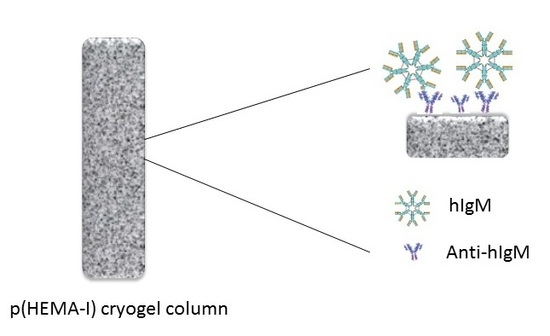
Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M
Abstract
:1. Introduction
2. Results and Discussion
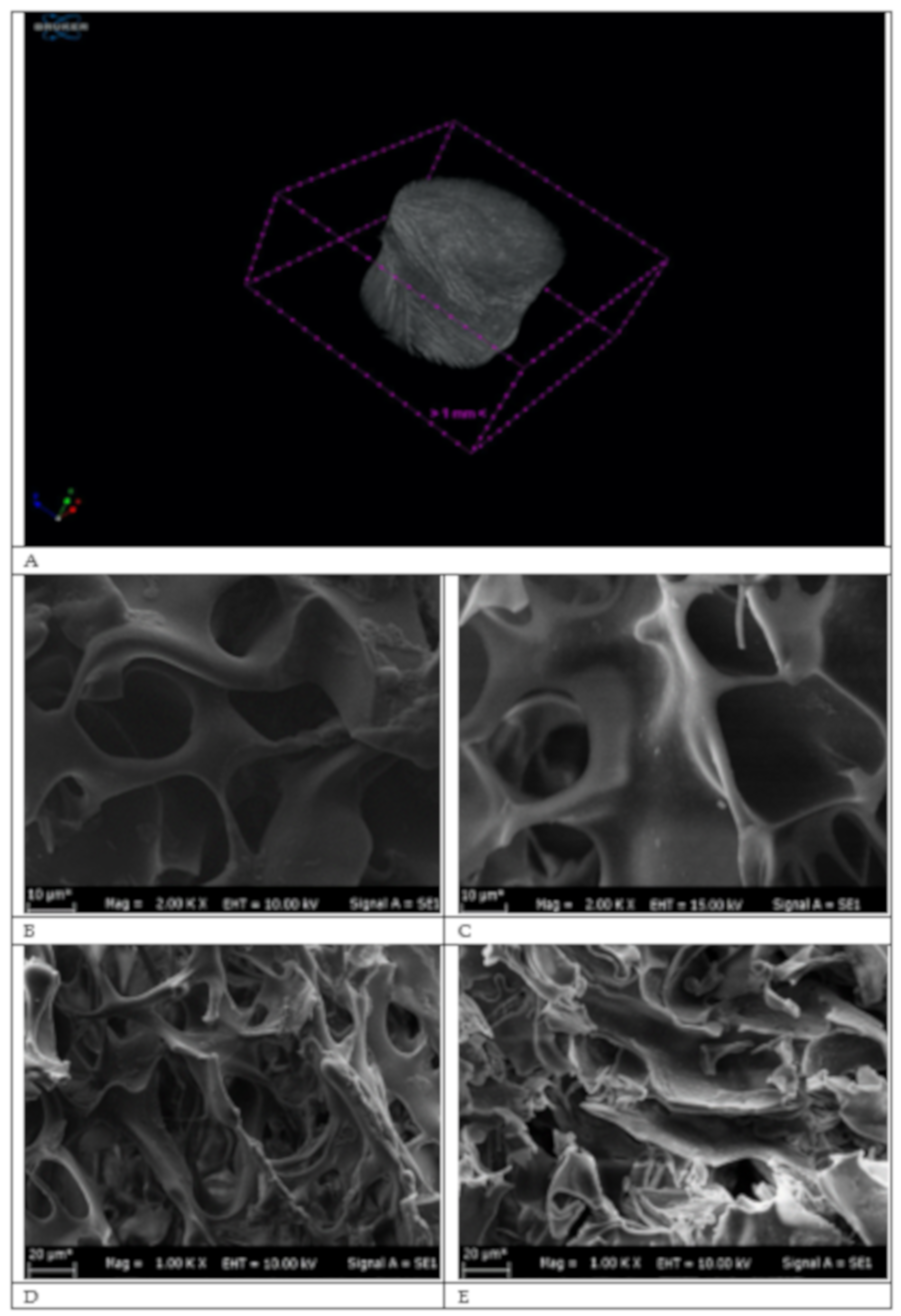
2.1. Characterization
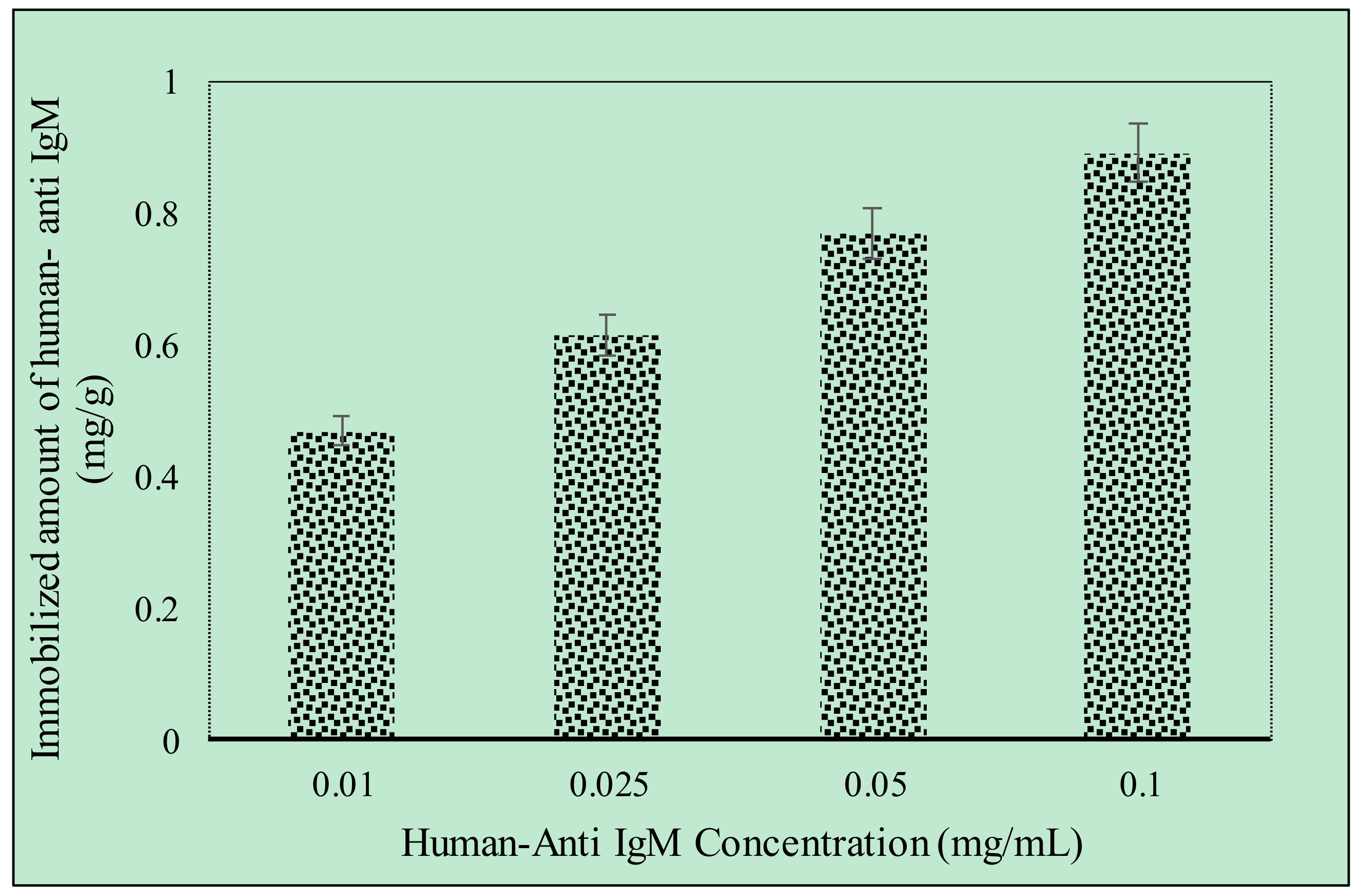
2.2. Effect of Different Concentrations of Anti-hIgM on Higm Binding
2.3. Effect of Adsorption Parameters on hIgM Binding
2.4. hIgM Purification from Artificial Human Plasma
2.5. Literature Comparison with the Present Study
2.6. Reusability of the p(HEMA-I) Column
3. Conclusions
4. Materials and methods
4.1. Materials
4.2. Preparation of p(HEMA-I) Cryogel Column
4.3. Preparation of Immunoaffinity p(HEMA-I) Cryogel Column
4.4. Characterization Studies
4.4.1. Properties of P(HEMA) and P(HEMA-I) Cryogel Columns Swollen in Water
4.4.2. Characterization of the p(HEMA) and the p(HEMA-I) cryogel columns
4.5. Purification of hIgM from Aqueous Solution
4.6. hIgM Purification from Artificial Plasma
4.7. Reusability of Cryogel Column
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gautam, S.; Loh, C.H. Immunoglobulin M purification-challenges and perspectives. Biotechnol. Adv. 2011, 29, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Winau, F.; Winau, R. Emil von Behring and Serum therapy. Microbes. Infect. 2002, 4, 185–188. [Google Scholar] [CrossRef]
- Chames, P.; Regenmortel, M.V.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Brit. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.C.A.; Silva, C.S.O.; Taipa, M.A. Affinity-based methodologies and ligands for antibody purification: Advances and perspectives. J. Chromatogr. A 2007, 1160, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Bereli, N.; Senel, S. Preparation and characterization of thiophilic cryogels with 2-mercapto ethanol as the ligand for IgG purification. Colloid. Surfaces. B. 2014, 113, 261–268. [Google Scholar] [CrossRef]
- Luo, Y.D.; Lin, D.Q.; Yuan, X.M.; Shi, W.; Yao, S.J.; Lin, D.Q. Selectivity evaluation and separation of human immunoglobulin G, Fab and Fc fragments with mixed-mode resins. J. Chromatogr. B 2017, 1040, 105–111. [Google Scholar] [CrossRef]
- Tong, H.F.; Lin, D.Q.; Yao, S.J. Purification of immunoglobulin IgY with hydrophobic charge induction chromatography. Chin. J. Chem. Eng. 2011, 62, 1574–1580. [Google Scholar]
- Luo, Y.; Zhang, Q.; Yao, S.; Lin, D. Adsorption behaviors of avian immunoglobulins and purification of immunoglobulin Y from chicken serum with mixed-mode resins. Chin J. Chem. Eng. 2019, 27, 514–518. [Google Scholar] [CrossRef]
- Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 2000, 37, 1141–1149. [Google Scholar] [CrossRef]
- Liu, Z.; Gurgel, P.V.; Carbonell, R.G. Effects pf peptide and elution pH on affinity chromatographic purification of human immunoglobulibs A and M. J. Chroma. A. 2011, 1218, 8344–8352. [Google Scholar] [CrossRef]
- Vollmers, H.P.; Hensel, F.; Hermann, R.; Dämmrich, J.; Wozniak, E.; Gessner, P.; Müller-Hermelink, H.K. Tumor-specific apoptosis induced by the human monoclonal antibody SC-1: A New therapeutical approach for stomach cancer. Oncol. Rep. 1998, 5, 35–75. [Google Scholar] [CrossRef] [PubMed]
- Brandlein, S.; Pohle, T.; Vollmers, C.; Wozniak, E.; Ruoff, N.; Müller-Hermelink, H.K.; Vollmers, H.P. CFR-1 receptor as target for tumor-specific apoptosis induced by the natural human monoclonal antibody PAM-1. Oncol. Rep. 2004, 11, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Brandlein, S.; Rauschert, N.; Rasche, L.; Dreykluft, A.; Hensel, F.; Conzelmann, E.; Müller-Hermelink, H.K.; Vollmers, H.P. The human IgM antibody SAM-6 induces tumor-specific apoptosis with oxidized low-density lipoprotein. Mol. Cancer. Ther. 2007, 6, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.D.; Zhang, Q.L.; Yao, S.J.; Lin, D.Q. Evaluation of adsorption selectivity of immunoglobulins M, A and G and purification of immunoglobulin M with mixed-mode resins. J. Chromatogr. A 2018, 1533, 77–86. [Google Scholar] [CrossRef]
- Roque, A.C.A.; Lowe, C.R.; Taipa, M.A. Antibodies and genetically engineered related molecules: Production and purification. Biotechnol. Prog. 2004, 20, 639–654. [Google Scholar] [CrossRef]
- Liu, Z.; Gurgel, P.V.; Carbonell, R.G. Purification of human immunoglobulins A, G and M from Cohn fraction II/III by small peptide affinity chromatography. J. Chromatog. A 2012, 1262, 169–179. [Google Scholar] [CrossRef]
- Tscheliessnig, A.; Ong, D.; Lee, J.; Pan, S.; Satianegara, G.; Schriebl, K.; Choo, A.; Jungbauer, A. Engineering of a two-step purification strategy for a panel of monoclonal immunoglobulin M directed against undifferetiated embryonic stem cells. J. Chromatogr. A 2009, 1216, 7851–7864. [Google Scholar] [CrossRef]
- Gagnon, P. Improved antibody aggregate removal by hydroxyapatite chromatography in the presence of polyethylene glycol. J. Immunol. Meth. 2008, 336, 222–228. [Google Scholar] [CrossRef]
- Lee, J.; Tscheliessing, A.; Chen, A.; Chen, A.; Lee, Y.Y.; Adduci, G.; Choo, A.; Jungbauer, A. Adaptation of hybridomas to protein-free media results in a simplified two-step immunoglobulin M purification process. J. Chromatogr. A 2009, 1216, 2683–2688. [Google Scholar] [CrossRef]
- Clamont, G.R.; Plata, M.C.C.; Zamudio, R.G.; Moreno, L.V. Novel hydrophobic interaction chromatography matrix for specific isolation and simple elution of immunoglobulins (A, G, and M) from porcine serum. J. Chromatogr. A 2006, 1122, 28–34. [Google Scholar] [CrossRef]
- Neff, S.; Jungauer, A. Monolith peptide affinity chromatography for quantification of immunoglobulin M. J. Chromatogr. A 2011, 1218, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Palombo, G.; Verdoliva, A.; Fassina, G. Affinity purification of immunoglobulin M using a novel synthetic ligand. J. Chromatogr. B 1998, 715, 137–145. [Google Scholar] [CrossRef]
- Liu, Z.; Gurgel, P.V.; Carbonell, R.G. Affinity chromatographic purification of human immunoglobulin M from human B lymphocyte cell culture supernatant. Biochem. Eng. J. 2013, 70, 63–70. [Google Scholar] [CrossRef]
- Göktürk, I.; Perçin, I.; Denizli, A. Catalase purification from rat liver with iron-chelated poly(hydroxyethyl methacrylate-N-methacryloyl-(l)-glutamic acid) cryogel discs. Prep Biochem. Biotechnol. 2016, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Saçlıgil, D.; Şenel, S.; Yavuz, H.; Denizli, A. Purification of transferrin by magnetic immunoaffinity beads. J. Sep. Sci. 2015, 38, 2729–2736. [Google Scholar]
- Yao, K.; Wen, K.; Shan, X.W.S.; Penga, T.; Wang, J.; Jianga, H.; Shao, B. Development of an immunoaffinity column for the highly sensitive analysis of bisphenol A in 14 kinds of foodstuffs using ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2018, 1080, 50–58. [Google Scholar] [CrossRef]
- Cetin, K.; Denizli, A. Immunoaffinity microcryogels for purification of transferrin. J. Chromatogr. B 2019, 1114, 5–12. [Google Scholar] [CrossRef]
- Denizli, A. Preparation of immuno-affinity membranes for cholesterol removal from human plasma. J. Chromatogr. B 2002, 772, 357–367. [Google Scholar] [CrossRef]
- Moser, A.C.; Hage, D. Immunoaffinity chromatography: An introduction to applications and recent developments. Bioanalysis 2010, 2, 769–790. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Kong, F. Separation of antigens and antibodies by immunoaffinity chromatography. Pharm. Biol. 2012, 50, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. Appl. Sci. 2019, 9, 553–575. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, G.R.F.; Gandolfi, O.R.R.; Santos, L.S.; Bonomo, R.C.F.; Velosoa, C.M.; Veríssimo, L.A.A.; Fontan, R.C.I. Immobilization of sugars in supermacroporous cryogels for the purification of lectins by affinity chromatography. J. Chromatogr. B 2017, 1068–1069, 71–77. [Google Scholar]
- Santos, M.; Brito, A.; Boto, R.; Sousa, P.; Almeida, C.; Cru, C.; Tomaz, C. Influenza DNA vaccine purification using pHEMA cryogel support. Sep. Purif. Technol. 2018, 192–198. [Google Scholar] [CrossRef]
- Binbin, W.; Li, J.; Ma, F.; Yu, N.; Zhang, W.; Jiang, L.; Wei, H. Preparation and properties of cryogel based on poly(2-hydroxyethyl methacrylate-co-glycidyl methacrylate). Langmuir 2019, 35, 3284–3294. [Google Scholar]
- Bakhshpour, M.; Yavuz, H.; Denizli, A. Controlled release of mitomycin C from PHEMAH–Cu(II) cryogel membranes. Artif. Cell. Nanomed. B 2018, 46, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 50. Cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Attieh-Daoud, M.; Chaib, H.; Armutcu, C.; Uzun, L.; Elkak, A.; Denizli, A. Immunoglobulin g purification from bovine serum with pseudo-specific macroporous cryogels. Sep. Purif. Technol. 2013, 118, 816–822. [Google Scholar] [CrossRef]
- Derazshamshir, A.; Baydemir, G.; Yılmaz, F.; Bereli, N.; Denizli, A. Preparation of cryogel columns for depletion of hemoglobin from human blood. Artif. Cell. Nanomed. B 2016, 44, 3–792. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Zhang, Q.; Zhong, Q.; Liu, L.; Gao, H.; Meng, R.; Yao, J. Macroporous cellulose-based cryogels with tunable porous structure and surface functional groups. Fibers Polym. 2016, 17, 712–720. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei Loghin, D.F.; Cocarta, A.I.; Doroftei, M. Multi-stimuli-responsive semi-IPN cryogels with native and anionic potato starch entrapped in poly(N,N-dimethylaminoethyl methacrylate) matrix and their potential in drug delivery. React. Funct. Polym. 2016, 105, 66–77. [Google Scholar] [CrossRef]
- Brne, P.; Podgorhik, A.; Benčina, K.; Gabor, B.; Štrancar, S.; Peterka, A. Fast and efficient separation of immunoglobulin M from immunoglobulin G using short monolithic columns. J. Chromatogr. A 2007, 120, 125. [Google Scholar] [CrossRef] [PubMed]
- Hennicke, J.; Reinhart, D.; Altmann, F.; Kunert, R. Impact of temperature and pH on recombinant human IgM quality attributes and productivity. New Biotechnol. 2019, 50, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hennicke, J.; Lastin, A.M.; Reinhart, D.; Grünwald-Gruber, K.; Altmann, F.; Kunert, R. Glycan profile of CHO derived IgM purified by highly efficient single step affinity chromatography. Anal. Biochem. 2017, 539, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, S.M. An overview of purification methods for proteins. Int. J. Appl. Res. 2015, 1, 12–450. [Google Scholar]
- Bereli, N.; Ertürk, G.; Tümer, M.A.; Say, R.; Denizli, A. Oriented immobilized anti-hIgG via Fc fragment-imprinted PHEMA cryogel for IgG purification. Biomed. Chromatogr. 2013, 27, 599–607. [Google Scholar] [CrossRef]
- Saylan, Y.; Bereli, N.; Uzun, L.; Denizli, A. Monolithic boronate affinity columns for IgG separation. Sep. Sci. Technol. 2014, 49, 1555–1565. [Google Scholar] [CrossRef]
- Sedlačík, T.; Proks, V.; Šlouf, M.; Dušková-Smrčkova, M.; Studenovská, H.; Rypáček, F. Macroporous Biodegradable Cryogels of Synthetic Poly(α-amino acids). Biomacromolecules 2015, 16, 3455–3465. [Google Scholar]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.-R.; Gomez, D.; Mikhalovsky, S.; Galaev, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter 2005, 1, 303–309. [Google Scholar] [CrossRef]
- Douglas, L.V.M.; Vermeulen, T. Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 1981, 85, 3247–3250. [Google Scholar]
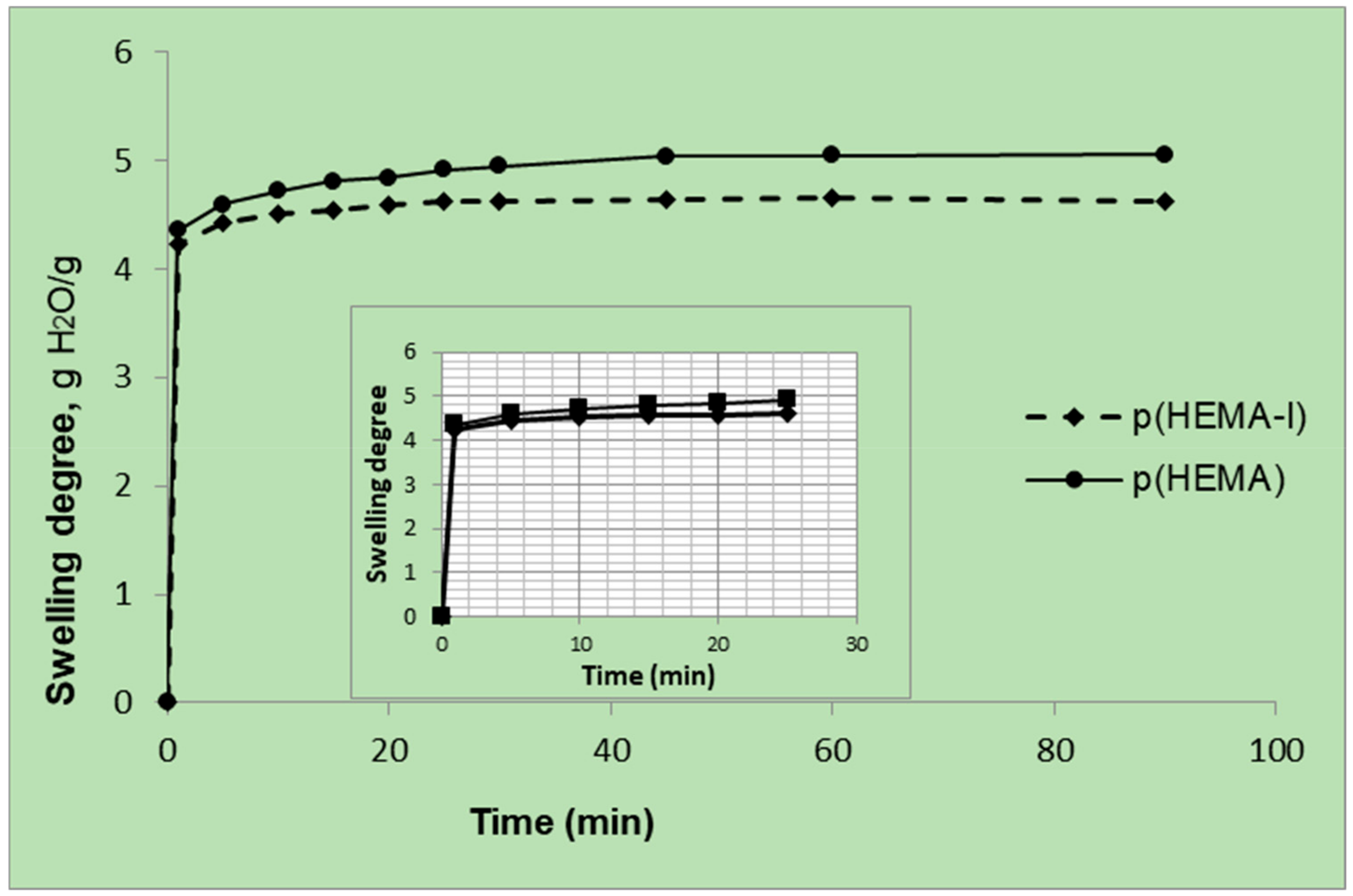
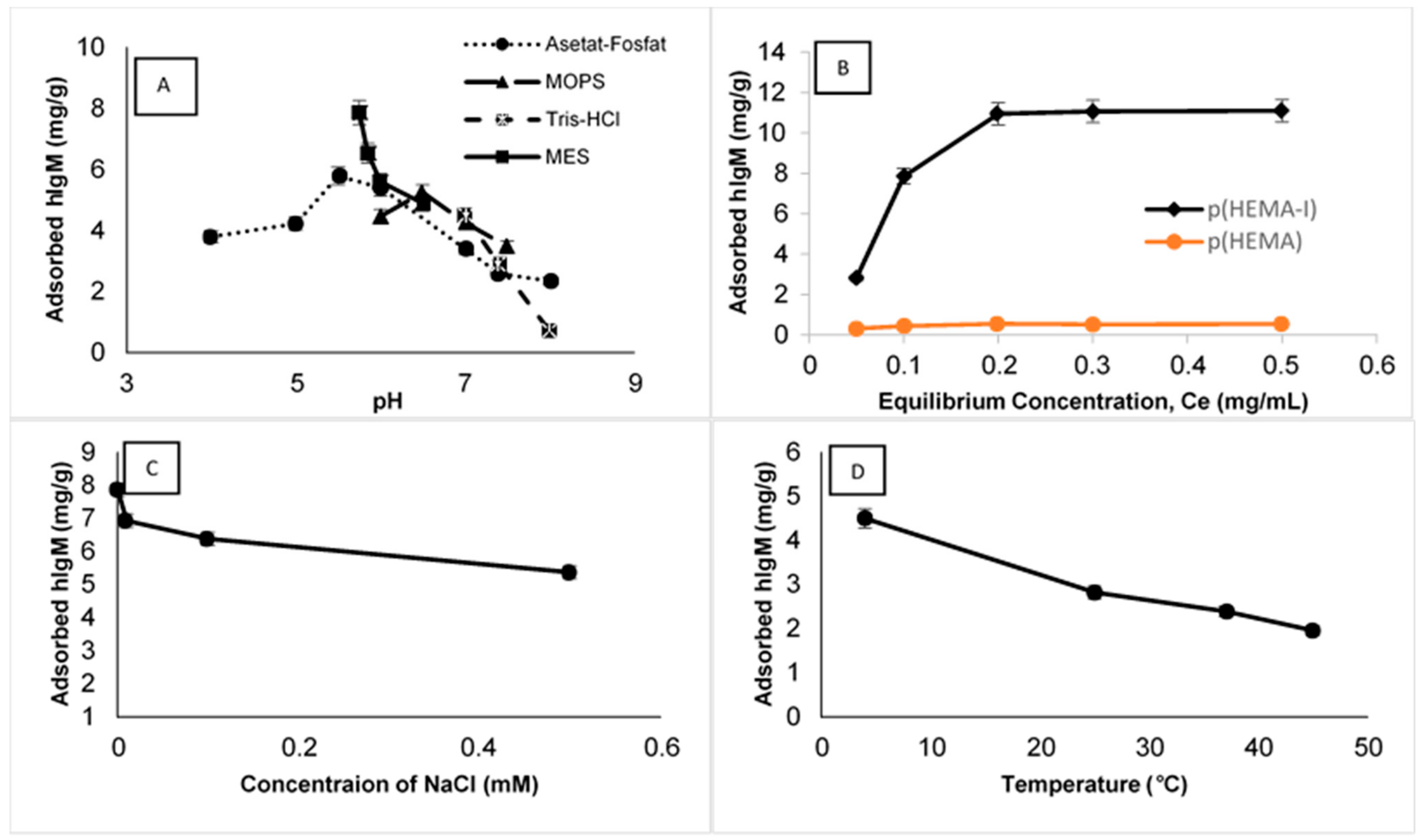
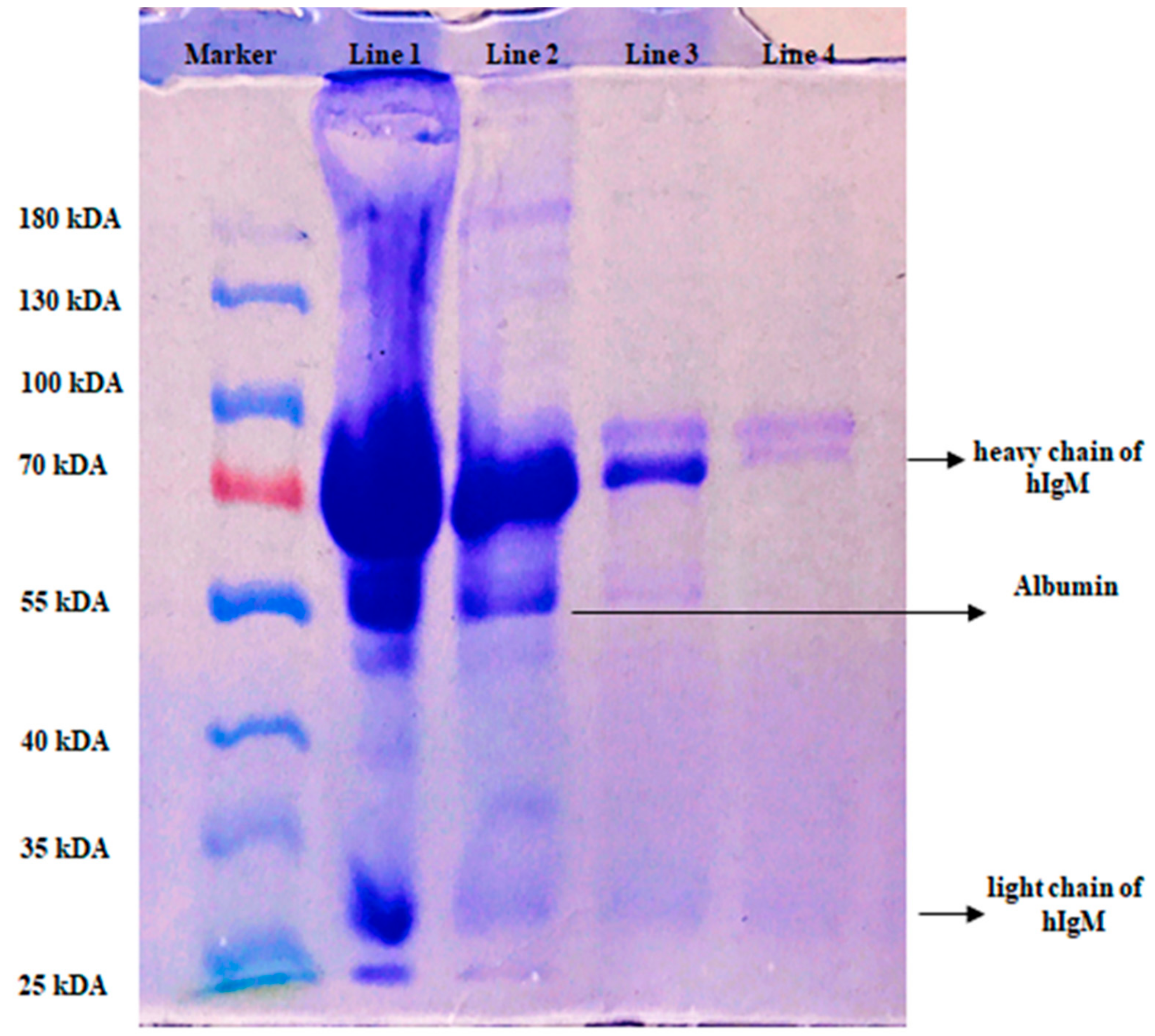
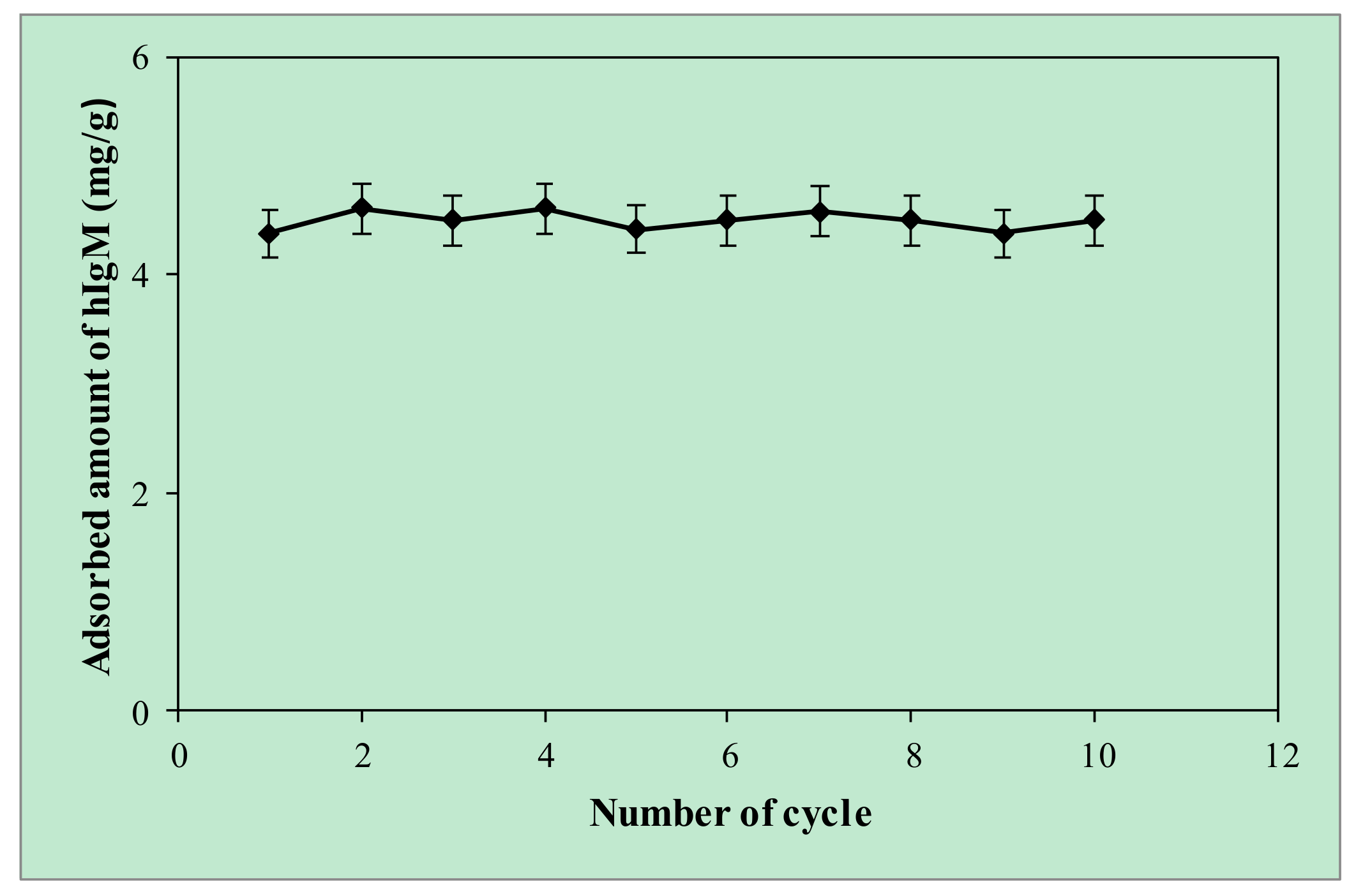
| Cryogel Samples | Swelling Degree (gH2O/g Cryogel) | Macroporosity (Volume %) |
|---|---|---|
| p(HEMA) | 5.05 | 89.6 |
| p(HEMA-I) | 4.63 | 88.1 |
| Experimental | Langmuir Constants | Freundlich Constants | Langmuir–Freundlich Constants | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | Qmax (mg/g) | bL (mL/mg) | R2 | Kf | n | R2 | Qmax (mg/g) | KLF (mL/mg) | R2 | |
| 11.10 | 11.83 | 45.37 | 0.99 | 11.24 | 75.18 | 0.89 | 10.1 | 1.1 | 0.97 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshpour, M.; Topcu, A.A.; Bereli, N.; Alkan, H.; Denizli, A. Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M. Gels 2020, 6, 4. https://doi.org/10.3390/gels6010004
Bakhshpour M, Topcu AA, Bereli N, Alkan H, Denizli A. Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M. Gels. 2020; 6(1):4. https://doi.org/10.3390/gels6010004
Chicago/Turabian StyleBakhshpour, Monireh, Aykut Arif Topcu, Nilay Bereli, Huseyin Alkan, and Adil Denizli. 2020. "Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M" Gels 6, no. 1: 4. https://doi.org/10.3390/gels6010004
APA StyleBakhshpour, M., Topcu, A. A., Bereli, N., Alkan, H., & Denizli, A. (2020). Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M. Gels, 6(1), 4. https://doi.org/10.3390/gels6010004