Low-Cost Microfluidic Sensors with Smart Hydrogel Patterned Arrays Using Electronic Resistive Channel Sensing for Readout
Abstract
1. Introduction
2. Results and Discussion
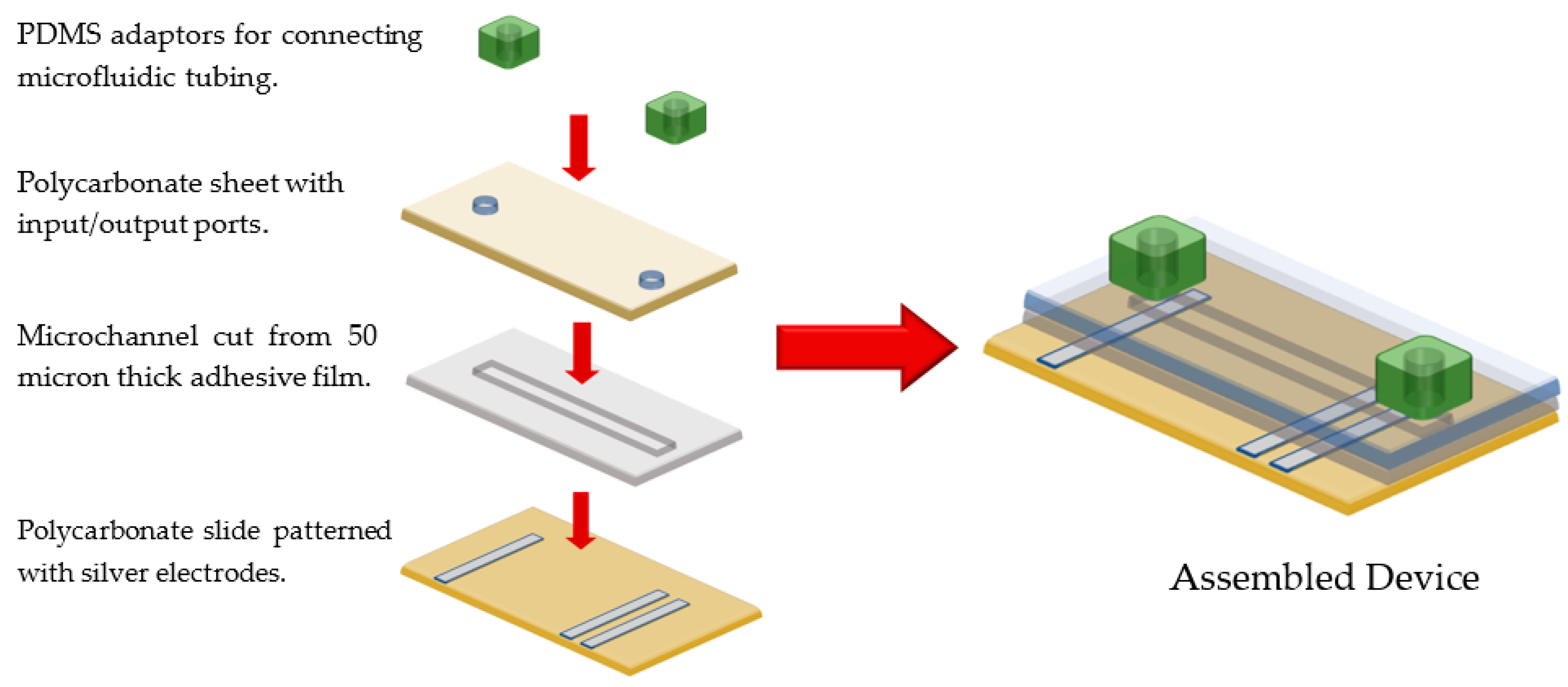
2.1. Fabrication of Microfluidic Channels
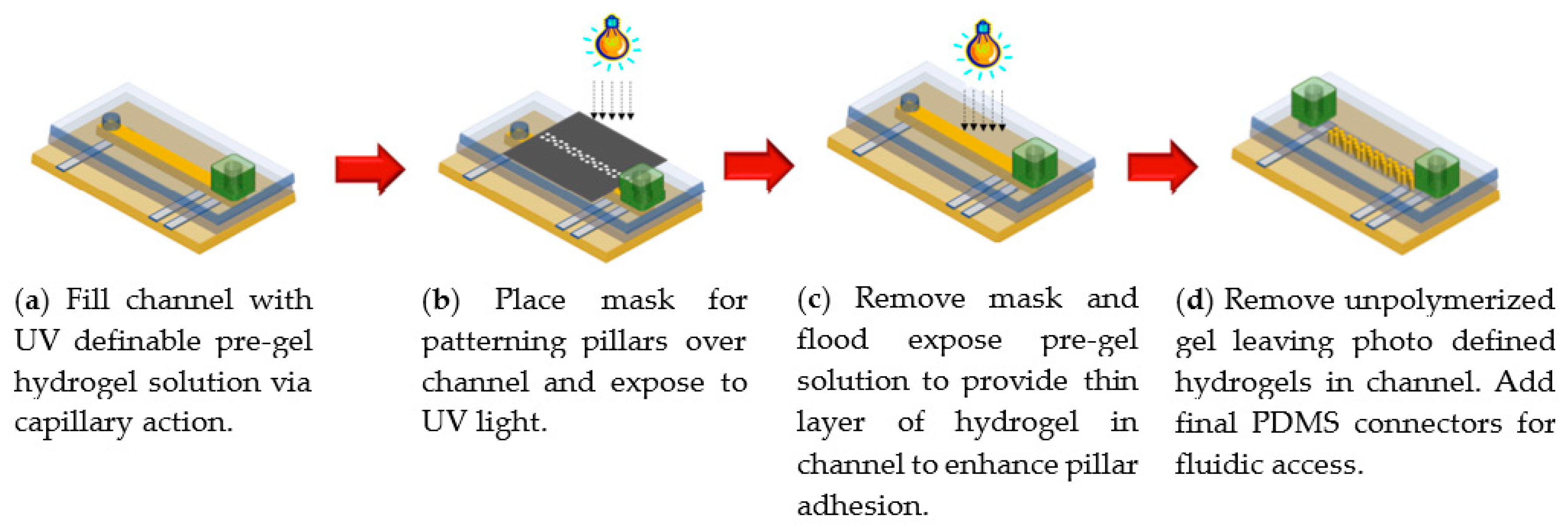
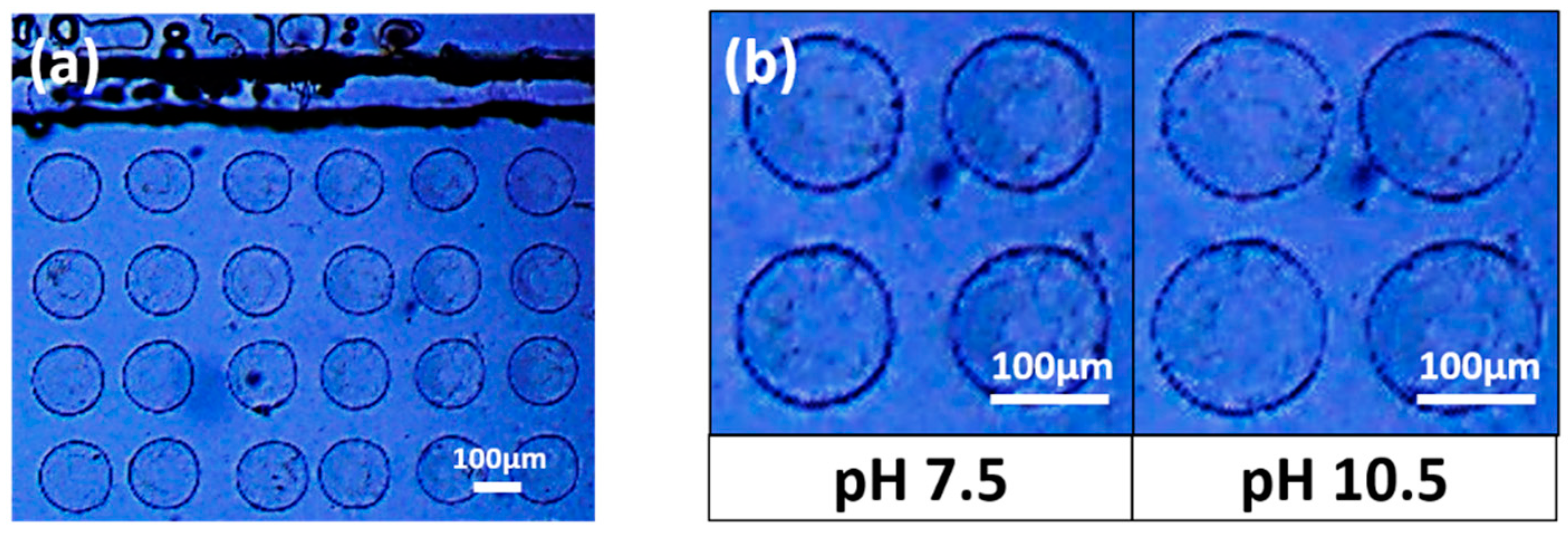
2.2. UV Photopolymerization of Smart Hydrogel Pillar Arrays within Microfluidic Channels
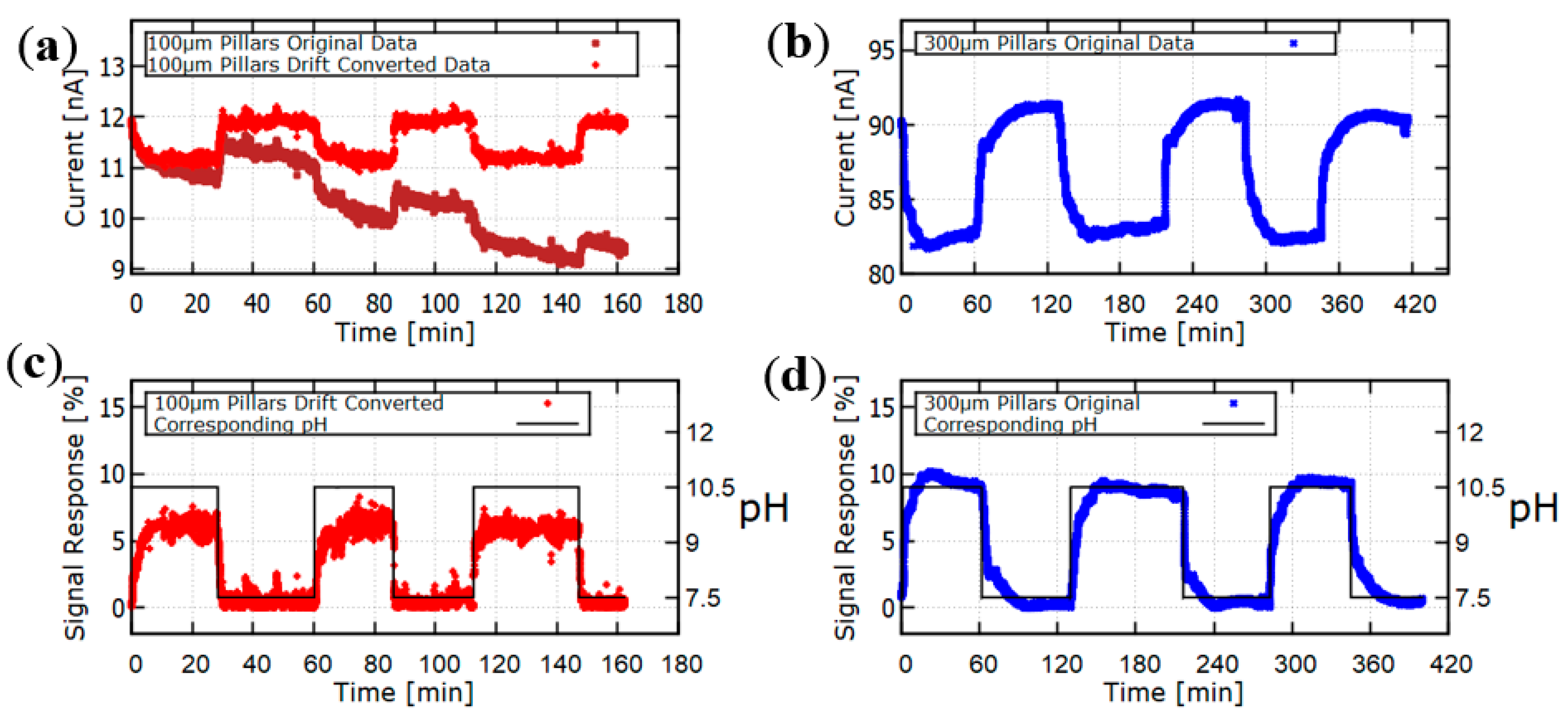
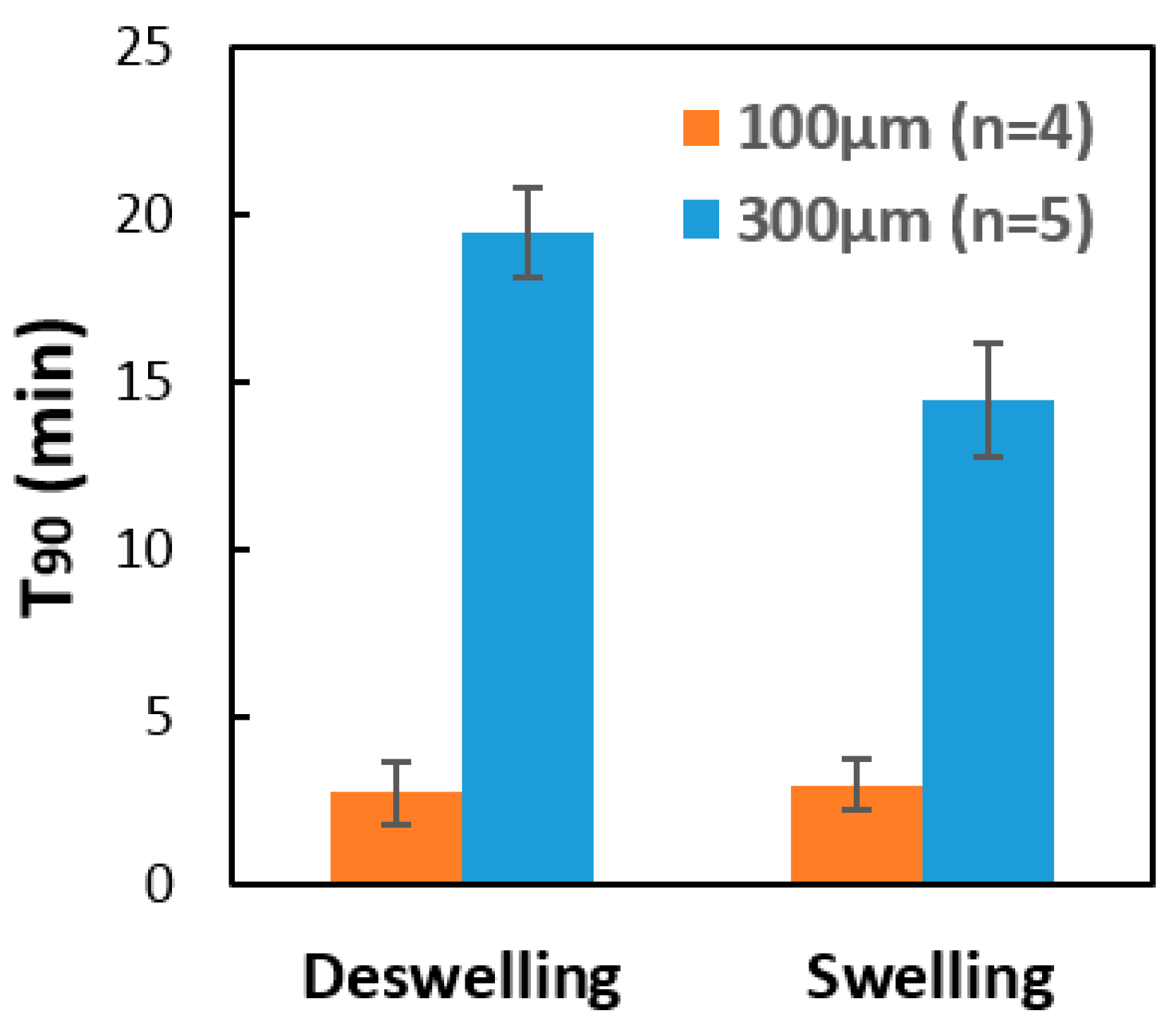
2.3. Response of the Hydrogel Pillars to Cyclic Changes in pH
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Gehrke, S.H. Synthesis, equilibrium swelling, kinetics, permeability and applications of environmentally responsive gels. In Responsive Gels: Volume Transitions II; Springer: Berlin, Germany, 1993; pp. 81–144. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.J. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliv. Rev. 2004, 56, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Hatch, A.; Hansmann, G.; Murthy, S.K. Engineered alginate hydrogels for effective microfluidic capture and release of endothelial progenitor cells from whole blood. Langmuir 2011, 27, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, B.; Yoon, H.; Suh, K.Y. Stimuli-responsive hydrogel patterns for smart microfluidics and microarrays. Analyst 2013, 138, 6230–6242. [Google Scholar]
- Saleh, O.A.; Sohn, L.L. An artificial nanopore for molecular sensing. Nano Lett. 2003, 3, 37–38. [Google Scholar] [CrossRef]
- Bartholomeusz, D.; Boutté, R.; Gale, B. Xurography: Microfluidic prototyping with a cutting plotter. In Lab on a Chip Technology: Fabrication and Microfluidics; Caister Academic Press: Poole, UK, 2009; Volume 1, pp. 65–82. [Google Scholar]
- Mohanty, S.K.; Kim, D.; Beebe, D.J. Do-it-yourself microelectrophoresis chips with integrated sample recovery. Electrophoresis 2006, 27, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Magda, J.J.; Tathireddy, P. Manipulation of the Isoelectric Point of Polyampholytic Smart Hydrogels In Order to Increase the Range and Selectivity of Continuous Glucose Sensors. Sens. Actuators B 2018, 255, 1057–1063. [Google Scholar] [CrossRef]
- Lin, G.; Chang, S.; Hao, H.; Tathireddy, P.; Orthner, M.; Magda, J.; Solzbacher, F. Osmotic swelling pressure response of smart hydrogels suitable for chronically implantable glucose sensors. Sens. Actuators B Chem. 2010, 144, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2016. Available online: https://imagej.nih.gov/ij/ (accessed on 15 February 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, H.-Y.; Farhoudi, N.; Reiche, C.F.; Körner, J.; Mohanty, S.; Solzbacher, F.; Magda, J. Low-Cost Microfluidic Sensors with Smart Hydrogel Patterned Arrays Using Electronic Resistive Channel Sensing for Readout. Gels 2018, 4, 84. https://doi.org/10.3390/gels4040084
Leu H-Y, Farhoudi N, Reiche CF, Körner J, Mohanty S, Solzbacher F, Magda J. Low-Cost Microfluidic Sensors with Smart Hydrogel Patterned Arrays Using Electronic Resistive Channel Sensing for Readout. Gels. 2018; 4(4):84. https://doi.org/10.3390/gels4040084
Chicago/Turabian StyleLeu, Hsuan-Yu, Navid Farhoudi, Christopher F. Reiche, Julia Körner, Swomitra Mohanty, Florian Solzbacher, and Jules Magda. 2018. "Low-Cost Microfluidic Sensors with Smart Hydrogel Patterned Arrays Using Electronic Resistive Channel Sensing for Readout" Gels 4, no. 4: 84. https://doi.org/10.3390/gels4040084
APA StyleLeu, H.-Y., Farhoudi, N., Reiche, C. F., Körner, J., Mohanty, S., Solzbacher, F., & Magda, J. (2018). Low-Cost Microfluidic Sensors with Smart Hydrogel Patterned Arrays Using Electronic Resistive Channel Sensing for Readout. Gels, 4(4), 84. https://doi.org/10.3390/gels4040084




