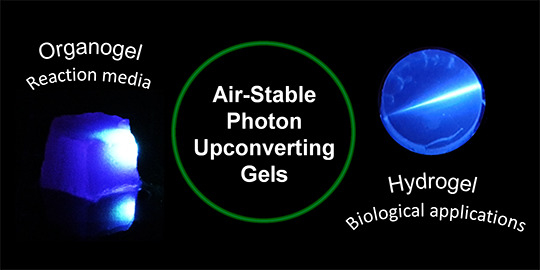
Recent Progress in Photon Upconverting Gels
Abstract
:1. Introduction
2. Upconverting Organogels
3. Upconverting Ionogels
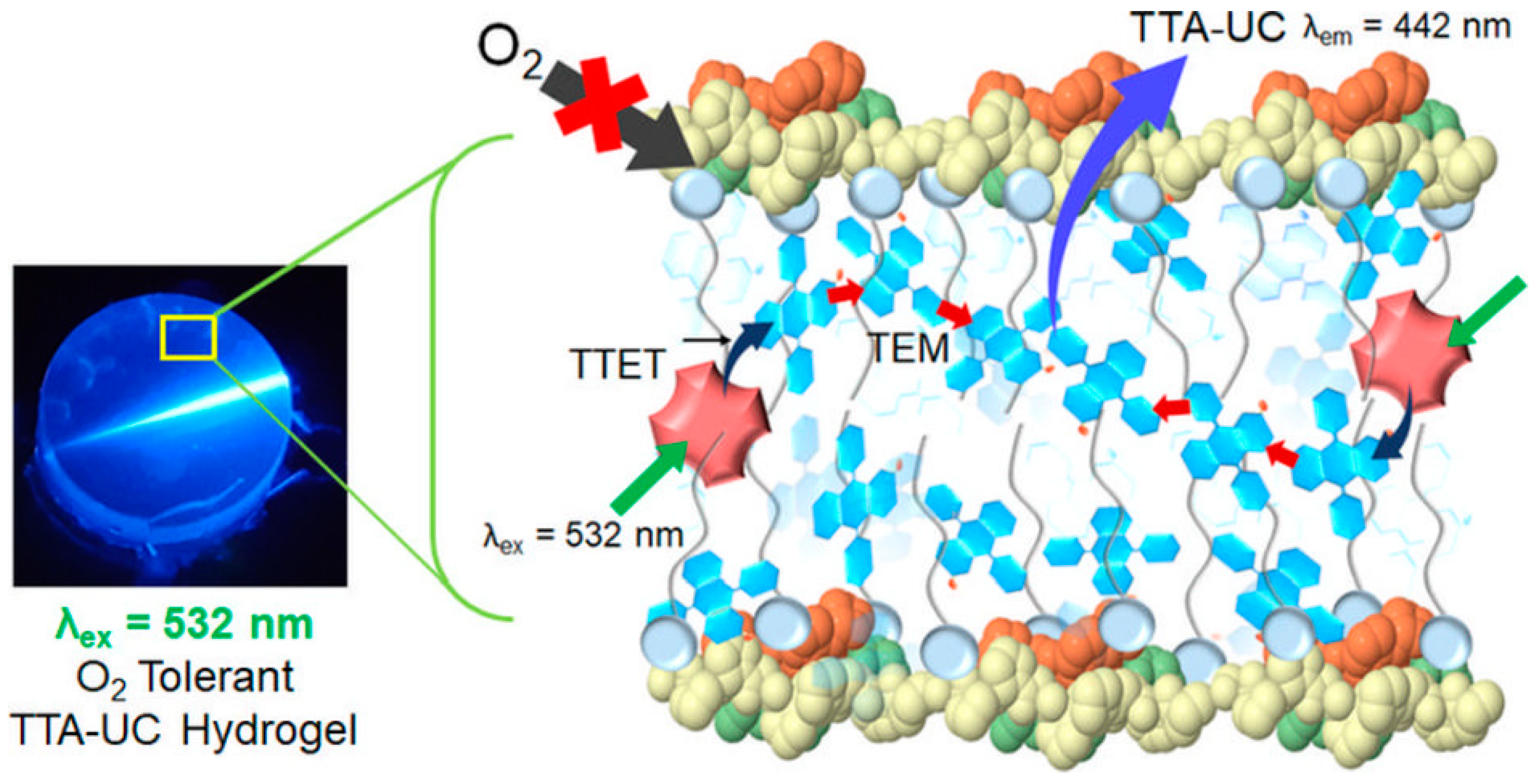
4. Upconverting Hydrogels
5. Conclusions and Future Possibilities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Ji, S.M.; Guo, H.M. Triplet-triplet annihilation based upconversion: From triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 2011, 1, 937–950. [Google Scholar] [CrossRef]
- Monguzzi, A.; Tubino, R.; Hoseinkhani, S.; Campione, M.; Meinardi, F. Low power, non-coherent sensitized photon up-conversion: Modeling and perspectives. Phys. Chem. Chem. Phys. 2012, 14, 4322–4332. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F.Y. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Filatov, M.A.; Baluschev, S.; Landfesterc, K. Protection of densely populated excited triplet state ensembles against deactivation by molecular oxygen. Chem. Soc. Rev. 2016, 45, 4668–4689. [Google Scholar] [CrossRef]
- Wu, M.; Congreve, D.N.; Wilson, M.W.B.; Jean, J.; Geva, N.; Welborn, M.; Voorhis, T.V.; Bulović, V.; Bawendi, M.G.; Baldo, M.A. Solid-state infrared-to-visible upconversion sensitized by colloidal nanocrystals. Nat. Photonics 2016, 10, 31–34. [Google Scholar] [CrossRef]
- Börjesson, K.; Rudquist, P.; Gray, V.; Moth-Poulsen, K. Photon upconversion with directed emission. Nat. Commun. 2016, 7, 12689. [Google Scholar] [CrossRef] [PubMed]
- Yanai, N.; Kimizuka, N. New triplet sensitization routes for photon upconversion: Thermally activated delayed fluorescence molecules, inorganic nanocrystals, and singlet-to-triplet absorption. Acc. Chem. Res. 2017, 50, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Tang, M.L. Designing transmitter ligands that mediate energy transfer between semiconductor nanocrystals and molecules. J. Am. Chem. Soc. 2017, 139, 9412–9418. [Google Scholar] [CrossRef]
- Han, J.; Duan, P.; Li, X.; Liu, M. Amplification of circularly polarized luminescence through triplet-triplet annihilation-based photon upconversion. J. Am. Chem. Soc. 2017, 139, 9783–9786. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, P. Energy up-conversion by low-power excitation: New applications of an old concept. Chem. Eur. J. 2011, 17, 9560–9564. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.P.; Hanson, K. Harnessing molecular photon upconversion in a solar cell at sub-solar irradiance: Role of the redox mediator. J. Am. Chem. Soc. 2017, 139, 10988–10991. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.-H. Encapsulated triplet-triplet annihilation-based upconversion in the aqueous phase for sub-band-gap semiconductor photocatalysis. J. Am. Chem. Soc. 2012, 134, 17478–17481. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, S.; Monguzzi, A.; Pedrini, J.; Sassi, M.; Villa, C.; Torrente, Y.; Marotta, R.; Meinardi, F.; Beverina, L. Self-assembled dual dye-doped nanosized micelles for high-contrast up-conversion bioimaging. Adv. Funct. Mater. 2016, 26, 8447–8454. [Google Scholar] [CrossRef]
- Van Rixel, V.H.S.; Siewert, B.; Hopkins, S.L.; Askes, S.H.C.; Busemann, A.; Siegler, M.A.; Bonnet, S. Green light-induced apoptosis in cancer cells by a tetrapyridyl ruthenium prodrug offering two trans coordination sites. Chem. Sci. 2016, 7, 4922–4929. [Google Scholar] [CrossRef]
- Grewer, C.; Brauer, H.-D. Mechanism of the triplet-state quenching by molecular oxygen in solution. J. Phys. Chem. 1994, 98, 4230–4235. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Wu, W.; Wu, W.; Guo, H.; Sun, J.; Sun, H.; Liu, Y.; Li, Q.; Huang, L. Transition metal complexes with strong absorption of visible light and long-lived triplet excited states: From molecular design to applications. RSC Adv. 2012, 2, 1712–1728. [Google Scholar] [CrossRef]
- Kruft, B.I.; Greer, A. Photosensitization reactions in vitro and in vivo. Photochem. Photobiol. 2011, 87, 1204–1213. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Zhan, C.; Barhoumi, A.; Yang, T.; Wylie, R.G.; Armstrong, P.A.; Kohane, D.S. Efficient triplet-triplet annihilation-based upconversion for nanoparticle phototargeting. Nano Lett. 2015, 15, 6332–6338. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Song, H.S.; Conde, J.; Kim, H.; Artzi, N.; Kim, J.-H. Dual-color emissive upconversion nanocapsules for differential cancer bioimaging in vivo. ACS Nano 2016, 10, 1512–1521. [Google Scholar] [CrossRef]
- Vadrucci, R.; Monguzzi, A.; Saenz, F.; Wilts, B.D.; Simon, Y.C.; Weder, C. Nanodroplet-Containing Polymers for Efficient Low-Power Light Upconversion. Adv. Mater. 2017, 29, 1702992. [Google Scholar] [CrossRef] [PubMed]
- Wohnhaas, C.; Turshatov, A.; Mailänder, V.; Lorenz, S.; Baluschev, S.; Miteva, T.; Landfester, K. Annihilation upconversion in cells by embedding the dye system in polymeric nanocapsules. Macromol. Biosci. 2011, 11, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Monguzzi, A.; Frigoli, M.; Larpent, C.; Tubino, R.; Meinardi, F. Low-power-photon up-conversion in dual-dye-loaded polymer nanoparticles. Adv. Funct. Mater. 2012, 22, 139–143. [Google Scholar] [CrossRef]
- Kang, J.-H.; Reichmanis, E. Low-threshold photon upconversion capsules obtained by photoinduced interfacial polymerization. Angew. Chem. Int. Ed. 2012, 51, 11841–11844. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Yanai, N.; Kimizuka, N. Photon upconverting liquids: Matrix-free molecular upconversion systems functioning in air. J. Am. Chem. Soc. 2013, 135, 19056–19059. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Yanai, N.; Monguzzi, A.; Kimizuka, N. Highly efficient photon upconversion in self-assembled light-harvesting molecular systems. Sci. Rep. 2015, 5, 10882. [Google Scholar] [CrossRef] [PubMed]
- Hisamitsu, S.; Yanai, N.; Kimizuka, N. Photon upconverting ionic liquids: Effective triplet energy migration in contiguous ionic chromophore arrays. Angew. Chem. Int. Ed. 2015, 54, 11550–11554. [Google Scholar] [CrossRef] [PubMed]
- Yanai, N.; Kimizuka, N. Recent emergence of photon upconversion based on triplet energy migration in molecular assemblies. Chem. Commun. 2016, 52, 5354–5370. [Google Scholar] [CrossRef]
- Kimizuka, N.; Yanai, N.; Morikawa, M.-A. Photon upconversion and molecular energy storage by maximizing the potential of molecular self-assembly. Langmuir 2016, 32, 12304–12322. [Google Scholar] [CrossRef]
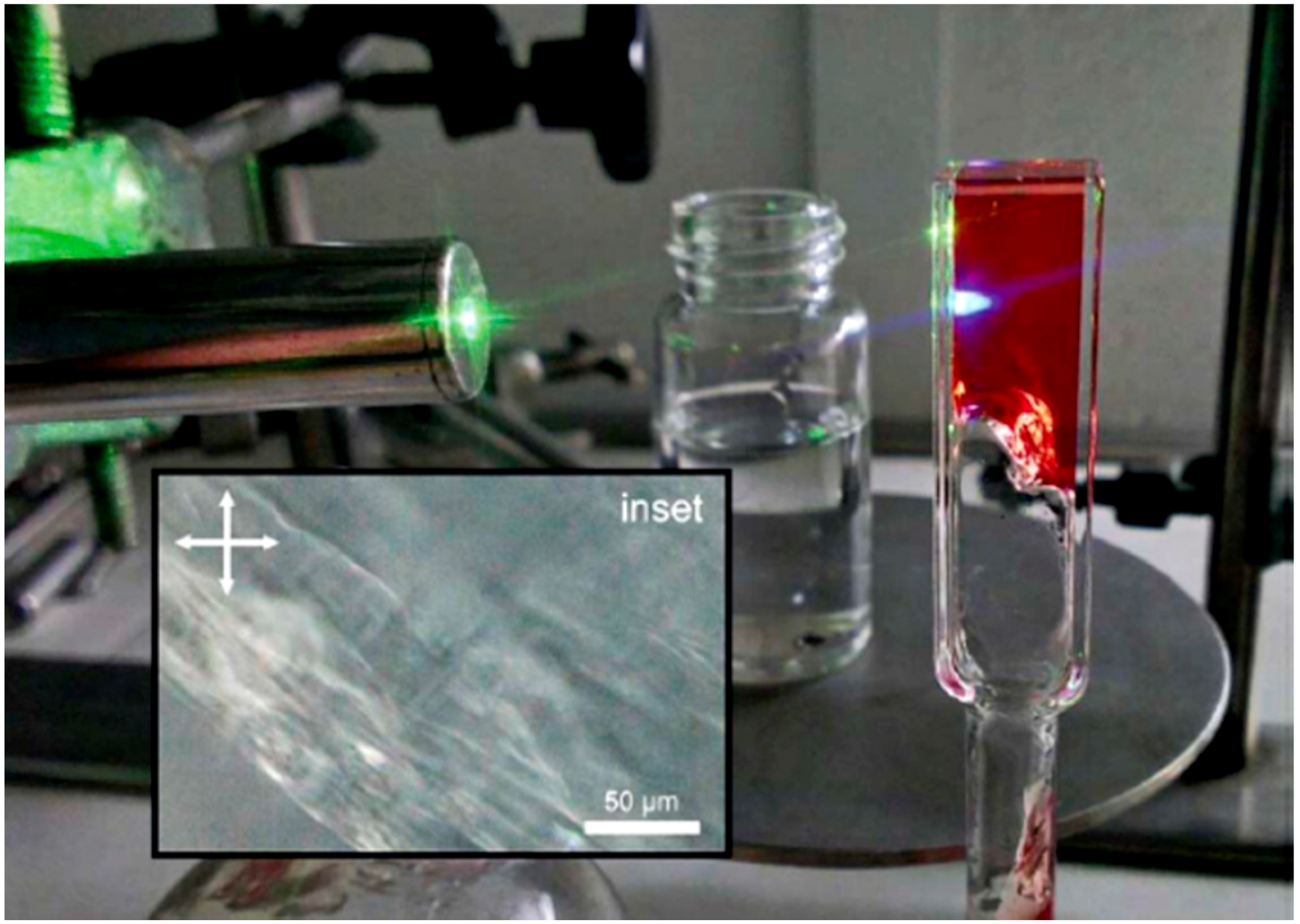
- Häring, M.; Pérez-Ruiz, R.; von Wangelin, A.J.; Díaz Díaz, D. Intragel photoreduction of aryl halides by green-to-blue upconversion under aerobic conditions. Chem. Commun. 2015, 51, 16848–16851. [Google Scholar] [CrossRef]
- Häring, M.; Abramov, A.; Okumura, K.; Ghosh, I.; König, B.; Yanai, N.; Kimizuka, N.; Díaz Díaz, D. Air-sensitive photoredox catalysis performed under aerobic conditions in gel networks. J. Org. Chem. 2018, 83, 7928–7938. [Google Scholar] [CrossRef]
- Díaz Díaz, D.; Saldías, C. Photon upconversion in supramolecular gels and synthetic application. Curr. Org. Chem. 2018, 22, 2223–2228. [Google Scholar] [CrossRef]
- Sripathy, K.; MacQueen, R.W.; Peterson, J.R.; Cheng, Y.Y.; Dvořák, M.; McCamey, D.R.; Treat, N.D.; Stingelin, N.; Schmidt, T.W. Highly efficient photochemical upconversion in a quasi-solid organogel. J. Mater. Chem. C 2015, 3, 616–622. [Google Scholar] [CrossRef]
- Vadrucci, R.; Weder, C.; Simon, Y.C. Organogels for low-power light upconversion. Mater. Horiz. 2015, 2, 120–124. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, B.; Yang, T.; Yang, Y.; Shen, Z.; Yao, P.; Li, F. A general strategy for biocompatible, high-effective upconversion nanocapsules based on triplet-triplet annihilation. J. Am. Chem. Soc. 2013, 135, 5029–5037. [Google Scholar] [CrossRef]
- Mongin, C.; Golden, J.H.; Castellano, F.N. Liquid peg polymers containing antioxidants: A versatile platform for studying oxygen-sensitive photochemical processes. ACS Appl. Mater. Interfaces 2016, 8, 24038–24048. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor-acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. [Google Scholar] [CrossRef]
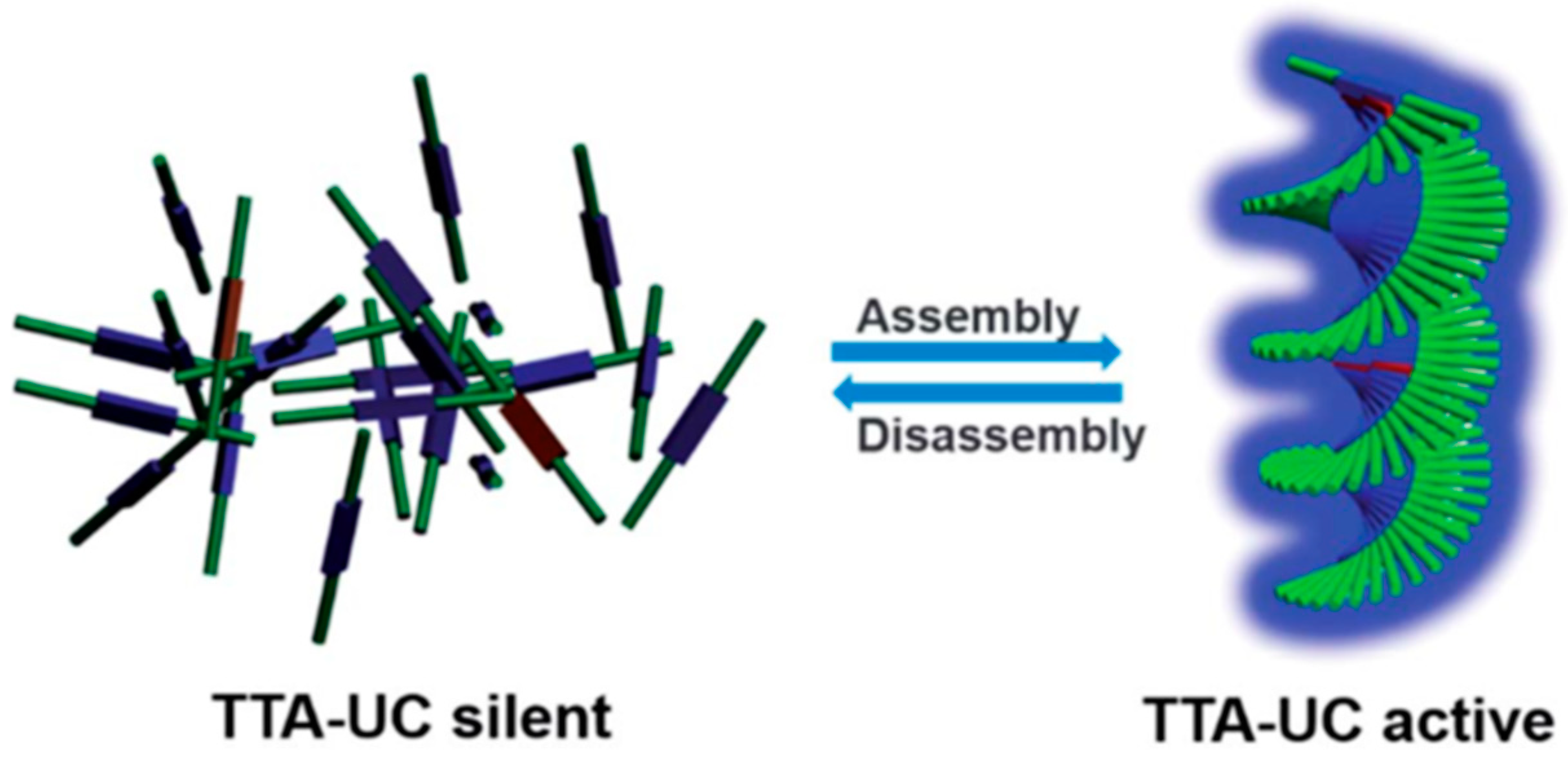
- Duan, P.; Asthana, D.; Nakashima, T.; Kawai, T.; Yanai, N.; Kimizuka, N. All-or-none switching of photon upconversion in self-assembled organogel systems. Faraday Discuss 2017, 196, 305–316. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvent for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Kimizuka, N.; Nakashima, T. Spontaneous self-assembly of glycolipid bilayer membranes in sugar-philic ionic liquids and formation of ionogels. Langmuir 2001, 17, 6759–6761. [Google Scholar] [CrossRef]
- Murakami, Y.; Himuro, Y.; Ito, T.; Morita, R.; Niimi, K.; Kiyoyanagi, N. Transparent and nonflammable ionogel photon upconverters and their solute transport properties. J. Phys. Chem. B 2016, 120, 748–755. [Google Scholar] [CrossRef] [PubMed]
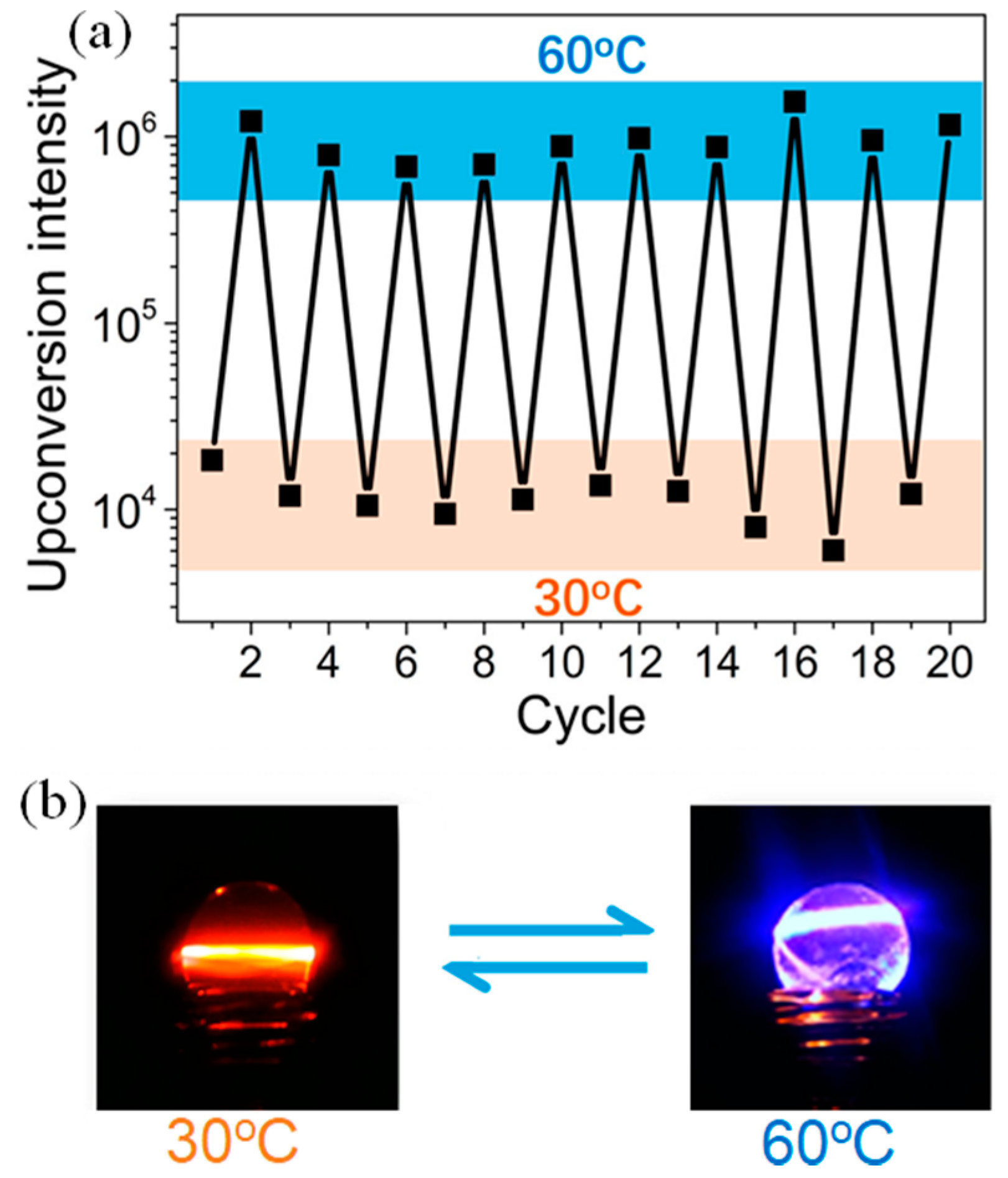
- Ye, C.; Ma, J.; Chen, S.; Ge, J.; Yang, W.; Zheng, Q.; Wang, X.; Liang, Z.; Zhou, Y. Eco-friendly solid-state upconversion hydrogel with thermoresponsive feature as the temperature indicator. J. Phys. Chem. C 2017, 121, 20158–20164. [Google Scholar] [CrossRef]
- Bharmoria, P.; Hisamitsu, S.; Nagatomi, H.; Ogawa, T.; Morikawa, M.; Yanai, N.; Kimizuka, N. A simple and versatile platform for air-tolerant photon upconverting hydrogels by biopolymer-surfactant-chromophore co-assembly. J. Am. Chem. Soc. 2018, 140, 10848–10855. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharmoria, P.; Yanai, N.; Kimizuka, N. Recent Progress in Photon Upconverting Gels. Gels 2019, 5, 18. https://doi.org/10.3390/gels5010018
Bharmoria P, Yanai N, Kimizuka N. Recent Progress in Photon Upconverting Gels. Gels. 2019; 5(1):18. https://doi.org/10.3390/gels5010018
Chicago/Turabian StyleBharmoria, Pankaj, Nobuhiro Yanai, and Nobuo Kimizuka. 2019. "Recent Progress in Photon Upconverting Gels" Gels 5, no. 1: 18. https://doi.org/10.3390/gels5010018
APA StyleBharmoria, P., Yanai, N., & Kimizuka, N. (2019). Recent Progress in Photon Upconverting Gels. Gels, 5(1), 18. https://doi.org/10.3390/gels5010018