Improved Water Barrier Properties of Calcium Alginate Capsules Modified by Silicone Oil
Abstract
:1. Introduction
2. Results
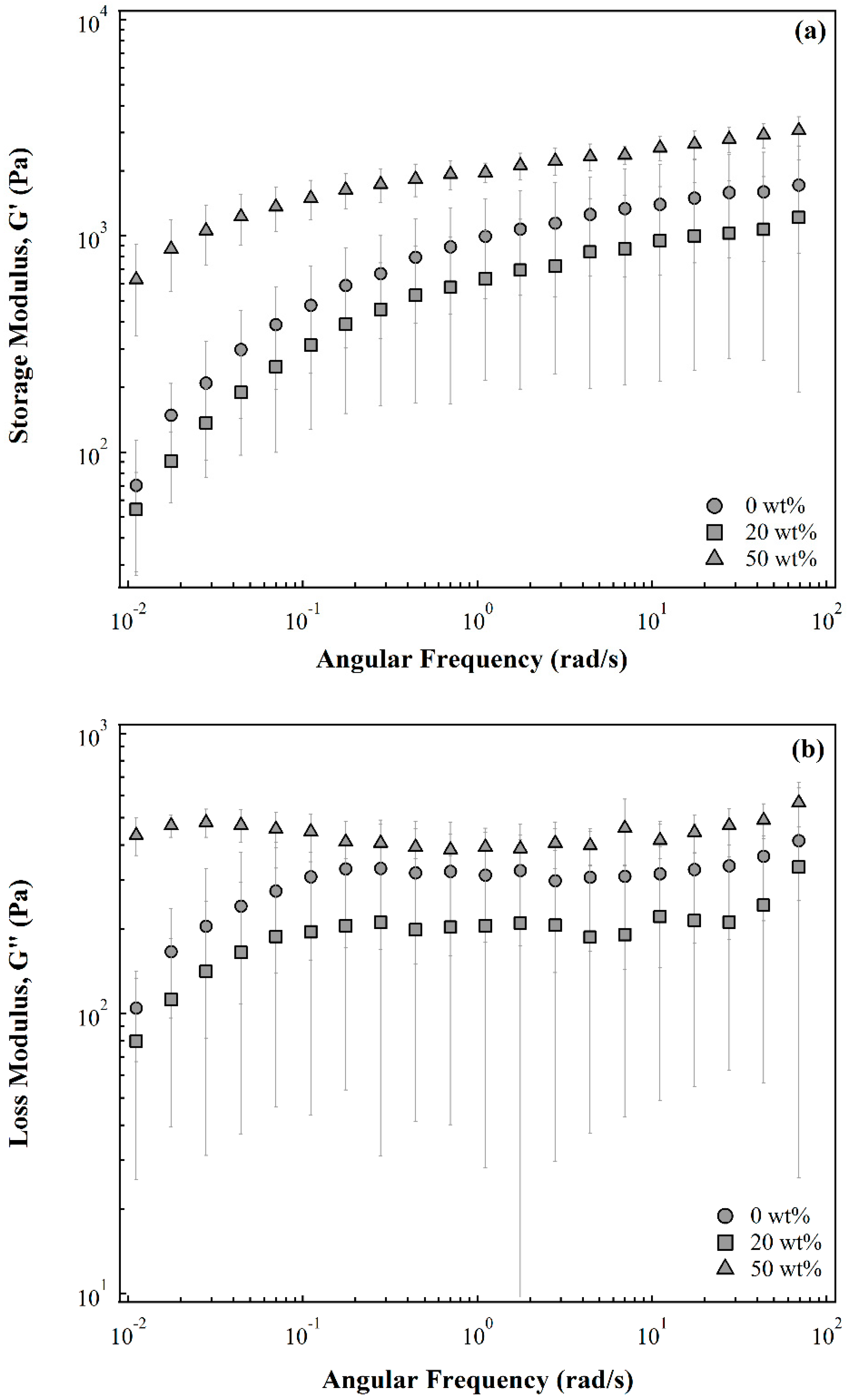
2.1. Membrane Rheological Behavior
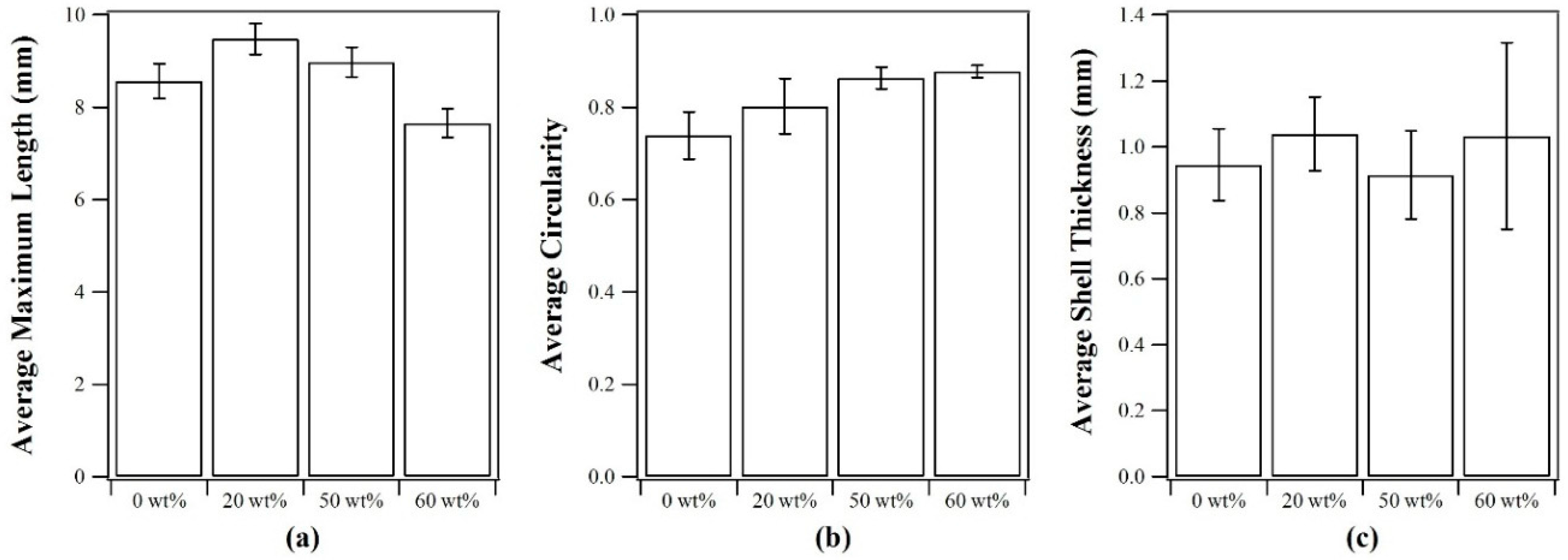
2.2. Capsule Properties
2.3. Capsule Drying
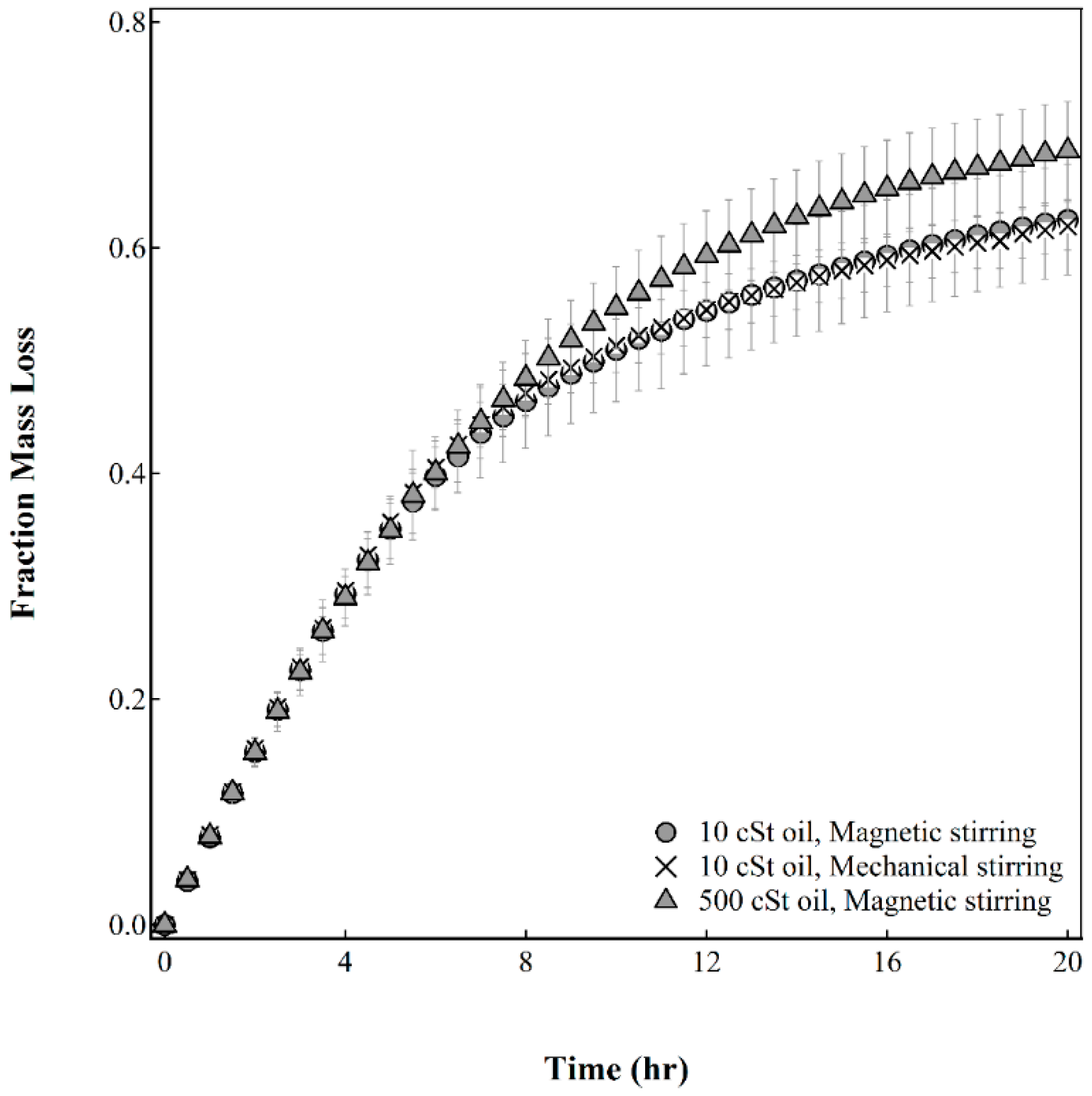
Effect of Process Parameters on Capsule Drying
3. Conclusions
4. Materials and Methods
4.1. Solution and Emulsion Preparation
4.2. Capsule Fabrication
4.3. Capsule Size Measurements
4.4. Rheological Measurements
4.5. Drying Measurements
4.6. Emulsion Droplet Size Distribution Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.-H.; Romanowsky, M.; Shum, H.C.; Sun, B.; Utada, A.S.; Windbergs, M.; et al. 25th Anniversary Article: Double Emulsion Templated Solid Microcapsules: Mechanics And Controlled Release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. Int. J. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Controlled Release 2010, 146, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.A.; Joung, M.J.; Ahn, S.D.; Kim, G.H.; Kang, S.-Y.; You, I.-K.; Oh, J.; Myoung, H.J.; Baek, K.H.; Suh, K.S. Microcapsules as an electronic ink to fabricate color electrophoretic displays. Synth. Met. 2005, 151, 181–185. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E.; Lyons, H.; Pembroke, T.; Byrne, H.; Murray, B.; Hall, M. The characterisation of a novel, covalently modified, amphiphilic alginate derivative, which retains gelling and non-toxic properties. J. Colloid Interface Sci. 2006, 298, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Mørch, Ý.A.; Donati, I.; Strand, B.L. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Bremond, N.; Santanach-Carreras, E.; Chu, L.-Y.; Bibette, J. Formation of liquid-core capsules having a thin hydrogel membrane: Liquid pearls. Soft Matter 2010, 6, 2484. [Google Scholar] [CrossRef]
- Kim, C.; Chung, S.; Kim, Y.E.; Lee, K.S.; Lee, S.H.; Oh, K.W.; Kang, J.Y. Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid. Lab Chip 2011, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.C.; Tsao, I.-F.; Sakoda, A.; Wang, H.Y. Techniques for preparing hydrogel membrane capsules. Biotechnol. Tech. 1988, 2, 271–276. [Google Scholar] [CrossRef]
- Liu, L.; Wu, F.; Ju, X.-J.; Xie, R.; Wang, W.; Niu, C.H.; Chu, L.-Y. Preparation of monodisperse calcium alginate microcapsules via internal gelation in microfluidic-generated double emulsions. J. Colloid Interface Sci. 2013, 404, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Nussinovitch, A. Hydrocolloid carriers with filler inclusion for diltiazem hydrochloride release. J. Pharm. Sci. 2007, 96, 168–178. [Google Scholar] [CrossRef] [PubMed]
- López-Córdoba, A.; Deladino, L.; Martino, M. Release of yerba mate antioxidants from corn starch–alginate capsules as affected by structure. Carbohydr. Polym. 2014, 99, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.; Justice, R.; Schaefer, D.; Mark, J. Calcium Alginate Barrier Films Modified by Montmorillonite Clay. J. Macromol. Sci. B Phys. 2005, 44, 821–831. [Google Scholar] [CrossRef]
- Degen, P.; Leick, S.; Siedenbiedel, F.; Rehage, H. Magnetic switchable alginate beads. Colloid Polym. Sci. 2012, 290, 97–106. [Google Scholar] [CrossRef]
- Wah Chan, L.; Liu, X.; Heng, P.W.S. Liquid phase coating to produce controlled-release alginate microspheres. J. Microencapsul. 2005, 22, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Patchan, M.W.; Baird, L.M.; Rhim, Y.-R.; LaBarre, E.D.; Maisano, A.J.; Deacon, R.M.; Xia, Z.; Benkoski, J.J. Liquid-Filled Metal Microcapsules. ACS Appl. Mater. Interfaces 2012, 4, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, A.; Debeaufort, F.; Bonnotte, A.; Voilley, A. Influence of alginate emulsion-based films structure on its barrier properties and on the protection of microencapsulated aroma compound. Food Hydrocoll. 2009, 23, 2116–2124. [Google Scholar] [CrossRef]
- Chan, E.-S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Abang, S.; Chan, E.-S.; Poncelet, D. Effects of process variables on the encapsulation of oil in ca-alginate capsules using an inverse gelation technique. J. Microencapsul. 2012, 29, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Renard, D.; Davy, J.; Marquis, M.; Poncelet, D. Oil core microcapsules by inverse gelation technique. J. Microencapsul. 2015, 32, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.-S.; Lee, B.-B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Saadevandi, B.A.; Zakin, J.L. A Study of Silicone Oil-in-Water Emulsions. Chem. Eng. Commun. 1996, 156, 227–246. [Google Scholar] [CrossRef]
- Aranberri, I.; Binks, B.P.; Clint, J.H.; Fletcher, P.D.I. Evaporation rates of water from concentrated oil-in-water emulsions. Langmuir 2004, 20, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Zukas, B.G. Encapsulation of Anti-Traction Material. Msc Thesis, Univeristy of New Hampshire, Durham, NH, USA, December 2014. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bron, J.L.; Vonk, L.A.; Smit, T.H.; Koenderink, G.H. Engineering alginate for intervertebral disc repair. J. Mech. Behav. Biomed. Mater. 2011, 4, 1196–1205. [Google Scholar] [CrossRef] [PubMed]


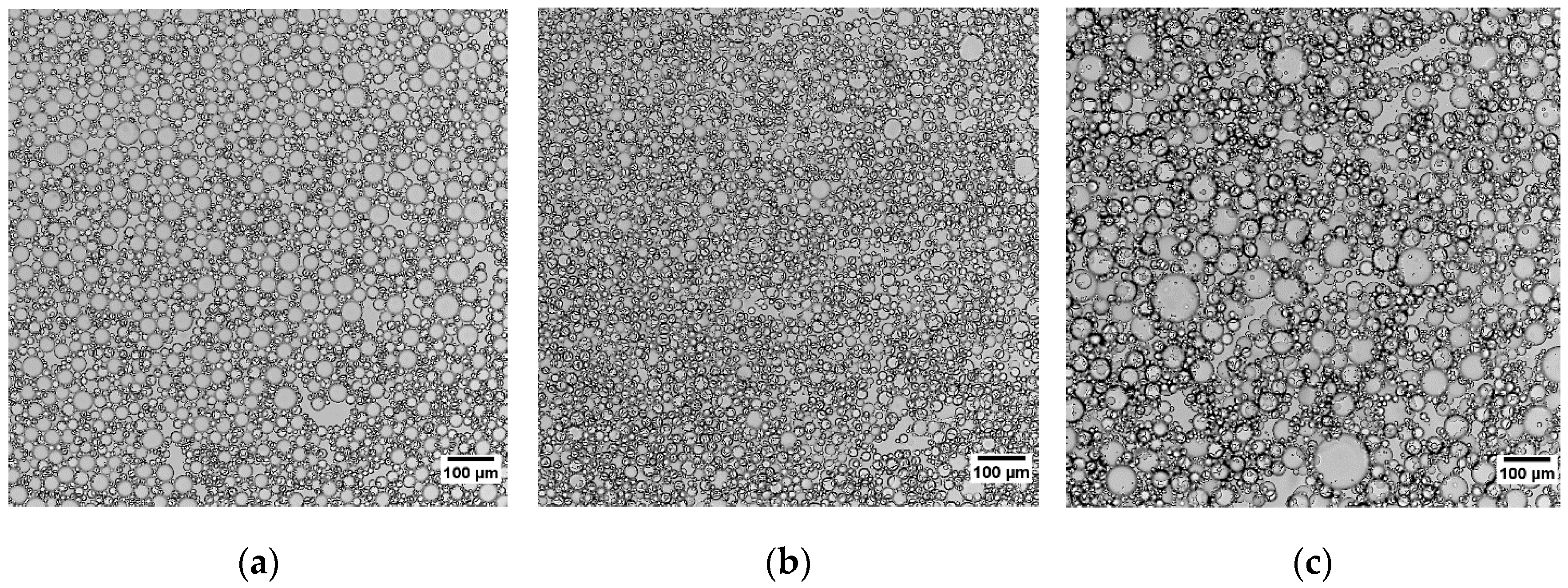
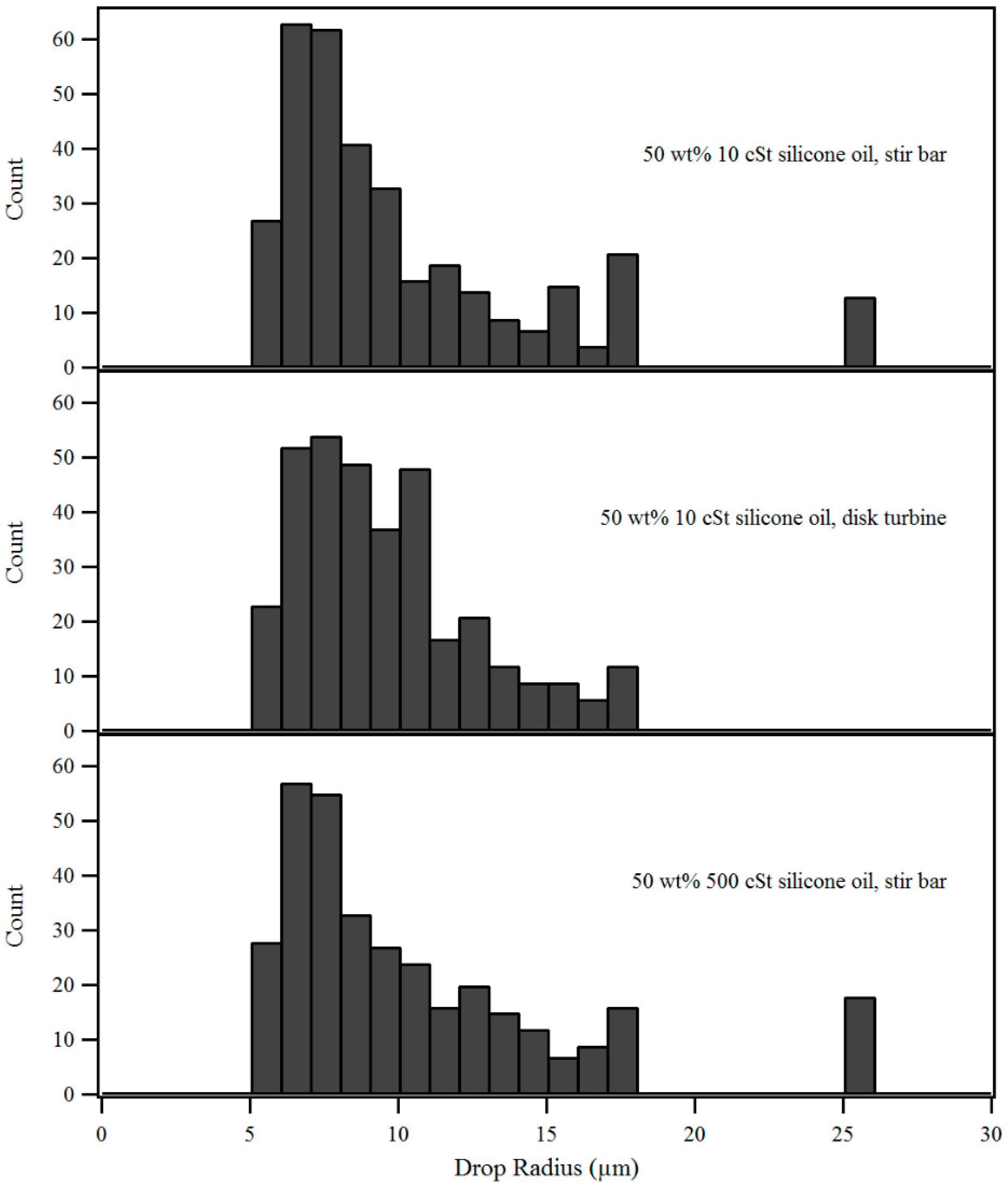
| Emulsion | Median Radius (µm) | IQR (µm) | Mean (µm) | Std. Dev. (µm) |
|---|---|---|---|---|
| 50 wt% 10 cSt, stir bar | 8.52 | 5.14 | 10.3 | 5.02 |
| 50 wt% 10 cSt, disk turbine | 8.91 | 3.82 | 9.57 | 3.04 |
| 50 wt% 500 cSt, stir bar | 9.11 | 6.04 | 11.3 | 6.34 |
| Solution | Viscosity (mPa.s) |
|---|---|
| 10% Dextran Solution | 33 ± 3 |
| 0.5 wt% Alginate Solution | 30.7 ± 0.1 (zero shear rate) |
| 10 cSt Silicone Oil | 9.7 ± 0.1 |
| 500 cSt Silicone Oil | 505 ± 4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zukas, B.G.; Gupta, N.R. Improved Water Barrier Properties of Calcium Alginate Capsules Modified by Silicone Oil. Gels 2016, 2, 14. https://doi.org/10.3390/gels2020014
Zukas BG, Gupta NR. Improved Water Barrier Properties of Calcium Alginate Capsules Modified by Silicone Oil. Gels. 2016; 2(2):14. https://doi.org/10.3390/gels2020014
Chicago/Turabian StyleZukas, Brian G., and Nivedita R. Gupta. 2016. "Improved Water Barrier Properties of Calcium Alginate Capsules Modified by Silicone Oil" Gels 2, no. 2: 14. https://doi.org/10.3390/gels2020014
APA StyleZukas, B. G., & Gupta, N. R. (2016). Improved Water Barrier Properties of Calcium Alginate Capsules Modified by Silicone Oil. Gels, 2(2), 14. https://doi.org/10.3390/gels2020014






