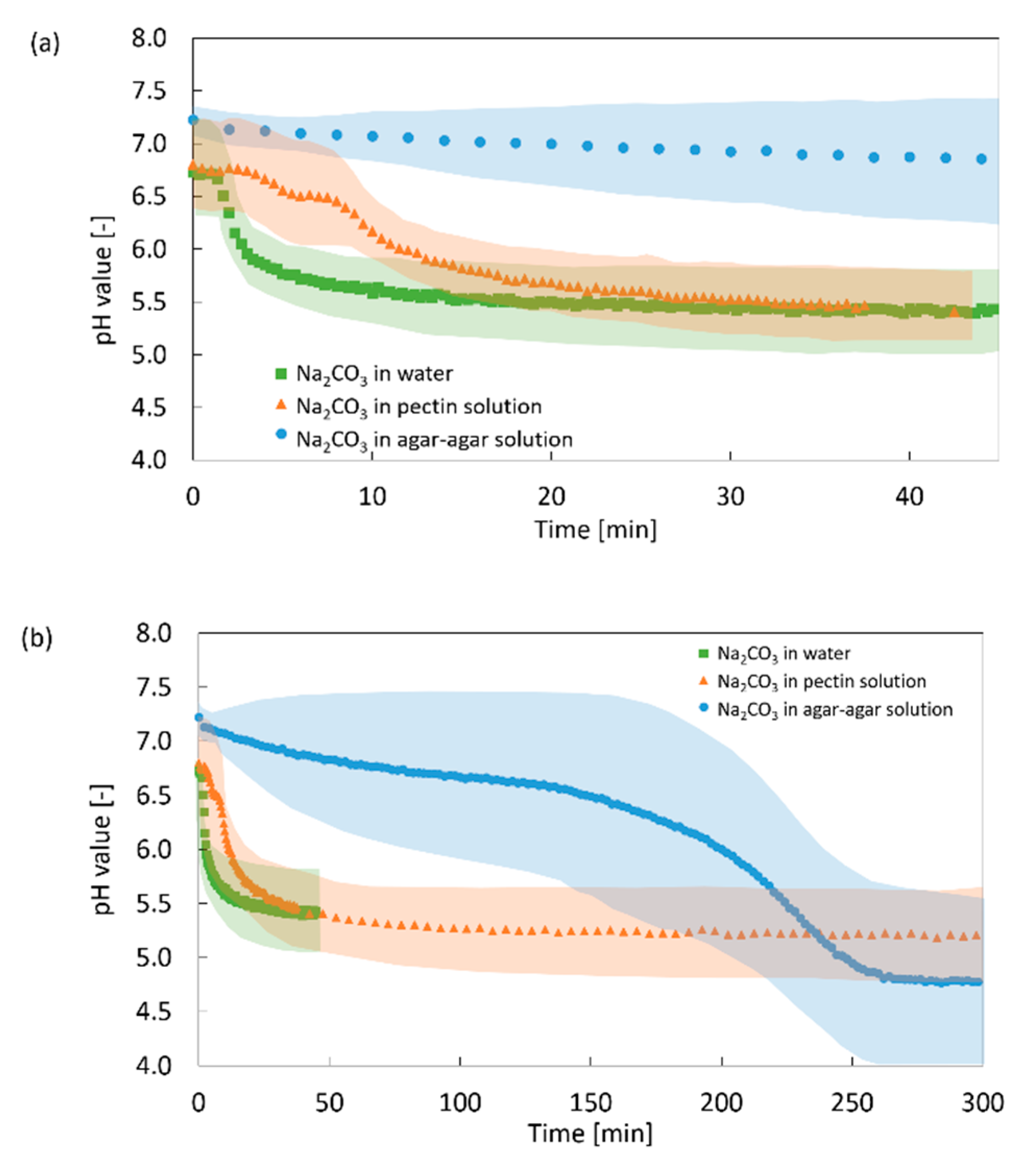
2.2.1. Impact of the Additional Carbonate
2.2.2. Impact of the Solution Viscosity
3. Conclusions
4. Materials
5. Methods
5.1. Preparation of Stock Solutions
5.2. CO2-Induced Gelation
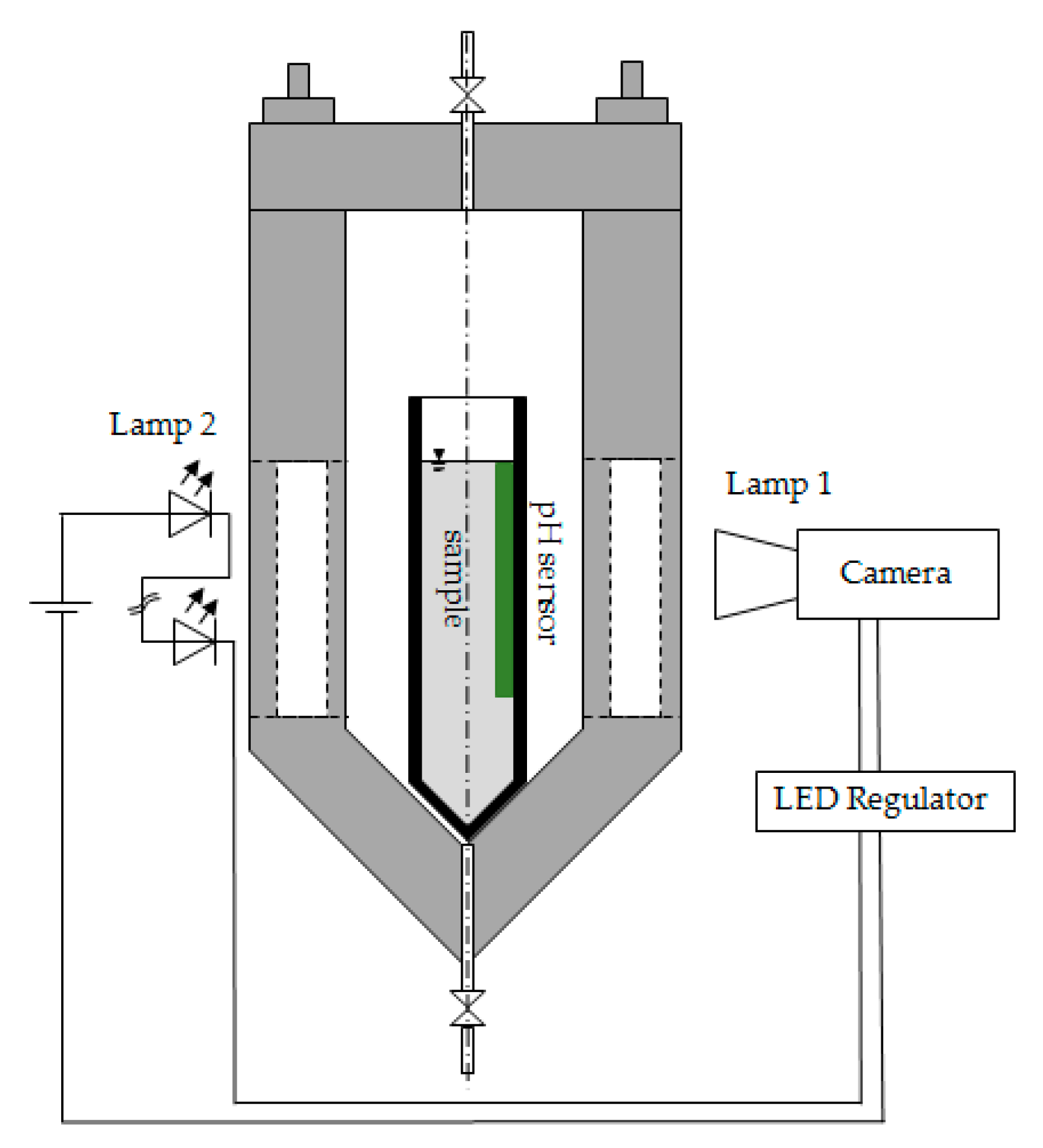
5.3. Visual Observation and Evaluation of the Gelation Progress
5.4. In Situ pH Measurement
5.5. Viscosity Measurement
Author Contributions
Funding
Conflicts of Interest
References
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of Biopolymer Aerogels Using Green Solvents. J. Vis. Exp. 2016, 113. [Google Scholar] [CrossRef] [Green Version]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Raman, S.P.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- Partap, S.; Rehman, I.; Jones, J.R.; Darr, J.A. “Supercritical Carbon Dioxide in Water” Emulsion-Templated Synthesis of Porous Calcium Alginate Hydrogels. Adv. Mater. 2006, 18, 501–504. [Google Scholar] [CrossRef]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon dioxide induced silk protein gelation for biomedical applications. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; McHugh, M.A. CO2-assisted synthesis of silk fibroin hydrogels and aerogels. Acta Biomater. 2014, 10, 4419–4424. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Dehghani, F.; Annabi, N.; Valtchev, P.; Mithieux, S.M.; Weiss, A.S.; Kazarian, S.G.; Tay, F.H. Effect of dense gas CO2 on the coacervation of elastin. Biomacromolecules 2008, 9, 1100–1105. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, F.; Jiang, J.; Wang, X.; Hou, Z.; Gao, Y. Structural and conformational modification of whey proteins induced by supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2012, 12, 32–37. [Google Scholar] [CrossRef]
- Muljana, H.; Picchioni, F.; Heeres, H.J.; Janssen, L.P.B.M. Supercritical carbon dioxide (scCO2) induced gelatinization of potato starch. Carbohydr. Polym. 2009, 78, 511–519. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Ismail, M.; Farid, M.M.; Ji, H.; Mao, W.; Gao, J.; Li, C. Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem. 2017, 227, 219–226. [Google Scholar] [CrossRef]
- Zubal, L.; Bonani, W.; Maniglio, D.; Ceccato, R.; Renciuk, D.; Hampl, A.; Migliaresi, C.; Jancar, J.; Vojtova, L. Soluble collagen dissolution and assembling in pressurized carbon dioxide water solutions. Express Polym. Lett. 2018, 12, 159–170. [Google Scholar] [CrossRef]
- Alnaief, M.; Mohammad, B.; Aljarrah, M.; Obaidat, R.M. Preparation and preliminary characterization of hybrid alginate—Carrageenan aerogel: Effect of gelation methods. Jordan J. Phys. 2019, 12, 79–86. [Google Scholar]
- Groult, S.; Budtova, T. Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef]
- Butler, J.N. Carbon Dioxide Equilibria and Their Applications; Lewis Publishers INC: Chelsea, MI, USA, 1991. [Google Scholar]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochim. Cosmochim. Acta. 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilib. 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Miller, J.P. A portion of the system calcium carbonate-carbon dioxide-water with geological implications. Am. J. Sci. 1952, 250, 161–203. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Martín, A.; Florusse, L.J.; Peters, C.J.; Cocero, M.J. The influence of Na2SO4 on the CO2 solubility in water at high pressure. Fluid Phase Equilib. 2005, 238, 220–228. [Google Scholar] [CrossRef]
- Jeon, P.R.; Lee, C.-H. Prediction of CO2 solubility in multicomponent electrolyte solutions up to 709 bar: Analogical bridge between hydrophobic solvation and adsorption model. Chem. Eng. J. 2020, 389, 123459. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef]
- Hart, P.W.; Colson, G.W.; Burris, J. Application of carbon dioxide to reduce water-side lime scale in heat exchangers. J. Sci. Technol. For. Prod. Process. 2011, 1, 67–70. [Google Scholar]
- Frear, G.L.; Johnston, J. The solubility of calcium carbonate (calcite) in certain aqueous solutions at 25°. J. Am. Chem. Soc. 1929, 51, 2082–2093. [Google Scholar] [CrossRef]
- Letcher, T.M. (Ed.) Developments and Applications in Solubility; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar] [CrossRef] [Green Version]
- Oechsle, A.L.; Lewis, L.; Hamad, W.Y.; Hatzikiriakos, S.G.; MacLachlan, M.J. CO2-Switchable Cellulose Nanocrystal Hydrogels. Chem. Mater. 2018, 30, 376–385. [Google Scholar] [CrossRef]
- Baehr, H.D.; Stephan, K. Heat and Mass Transfer, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Meyssami, B.; Balaban, M.; Teixeira, A. Prediction of pH in model systems pressurized with carbon dioxide. Biotechnol. Prog. 1992, 8, 149–154. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Elmanovich, I.V.; Kononevich, Y.N.; Gallyamov, M.O.; Muzafarov, A.M. A biphase H2O/CO2 system as a versatile reaction medium for organic synthesis. RSC Adv. 2015, 5, 103573–103608. [Google Scholar] [CrossRef]

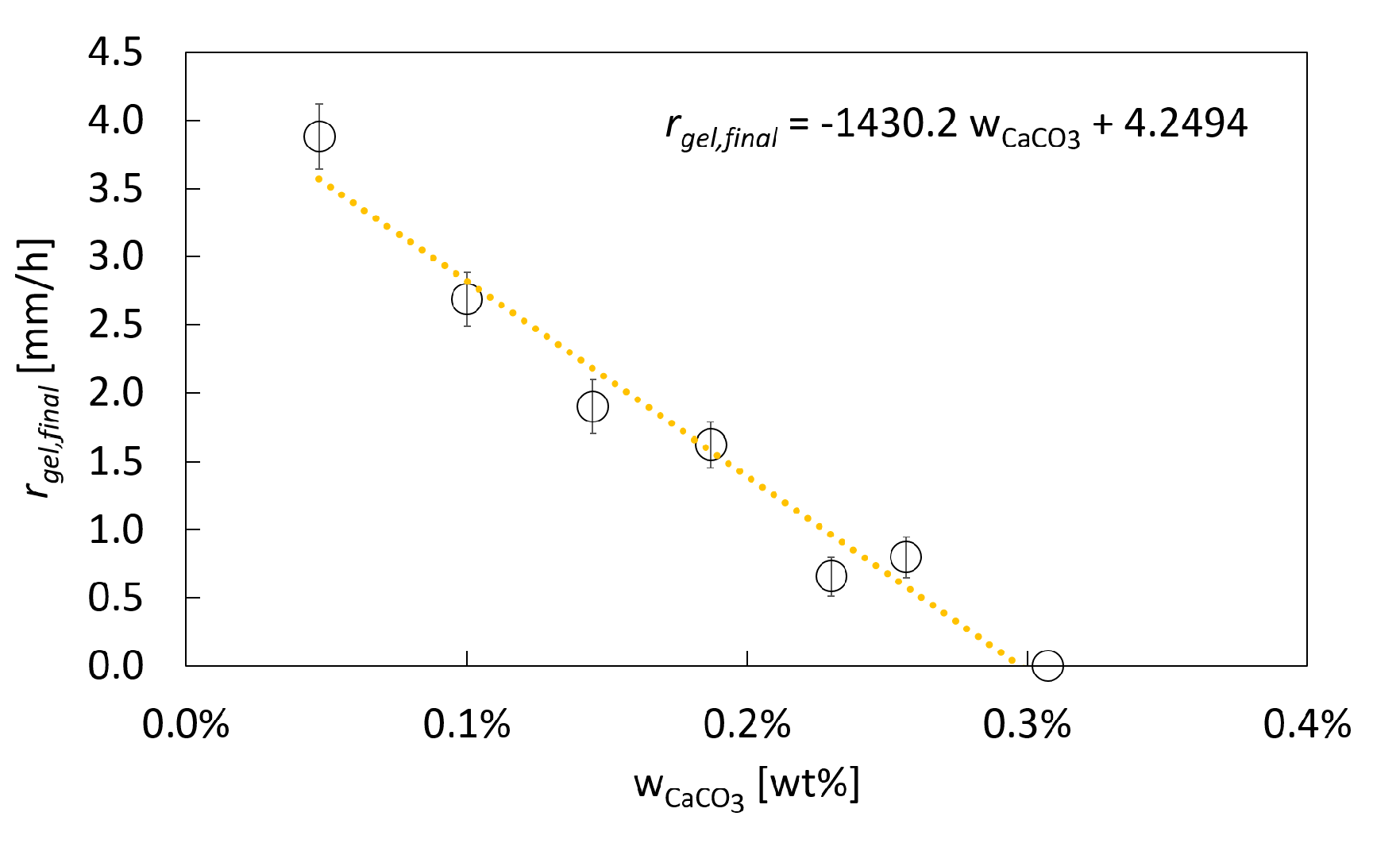

| Pressure of CO2 Atmosphere (Bar) | Solubility of CO2 in Pure Water (mol/m3) |
|---|---|
| 20 | 660 |
| 37 | 1221 |
| 48 | 1584 |
| 58 | 1914 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preibisch, I.; Ränger, L.-M.; Gurikov, P.; Smirnova, I. In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems. Gels 2020, 6, 28. https://doi.org/10.3390/gels6030028
Preibisch I, Ränger L-M, Gurikov P, Smirnova I. In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems. Gels. 2020; 6(3):28. https://doi.org/10.3390/gels6030028
Chicago/Turabian StylePreibisch, Imke, Lena-Marie Ränger, Pavel Gurikov, and Irina Smirnova. 2020. "In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems" Gels 6, no. 3: 28. https://doi.org/10.3390/gels6030028
APA StylePreibisch, I., Ränger, L.-M., Gurikov, P., & Smirnova, I. (2020). In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems. Gels, 6(3), 28. https://doi.org/10.3390/gels6030028