Capillary Gradient Gel Electrophoresis
Abstract
1. Introduction
2. Fundamental Principles of Capillary Gradient Gel Electrophoresis
2.1. Physical and Chemical Basis of Separation
2.2. Background of Gradient Gels
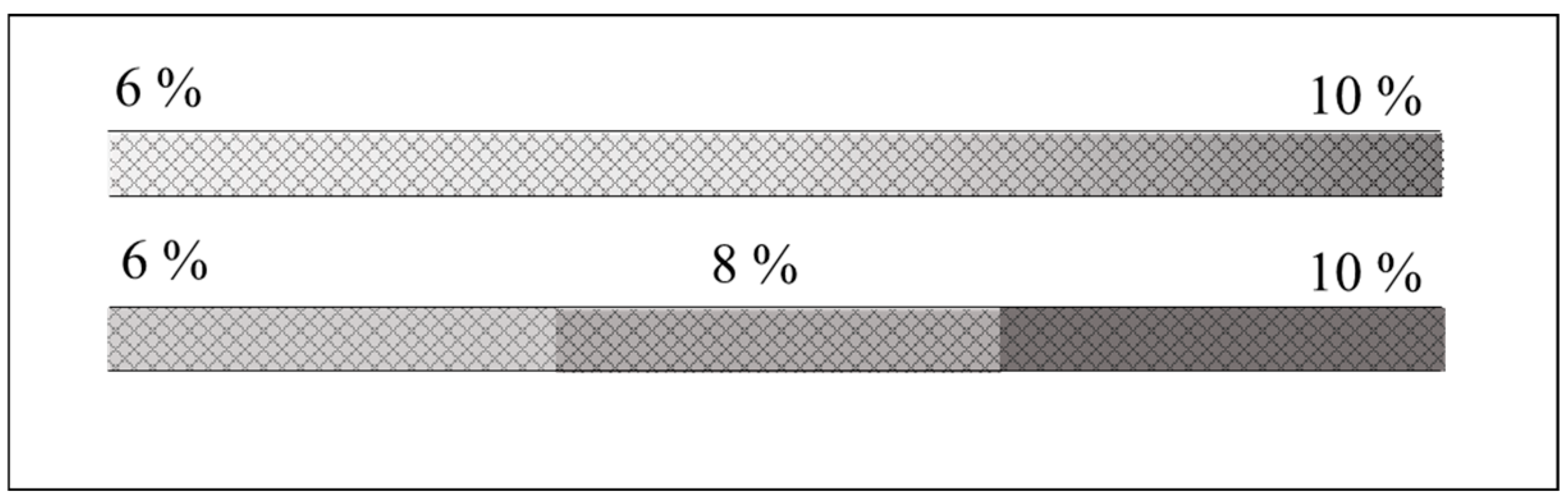
2.3. Capillary Format Adaptations for Pore-Size Gradient Gels
2.4. Gels and Sieving Matrices
3. Instrumentation
3.1. Instrumental Architecture and System Components
3.2. Sample Introduction Techniques
3.3. Thermal Management and Control of Joule Heating
3.4. Detection Strategies and Data Acquisition
4. Method Development
4.1. Technical Challenges in Gradient Matrix Preparation, Capillary Filling, and Polymerization
4.2. Method Optimization and Quality Assessment
5. Applications in Bioanalytical Chemistry
5.1. Nucleic Acids
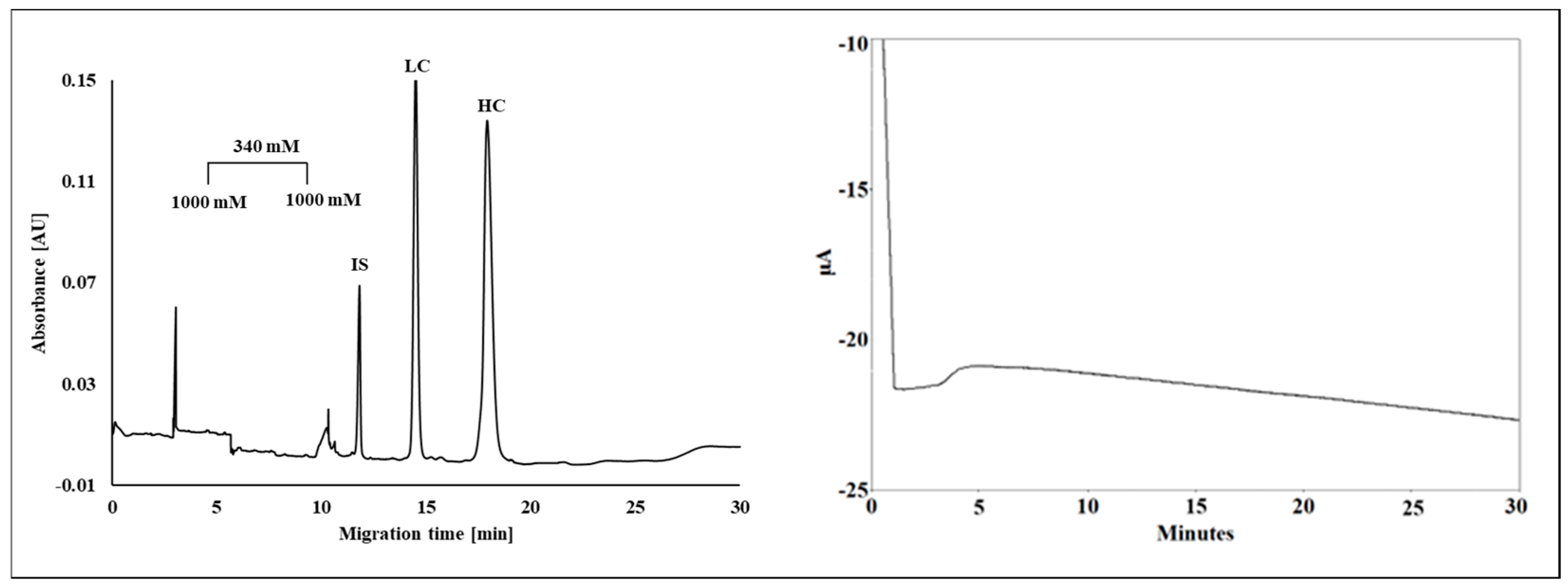
5.2. Proteins and Peptides
5.3. Emerging Directions in Microfluidic and Integrated Bioanalysis
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGE | Capillary Gel Electrophoresis |
| CGGE | Capillary Gradient Gel Electrophoresis |
| EOF | Electroosmotic flow |
| LIF | Laser-induced fluorescence |
| MW | Molecular weight |
| MS | Mass spectrometry |
| SDS-CGE | Sodium dodecyl sulfate capillary gel electrophoresis |
References
- Tiselius, A. A new apparatus for electrophoretic analysis of colloidal mixtures. Trans. Faraday Soc. 1937, 33, 524–531. [Google Scholar] [CrossRef]
- Andrews, A.T. Electrophoresis: Theory, Techniques, and Biochemical and Clinical Applications; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Chrambach, A.; Rodbard, D. Polyacrylamide gel electrophoresis. Science 1971, 172, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, J.W.; Lukacs, K.D. Capillary Zone Electrophoresis. Science 1983, 222, 266–272. [Google Scholar] [CrossRef]
- Hjertén, S. High-performance electrophoresis: The electrophoretic counterpart of high-performance liquid chromatography. J. Chromatogr. A 1983, 270, 1–6. [Google Scholar] [CrossRef]
- Guttman, A.; Hajba, L. Capillary Gel Electrophoresis; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Cohen, A.S.; Najarian, D.R.; Paulus, A.; Smith, J.A.; Karger, B.L. Rapid separation and purification of oligonucleotides by high-performance capillary gel electrophoresis. Proc. Natl. Acad. Sci. USA 1988, 85, 9660–9663. [Google Scholar] [CrossRef]
- Cohen, A.S.; Karger, B.L. High-performance sodium dodecyl sulfate polyacrylamide gel capillary electrophoresis of peptides and proteins. J. Chromatogr. A 1987, 397, 409–417. [Google Scholar] [CrossRef]
- Guttman, A. Capillary Electrophoresis Using Replaceable Gels. Patent #US5421980A, 6 June 1995. [Google Scholar]
- Karger, B.L.; Guttman, A. DNA sequencing by CE. Electrophoresis 2009, 30, S196–S202. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D.; Kapadia, G.; Chrambach, A. Pore gradient electrophoresis. Anal. Biochem. 1971, 40, 135–157. [Google Scholar] [CrossRef]
- Wang, C.C.; Beale, S.C. Preparation of linear polyacrylamide gel step gradients for capillary electrophoresis. J. Chromatogr. A 1996, 756, 245–253. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, F.; Baba, Y. Stepwise gradient of linear polymer matrices in microchip electrophoresis for high-resolution separation of DNA. Electrophoresis 2002, 23, 2341–2346. [Google Scholar] [CrossRef]
- Qiao, Y.; Ding, L.; Huang, C.; Chen, Y.; Wen, T.; Tu, J. A microchip electrophoresis method for rapid nucleic acid extraction and bacteria detection. Talanta 2026, 296, 128493. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G.; Gelfi, C. Capillary electrophoresis of DNA for molecular diagnostics. Electrophoresis 1997, 18, 1709–1714. [Google Scholar] [CrossRef]
- Offord, R.E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature 1966, 211, 591–593. [Google Scholar] [CrossRef]
- Ferguson, K.A. Starch-gel electrophoresis—Application to the classification of pituitary proteins and polypeptides. Metabolism 1964, 13, 985–1002. [Google Scholar] [CrossRef]
- Walker, J.M. Gradient SDS Polyacrylamide Gel Electrophoresis. Methods Mol. Biol. 1984, 1, 57–61. [Google Scholar]
- Rickwood, D. Gel Electrophoresis of Nucleic Acids: A Practical Approach; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Rickwood, D. Gel Electrophoresis of Proteins: A Practical Approached; IRL Press Limited: Oxford, UK, 1981. [Google Scholar]
- Lo, C.T.; Throckmorton, D.J.; Singhb, A.K.; Herr, A.E. Photopolymerized diffusion-defined polyacrylamide gradient gels for on-chip protein sizing. Lab Chip 2008, 8, 1273–1279. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. Recent advances in column coatings for capillary electrophoresis of proteins. TrAC Trends Anal. Chem. 2017, 90, 38–44. [Google Scholar] [CrossRef]
- Yin, G.S.A.L.-F. Production of Polyacrylamide Gel Filled Capillaries for Capillary Gel Electrophoresis. Patent #US5141612A, 25 August 1992. [Google Scholar]
- Chung, M.; Kim, D.; Herr, A.E. Polymer sieving matrices in microanalytical electrophoresis. Analyst 2014, 139, 5635–5654. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Grochocki, W.; Kalsoom, U.; Alves, M.N.; Phung, S.C.; Rokh, M.T.; Cabot, J.M.; Ghiasvand, A.; Li, F.; Shallan, A.I.; et al. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2016–2018). Electrophoresis 2019, 40, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Höltje, J.-V.; Schwarz, U. Preparation of highly condensed polyacrylamide gel-filled capillaries with low detection background. J. Chromatogr. A 1994, 685, 121–129. [Google Scholar] [CrossRef]
- Widhalm, A.; Schwer, C.; Blaas, D.; Kenndler, E. Capillary zone electrophoresis with a linear, non-cross-linked polyacrylamide gel: Separation of proteins according to molecular mass. J. Chromatogr. A 1991, 549, 446–451. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, J.J.; Liu, S. Protein separation by capillary gel electrophoresis: A review. Anal. Chim. Acta 2012, 709, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Liu, S.; Pu, Q. Replaceable cross-linked polyacrylamide for high performance separation of proteins. J. Proteome Res. 2005, 4, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Ganzler, K.; Greve, K.S.; Cohen, A.S.; Karger, B.L.; Guttman, A.; Cooke, N.C. High-performance capillary electrophoresis of SDS-protein complexes using UV-transparent polymer networks. Anal. Chem. 1992, 64, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, M.; Shibukawa, A.; Nakagawa, T. Separation mechanism of pullulan solution-filled capillary electrophoresis of sodium dodecyl sulfate-proteins. Electrophoresis 1996, 17, 1584–1586. [Google Scholar] [CrossRef]
- Mitnik, L.; Salomé, L.; Viovy, J.L.; Heller, C. Systematic study of field and concentration effects in capillary electrophoresis of DNA in polymer solutions. J. Chromatogr. A 1995, 710, 309–321. [Google Scholar] [CrossRef]
- Barron, A.E.; Soane, D.S.; Blanch, H.W. Capillary electrophoresis of DNA in uncross-linked polymer solutions. J. Chromatogr. A 1993, 652, 3–16. [Google Scholar] [CrossRef]
- Fung, E.N.; Yeung, E.S. High-Speed DNA Sequencing by Using Mixed Poly(ethylene oxide) Solutions in Uncoated Capillary Columns. Anal. Chem. 1995, 67, 1913–1919. [Google Scholar] [CrossRef]
- Simò-Alfonso, E.; Conti, M.; Gelfi, C.; Righetti, P.G. Sodium dodecyl sulfate capillary electrophoresis of proteins in entangled solutions of poly(vinyl alcohol). J. Chromatogr. A 1995, 689, 85–96. [Google Scholar] [CrossRef]
- Liang, D.; Chu, B. High speed separation of DNA fragments by capillary electrophoresis in poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock polymer. Electrophoresis 1998, 19, 2447–2453. [Google Scholar] [CrossRef]
- Guttman, A.; Filep, C.; Karger, B.L. Fundamentals of Capillary Electrophoretic Migration and Separation of SDS Proteins in Borate Cross-Linked Dextran Gels. Anal. Chem. 2021, 93, 9267–9276. [Google Scholar] [CrossRef]
- Kartsova, L.; Makeeva, D.; Bessonova, E. Current status of capillary electrophoresis. J. Anal. Chem. 2020, 75, 1497–1513. [Google Scholar] [CrossRef]
- Hajba, L.; Jeong, S.; Chung, D.S.; Guttman, A. Capillary Gel Electrophoresis of Proteins: Historical overview and recent advances. TrAC Trends Anal. Chem. 2023, 162, 117024. [Google Scholar] [CrossRef]
- Breadmore, M.C. Electrokinetic and hydrodynamic injection: Making the right choice for capillary electrophoresis. Bioanalysis 2009, 1, 889–894. [Google Scholar] [CrossRef]
- Kitagishi, K. Sample injection. In Handbook of Capillary Electrophoresis Applications; Shintani, H., Polonský, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 17–24. [Google Scholar]
- Zhang, C.X.; Meagher, M.M. Sample Stacking Provides Three Orders of Magnitude Sensitivity Enhancement in SDS Capillary Gel Electrophoresis of Adeno-Associated Virus Capsid Proteins. Anal. Chem. 2017, 89, 3285–3292. [Google Scholar] [CrossRef]
- Voeten, R.L.C.; Ventouri, I.K.; Haselberg, R.; Somsen, G.W. Capillary Electrophoresis: Trends and Recent Advances. Anal. Chem. 2018, 90, 1464–1481. [Google Scholar] [CrossRef]
- Britz-McKibbin, P.; Terabe, S. High-sensitivity analyses of metabolites in biological samples by capillary electrophoresis using dynamic pH junction-sweeping. Chem. Rec. 2002, 2, 397–404. [Google Scholar] [CrossRef]
- Slater, G.W.; Mayer, P.; Grossman, P.D. Diffusion, Joule heating, and band broadening in capillary gel electrophoresis of DNA. Electrophoresis 1995, 16, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S. Joule heating and determination of temperature in capillary electrophoresis and capillary electrochromatography columns. J. Chromatogr. A 2004, 1037, 431–443. [Google Scholar] [CrossRef] [PubMed]
- García-Campaña, A.M.; Taverna, M.; Fabre, H. LIF detection of peptides and proteins in CE. Electrophoresis 2007, 28, 208–232. [Google Scholar] [CrossRef]
- Sarkozy, D.; Guttman, A. Analysis of Peptides and Proteins by Native and SDS Capillary Gel Electrophoresis Coupled to Electrospray Ionization Mass Spectrometry via a Closed-Circuit Coaxial Sheath Flow Reactor Interface. Anal. Chem. 2023, 95, 7082–7086. [Google Scholar] [CrossRef]
- Miksík, I.; Sedláková, P.; Mikulíková, K.; Eckhardt, A.; Cserhati, T.; Horváth, T. Matrices for capillary gel electrophoresis--a brief overview of uncommon gels. Biomed. Chromatogr. 2006, 20, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ulvik, A.; Refsum, H.; Ueland, P.M. Applications of short-chain polydimethylacrylamide as sieving medium for the electrophoretic separation of DNA fragments and mutation analysis in uncoated capillaries. Anal. Biochem. 1999, 276, 188–194. [Google Scholar] [CrossRef]
- Luckey, J.A.; Smith, L.M. Optimization of electric field strength for DNA sequencing in capillary gel electrophoresis. Anal. Chem. 1993, 65, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.A.; He, Y.; Guerrette, J.R.; Crihfield, C.L.; Bwanali, L. Simple, rapid, and reproducible capillary gel electrophoresis separation and laser-induced fluorescence detection of DNA topoisomers with unmodified fused silica separation capillaries. Anal. Bioanal. Chem. 2022, 414, 713–720. [Google Scholar] [CrossRef]
- Filep, C.; Guttman, A. Electromigration Dispersion in Sodium Dodecyl Sulfate Capillary Gel Electrophoresis of Proteins. Anal. Chem. 2022, 94, 13092–13099. [Google Scholar] [CrossRef] [PubMed]
- Durney, B.C.; Crihfield, C.L.; Holland, L.A. Capillary electrophoresis applied to DNA: Determining and harnessing sequence and structure to advance bioanalyses (2009–2014). Anal. Bioanal. Chem. 2015, 407, 6923–6938. [Google Scholar] [CrossRef]
- Camperi, J.; Chatla, K.; Freund, E.; Galan, C.; Lippold, S.; Guilbaud, A. Current Analytical Strategies for mRNA-Based Therapeutics. Molecules 2025, 30, 1629. [Google Scholar] [CrossRef]
- Bhimwal, R.; Rustandi, R.R.; Payne, A.; Dawod, M. Recent advances in capillary gel electrophoresis for the analysis of proteins. J. Chromatogr. A 2022, 1682, 463453. [Google Scholar] [CrossRef]
- Berlanda, S.F.; Breitfeld, M.; Dietsche, C.L.; Dittrich, P.S. Recent Advances in Microfluidic Technology for Bioanalysis and Diagnostics. Anal. Chem. 2021, 93, 311–331. [Google Scholar] [CrossRef]
- Crihfield, C.L.; Holland, L.A. Protein Sieving with Capillary Nanogel Electrophoresis. Anal. Chem. 2021, 93, 1537–1543. [Google Scholar] [CrossRef] [PubMed]


| Polymer | Repeating Unit | Viscosity | UV Cutoff | Typical Use |
|---|---|---|---|---|
| Dextran (borate cross-linked) | (C6H10O5)n | Moderate to high (tunable) | Low | Tunable and replaceable sieving matrix for CGE; widely used for SDS-protein separations, biopharmaceutical analysis, and method development requiring adjustable sieving properties |
| Polyacrylamide (linear, LPA) | (C3H5NO)n | Low to moderate | Moderate | Classical replaceable sieving matrix in CGE for DNA and proteins; ease of replenishment, and routine separations |
| Cross-linked polyacrylamide | (C3H5NO)n + cross-links | High | Moderate | High-resolution CGE tunable for wide MW ranges; preferred when enhanced sieving efficiency and improved resolution are required |
| Poly(vinyl alcohol) (PVA) | (C2H4O)n | Low | Low | Dynamic wall-coating polymer in CGE; used to suppress EOF and analyte–wall interactions, often combined with other sieving matrices |
| Hydroxyethyl cellulose (HEC) | Cellulose backbone with –CH2CH2OH substituents | Low to moderate | Low | Physical matrix in CGE; applied in DNA and protein separations where temperature-dependent viscosity control is beneficial |
| Poly(ethylene oxide) (PEO) | (C2H4O)n | Low to moderate | Low | Replaceable entanglement-based sieving matrix for CGE; suitable for DNA and protein separations with flexible tuning via polymer MW |
| Pullulan | (C6H10O5)n | Moderate | Low | Neutral polysaccharide sieving matrix in CGE; used for DNA and protein separations where low UV absorbance and mild polymer–analyte interactions are desired |
| Technical Challenge | Origin/Cause | Impact on CGGE | Reported Solutions |
|---|---|---|---|
| Gradient reproducibility | In-capillary gradient formation and polymerization | Run-to-run variability; limited robustness | Controlled polymerization protocols; degassing; surface-treated capillaries |
| Gel stability and lifetime | Mechanical stress and bubble formation in gradient gels | Short column lifetime; limited throughput | Optimized gel composition; pressure control; improved capillary coatings |
| Limited matrix replaceability | Cross-linked/high viscosity gradient gels | Reduced suitability for routine use | Step-gradient approaches; diffusion-based gradients; replaceable polymer systems |
| High viscosity of gradient gels | Dense sieving matrices | Difficult capillary loading; pressure requirements | Pressure-assisted filling; temperature-controlled loading |
| Gradient relaxation | Diffusion between gradient zones | Loss of gradient sharpness | Optimized gradient length; minimized equilibration time |
| Coupling to MS | Gel and detergent incompatibility with MS | No CGGE–MS implementation | Gel-free detection zones; partial gel removal; specialized CE–MS interfaces |
| Limited quantitative robustness | Gradient instability and detector constraints | Challenges in validation and quantitation | Temperature stabilization; internal standards; optimized detection conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Guttman, A.; Auer, F. Capillary Gradient Gel Electrophoresis. Gels 2026, 12, 29. https://doi.org/10.3390/gels12010029
Guttman A, Auer F. Capillary Gradient Gel Electrophoresis. Gels. 2026; 12(1):29. https://doi.org/10.3390/gels12010029
Chicago/Turabian StyleGuttman, Andras, and Felicia Auer. 2026. "Capillary Gradient Gel Electrophoresis" Gels 12, no. 1: 29. https://doi.org/10.3390/gels12010029
APA StyleGuttman, A., & Auer, F. (2026). Capillary Gradient Gel Electrophoresis. Gels, 12(1), 29. https://doi.org/10.3390/gels12010029








