Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring
Abstract
1. Introduction
2. Results and Discussion
2.1. Gel Formation
2.2. Isothermal Rheological Measurements
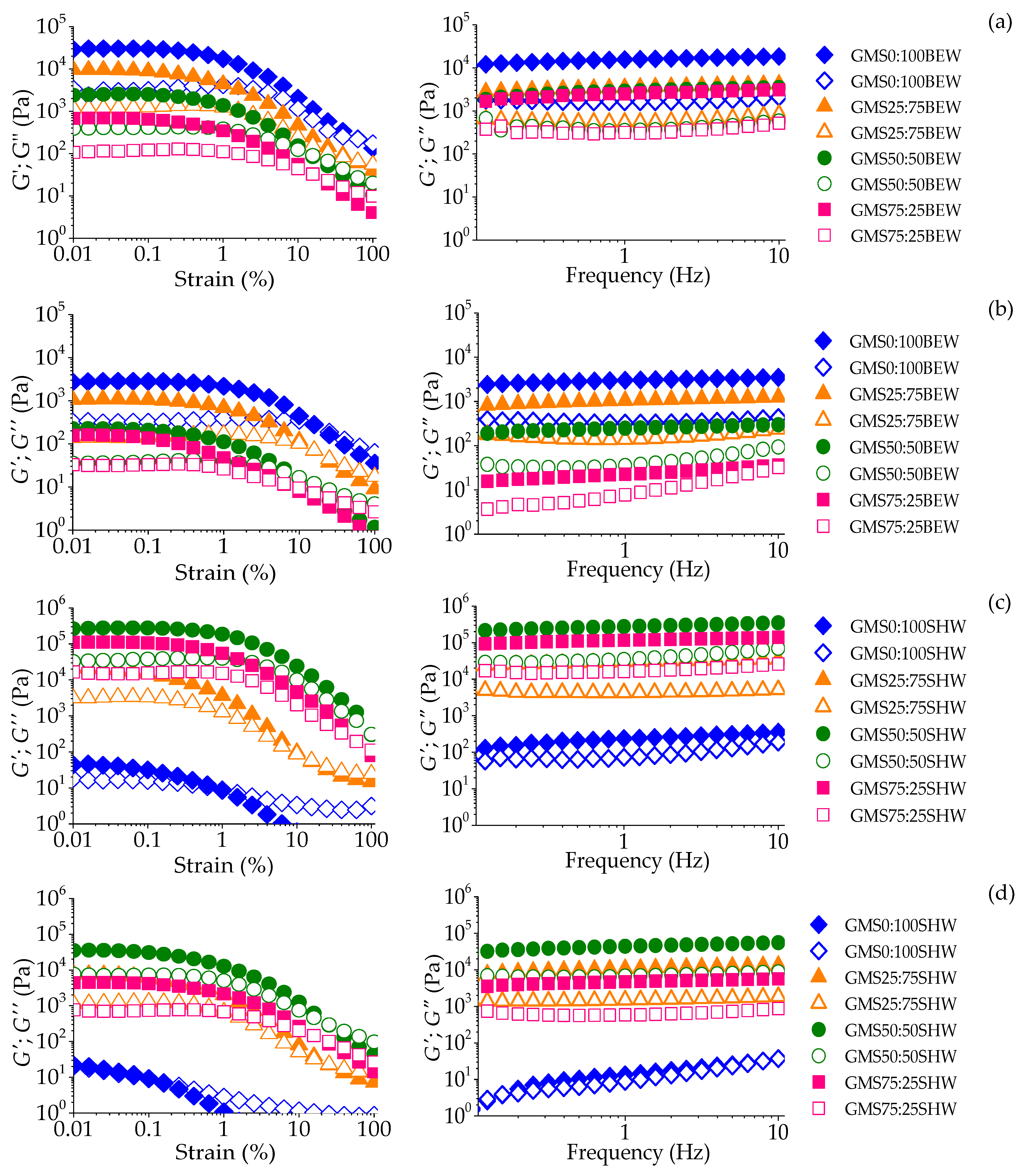
2.2.1. Strain Sweep
2.2.2. Frequency Sweep
2.2.3. Thixotropy
2.3. Non-Isothermal Measurements
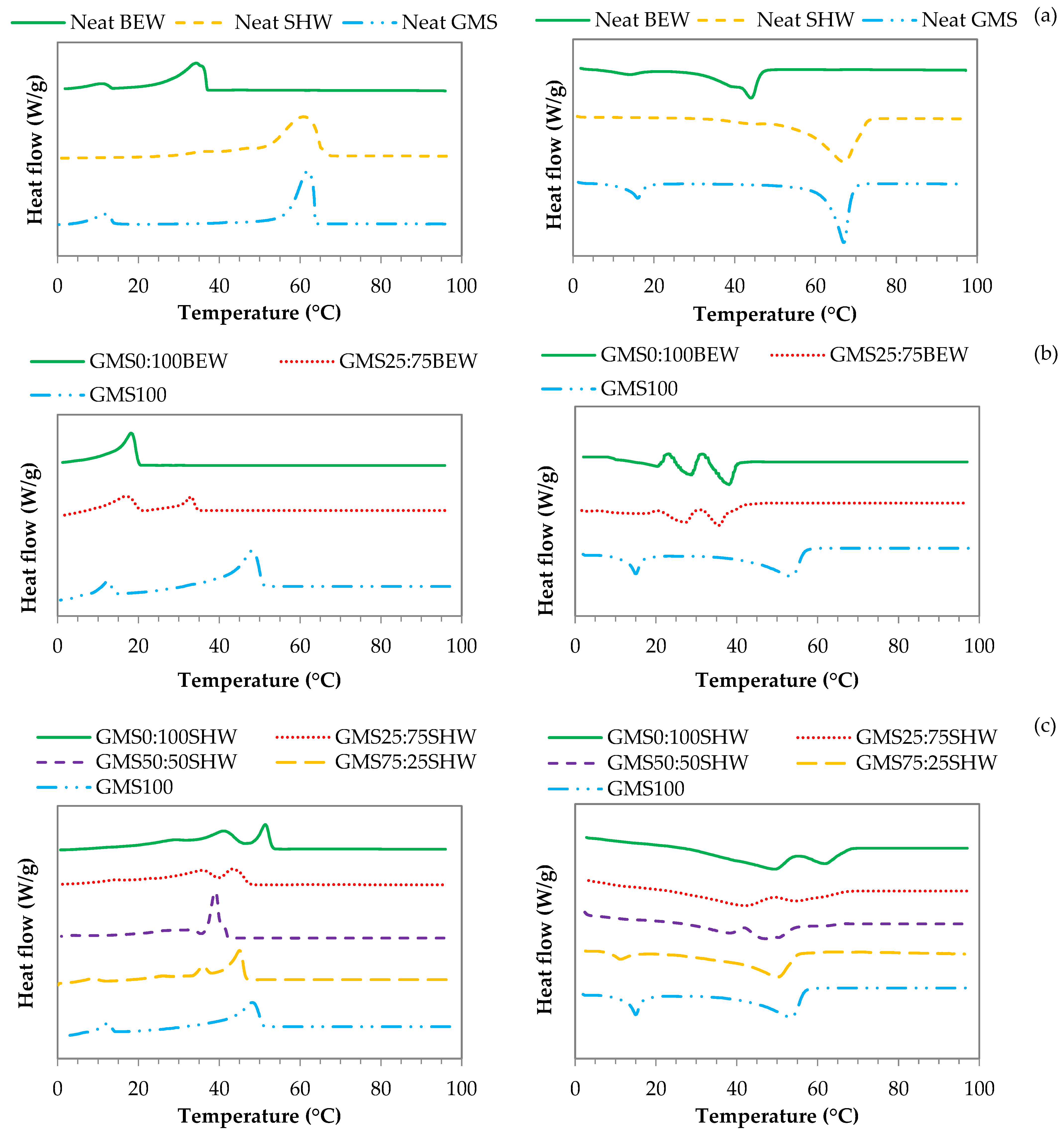
2.3.1. Differential Calorimetry Scanning-DSC
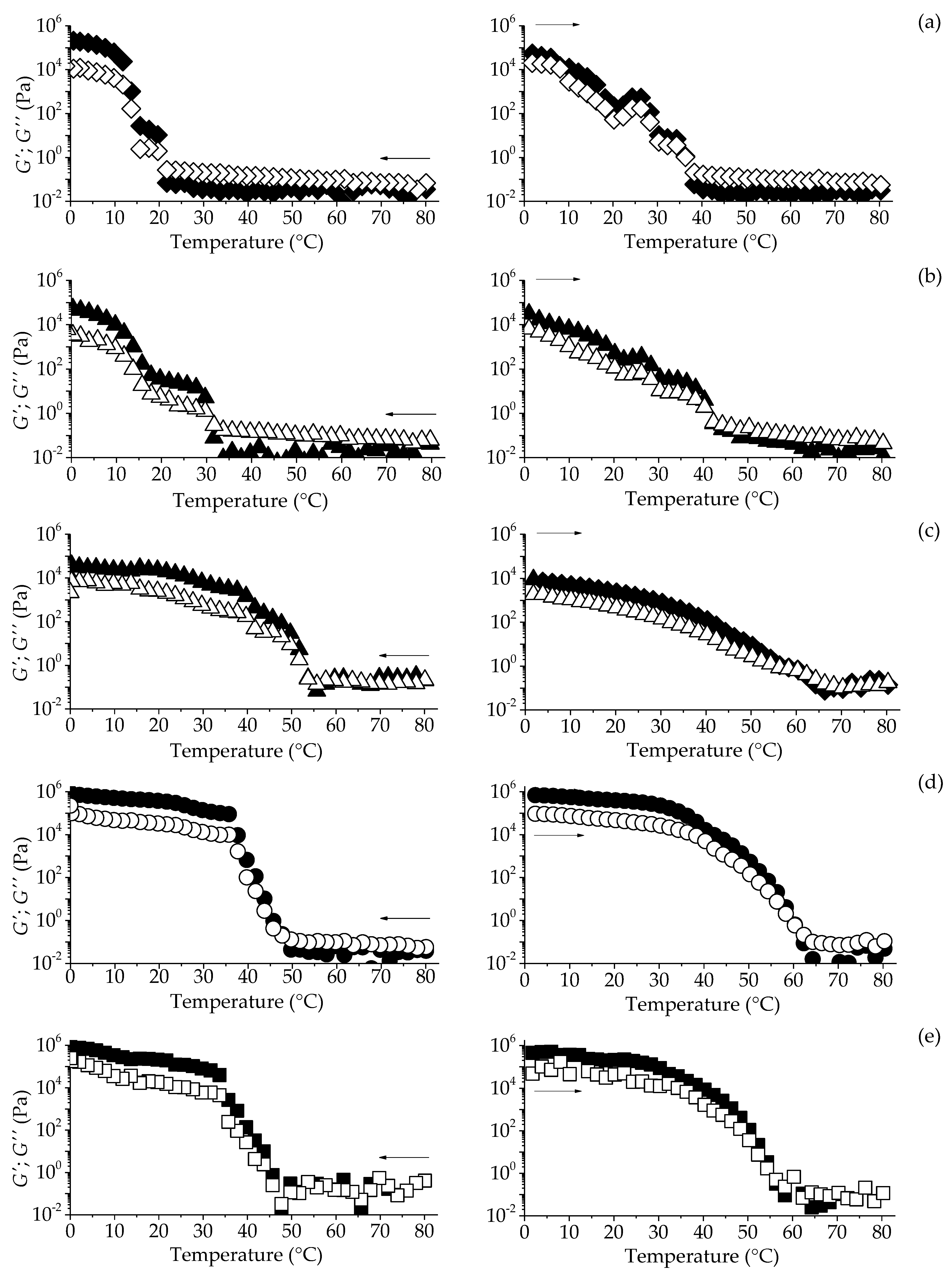
2.3.2. Temperature Sweep-From Rheological Measurements
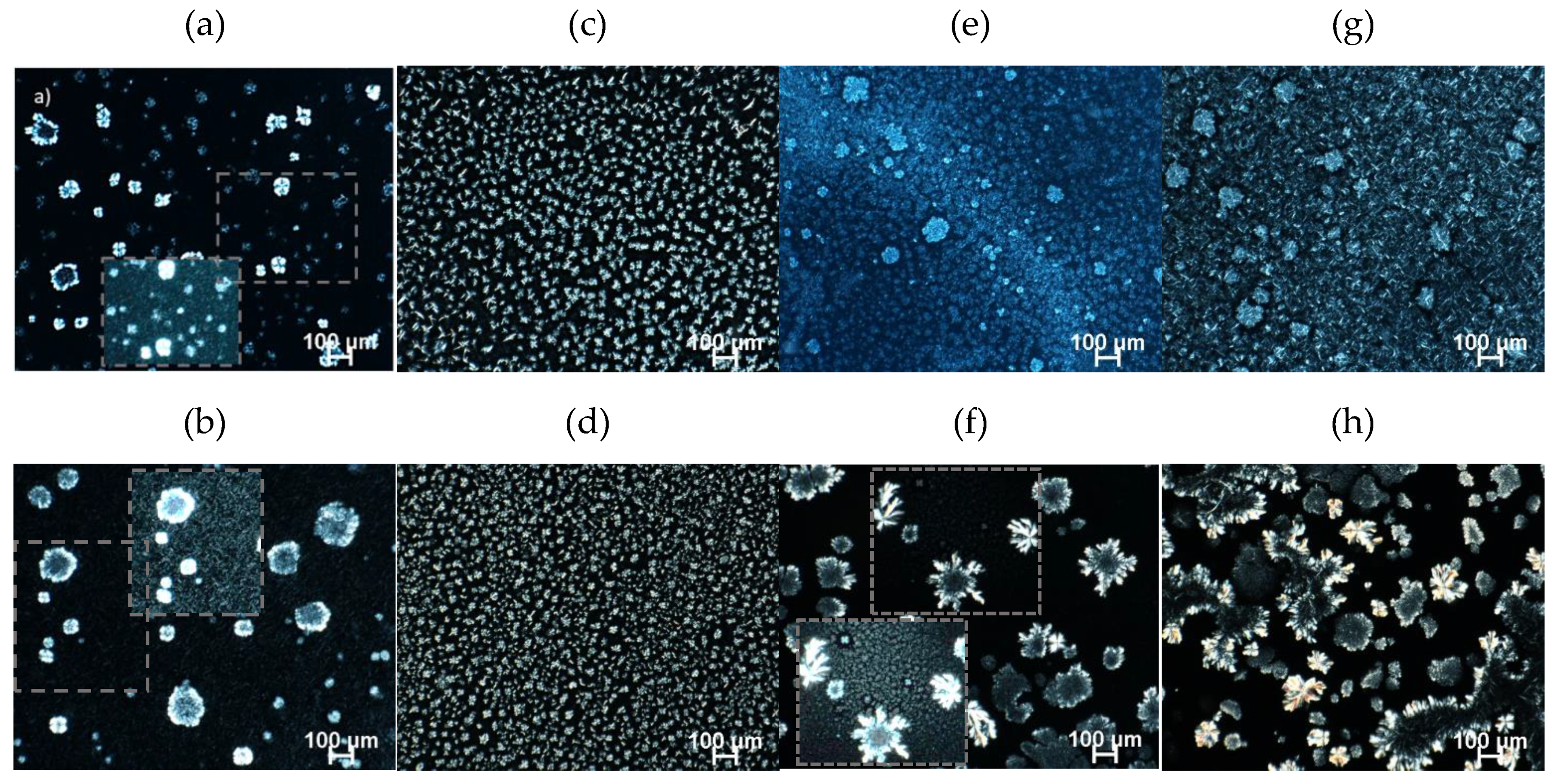
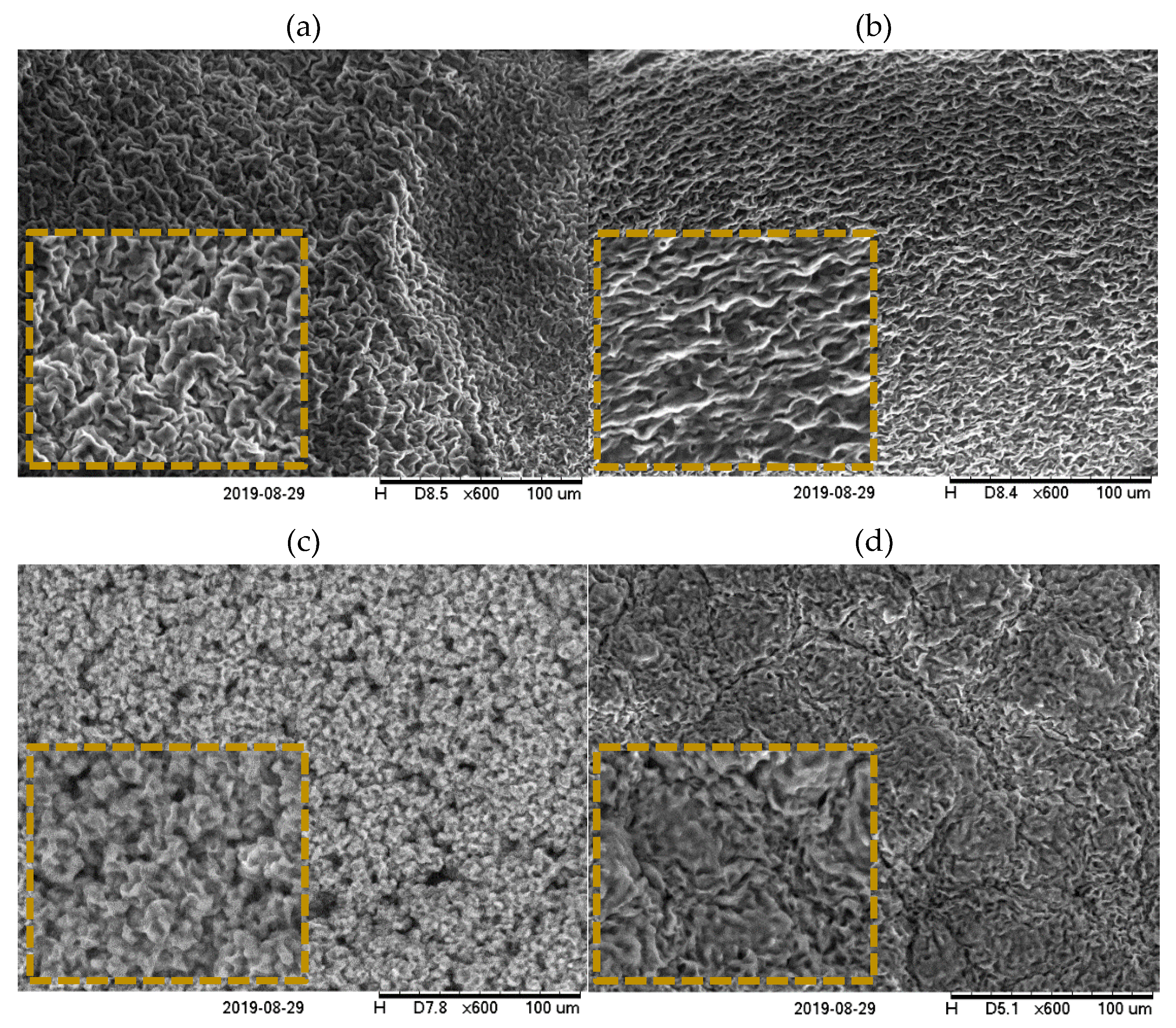
2.4. Microstructure
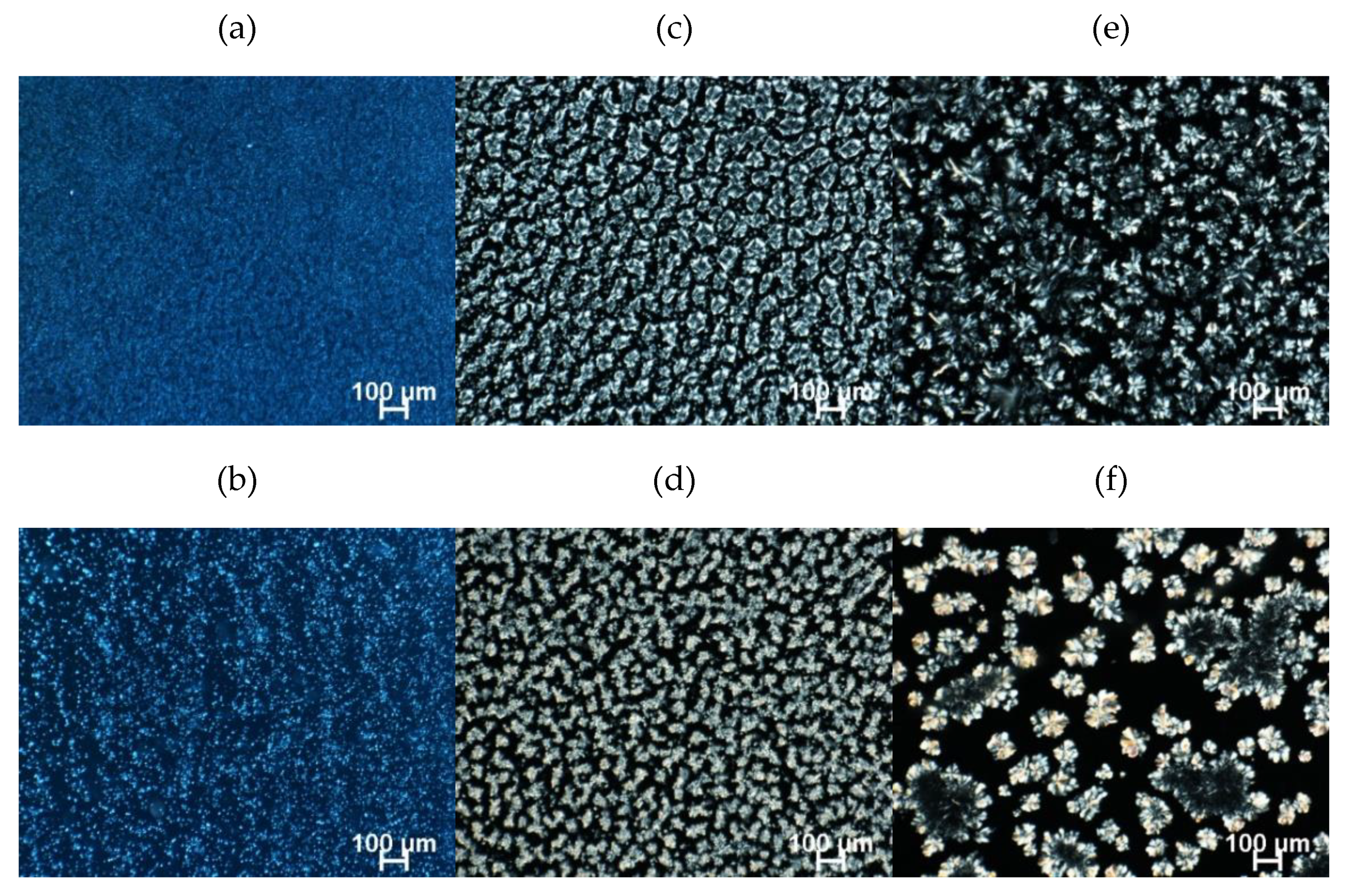
2.4.1. Polarized Light Microscopy (PLM)
2.4.2. Scanning Electron Microscopy (SEM)
2.5. Oil Binding Capacity (OBC)
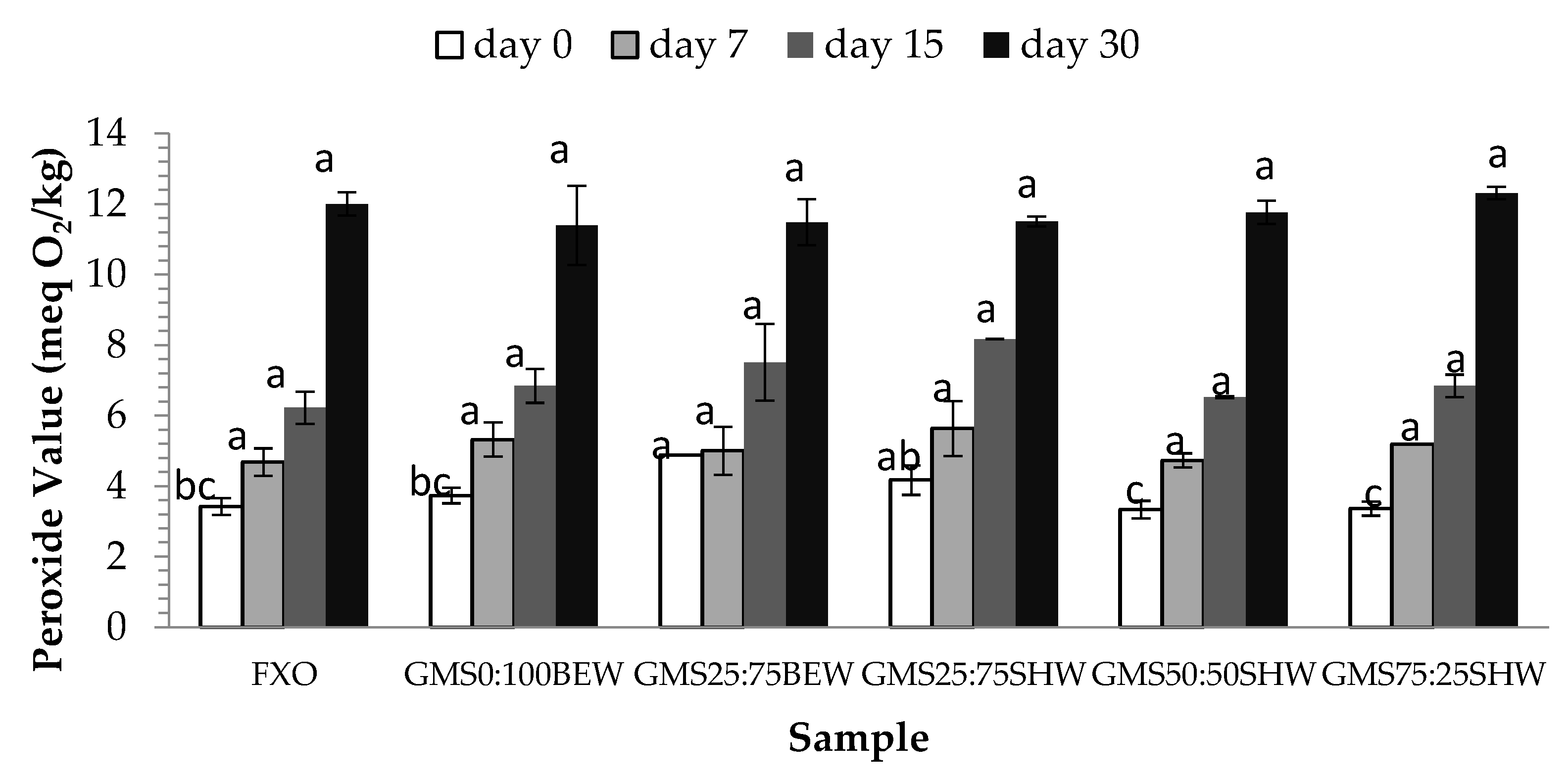
2.6. Oxidative Stability (OS)
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Preparation of Oleogels
4.3. Characterization of oleogels
4.3.1. Rheological Measurements
- Strain and frequency sweeps (isothermal measurements)
- Thixotropy (isothermal measurement)
- Temperature sweeps (non-isothermal measurements)
4.3.2. Differential Scanning Calorimetry (DSC)
4.3.3. Microstructure
- Polarized light microscopy (PLM)
- Scanning Electron Microscopy (SEM)
4.3.4. Oil Binding Capacity (OBC)
4.3.5. Oxidative Stability (OS)
4.3.6. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romagny, S.; Ginon, E.; Salles, C. Impact of reducing fat, salt and sugar in commercial foods on consumer acceptability and willingness to pay in real tasting conditions: A home experiment. Food Qual. Prefer. 2017, 56, 164–172. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.É.; Ferraz, A.C.G.; Portovedo, M.; Reginato, A.; Stahl, M.A.; Ignacio-Souza, L.M.; Chan, K.L.; Torsoni, A.S.; Torsoni, M.A.; Ribeiro, A.P.B.; et al. Interesterified soybean oil promotes weight gain, impaired glucose tolerance and increased liver cellular stress markers. J. Nutr. Biochem. 2018, 59, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Öğütcü, M.; Yilmaz, E. Characterization of Hazelnut Oil Oleogels Prepared with Sunflower and Carnauba Waxes. Int. J. Food Prop. 2015, 18, 1741–1755. [Google Scholar] [CrossRef]
- Patel, A.R. Alternative Routes to Oil Structuring; Springer: New York, NY, USA, 2015; ISBN 978-3-319-19137-9. [Google Scholar]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Anderson, J.; Felker, F.C.; Hwang, H. Physical Properties of Beeswax, Sunflower Wax, and Candelilla Wax Mixtures and Oleogels. J. Am. Oil Chem. Soc. 2019, 96, 1125–1142. [Google Scholar] [CrossRef]
- Palla, C.; de Vicente, J.; Carrín, M.E.; Gálvez Ruiz, M.J. Effects of cooling temperature profiles on the monoglycerides oleogel properties: A rheo-microscopy study. Food Res. Int. 2019, 125, 108613. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Sterol-based oleogels’ characterization envisioning food applications. J. Sci. Food Agric. 2019, 99, 3318–3325. [Google Scholar] [CrossRef]
- Harris, L.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. Gelation of oil using combination of different free fatty acids. Food Struct. 2019, 21, 100121. [Google Scholar] [CrossRef]
- Valoppi, F.; Calligaris, S.; Marangoni, A.G. Structure and physical properties of oleogels containing peanut oil and saturated fatty alcohols. Eur. J. Lipid Sci. Technol. 2017, 119, 1600252. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Marangoni, A.G. Internal and external factors affecting the stability of glycerol monostearate structured emulsions. RSC Adv. 2015, 5, 93108–93116. [Google Scholar] [CrossRef]
- Callau, M.; Sow-Kébé, K.; Nicolas-Morgantini, L.; Fameau, A.-L. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J. Colloid Interface Sci. 2020, 560, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R. A colloidal gel perspective for understanding oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Raygan, F.; Taghizadeh, M.; Mirhosseini, N.; Akbari, E.; Bahmani, F.; Memarzadeh, M.R.; Sharifi, N.; Jafarnejad, S.; Banikazemi, Z.; Asemi, Z. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: A randomized, double-blinded, placebo-controlled trial. Phyther. Res. 2019, ptr.6393. [Google Scholar] [CrossRef] [PubMed]
- Buckner, A.L.; Buckner, C.A.; Montaut, S.; Lafrenie, R.M. Treatment with flaxseed oil induces apoptosis in cultured malignant cells. Heliyon 2019, 5, e02251. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Linghu, K.; Huang, S.; Battino, M.; Georgiev, M.I.; Zengin, G.; Li, D.; Deng, Y.; Wang, Y.T.; Cao, H. Flaxseed extract induces apoptosis in human breast cancer MCF-7 cells. Food Chem. Toxicol. 2019, 127, 188–196. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Veiga, C.B.; Bessi, M.P.; Dátilo, M.N.; Sant’Ana, M.R.; Rodrigues, P.B.; de Moura, L.P.; da Silva, A.S.R.; Santos, G.A.; Catharino, R.R.; et al. Unsaturated fatty acids from flaxseed oil and exercise modulate GPR120 but not GPR40 in the liver of obese mice: a new anti-inflammatory approach. J. Nutr. Biochem. 2019, 66, 52–62. [Google Scholar] [CrossRef]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter 2011, 7, 2734–2743. [Google Scholar] [CrossRef]
- Swe, M.T.H.; Asavapichayont, P. Effect of silicone oil on the microstructure, gelation and rheological properties of sorbitan monostearate–sesame oil oleogels. Asian J. Pharm. Sci. 2018, 13, 485–497. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Sintang, M.D.B.; Danthine, S.; Van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: a Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2017, 12, 97–108. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 3rd ed.; Vincentz Network GmbH & Co. KG: Hanover, Germany, 2011; ISBN 3866308647. [Google Scholar]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.F.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and mechanical properties of organogels: Role of oil and gelator molecular structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bin Sintang, M.D.; Rimaux, T.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Oil structuring properties of monoglycerides and phytosterols mixtures. Eur. J. Lipid Sci. Technol. 2017, 119, 1500517. [Google Scholar] [CrossRef]
- Uhlherr, P.H.T.; Guo, J.; Tiu, C.; Zhang, X.-M.; Zhou, J.Z.-Q.; Fang, T.-N. The shear-induced solid–liquid transition in yield stress materials with chemically different structures. J. Nonnewton. Fluid Mech. 2005, 125, 101–119. [Google Scholar] [CrossRef]
- Doan, C.D.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the Oil-Gelling Properties of Natural Waxes in Rice Bran Oil: Rheological, Thermal, and Microstructural Study. J. Am. Oil Chem. Soc. 2015, 92, 801–811. [Google Scholar] [CrossRef]
- López-Martínez, A.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Comparing the crystallization and rheological behavior of organogels developed by pure and commercial monoglycerides in vegetable oil. Food Res. Int. 2014, 64, 946–957. [Google Scholar] [CrossRef]
- Xia, D.; Cui, F.; Gan, Y.; Mu, H.; Yang, M. Design of Lipid Matrix Particles for Fenofibrate: Effect of Polymorphism of Glycerol Monostearate on Drug Incorporation and Release. J. Pharm. Sci. 2014, 103, 697–705. [Google Scholar] [CrossRef]
- Tavernier, I.; Doan, C.D.; Van De Walle, D.; Danthine, S.; Rimaux, T.; Dewettinck, K. Sequential crystallization of high and low melting waxes to improve oil structuring in wax-based oleogels. RSC Adv. 2017, 7, 12113–12125. [Google Scholar] [CrossRef]
- Pérez-Monterroza, E.J.; Márquez-Cardozo, C.J.; Ciro-Velásquez, H.J. Rheological behavior of avocado (Persea americana Mill, cv. Hass) oleogels considering the combined effect of structuring agents. LWT - Food Sci. Technol. 2014. [Google Scholar]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Chen, C.H.; Terentjev, E.M. Aging and Metastability of Monoglycerides in Hydrophobic Solutions. Langmuir 2009, 25, 6717–6724. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.I.; Marangoni, A.G. Plant wax crystals display platelet-like morphology. Food Struct. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Patel, A.R.; Schatteman, D.; De Vos, W.H.; Dewettinck, K. Shellac as a natural material to structure a liquid oil-based thermo reversible soft matter system. RSC Adv. 2013, 3, 5324. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. The use of cooling rate to engineer the microstructure and oil Binding capacity of wax crystal networks. Food Biophys. 2015, 10, 456–465. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Wang, Q.; Decker, E.A.; Rao, J.; Chen, B. A combination of monoacylglycerol crystalline network and hydrophilic antioxidants synergistically enhances the oxidative stability of gelled algae oil. Food Funct. 2019, 10, 315–324. [Google Scholar] [CrossRef]
- Nykter, M.; Kymäläinen, H.-R.; Gates, F. Quality characteristics of edible linseed oil. Agric. Food Sci. 2006, 15, 402–413. [Google Scholar] [CrossRef]
- Codex standard for named vegetable oils - CX-STAN 210 - 1999. Available online: https://mvo.nl/media/voedselveiligheid/codex_standard_named_vegetable_oils.pdf (accessed on 21 October 2019).
- Miao, S.; Lin, D. Monoglycerides: Categories, Structures, Properties, Preparations, and Applications in the Food Industry. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128140260. [Google Scholar]
- Picone, C.S.F.; Cunha, R.L. Influence of pH on formation and properties of gellan gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef]
- Okuro, P.K.; Malfatti-Gasperini, A.A.; Vicente, A.A.; Cunha, R.L. Lecithin and phytosterols-based mixtures as hybrid structuring agents in different organic phases. Food Res. Int. 2018, 111, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.C.; Marangoni, A.G. Characterization of the Nanoscale in Triacylglycerol Crystal Networks. Cryst. Growth Des. 2010, 10, 3327–3333. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Cunha, R.L.; Vicente, A.A. Fortified beeswax oleogels: Effect of β-carotene on the gel structure and oxidative stability. Food Funct. 2017, 8, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
| Initial Viscosity (Pa.s) | Viscosity Recovery (%) | |||
|---|---|---|---|---|
| Sample | 5 °C | 25 °C | 5 °C | 25 °C |
| GMS0:100BEW | 460.5 ± 10.6 a;A | 279.7 ± 16.5 a;B | 32.7 ± 0.6 b;B | 77.5 ± 2.3 a;A |
| GMS25:75BEW | 155.3 ± 27.1 b;A | 103 ± 4.2 b;A | 56.5 ± 5.0 a;A | 26.7 ± 3.7 b;B |
| GMS25:75SHW | 478 ± 33.9 c;A | 167 ± 24 c;B | 13.1 ± 0.6 b;A | 6.1 ± 0.7 a;B |
| GMS50:50SHW | 1465 ± 134.4 a;A | 975 ± 34 a | 27.8 ± 0.7 a; | 11.2 ± 3.1 a;B |
| GMS75:25SHW | 980.5 ± 21.9 b;A | 257.7 ± 10.7 b;B | 10.4 ± 1.9 b;A | 10.5 ±1.5 a;A |
| Sample | ΔHmI (J/g) | ΔHmII (J/g) | TC1, onset (°C) | Tsol-gel (°C) | Tgel-sol (°C) |
|---|---|---|---|---|---|
| GMS0:100BEW | 8.51 ± 1.58 c;A | 7.91 ± 0.38 b;A | 19.83 ± 0.13 c | 20.70 ± 0.00 b | 37.30 ± 0.00 b |
| GMS25:75BEW | 9.73 ± 2.28 ab;A | 8.21 ± 0.58 b;A | 33.32 ± 1.61 b | 30.70 ± 0.00 a | 42.30 ± 1.41 a |
| GMS100 | 18.78 ± 2.96 a;A | 16.01 ± 0.83 a;A | 50.47 ± 0.35 a | - | - |
| GMS0:100SHW | 20.72 ± 0.43 a;A | 20.87 ± 1.34 a;A | 51.47 ± 2.04 a | - | - |
| GMS25:75SHW | 18.88 ± 0.84 a;A | 16.58 ± 1.12 b;A | 48.90 ± 2.68 ab | 53.70 ± 1.41 a | 61.97 ± 3.06 a |
| GMS50:50SHW | 20.36 ± 2.81 a;A | 13.65 ± 0.66 b;A | 42.78 ± 1.29 c | 45.37 ± 3.06 c | 60.30 ± 1.41 a |
| GMS75:25SHW | 20.32 ± 0.11 a;A | 14.16 ± 0.65 b;B | 46.65 ± 0.37 ab | 46.70 ± 0.00 ab | 59.30 ± 2.00 a |
| GMS100 | 18.78 ± 2.96 a;A | 16.01 ± 0.83 b;A | 50.47 ± 0.35 a | - | - |
| Neat BEW | 137 ± 0.4 | 100.8 ± 0.8 | 37.07 ± 0.15 | - | - |
| Neat SHW | 209 ± 12 | 187.8 ± 3.8 | 65.31 ± 0.26 | - | - |
| Neat GMS | 137 ± 0.4 | 100.9 ± 0.8 | 64.02 ± 0.25 | - | - |
| OBC (%) | ||
|---|---|---|
| Sample | 5 °C | 25 °C |
| GMS0:100BEW | 100.0 ± 0.0 a; A | 96.7 ± 1.2 a; B |
| GMS25:75BEW | 92.7 ± 0.6 b; A | 65.1 ± 4.3 b; B |
| GMS25:75SHW | 94.3 ± 6.5 a; A | 81.9 ± 6.9 b; A |
| GMS50:50SHW | 100 ± 0.0 a; A | 93.4 ± 0.7 a; B |
| GMS75:25SHW | 99.7 ± 0.2 a; A | 58.2 ± 2.4 c; B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6, 5. https://doi.org/10.3390/gels6010005
Barroso NG, Okuro PK, Ribeiro APB, Cunha RL. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels. 2020; 6(1):5. https://doi.org/10.3390/gels6010005
Chicago/Turabian StyleBarroso, Noadia G., Paula K. Okuro, Ana P. B. Ribeiro, and Rosiane L. Cunha. 2020. "Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring" Gels 6, no. 1: 5. https://doi.org/10.3390/gels6010005
APA StyleBarroso, N. G., Okuro, P. K., Ribeiro, A. P. B., & Cunha, R. L. (2020). Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels, 6(1), 5. https://doi.org/10.3390/gels6010005






