Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels
Abstract
1. Introduction
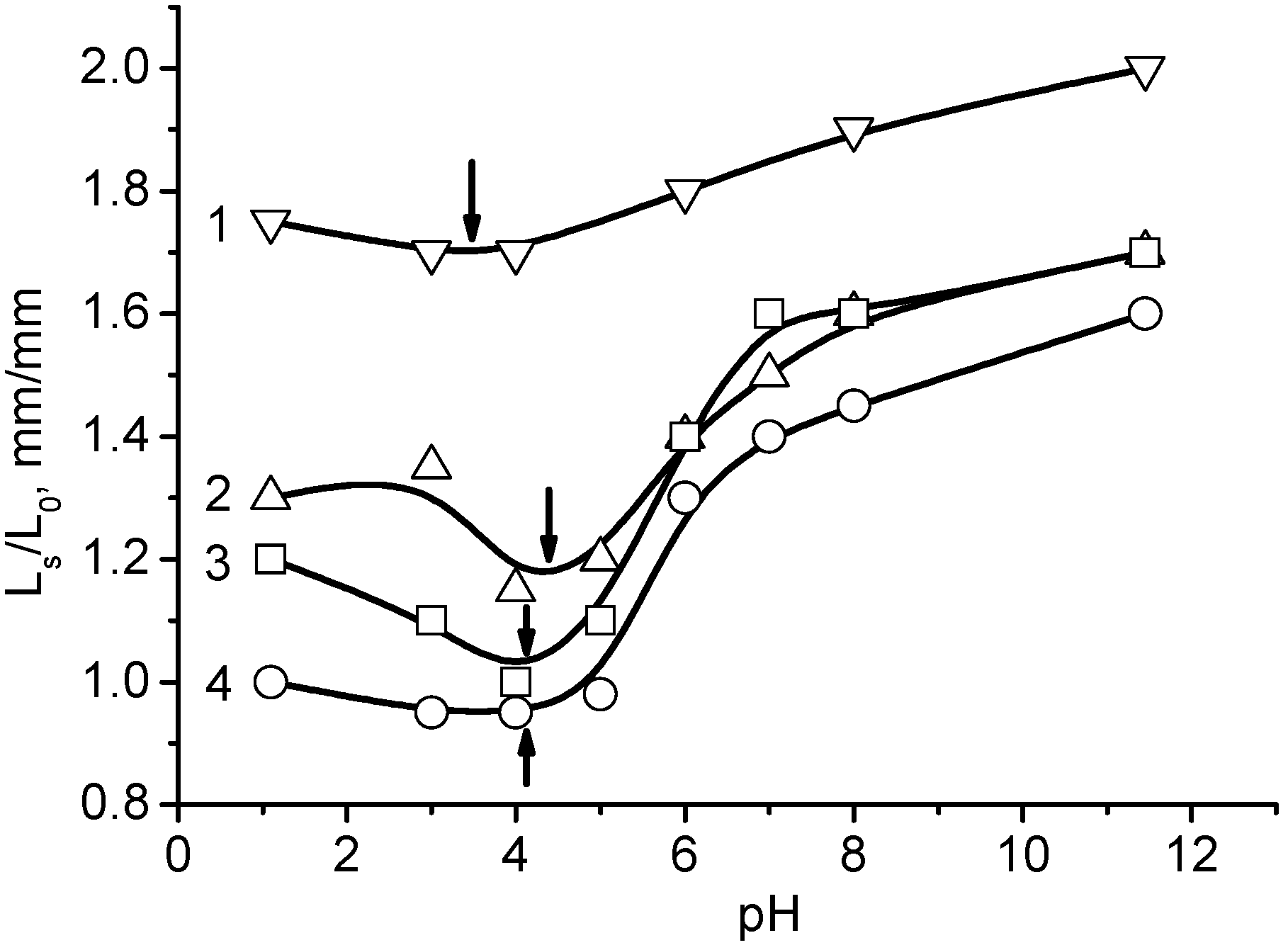
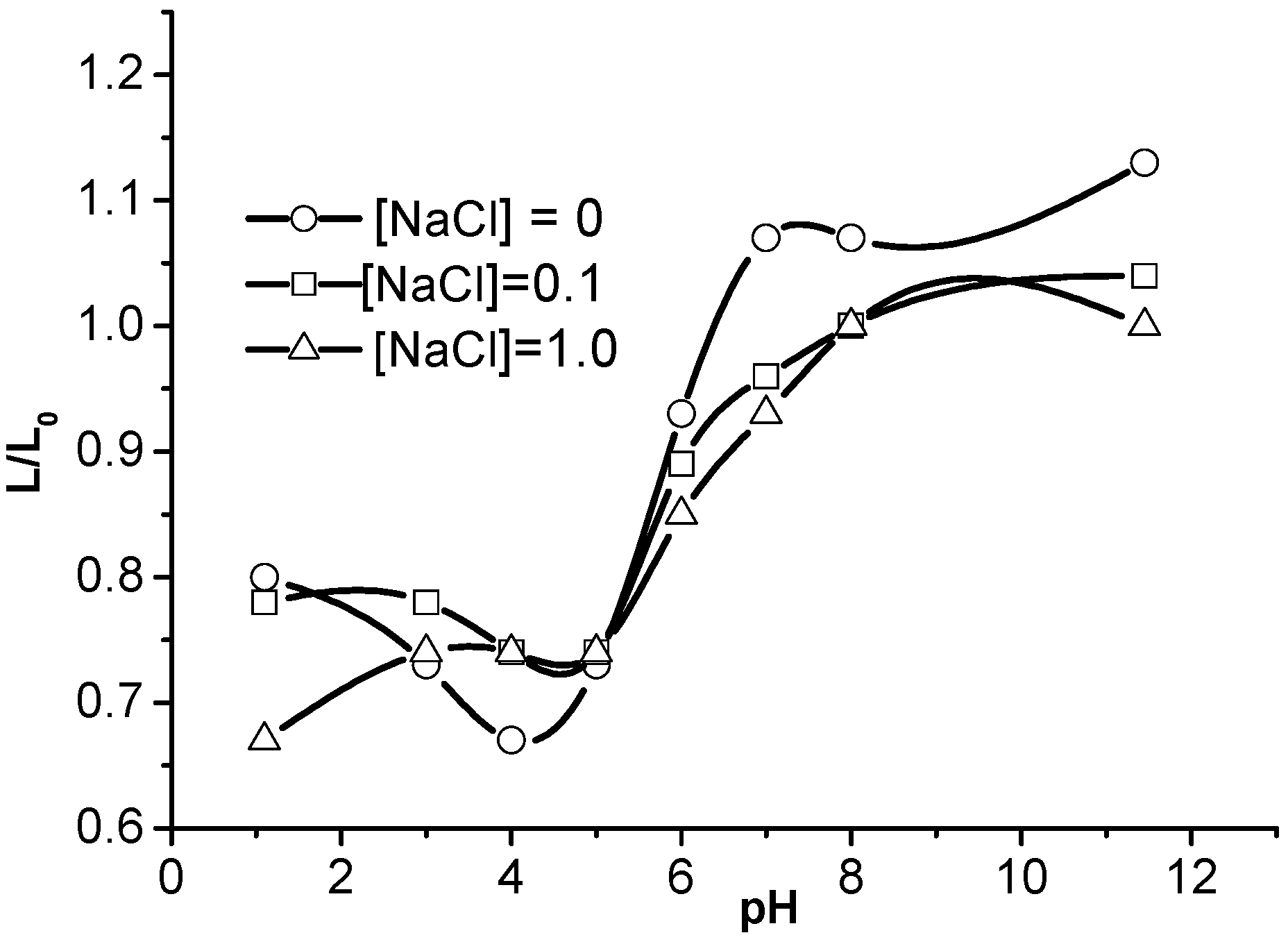
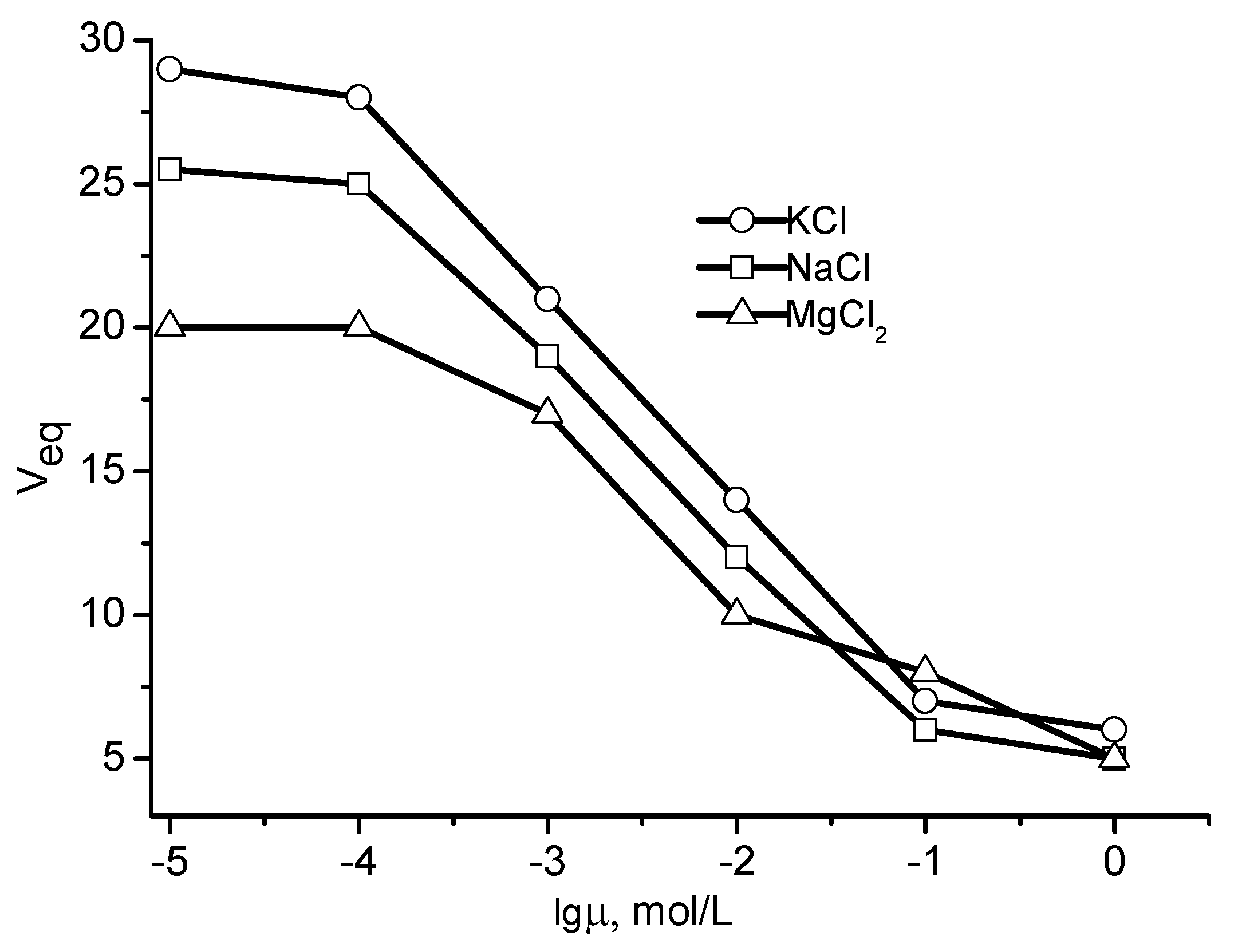
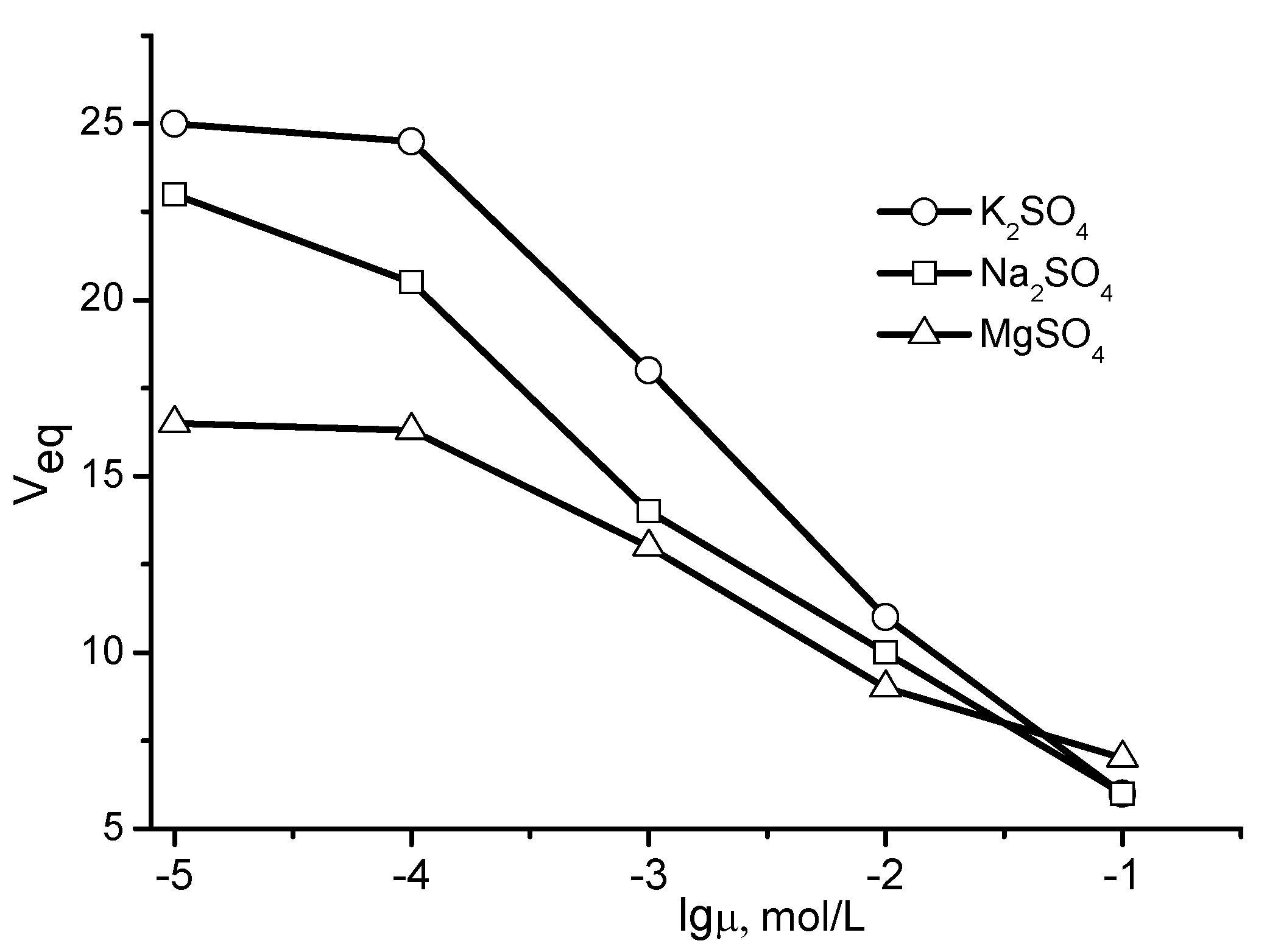
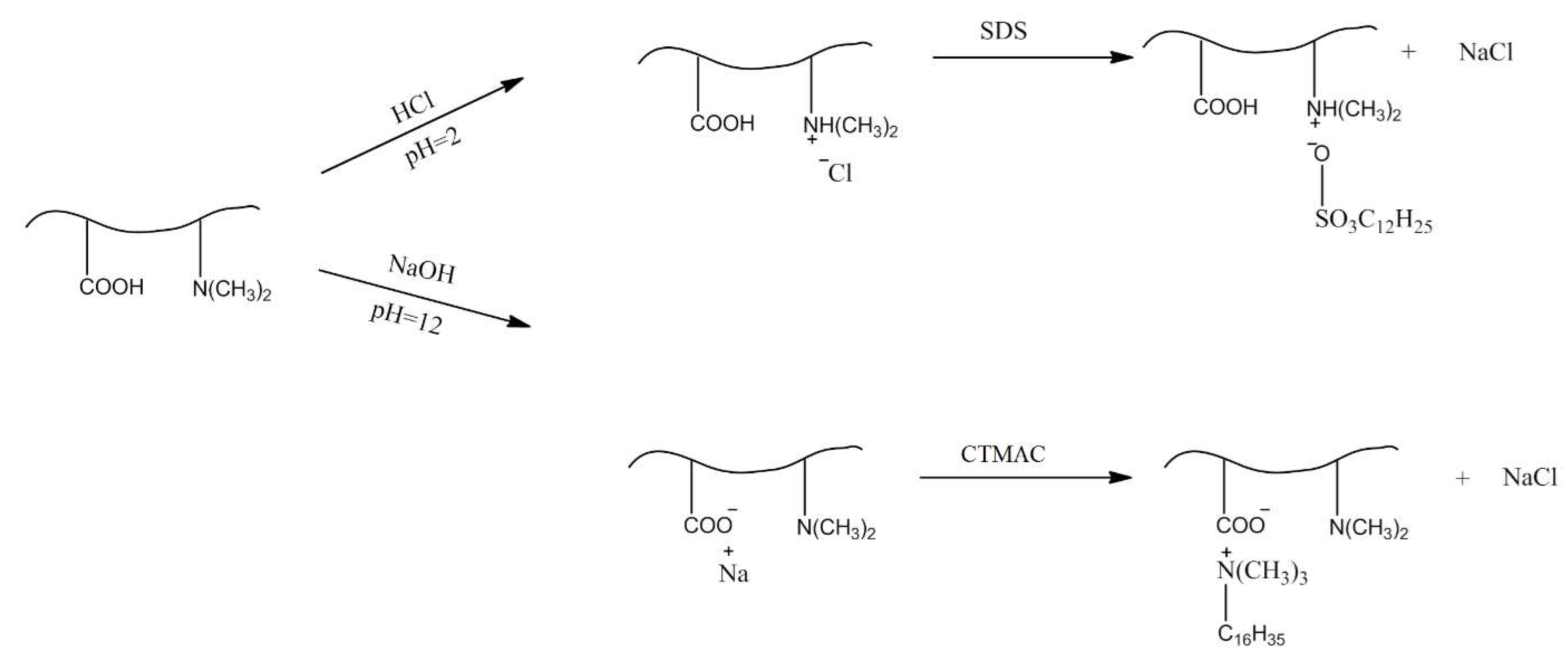
2. Physicochemical Properties of Polyampholyte Cryogels
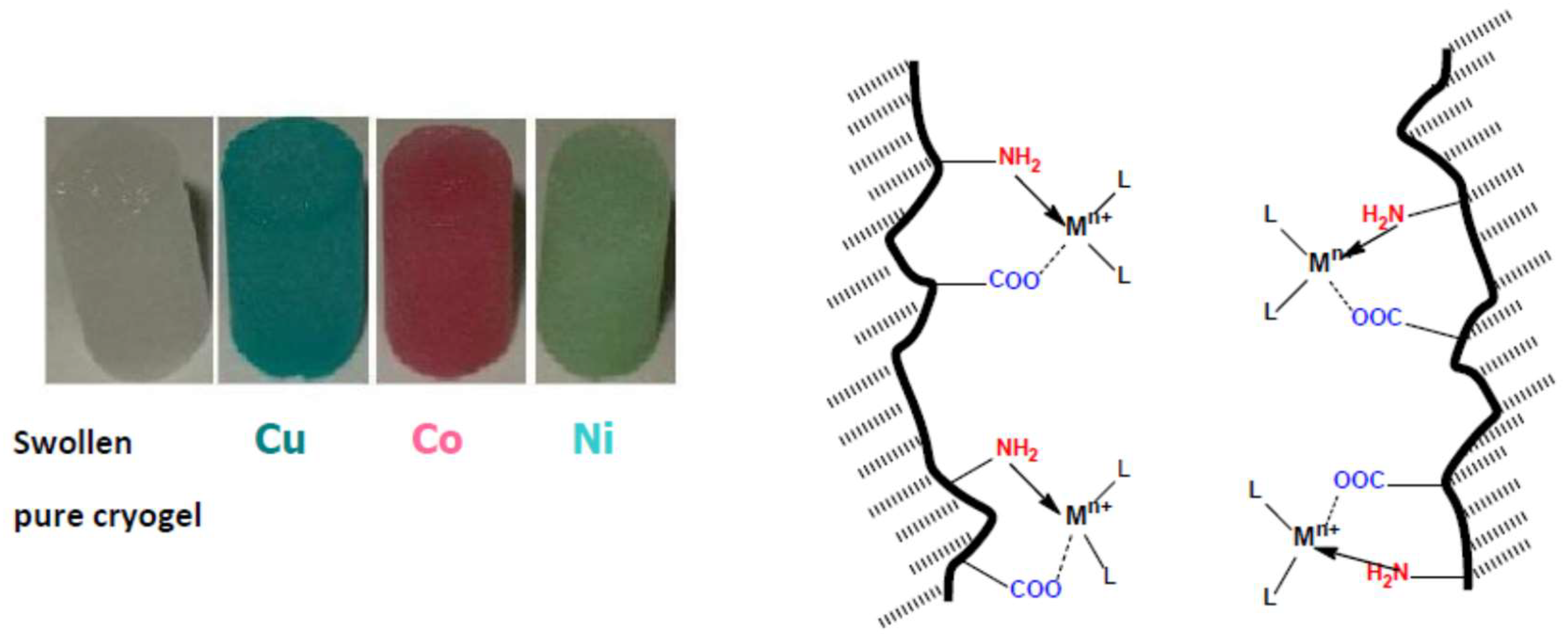
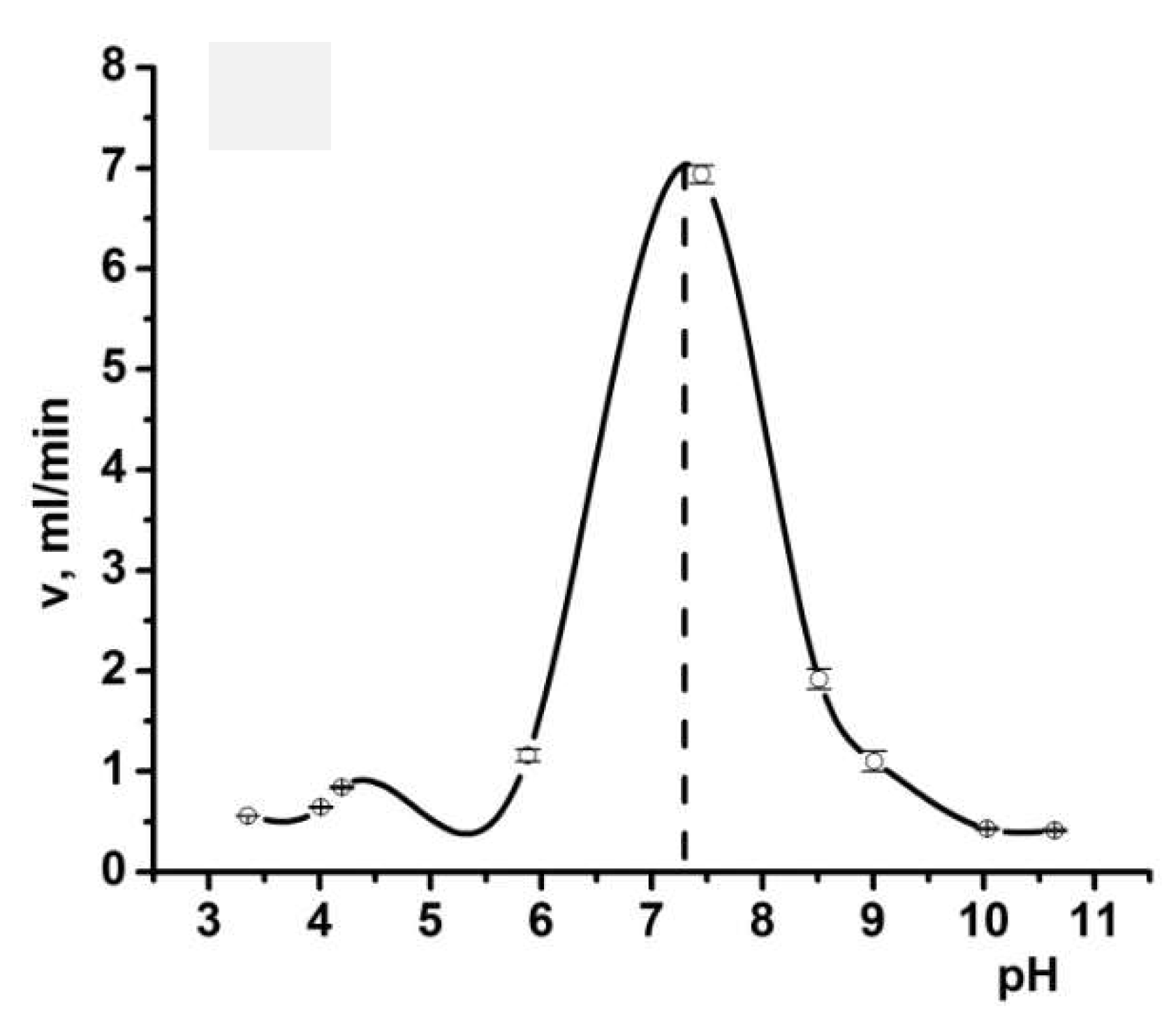
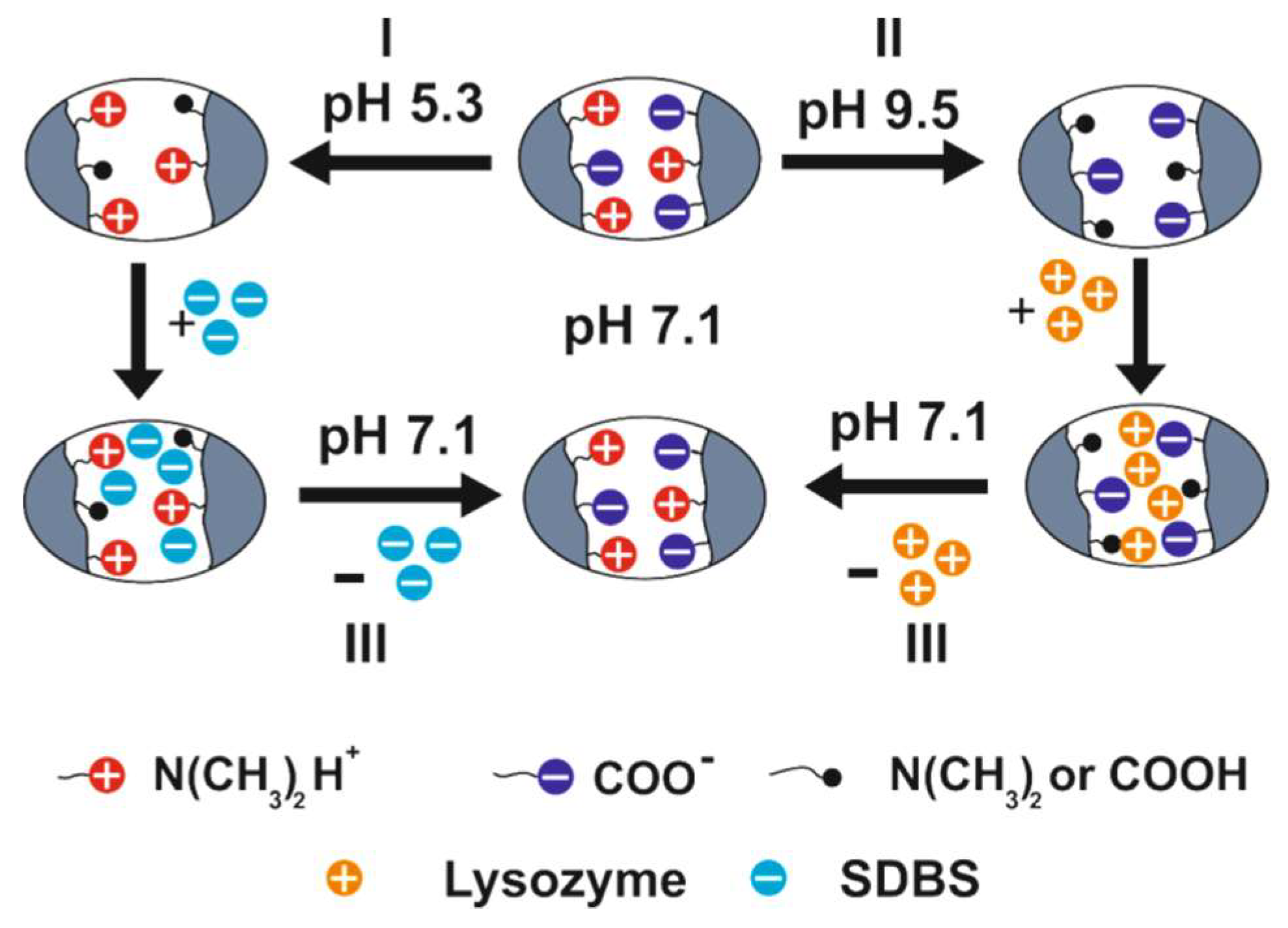
3. Complexation of Polyampholyte Cryogels with Transition Metal Ions, Dyes, Surfactants, and Proteins
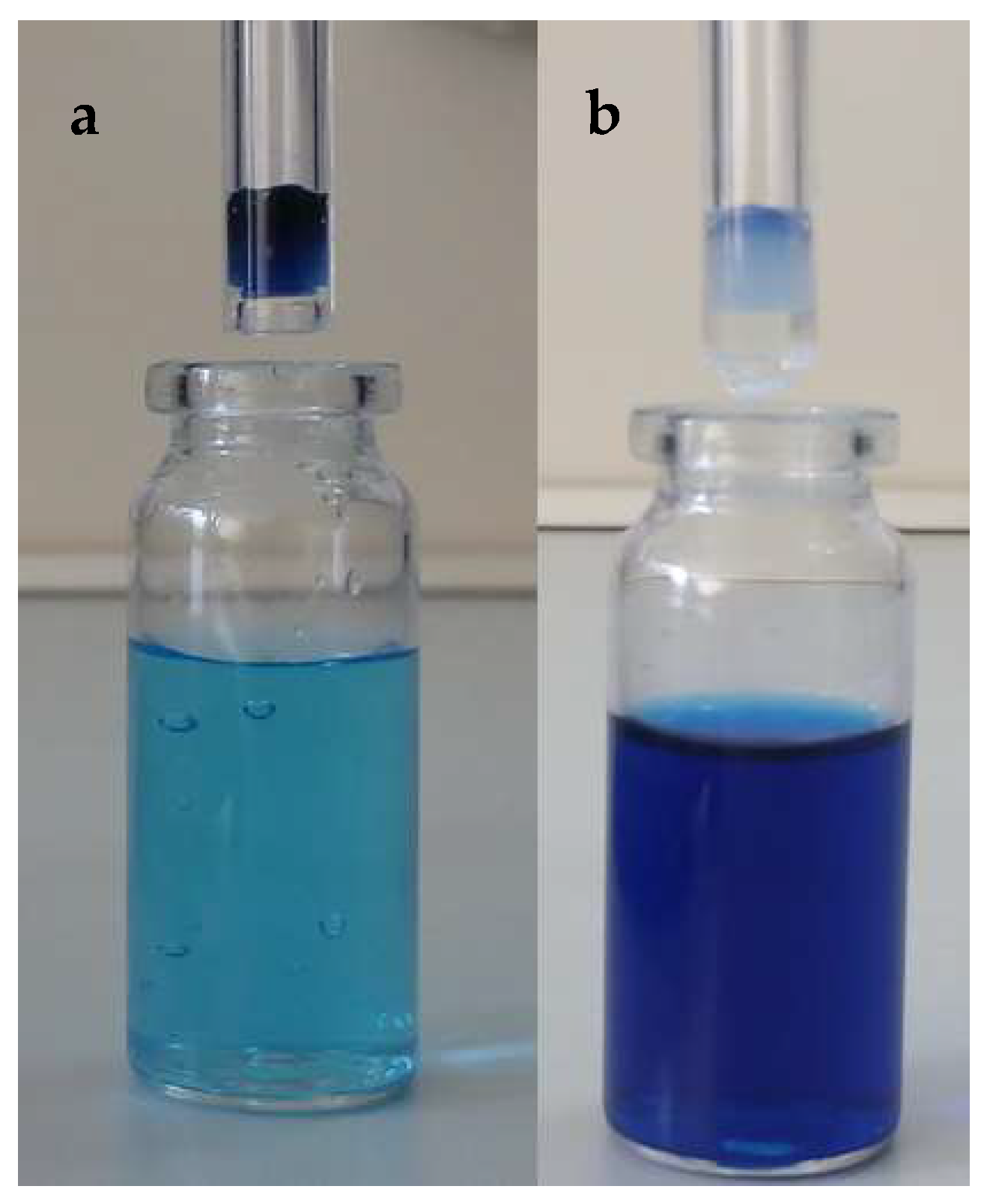
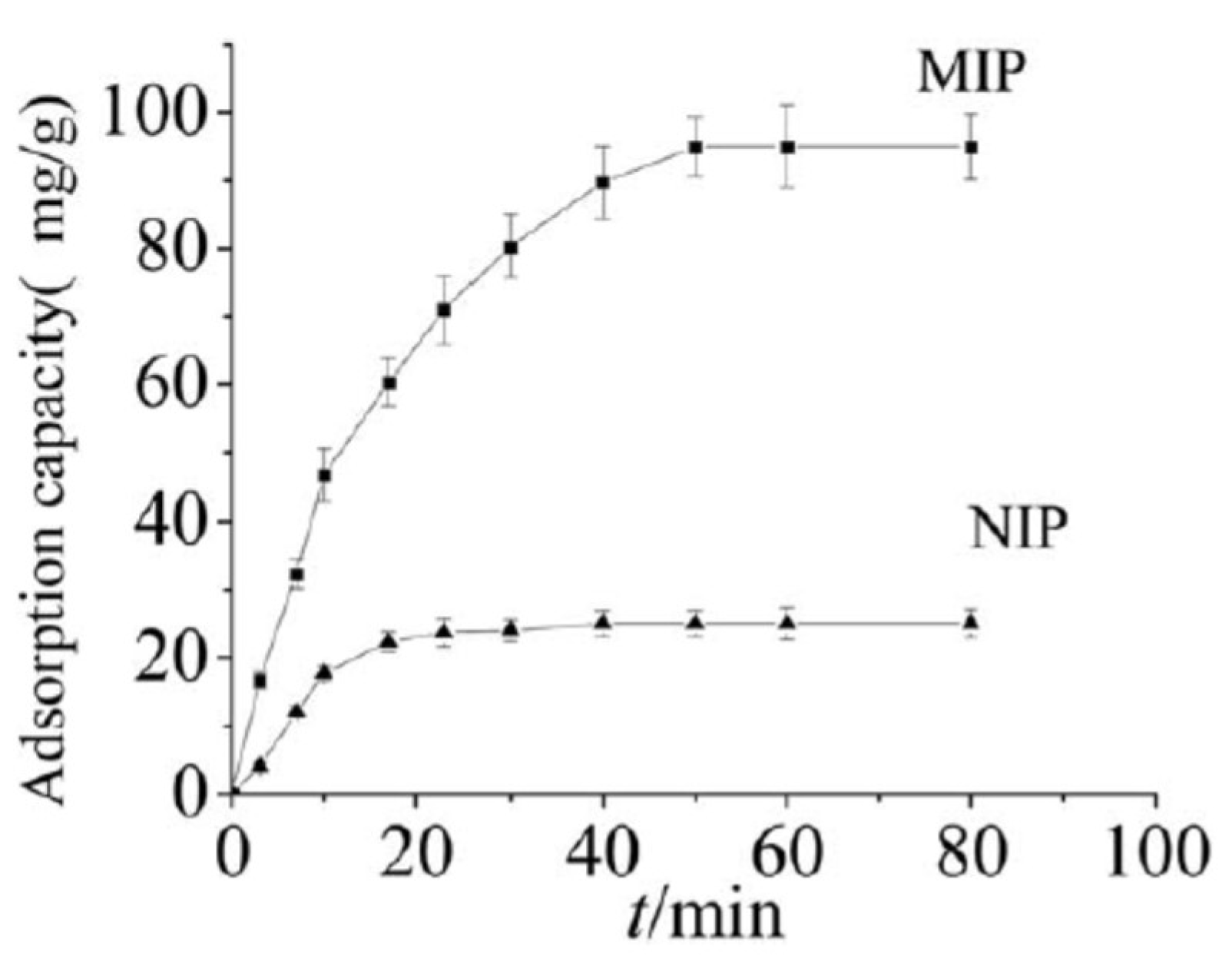
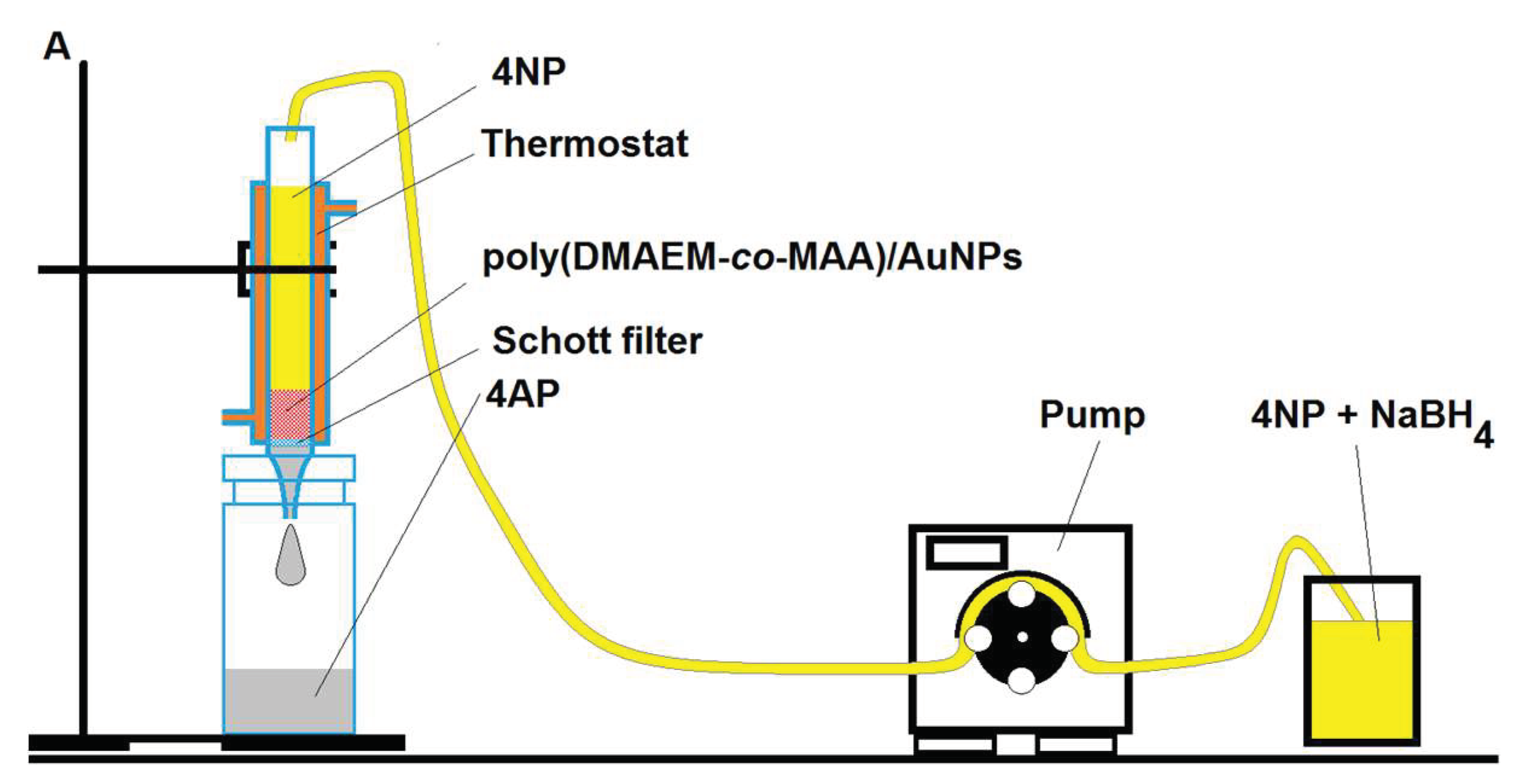
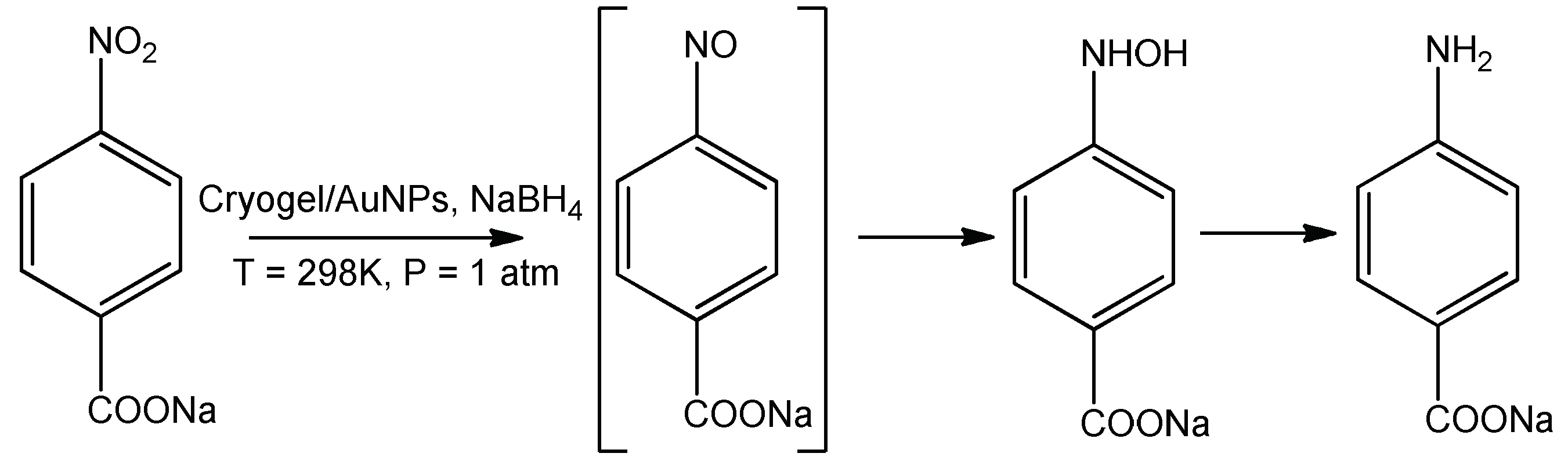
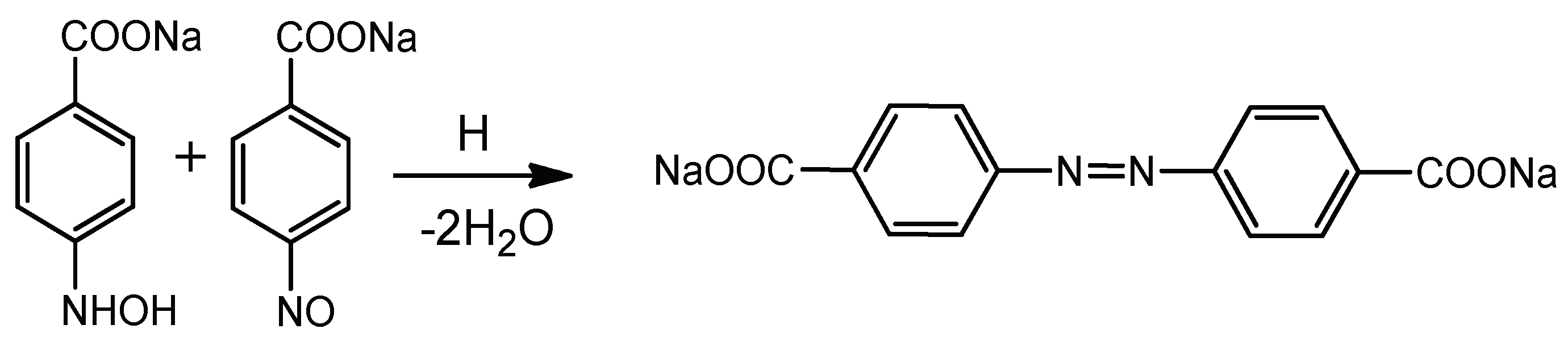
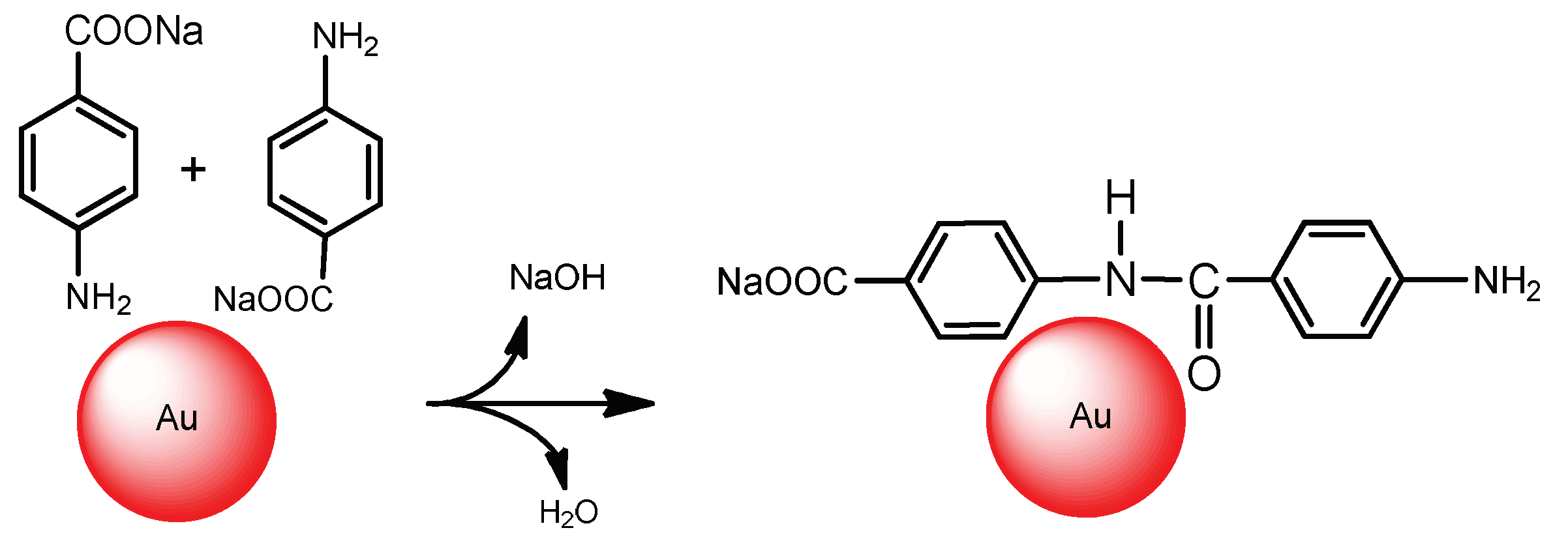
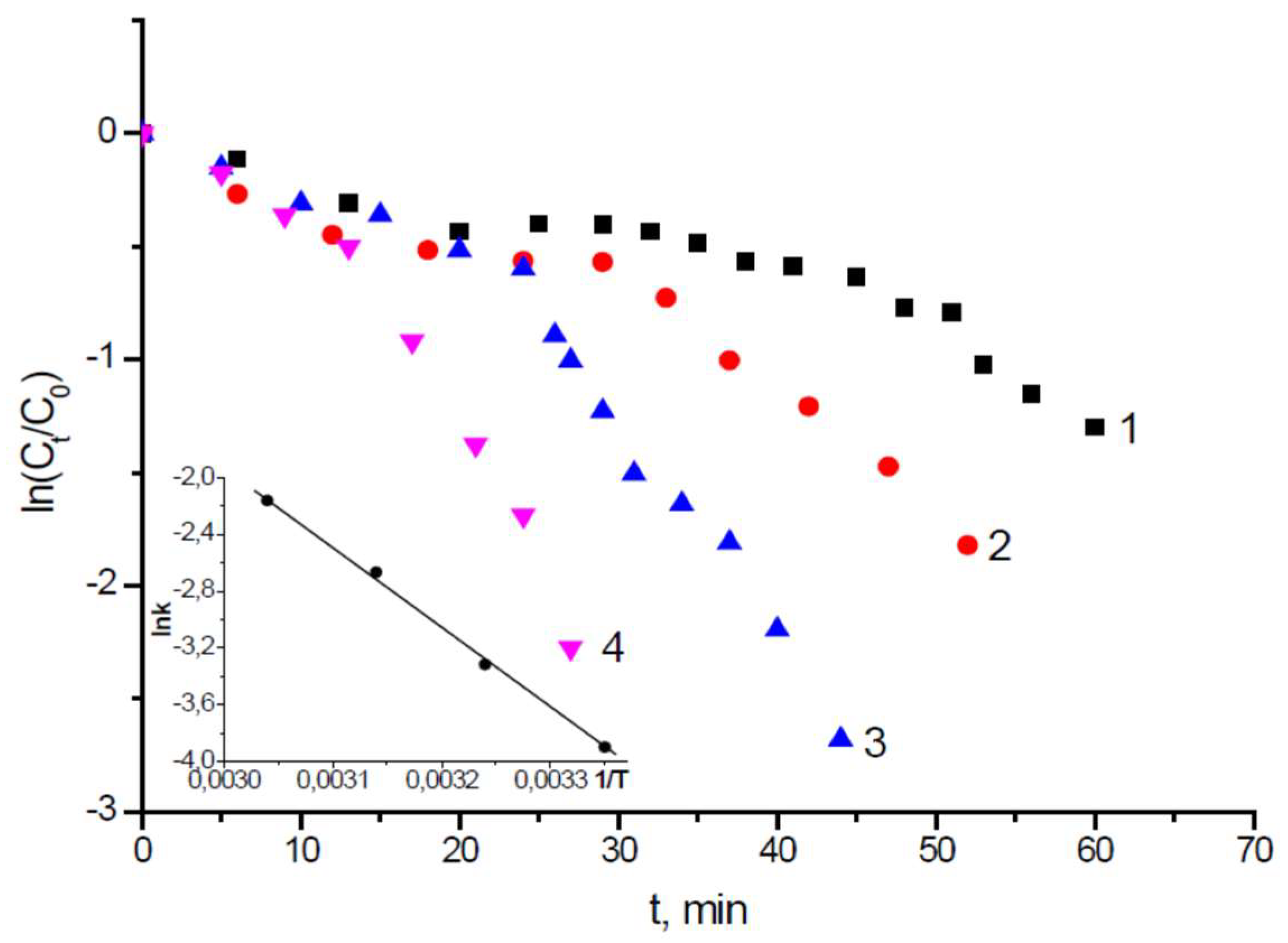
4. Immobilization of Metal Nanoparticles within Polyampholyte Cryogel Matrix and Evaluation of Their Catalytic Activity
5. Comparative Analysis of Polyampholyte Cryogels with Nonionic, Anionic and Cationic Precursors
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Allylamine |
| AAc | Acrylic acid |
| AAm | Acrylamide |
| ACG | Amphoteric cryogel |
| AMPS | 2-Acrylamido-2-methyl-1-propanesulfonic acid |
| 4-AP | 4-Aminophenol |
| AuNPs | Gold nanoparticles |
| BSA | Bovine serum albumin |
| CTMAC | Cetyltrimethylammonium chloride |
| DEAAm | N,N-diethylacrylamide |
| DEAEM | 2-(Diethylamino)ethylmethacrylate |
| DMAAm | N,N-dimethylacrylamide |
| DS | Disulfide |
| DTT | d,l-dithiotreitol |
| Ea | Activation energy |
| HEMA | 2-Hydroxyethylmethacrylate |
| IAc | Itaconic acid |
| IEP | Isoelectric point |
| IF | Imprinting factor |
| IIP | Isoionic point |
| µ | Ionic strength |
| MAA | Methacrylic acid |
| MB | Methylene blue |
| MBAA | N,N-methylenebisacrylamide |
| METMAC | [2-(methacryloyloxy)ethyl]trimethylammonium chloride |
| MIP | Molecularly imprinted polyampholyte cryogels |
| MIPAHs | Molecularly imprinted polyampholyte hydrogels |
| MO | Methyl orange |
| n | Characteristic exponent describing the mode of penetrant (water) transport mechanism |
| 4-NA | 4-Nitroaniline |
| NIP | Non-imprinted polyampholyte cryogels |
| NIPAHs | Non-imprinted polyampholyte hydrogels |
| NIPAM | N-isopropylacrylamide |
| 2-NP | 2-Nitrophenol |
| 4-NP | 4-Nitrophenol |
| P(AA-co-AAc-co-AAm) | Allylamine-co-acrylic acid-co-acrylamide |
| P(AA-co-MAA-co-AAm) | Allylamine-co-methacrylic acid-co-acrylamide |
| p-ABA | p-Aminobenzoic acid |
| P(AMPSNa-co-APTAC) | 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt-co-(3-acrylamidopropyl)trimethylammonium chloride |
| P(DAA-co-AAc) | Diallylamine-co-acrylic acid |
| P(DMAEM-co-AMPS) | N,N-dimethylaminoethylmethacrylate-co-2-acrylamido-2-methyl-1-propanesulfonic acid |
| P(DMAEM-co-MAA) | N,N-dimethylaminoethylmethacrylate-co-methacrylic acid |
| PdNPs | Palladium nanoparticles |
| PEO | Poly(ethylene oxide) |
| pHIEP | Isoelectric pH |
| pKa | Ionization constant of acidic group |
| pKb | Ionization constant of basic group |
| PMODMSPA | Poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl) ammonium hydroxide} |
| p-NBA | p-Nitrobenzoic acid |
| polyHEMA | Poly(2-hydroxyethylmethacrylate) |
| PVA | Poly(vinyl alcohol) |
| PVP | Poly(N-vinylpyrrolidone) |
| qm | Adsorption capacity |
| SDBS | Sodium dodecylbenzenesulfonate |
| SEM | Scanning electron microscope |
| TOF | Turnover frequency |
| TON | Turnover number |
| Veq | Equilibrium swelling |
References
- Lozinsky, V.I. Cryostructuring of polymeric systems. 50. Cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I. A brief history of polymeric cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 48–102. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis, structure-property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Okay, O. (Ed.) Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Springer: Cham, Switzerland, 2014; 330p, ISBN 978-3-319-05845-0. [Google Scholar]
- Kumar, A. (Ed.) Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016; 480p, ISBN 978-1-4822-281-6. [Google Scholar]
- Liu, C.; Tong, G.; Tan, Z.; Quan, C.; Zhang, C. Polymeric cryogel: Preparation, properties and biomedical applications. Prog. Chem. 2014, 26, 1190–1201. [Google Scholar]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Nakayama, A.; Kokufuta, E. Preparation and characterization of thermosensitive polyampholyte nanogels. Langmuir 2003, 19, 3178–3184. [Google Scholar] [CrossRef]
- Deng, L.; Zhai, Y.; Guo, S.; Jin, F.; Xie, Z.; He, X.; Dong, A. Investigation on properties of P(MAA-co-DMAEM)-g-EG) polyampholyte nanogels. J. Nanopart. Res. 2009, 11, 365–374. [Google Scholar] [CrossRef]
- Tan, B.H.; Ravi, P.; Tam, K.C. Synthesis and characterization of novel pH-responsive polyampholyte microgels. Macromol. Rapid Commun. 2006, 27, 522–528. [Google Scholar] [CrossRef]
- Christodoulakis, K.E.; Vamvakaki, M. Amphoteric core-shell microgels. Langmuir 2010, 26, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Schachschal, S.; Balaceanu, A.; Melian, C.; Demco, D.E.; Eckert, T.; Richetring, W.; Pich, A. Polyampholyte microgels with anionic core and cationic shell. Macromolecules 2010, 43, 4331–4339. [Google Scholar] [CrossRef]
- Ni, H.; Kawaguchi, H.; Endo, T. Preparation of amphoteric microgels of poly(acrylamide/methacrylic acid/dimethylaminoethylmethacrylate) with a novel pH-volume transition. Macromolecules 2007, 40, 6370–6376. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Nuraje, N.; Khutoryanskiy, V.V. Amphoteric nano-, micro-, and macrogels, membranes, and thin films. Soft Matter 2012, 8, 9302–9321. [Google Scholar] [CrossRef]
- Klivenko, A.N.; Tatykhanova, G.S.; Mun, G.A.; Kudaibergenov, S.E. Synthesis and physicochemical properties of macroporous cryogels. Int. J. Biol. Chem. 2015, 8, 52–60. [Google Scholar] [CrossRef]
- Yang, C.; Liu, G.F.; Zhou, X.L.; Liu, Y.R.; Wang, J.; Tian, L.L.; Hu, X.Y.; Wang, Y.Y. Polyacrylamide based cryogels as catalysts for biodiesel. Catal. Lett. 2015, 145, 1778–1783. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Adilov, Z.; Berillo, D.; Tatykhanova, G.; Sadakbaeva, Z.; Abdullin, K.; Galaev, I. Novel macroporous amphoteric gels: Preparation and characterization. eXPRESS Polym. Lett. 2012, 6, 346–353. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Sadakbaeva, Z.; Berillo, D.; Galaev, I. Structure and swelling behavior of novel amphoteric cryogels. In Proceedings of the Macro- and Supramolecular Architectures and Materials (MAM), MAM-10, Montego Bay, Jamaica, 15–20 August 2010. [Google Scholar]
- Yang, C.; Zhou, X.L.; Liu, Y.R.; Wang, J.; Tian, L.L.; Zhang, Y.; Hu, X.Y. Charge group energetically enhance protein imprinting in amphoteric polyacrylamide cryogels. J. Appl. Polym. Sci. 2016, 133, 43851. [Google Scholar] [CrossRef]
- Klivenko, A.N.; Bolat, A.; Kudaibergenov, S.E.; Mun, G.A. Synthesis and characterization of amphoteric cryogels based on methacrylic acid and N,N-dimethylaminoethylmethacrylate. Bull. Natl. Eng. Acad. 2014, 3, 85–91. [Google Scholar]
- Musabaeva, B.H.; Kudaibergenov, S.E.; Orazzhanova, L.K.; Iminova, D.E.; Bayakhmetova, B.B. Determination of the microstruature, the swelling degree and dynamics of cryogels based on methacrylic acid, dimethylaminoethylmethacrylate and acrylamide. Bull. Karaganda State Univ. Ser. Chem. 2016, 3, 8–15. [Google Scholar]
- Boyaci, T.; Orakdogen, N. pH-responsive poly(N,N-dimethylaminoethyl methacrylate-co-2-acrylamido-2-methyl-propanosulfonic acid) cryogels: Swelling, elasticity and diffusive properties. RSC Adv. 2015, 94, 77235–77247. [Google Scholar] [CrossRef]
- Shakhvorostov, A.; Toleutay, G.; Kudaibergenov, S. Synthesis, physicochemical and complexation properties of “quenched” polyampholytes based on fully charged monomers. In Proceedings of the Materials of the International Conference “Polyelectrolytes in Chemistry, Biology and Technology”, Singapore, 12–14 March 2018. [Google Scholar]
- Kudaibergenov, S.; Shakhvorostov, A.; Dauletbekova, M.; Toleutay, G.; Nurakhmetova, Z.; Kudaibergenova, G. Solution and volume-phase properties of linear and crosslinked “quenched” polyampholytes. In Proceedings of the Materials of the 12-th International Symposium on Polyelectrolytes ISP, Wageningen, The Netherlands, 27–31 August 2018; p. 102. [Google Scholar]
- Sener, G.; Krebs, M.D. Zwitterionic cryogels for sustained release of proteins. RSC Adv. 2016, 6, 29608–29611. [Google Scholar] [CrossRef]
- Kudaibergenov, S. Polyampholytes: Synthesis, Characterization and Application; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; 220p, ISBN 978-1-4613-5165-8. [Google Scholar]
- Tatykhanova, G.; Sadakbayeva, Z.; Berillo, D.; Galaev, I.; Abdullin, K.; Adilov, Z.; Kudaibergenov, S. Metal complexes of amphoteric cryogels based on allylamine and methacrylic acid. Macromol. Symp. 2012, 317–318, 7–17. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Jaeger, W.; Laschewsky, A. Polymeric betaines: Synthesis, characterization and application. Adv. Polym. Sci. 2006, 201, 157–224. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Tatykhanova, G.S.; Klivenko, A.N. Complexation of macroporous amphoteric cryogels based on N,N-dimethylaminoethylmethacrylate and methacrylic acid with dyes, surfactant, and protein. J. Appl. Polym. Sci. 2016, 133, 43784. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.; Abylgazina, L.; Poulopoulos, S.G.; Inglezakis, V.J. Novel Amphoteric cryogels for Cd2+ ions removal from aqueous solutions. Key Eng. Mater. 2018, 775, 376–382. [Google Scholar] [CrossRef]
- Veleshko, I.E.; Nikonorov, V.V.; Veleshko, A.N.; Rumyantseva, E.V.; Mikhailov, S.N.; Lozinsky, V.I.; Ivanov, R.V.; Gal’braikh, L.S.; Kil’deeva, N.R. Sorption of Eu(III) from solutions of covalently cross-linked chitosan cryogels. Fibre Chem. 2011, 42, 364–368. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; Al-Hussain, S.A.; Al-Lohedan, H.A.; Tawfeek, A.M.; Hashem, A.I. New crosslinked poly (ionic liquid) cryogels for fast removal of methylene blue from waste water. React. Funct. Polym. 2018, 131, 420. [Google Scholar] [CrossRef]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Doroftei, F. Macroporous composite IPN hydrogels based on poly(acrylamide) and chitosan with tuned swelling and sorption of cationic dyes. Chem. Eng. J. 2012, 204–206, 198–209. [Google Scholar] [CrossRef]
- Nerapusri, V.; Keddie, J.L.; Vincent, B.; Bushnak, L.A. Absorption of cetylpyridinium chloride into poly(N-isopropylacrylamide)-based microgel particles, in dispersion and as surface-deposited monolayers. Langmuir 2007, 23, 9572–9577. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Nuraje, N. Intra- and interpolyelectrolyte complexes of polyampholytes. Polymers 2018, 10, 1146. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Koetz, J.; Nuraje, N. Nanostructured hydrophobic polyampholytes: Self-assembly, stimuli-sensitivity, and application. Adv. Compos. Hybrid Mater. 2018, 1, 649–684. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Zhaimina, G.M.; Bekturov, E.A. Recovery of Transition Metal Ions by Polyampholytes; Author Certificate of the USSR No. 1231810; Moscow, Russia, 1984; pp. 1–5. [Google Scholar]
- Bekturov, E.A.; Kudaibergenov, S.E.; Saltybaeva, S.S.; Kyaturka, V.G.; Radzhunas, L.V. Study of complex formation properties of synthetic polyampholyte based on N,N-dimethylaminoethylmethacrylate and methacrylic acid. Izvestia Akademii nauk Respubliki Kazakhstan Seria khimicheskaia 1990, 1, 33–38. [Google Scholar]
- Kudaibergenov, S.E.; Bekturov, E.A. Influence of the coil-globule confromational transition in polyampholytes on sorption and desorption of polyelectrolytes and human serum albumin. Vysokomol. Soedin. Ser. A 1989, 31, 2614–2617. [Google Scholar]
- Kudaibergenov, S.E. Complexation Reactions with Participation of Synthetic Polyampholytes. Ph.D. Thesis, Moscow State University, Moscow, Russia, 1991. [Google Scholar]
- Janiak, D.S.; Ayyub, O.B.; Kofinas, P. Effects of charge density on the recognition properties of molecularly imprinted polyampholyte hydrogels. Polymer 2010, 51, 665–670. [Google Scholar] [CrossRef]
- Lago, M.A.; Grinberg, V.Y.; Burova, T.V.; Concheiro, A.; Alvarez-Lorenzo, C. Ionic and polyampholyte N-isopropylacrylamide-based hydrogels prepared in the presence of imprinting ligands: Stimuli-responsiveness and adsorption/release properties. J. Funct. Biomater. 2011, 2, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.; Ibrayeva, Z.; Yashkarova, M.; Kabdrakhmanova, S.; Tatykhanova, G.S. Composite Hydrogel Materials. In Advanced Separations by Specialized Sorbents; Dragan, E., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–37. [Google Scholar]
- Huang, J.T.; Zhang, J.; Zhang, J.Q.; Zheng, S.H. Template imprinting amphoteric polymer for the recognition of proteins. J. Appl. Polym. Sci. 2005, 95, 358–361. [Google Scholar] [CrossRef]
- Haag, S.L.; Barnards, M.T. Polyampholyte hydrogels in biomedical applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef]
- Zuric, K.M.; Bernards, M. Recent biomedical advances with polyampholyte polymers. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Su, E.; Okay, O. Polyampholyte hydrogels formed via electrostatic and hydrophobic interactions. Eur. Polym. J. 2017, 88, 191–204. [Google Scholar] [CrossRef]
- Kanazawa, R.; Sasaki, A.; Tokuyama, H. Preparation of dual temperature/pH-sensitive polyampholyte gels and investigation of their protein adsorption behaviors. Sep. Purif. Technol. 2012, 96, 26–32. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I.; Gierszewska, M. Designing novel macroporous composite hydrogels based on methacrylic acid copolymers and chitosan and in vitro assessment of lysozyme controlled delivery. Colloids Surf. B Biointerfaces 2016, 139, 33–41. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, X.L.; Liu, Y.R.; Zhang, Y.; Yan, Y.N. Extensive imprinting adaptability of polyacrylamide-based amphoteric cryogels against protein molecules. Chin. J. Anal. Chem. 2016, 44, 1322–1327. [Google Scholar] [CrossRef]
- Ou, S.H.; Wu, M.C.; Chou, T.C.; Liu, C.C. Polyacrylamide gels with electrostatic functional groups for the molecular imprinting of lysozyme. Anal. Chim. Acta 2004, 504, 163–166. [Google Scholar] [CrossRef]
- Ying, X.; Zhu, X.; Li, D.; Li, X. Preparation and specific recognition of protein macromolecularly imprinted polyampholyte hydrogel. Talanta 2019, 192, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Tang, K.; Verpoort, F.; Sun, T. Polymer-based stimuli-responsive recyclable catalytic systems for organic synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Kudaibergenov, S.E. Catalysis by Polymers; Huthig & Wepf Verlag Zug: Heidelberg, Germany, 1996; 153p. [Google Scholar]
- Dolya, N.A.; Kudaibergenov, S.E. Catalysis by thermoresponsive polymers. In Temperature-Responsive Polymers: Chemistry, Properties and Applications; Khutroyanskiy, V.V., Georgiu, T.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 357–377. [Google Scholar]
- Kudaibergenov, S.E.; Tatykhanova, G.S.; Selenova, B.S. Polymer Protected and gel immobilized gold and silver nanoparticles in catalysis. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1198–1211. [Google Scholar] [CrossRef]
- Sahiner, N.; Yildiz, S.; Sahiner, M.; Issa, Z.A.; Al-Lohedan, H. Macroporous cryogel metal nanoparticle composites for H2 generation from NaBH4 hydrolysis in seawater. Appl. Surf. Sci. 2015, 354, 388–396. [Google Scholar] [CrossRef]
- Yildiz, S.; Aktas, N.; Sahiner, N. Metal nanoparticle-embedded super porous poly(3-sulfopropyl methacrylate) cryogel for H2 production from chemical hydride hydrolysis. Int. J. Hydrog. Energy 2014, 39, 14690–14700. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. The use of superporous p(AAc (acrylic acid)) cryogels as support for Co and Ni nanoparticle preparation and as reactor in H2 production from sodium borohydride hydrolysis. Energy 2014, 71, 170–179. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. Energy and environmental usage of super porous poly(2-acrylamido-2-methyl-1-propanesulfonic acid) cryogel support. RSC Adv. 2014, 4, 23886–23897. [Google Scholar] [CrossRef]
- Sahiner, N.; Yildiz, S. Preparation of super porous poly(4-vinylpyridine) cryogel and their template metal nanoparticles composites for H2 production via hydrolysis reactions. Fuel Process Technol. 2014, 126, 324–331. [Google Scholar] [CrossRef]
- Ajmal, M.; Siddiq, M.; Sahiner, N. Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 5, 43873–43884. [Google Scholar] [CrossRef]
- Klivenko, A.N.; Tatykhanova, G.S.; Nuraje, N.; Kudaibergenov, S. Hydrogenation of p-nitrophenol by gold nanoparticles immobilized within macroporous amphoteric cryogel based on N,N-dimethylaminoethyl methacrylate and methacrylic acid. Bull. Karaganda Univ. Ser. Chem. 2015, 4, 10–15. [Google Scholar]
- Klivenko, A.N.; Yergazieva, E.; Kudaibergenov, S.E. Gold nanoparticles stabilized by amphoteric cryogel-perspective flow-through catalytic reactor for oxidation and reduction processes. Int. Conf. Nanomater. Appl. Prop. NAP 2016. [Google Scholar] [CrossRef]
- Tatykhanova, G.S.; Klivenko, A.N.; Kudaibergenova, G.M.; Kudaibergenov, S.E. Flow-through catalytic reactor based on amphoteric cryogels and gold nanoparticles. Macromol. Symp. 2016, 363, 49–56. [Google Scholar] [CrossRef]
- Aldabergenov, M.; Dauletbekova, M.; Shakhvorostov, A.; Toleutay, G.; Klivenko, A.; Kudaibergenov, S. Hydrogenation of nitroaromatic compounds by gold nanoparticles immobilized within macroporous amphoteric cryogels in aqueous solutions. J. Chem. Technol. Metall. 2018, 53, 17–26. [Google Scholar]
- Kudaibergenov, S.; Dauletbekova, M.; Toleutay, G.; Kabdrakhmanova, S.; Seilkhanov, T.; Abdullin, K. Hydrogenation of p-nitrobenzoic acid by gold and palladium nanoparticles immobilized within macroporous amphoteric cryogels in aqueous solution. J. Inorg. Organomet. Polym. 2018, 28, 2427–2438. [Google Scholar] [CrossRef]
- Litvin, E.F.; Freidlin, L.K.; Gurskii, R.N.; Istratova, R.V.; Vaisman, I.L. Investigation of the catalytic reduction of p-nitrobenzoic acid and its salt on Pd-, Rh-, and Ru-catalysts. Bull. Acad. Sci. USSR Chem. Sci. 1975, 24, 1620–1625. [Google Scholar] [CrossRef]
- Gainar, A.; Stevens, J.S.; Suljoti, E.; Xiao, J.; Golnak, R.; Aziz, E.F.; Schroeder, S.L.M. The structure of p-aminobenzoic acid in water: Studies combining UV-Vis, NEXAFS and RIXS spectroscopies. J. Phys. Conf. Ser. 2016, 712, 012034. [Google Scholar] [CrossRef]
- Muniz-Miranda, M. Application of the SERS spectroscopy to the study of catalytic reactions by means of mono and bimetallic nanoparticles. J. Anal. Bioanal. Tech. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Abe, Y.; Sato, N.; Fujimori, A.; Oishi, Y. Direct condensation polymerization of N-alkylated p-aminbenzoic acid and packing of rigid-rod main chains with flexible side chains. Polym. J. 2010, 42, 72–80. [Google Scholar] [CrossRef]
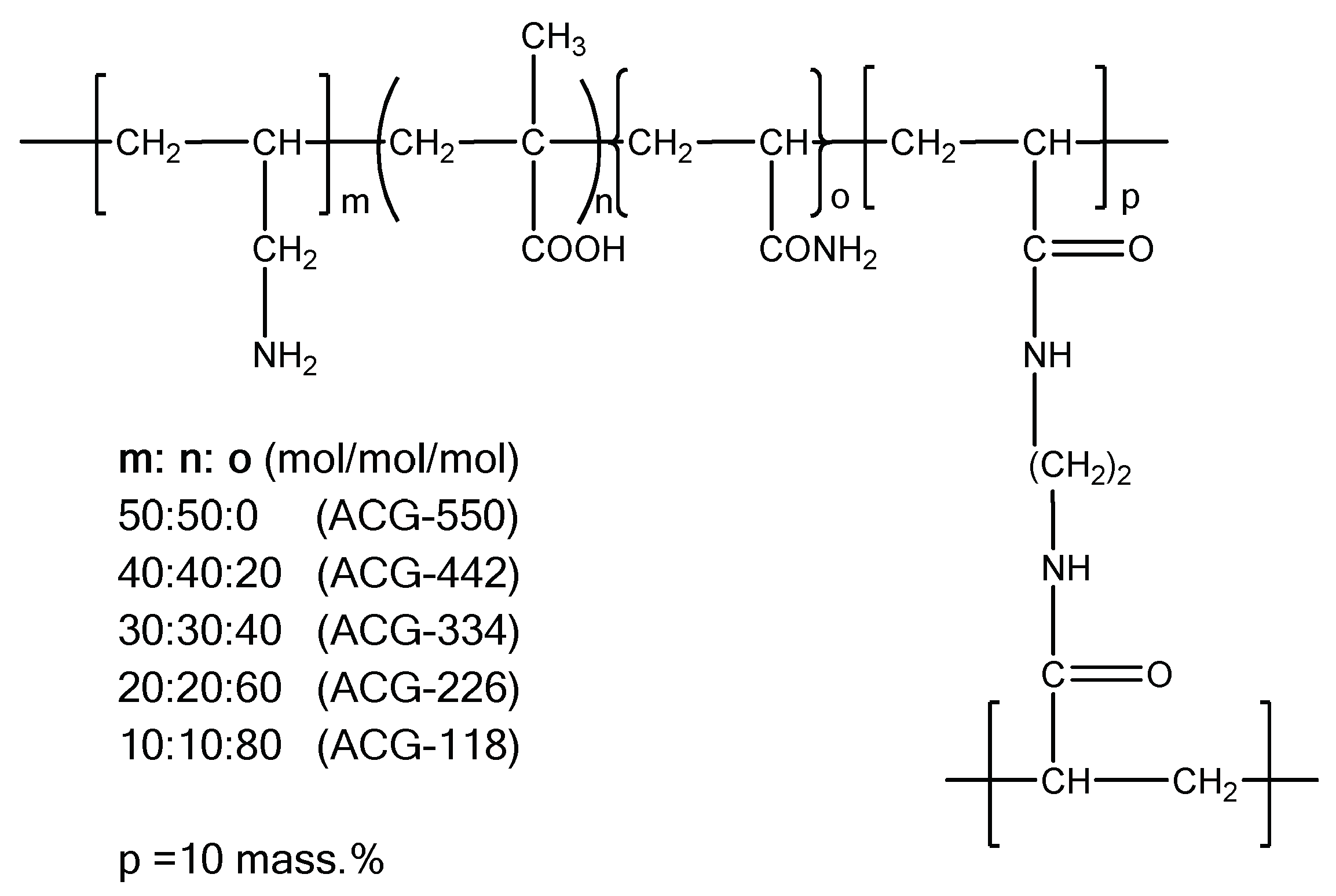
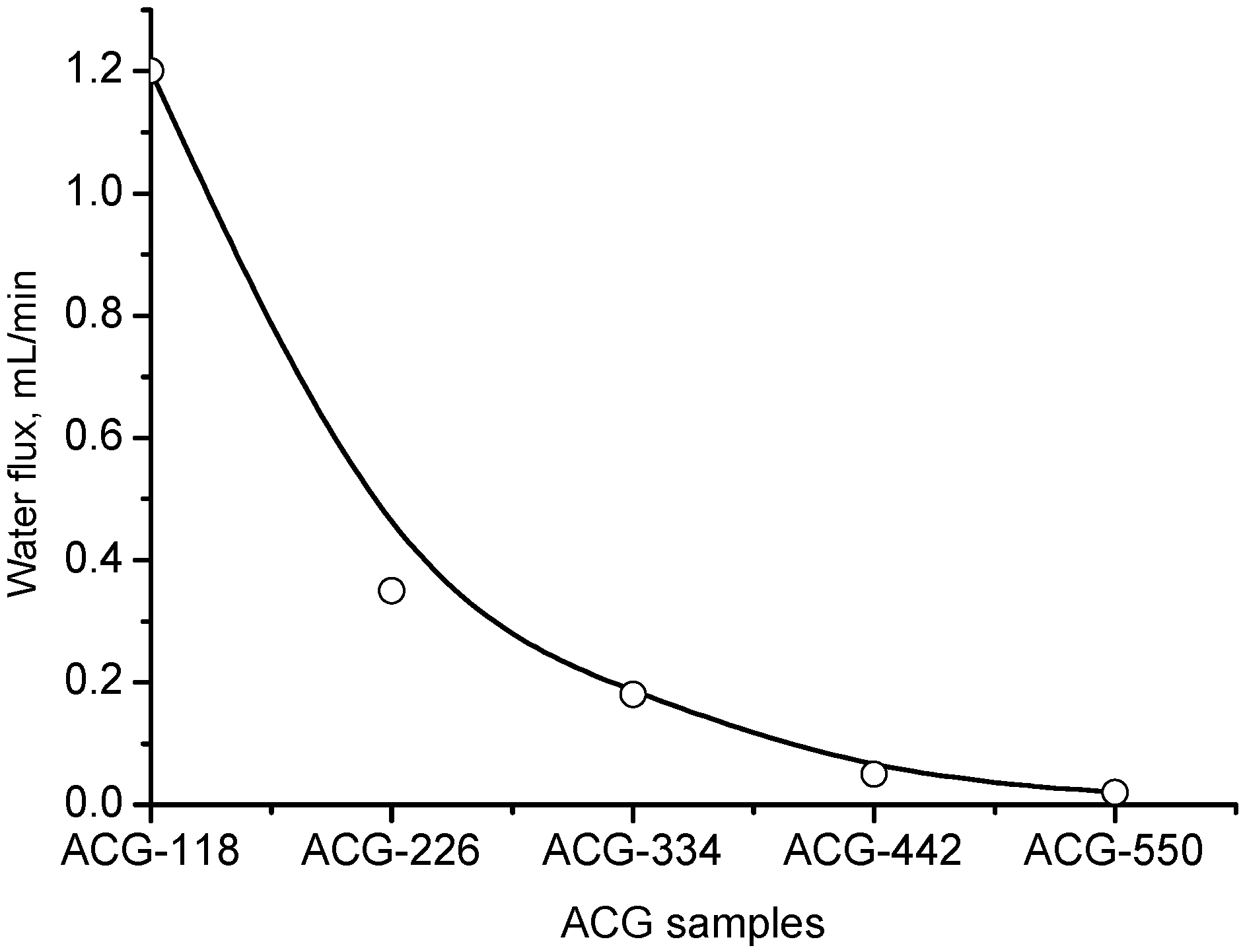
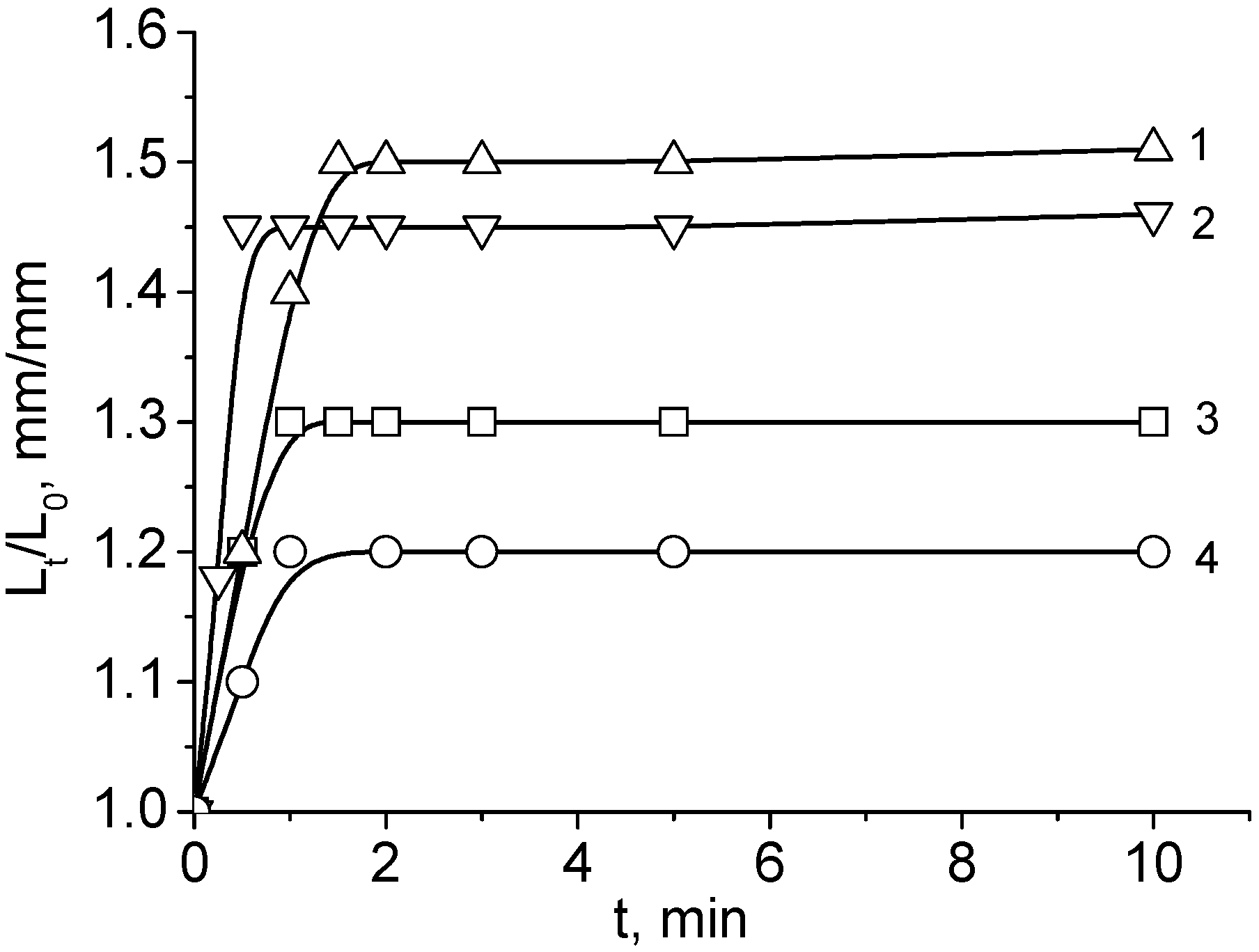
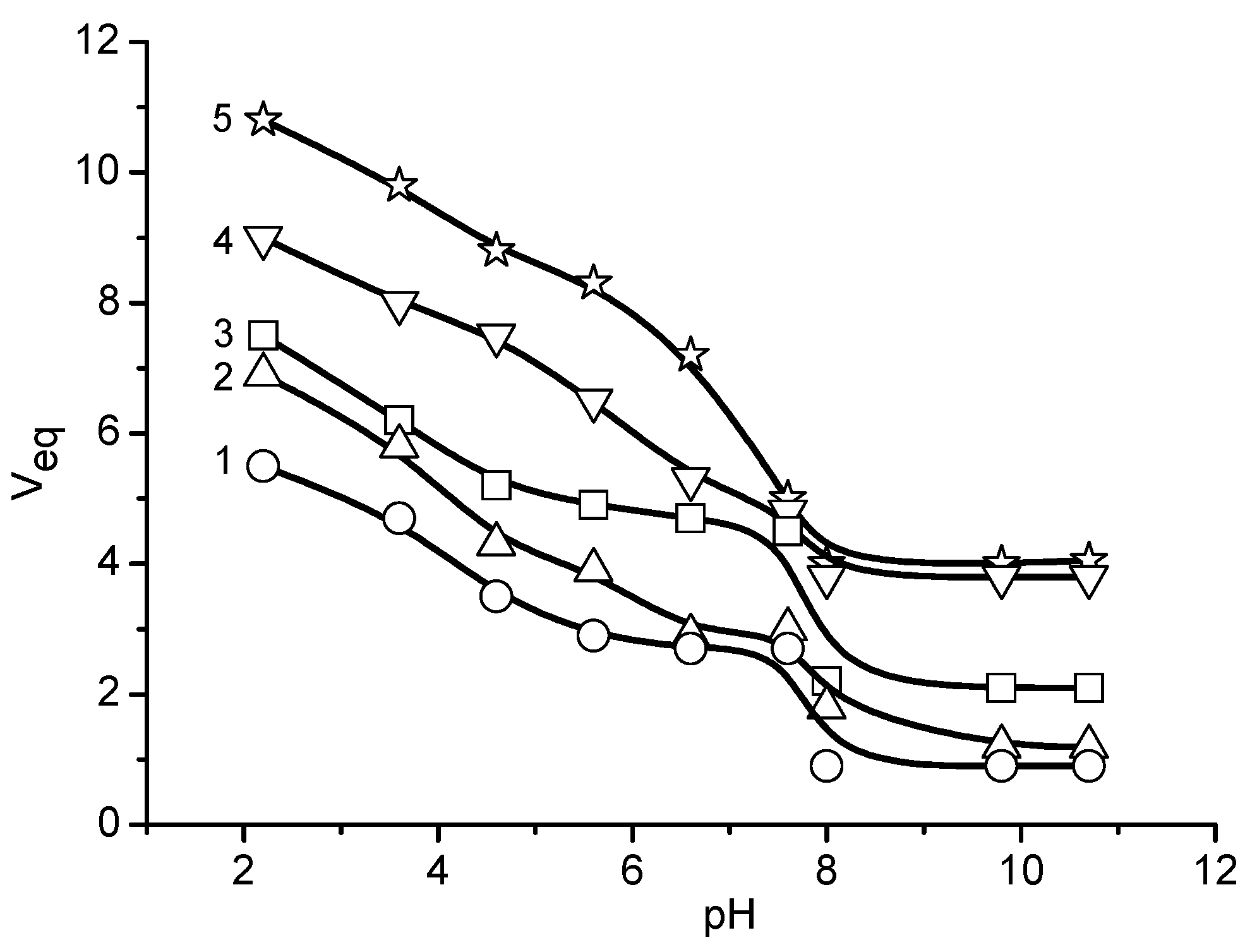



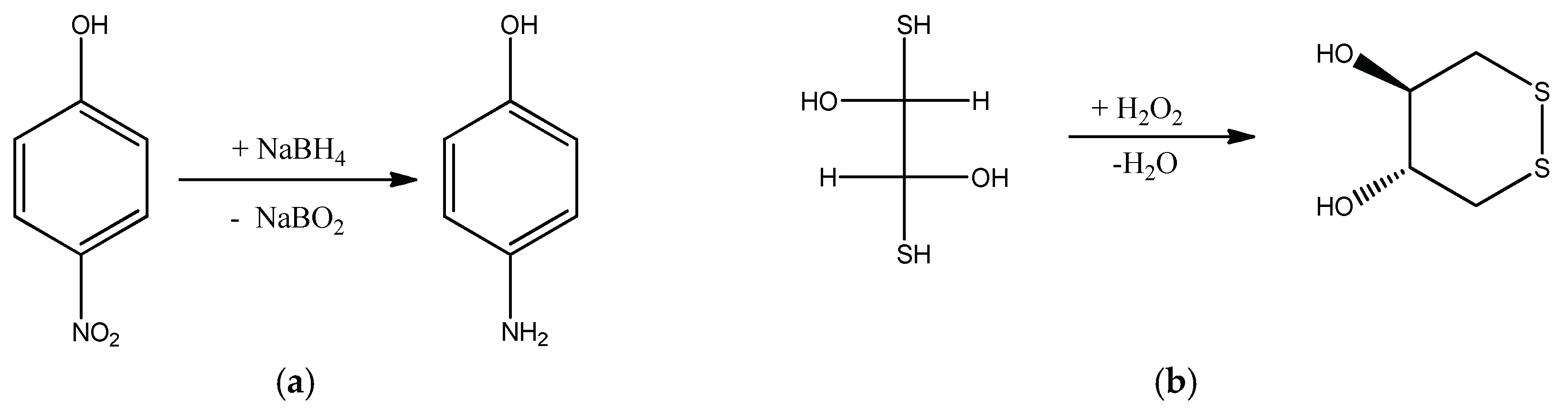
| Chemical Structure of Polyampholyte Cryogels * | Name Acronym | Refs |
|---|---|---|
 | allylamine-co-acrylic acid-co-acrylamide, P(AA-co-AAc-co-AAm) | [18] |
 | allylamine-co-methacrylic acid-co-acrylamide, P(AA-co-MAA-co-AAm) | [19,20] |
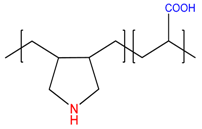 | diallylamine-co-acrylic acid, P(DAA-co-AAc) | [21] |
 | N,N-dimethylaminoethylmethacrylate-co-methacrylic acid, P(DMAEM-co-MAA) | [22,23] |
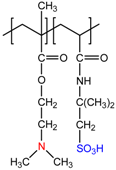 | N,N-dimethylaminoethylmethacrylate-co-2-acrylamido-2-methyl-1-propanesulfonic acid, P(DMAEM-co-AMPS) | [24] |
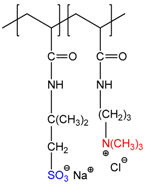 | 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt-co-(3-acrylamidopropyl)trimethylammonium chloride, P(AMPSNa-co-APTAC) | [25,26] |
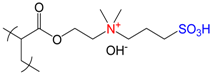 | Poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide}, PMODMSPA | [27] |
| Cryogels | –NH2, mol.% | –COOH, mol.% | pKb | pHIEP | ||
|---|---|---|---|---|---|---|
| Potentiometric Titration | Conductimetric Titration | Potentiometric Titration | Conductimetric Titration | |||
| ACG-550 | 46.7 | 41.0 | 53.3 | 59.0 | 5.44 | 4.0 |
| ACG-442 | 42.4 | 37.0 | 57.6 | 63.0 | 5.25 | 4.1 |
| ACG-334 | 43.3 | 33.3 | 56.7 | 66.6 | 5.78 | 4.2 |
| ACG-226 | - | 41.4 | - | 58.6 | 5.62 | 4.3 |
| ACG-118 | - | 38.5 | - | 61.5 | - | 3.5 |
| Transition Metal Ions | Concentration of Metal Ions, mol·L−1 | Adsorbed, % | Desorbed, % |
|---|---|---|---|
| Cu2+ | 10−3 | 99.9 | 51.4 |
| Ni2+ | 99.9 | 67.2 | |
| Co2+ | 99.9 | 62.0 |
| Substances | qm, mg·g−1 | pH of Adsorption |
|---|---|---|
| Lysozyme | 123 | 9.5 |
| MB | 8.3 | 9.5 |
| SDBS | 1313 | 5.3 |
| MO | 27.1 | 5.3 |
| Substance | Release, mg/% | ||
|---|---|---|---|
| pH | |||
| 7.1 (IEP) | 9.5 | 5.3 | |
| MB | 0.04/94 | 0.003/6 | - |
| MO | 0.13/94.5 | - | 0.0076/5.5 |
| Lysozyme | 1.72/98 | 0.035/2 | - |
| SDBS | 17.9/93.2 | - | 1.3/6.7 |
| Cycles | Conversion Degree, % | ||
|---|---|---|---|
| [p-NBA]:[NaBH4] = 1:50 mol/mol | [p-NBA]:[NaBH4] = 1:100 mol/mol | [p-NBA]:[NaBH4] = 1:200 mol/mol | |
| 1 | 0.95 | 100 | 100 |
| 2 | 39.02 | 89.84 | 100 |
| 3 | 34.73 | 82.77 | 100 |
| 4 | 38.10 | 78.48 | 100 |
| 5 | 38.40 | 78.78 | 100 |
| 6 | 39.63 | 78.78 | 100 |
| 7 | 39.94 | 80.01 | 100 |
| 8 | 39.94 | 84.34 | 100 |
| 9 | 40.55 | 84.92 | 100 |
| 10 | 44.23 | 84.62 | 100 |
| Average | 39.93 * | 84.28 | 100 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudaibergenov, S.E. Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels. Gels 2019, 5, 8. https://doi.org/10.3390/gels5010008
Kudaibergenov SE. Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels. Gels. 2019; 5(1):8. https://doi.org/10.3390/gels5010008
Chicago/Turabian StyleKudaibergenov, Sarkyt E. 2019. "Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels" Gels 5, no. 1: 8. https://doi.org/10.3390/gels5010008
APA StyleKudaibergenov, S. E. (2019). Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels. Gels, 5(1), 8. https://doi.org/10.3390/gels5010008





