Recent Advances in Injectable Hydrogels for Biomedical and Aesthetic Applications: Focus on Rheological Characteristics
Abstract
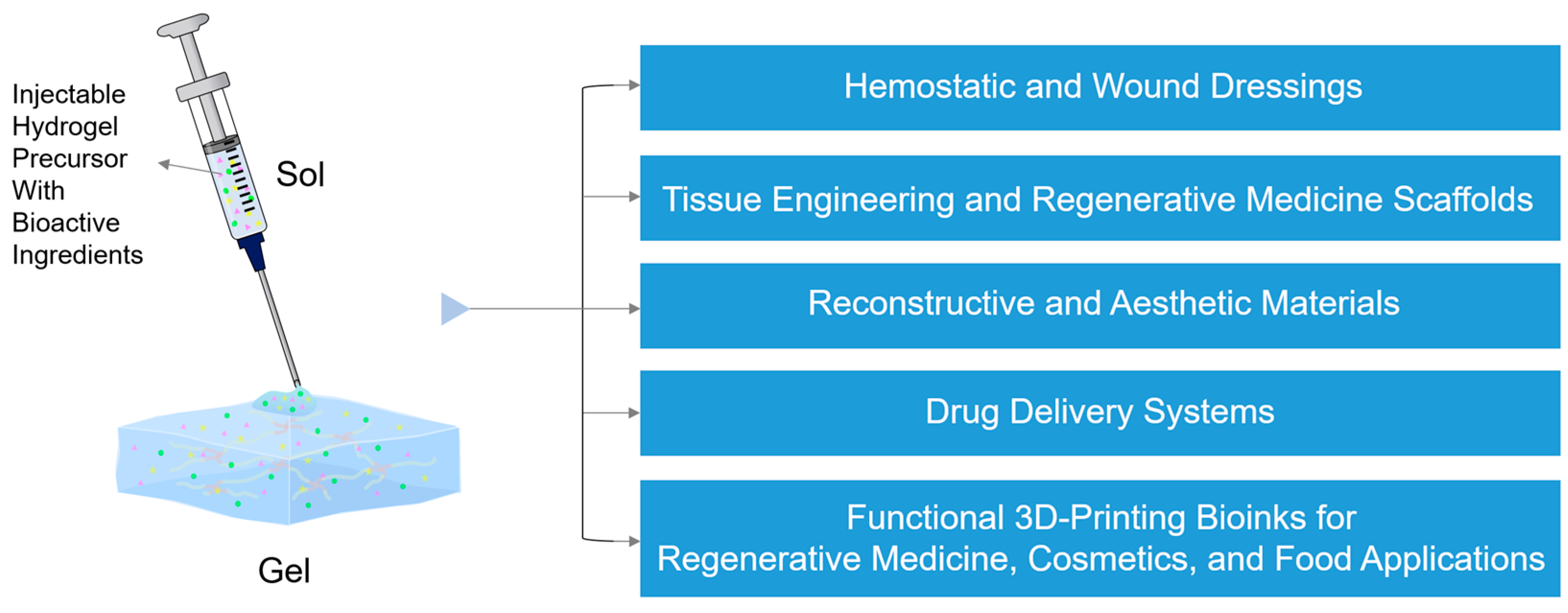
1. Introduction
2. Recent Advances in IHs and Their Rheological Characteristics Across Diverse Biomedical and Aesthetic Applications
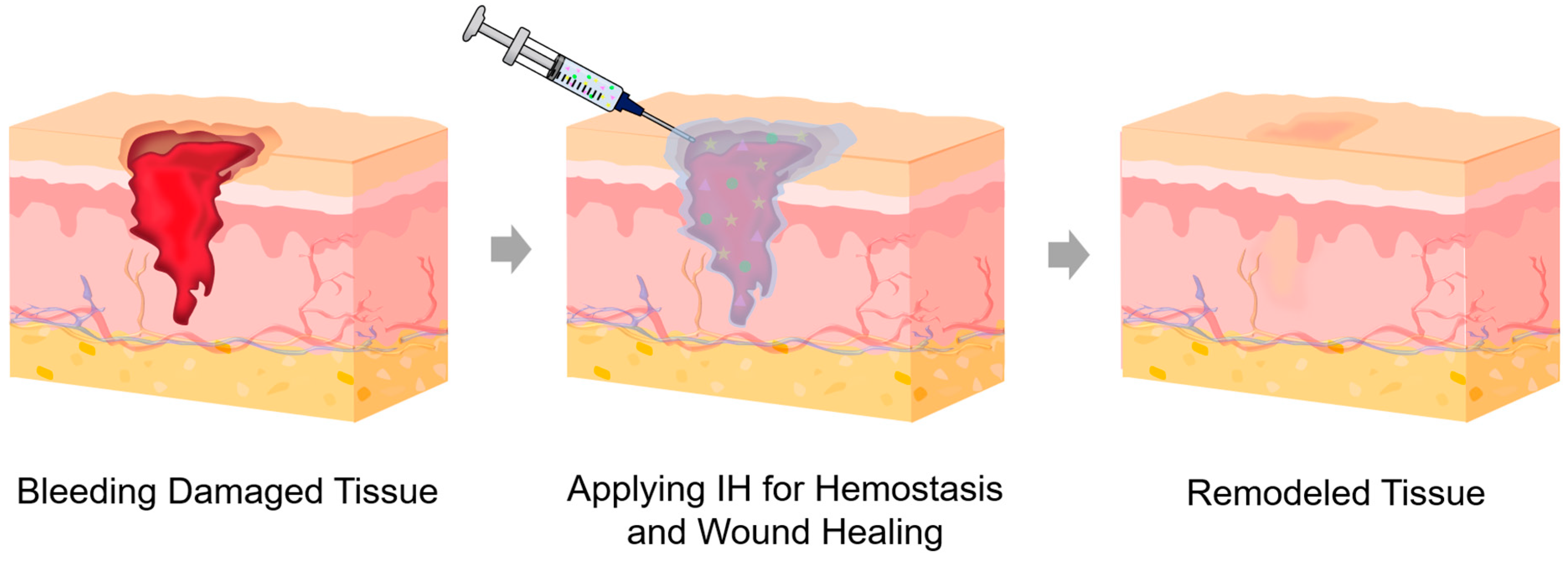
2.1. Hemostatic and Wound Dressings
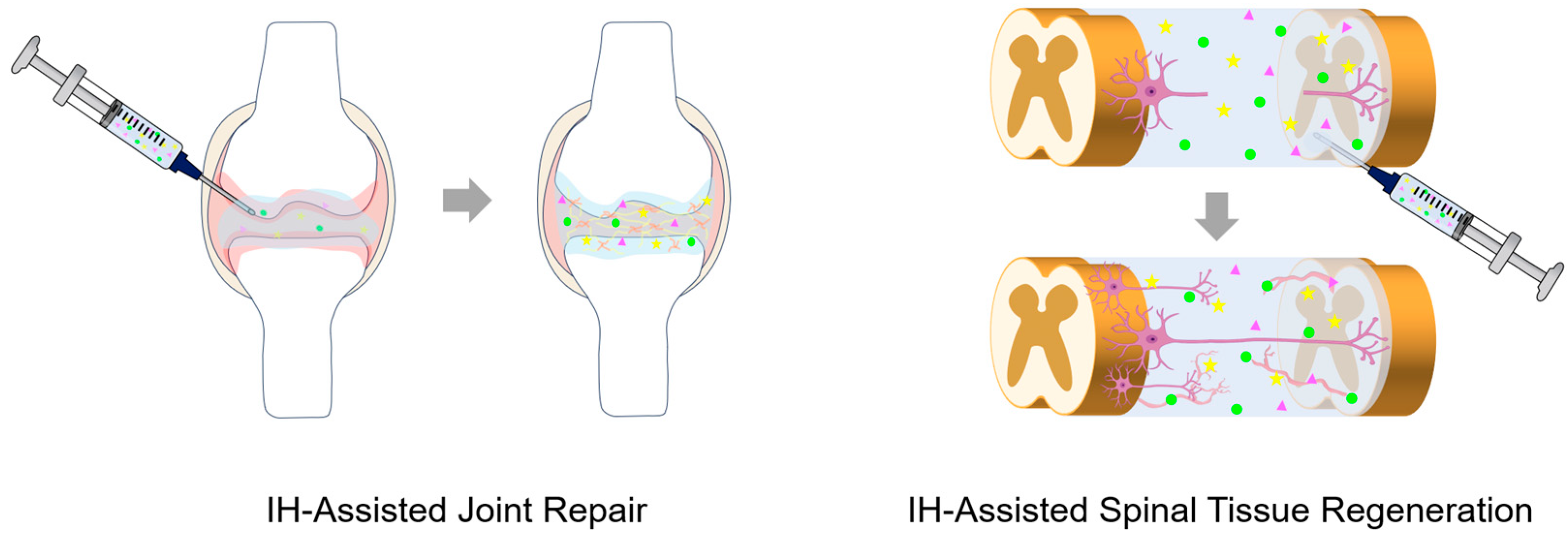
2.2. Tissue Engineering and Regenerative Medicine Scaffolds
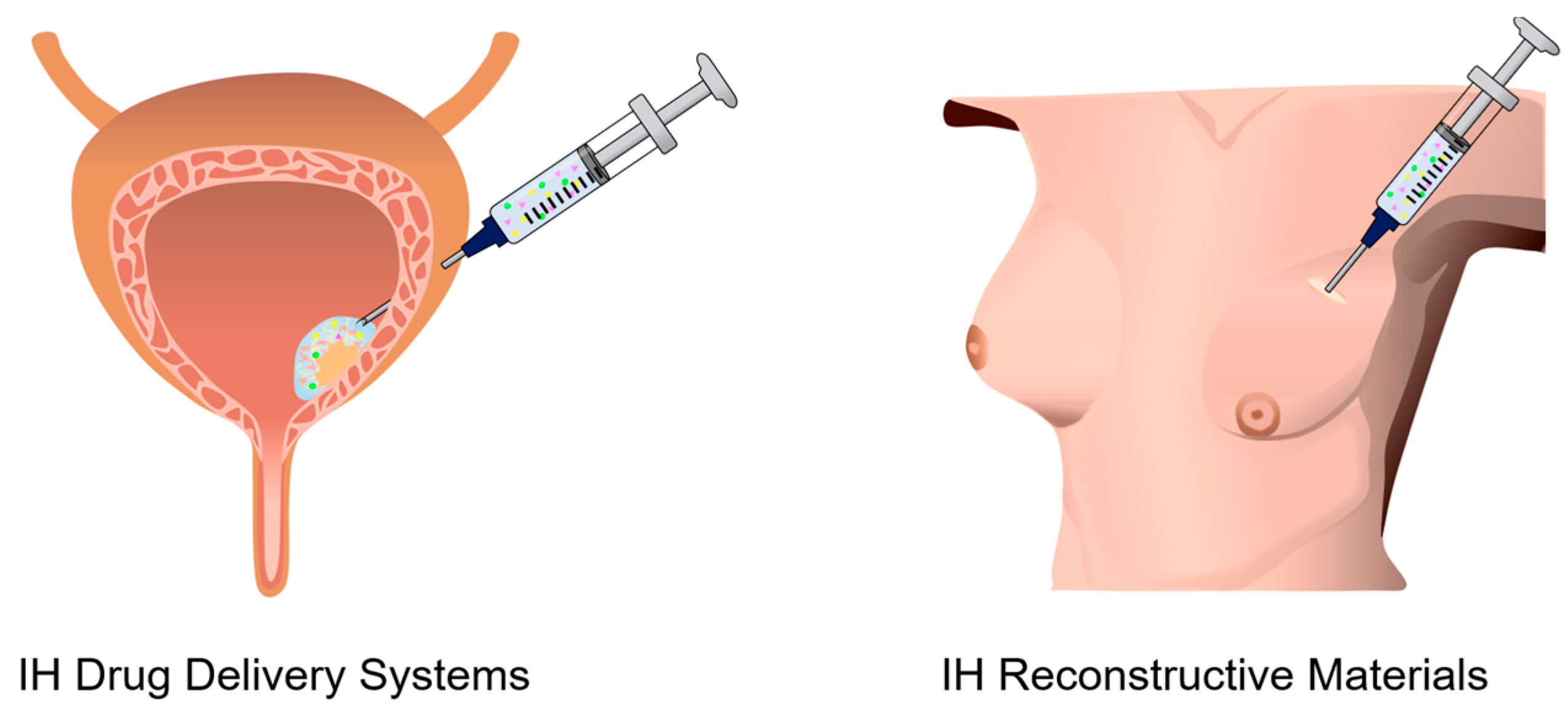
2.3. Drug Delivery Systems (DDSs)
2.4. Reconstructive Materials
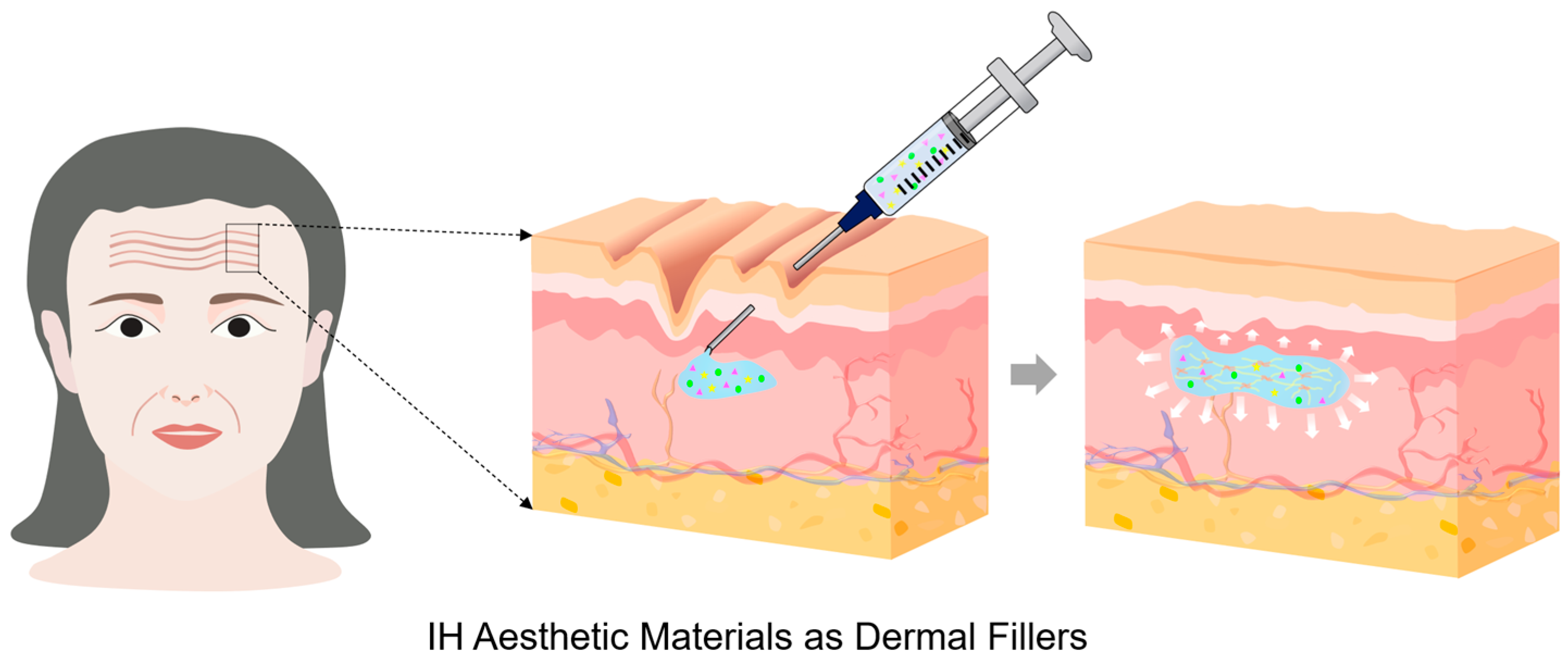
2.5. Aesthetic Materials
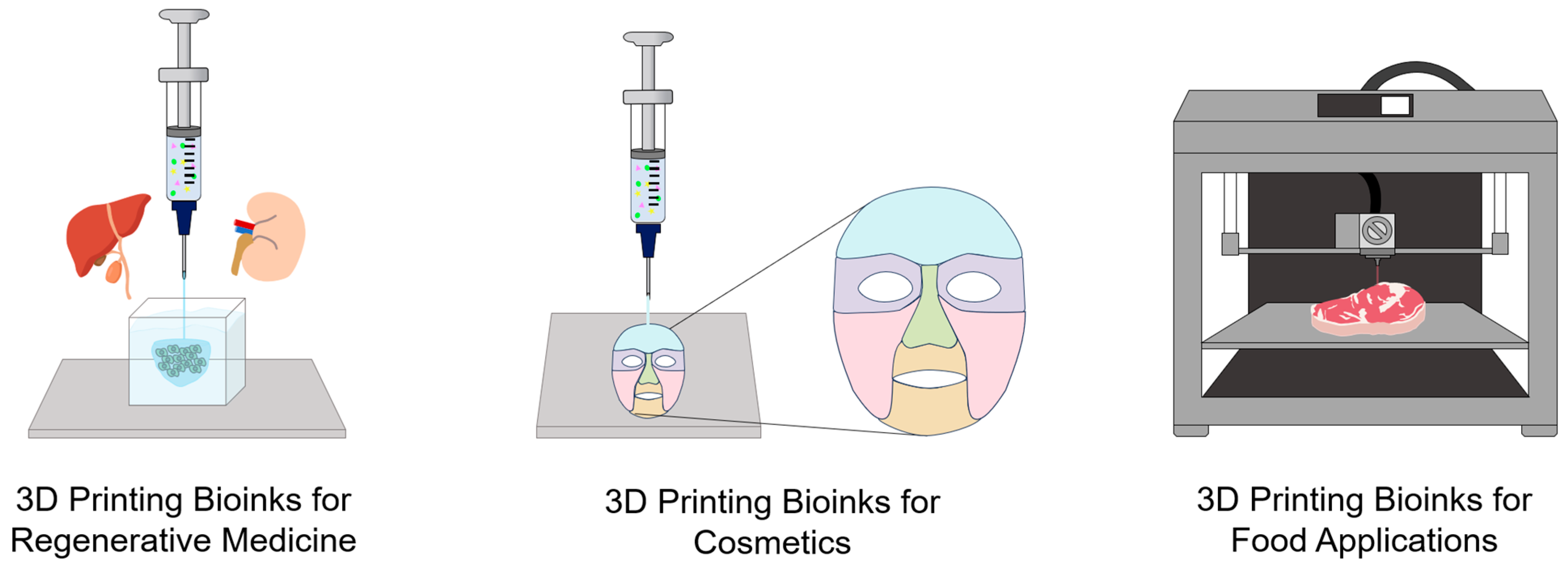
2.6. Functional Bioinks for 3D Printing
2.6.1. Bioinks in Tissue Engineering and Regenerative Medicine
2.6.2. Bioinks in Cosmetics
2.6.3. Bioinks in Food Applications
3. Conclusions and Challenges
| IH Name | Crosslinking Mechanisms/ Interactions | Features | Rheological Properties | Ref. |
|---|---|---|---|---|
| Hemostatic and Wound Dressings | ||||
| N,O-CMC/ OCS | CC, SBR/ | High equilibrium swelling ratio (qe = 10–20), degradability (50–60% within 2 weeks), nontoxicity, antibacterial properties via electrostatic membrane disruption, hemostatic, wound-healing efficacy | G′ > G″ (elastic-dominant behavior), G′↑ & tgel↓ with OCS conc.↑, tgel ≈ 133 s (m_CMC:OCS = 2:1); 5 s (m_CMC:OCS = 1:1 or 1:2) | [39] |
| AC | PC/HB | Self-healing, thermo-reversible stability, antibacterial properties, hemostatic, wound-healing efficacy | G′ > G″ (G′/G″ < 10), G′↓ with DS↑; G′↑ with AC conc. ↑, G′↓ on heating; recovered upon cooling, G′ = G″ at γ = 680%, G′: 570→130 Pa at γ ≈ 1200% (G′ < G″); returns to initial at γ ≈ 10%, shear thinning | [42] |
| γ-PGA-SH/HA-CHO | CC, PC, TAAR/ HB | High water content (85–95%), antioxidant activity, tissue adhesiveness, in vivo degradation in 3 days, wound-healing efficacy, adaptability | Tting the G′/G″ > 10 in the LVE region, G′↓ with polymer conc.↓, tgel ≈ 3 min, lower conc. (15 → 5 wt%) → faster relaxation, shear thinning | [44] |
| CMC/ Eu-EDTA | PC/MCCI | Biocompatibility, self-healing behavior, real-time pH monitoring (pH 4.5–7.5), ROS generation promoting angiogenesis, theragnostic (therapy + diagnosis) for wound care, diabetic wound healing | G′ > G″ (G′/G″ < 10), G′↑ with Eu-EDTA conc.↑ (81.3 ± 2.2 → 236.7 ± 10.0 Pa), G′ = G″ at γ ≈ 300–1200%, G′ & G″ rapid recovery < 1 s (γ: 1% ↔ 1300%), structural recovery (within 10 min), shear thinning | [45] |
| 4-arm PEG/PEI/ADH | CC, SBR, TTER/ | Hemostatic sealant, on-demand dissolution via CME-triggered thiol-thioester exchange, high tissue adhesion, tunable mechanical robustness, viscoelasticity, biodegradability, emergency hemostatic sealant | G′ > G″ (G′/G″ > 10), tgel↓ with PEI or 4-arm PEG crosslinker conc.↑ & ADH conc.↓, shear thinning | [46] |
| CQCS@gel (gel: DB-PEG2000) | CC, PC, SBR/HB, PI, II | Biocompatibility, antioxidant effect, broad-spectrum antibacterial, pH-responsive drug release, tissue-adhesive, hemostatic, wound-healing efficacy, self-healing | G′ > G″ (elastic-dominant behavior over 600 s), G′↑ with CQCS conc.↑, tgel < 30 s (CQCS > 1.5 wt%), G′: 245 Pa (healed in 30 min) → 341 Pa (healed in 3 h); recovers to 65% and 91% of initial (373 Pa) | [47] |
| Tissue Engineering and Regenerative Medicine Scaffolds | ||||
| CS/PEG-SiW | CC, PC, SBR/ EI | Excellent mechanical strength, good biodegradability (approximately 51% within 21 days), cytocompatibility, effective promotion of cartilage regeneration, excellent flexibility, self-healing behavior, cartilage tissue engineering | G′ > G″ (G′/G″ < 10), G′ & compressive strength↑ with SiW conc.↑ (1 → 2%), sol-gel transition time↓ via microgel recombination, shear thinning | [48] |
| CSMA-PEGDA-L | CC, SBR, PIC/ | High compressive strength, low cytotoxicity, excellent biodegradability, excellent therapeutic efficacy, intervertebral disc repair | Highest G′ with optimal 2 wt% CSMA, η↑ with CSMA conc.↑ (mixing↓ & crosslinking↓ at 3 wt%), tgel↓ with PEGDA conc.↑ (90 s → 36 s) | [49] |
| Cur@PDA@GelCA | PC/PI, HB | Exhibited excellent biocompatibility, antioxidant properties, enhanced tissue adhesion, good biodegradability (approximately 80% in 8 h), retinal tissue engineering | G′ > G″, G′ & compressive modulus↑ with GelCA conc.↑ (enhanced stiffness); with PDA and Cur@PDA NPs, tgel↑ with GelCA conc.↑, shear thinning | [50] |
| Lip/Cur + Toc@LCH, Lip/Cur + Toc@HCH | PC/HB | Excellent water absorption capacity, sustained drug release properties (10–25% for three weeks), antibacterial effect, excellent wound healing, dental tissue engineering | G′/G″ ≈ 10, G′, G″ & LVE region: Lip/Cur + Toc@LCH < Lip/Cur + Toc@HCH, η↑ with T↑ (24 °C → 40 °C), shear thinning | [51] |
| ACCP | CC, PC, SBR/ EI | Electroconductivity (up to 8.27 × 10−4 S· cm−1), biocompatibility, biodegradability, self-healing behavior, elastic modulus comparable to native sciatic nerve (33.5–66.8 kPa), peripheral nerve regeneration | G′ > G″ (G′/G″ > 10), G′↑ with CP conc.↑, G′ = G″ at γ ≈ 377.2%, G′ & G″ rapid recovery < 1 s (γ: 1% ↔ 500%), shear thinning | [56] |
| AHA/DTP | CC, PC, SBR/HB | Rapid gelation (~50 s), self-healing behavior (~ h), tissue-matching stiffness (2–20 kPa), biocompatibility, promotes neuronal differentiation, pH-responsive degradability, spinal cord injury | G′ > G″ (G′/G″ > 10), tgel < 50 s, G′ & G″ rapid recovery < 1 s (γ: 1% ↔ 450%), shear thinning | [57] |
| HA/PA | PC/HB | Optimized HA: PA weight ratio (4.5:1), porous interconnected microchannels (~25 μm), biocompatible, tissue adherence, regenerative medicine | G′ > G″ (elastic-dominant behavior, G′/G″ < 10), G′ & G″ rapid recovery < 1 s (γ: 50% ↔ 3000%), η0 = 64.85 Pa·s and tR = 31.65 s (HA:PA = 4.5:1), thixotropic behavior, shear thinning | [58] |
| Drug Delivery Systems | ||||
| HABP-ZnBCP@CH | CC, PC, CR/ EI | Excellent adaptability to defect shape, multiple ion-delivery, strong anti-inflammatory properties, strong bone-regenerative effects, excellent biodegradability, rheumatoid arthritis therapy | G′ > G″ (G′/G″ > 10), G′↑ with added HABP & ZnBCP, G′: HABP-ZnBCP@CH (6646 Pa) > CH (3164 Pa) | [62] |
| NSCs-cfGel (Gel: o-Dex/4-arm-PEG-NHNH2) | CC/HZB | Dual drug-loaded system (cetuximab, FTY720), biocompatibility, encapsulated NSCs, tunable elasticity (G′ < 7 kPa), porous microstructure, in vivo degradation (~18 days), controlled dual-drug release (~83% released within 36 h), dynamic reversibility, spinal cord injury | G′ > G″ (elastic-dominant behavior over 1500 s at 37 °C), tgel < 2 min (at 37 °C), time-dependent η (~600 Pa·s), fracture stress (~4200 Pa) | [63] |
| Gel/HMS-ROP/DEX (Gel: OHA/CMC-ADH) | CC, PC, SBR/ HB | Self-healing behavior, tissue-adhesiveness, mechanical resilience, high ROP loading capacity (≈77%), sustained drug release (>168 h), excellent injectability, biocompatibility, controlled degradation, long-acting postoperative pain management | G′ > G″, tgel < 35 s, G′ & G″ recovery < 900 s (γ: 1% ↔ 200%), G′ (74.7 ± 4.8) recovered to 12% of the initial, shear thinning | [64] |
| DFO-NP/HA/F127 | PC, MC/ | Thermosensitive gelation, biocompatibility, minimal burst release, sustained DFO release (>14 days), 4.3-fold longer half-life vs. free DFO, robust structural integrity, treatment of iron overload disorders | G′ > G″ (in T: 4–40 °C); G′ & η↑ with added F127, G′↑ to ~8.5 kPa (DFO-NP/HA/F127, at >30 °C), sol-gel transition behavior (at >30 °C), shear thinning | [65] |
| HA-CPP⊂CB [8] | PC/HGI | Biocompatibility, tunable porosity, reversible self-healing, localized and sustained cytokine release (CXCL13, LIGHT), promoted TLSs formation, antigen-specific T-cell activation, tumor growth inhibition, tumor immunotherapy | G′ > G″, G′ & G″: rapid recovery <1 s (γ: 1% ↔ 100%), tR = ~1 min, shear thinning | [66] |
| Gel@Cmab/PCZ (Gel: triblock copolymer PLGA–PEG–PLGA) | PC, MC/ | Biocompatibility, biodegradability, thermosensitive gelation, sustained and localized release of Cmab and PCZ (up to 70–80% for over 15 d), enhanced ADCC in CRC, increased NK-cell infiltration, colorectal cancer immunotherapy | G′↑ with T↑, G′ & G″: low & stable (at 15–30 °C), G′ & G″: sharply↑ near at 30 °C (G′↑ > G″↑), sol-gel transition (at ≈30 °C, over 2 min), shear thinning | [67] |
| Reconstructive Materials | ||||
| D-MA + HAMA (OcuPairTM) | CC, PIC/ | High transparency, biocompatibility, flexibility, hydration level (78–88% (similar to human cornea)), adheres to wet ocular tissue, withstands intraocular pressure > 80 mmHg, traumatic corneal injury | G′ > G′′ (elastic-dominant behavior after 40 s UV irradiation), tgel = 30–45 s (under UV light), η ≈ 5.0 × 103 Pa·s (D-MA: HAMA = 30:70, before UV light), shear thinning | [72] |
| LBL | PC, MS/HB | Thermosensitive gelation, self-assembly, soft-tissue-mimicking viscoelasticity, reversible self-healing behavior, prevention of water expulsion (during gelation), excellent biocompatibility, minimal inflammation, high adaptability, reconstructive body fillers | G′/G′′ < 10, G′↑ with LBL conc.↑ & ϕL↑, Tgel↑ with PEG length↑ & PNIPAM length ↓, sol–gel transition (at 37 °C), J-shaped stress–strain curve | [73] |
| IR820/Mgel (Gel: methylcellulose) | PC/ | Dual-function system (tumor recurrence prevention + breast tissue reconstruction), excellent biocompatibility, cell adhesion, long-term shape retention, sustained photothermal activity, breast reconstruction | G′ & G′′↑ with added PLGA M, sol-gel transition (at 29–31 °C), tgel ≈ 95 s (pure gel) & tgel ≈ 118 s (Mgel) | [74] |
| eTGF-β1 SH | /HB, HI | Excellent biocompatibility, eTGF-β1 content ≈ 5.6 wt%, sustained eTGF-β1 release (>30 days), promoted fibroblast proliferation, osteoblast maturation, osteogenic markers expression, complete alveolar bone regeneration, high structural stability | G′ > G″, shear thinning | [75] |
| PHM/Fe3+ | PC/MLCB, HB | High toughness: ≈1 MJ·m−3, fatigue resistance (retained performance after 1000 compression cycles), minimal inflammation, thinner fibrous capsule (vs. conventional implants), elasticity (similar to soft tissue), Ea ≈ 57 kJ·mol−1, rapid self-recovery, breast reconstruction | G′ > G″ (G′/G″ ≈ 10, 25–60 °C), G′↑ with angular frequency↑, shear thinning | [76] |
| Aesthetic Materials | ||||
| HA-DA | CC, MR/ | Biocompatibility, non-cytotoxicity, controlled enzymatic degradation, balanced regulation of metabolism (collagen), decreased COL1A1 and MMP1 expression, pH stability (~7.4), mechanically tunable, inflammation-modulating platform, anti-inflammatory potential, dermal filler | G′ > G″ (elastic-dominant behavior, G′/G′′ > 10), 80–90% recovery within 20–25 s after γ = 2000% (G′ ≈ 270 → 470 Pa), shear thinning | [81] |
| Sericin/ nHAP | PC, UIG/ | Rapid gelation (3–5 min), uniform porous structure (pore size ~17 μm), 10-fold swelling ratio, potent antioxidant, anti-inflammatory properties, collagen synthesis, angiogenesis, maintains tissue volume (for 8 weeks more), dermal filler | G′ > G″ (G′/G′′ < 10), tgel = 3–5 min, ηmax ≈ 102 Pa (0.5% of IH) and 100 Pa (0.25% of IH), shear thinning | [84] |
| PPBL | CC, PC/ BEB, HB | Self-healing behavior (96%), water retention by introducing multiple hydrogen-bonding sites, improved mechanical strength and toughness (tensile strength 502 kPa, elongation 630%), smooth injectability, dermal filler | G′ > G″ (G′/G′′ > 10), G′↑ added BA and DL, G′ & G″ rapid recovery < 1 s (γ: 1% ↔ 50, 500%), shear thinning | [85] |
| NHO/ NPLLA(T) | CC, SBR/ | Self-healing behavior (~95%), improved dermal thickness, collagen I/III deposition, minimal inflammation, biocompatibility, antioxidant effect, dermal filler | G′ (~1000 Pa) > G″, tgel↓ with NPLLA conc.↑ (115 → 84 s), self-healing behavior (~95% recovery), shear thinning | [86] |
| EVTS-Gel (Gel: PCL–PEG–PCL triblock copolymer) | Self-A | Thermosensitive gelation, biocompatibility, controlled degradation, stable collagen metabolism, reduced COL1A1 & MMP1 (low collagen turnover), anti-inflammatory (IL-6 and COX-2 inhibition), promoted collagen regeneration, dermal filler | η*, G′ & G″↑ with EV, sol-gel transition at ~32.6 °C, η*, G′ & G″: low at RT → increased up to 37 °C | [87] |
| Functional Bioinks for 3D Printing | ||||
| Bioinks in Tissue Engineering and Regenerative Medicine | ||||
| HAGA-HAMA | CC, PIC/ | pH-responsive η, easy extrusion (at 37 °C), tissue-adhesive, antioxidant properties, photo-crosslinked after printing and UV irradiation, self-healing behavior, tissue engineering | G′ > G″, 80–90% recovery within 20–25 s after γ = 2000% (G′ ≈ 270 → 470 Pa), shear thinning | [93] |
| HELP | CC/HZB | Tunable gelation via competitive aldehyde analogs and catalysts, minimized erosion (<3% over 14 days), high cell viability (>95%), mechanical resilience, biocompatibility, tissue engineering | G′ > G″ (G′/G′′ > 10), tgel = 3–5 min, ηmax ≈ 102 Pa (0.5% of IH) and 100 Pa (0.25% of IH), shear thinning | [94] |
| PEGDA/Co NW | CC, PC, PIC/ II | Porous and transparent structure (swelling ≈ 400%), controlled degradation (3 weeks), enhanced mechanical strength, slow Co2+ release modulated cellular redox balance, hypoxia-mimicking for chondrogenic differentiation of UMSCs, cartilage regeneration | G′ > G″ (G′/G′′ < 10), G′↑ added BA and DL, G′ & G″ rapid recovery < 1 s (γ: 1% ↔ 50, 500%), shear thinning | [95] |
| Bioinks in Cosmetics | ||||
| IC–SH–GL/IC–SH/IC–GL–CSLD | PC/II, HB, EC | Biocompatibility, non-irritation, high swelling properties, effectively reduced sebum secretion, improved skin moisture content, skincare hydrogel patch | G′ > G″ (G′/G′′ < 10), η ≈ 104–105 mPa·s, SH addition → η↑ & stiffness↑, GL addition → fluidity↑ & shear thinning | [97] |
| Gel/TA, Gel/TA–Met, Gel/TA–SA | CC, PC/HB | Anti-inflammatory, keratolytic functions, facial skin irritation, biocompatibility, customizable formulations, hydrogel patch for treating skin lesions | G′ > G″ elastic-dominant behavior), SA addition → disrupted crosslinking & Tgel↓, Met addition → enhanced H-bonding & stiffness↑, tgel < 25 s, thixotropic recovery, shear thinning | [99] |
| 3DP-NH | CC/II | Embedded CPT-loaded niosomes (~150 nm, EE 67–71%), Sustained CPT release (~61%/96 h), non-irritant, anti-acne, enhanced skin hydration, acne therapy | Flux≈11,4 ng/cm2/h, G′ > G″, gel strength = 0.57 kg (5.59 N, 28 kPa), stable extrudability = 0.29 kg (2.86 N) under 9–15 psi, shear thinning | [100] |
| Bioinks in Food Applications | ||||
| PSP–ALG | PC/HB, WB | Plant-based gelatin substitute, edible 3D-printing ink, gelatin-like texture (tuned by ALG & PSP), low cost, thermally stable system, 3D food printing (pork belly, arctic surfclams) | G′ > G″ (G′/G′′ ≈ 10), PSP-ALG hardness (at 2–3 wt% ALG) ≈ gelatin (0.5–2 N), thermal stability & hardness↑ with ALG conc.↑, hardness↓ with PSP conc.↑ (ALG-Ca2+ disruption), tgel < 5 min, shear thinning | [102] |
| Starch–mango/Starch–AX | PC/PE | Food-grade hydrocolloid inks, Tunable composition, optimal printing: 75–100% starch, nozzle 0.4 mm, speed 3–6 mm/s, mesh-structured network, gel-like, high thixotropy | G′/G′′ < 10, Starch content↑ → G′ & G″↑ (stronger shear thinning; η: rapid recovery (γ: 0–200-0 s−1) in ~30–40 s | [105] |
| HGIs (HGIs: litchi homogenate, κ-carrageenan, xanthan gum, bees wax) | /HB, HI | Food-grade hybrid gelator inks, smooth extrusion, high-precision & thermally stable 3D printing, overhang 53.5° angle, 85-layer stability (HGIs > HIs), enhanced mechanical strength, deformation resistance, superior self-support | tgel < ~125 s (HGIs), τy = 623 Pa, K = 2.92 × 105 Pa·sn, n = 0.12, viscosity recovery (~30 s, HGIs), G′ (HGIs) ≈ 10 times higher than HIs, shear thinning | [106] |
| Category | Polymer Type | Crosslinking Mechanism | G′ [Pa] | τy [Pa] | tgel [s] | Injectability Notes |
|---|---|---|---|---|---|---|
| Natural IHs | HA, Chitosan, Gelatin, Collagen, Silk, HAMA, GelMa, etc. | SBR, II, UV, HB | 50–500 | 10–60 | 5–180 | Excellent biocompatibility, moderate mechanical strength |
| Synthetic IHs | PEGDA, PVA, PNIPAM, PEG, PEI, etc. | CC, MR, UV, HB, HI | 300–3000 | 50–200 | <10–60 | Tunable strength, controllable degradation |
| Crosslinking Type | Crosslinking Mechanism | tgel [s] | G’ [Pa] | Pros | Cons |
|---|---|---|---|---|---|
| Physical Crosslinking | HB, II, HI | Fast (<10) | Low-mid’ (50–300) | Self-healing, Easily controllable | Weak Strength |
| Chemical Crosslinking | CC, SBR, MR | Slow–mid (20–180) | High (500–3000) | Strong network, High mechanical integrity | Irreversible (Exceptions exist) |
| Hybrid Crosslinking | Physical & Chemical Crosslinking (CC, II, HB, HI, MR, SBR) | Tunable | Tunable Range | Best Balance | Complex Synthesis |
| Application | Needle /Cannula Guage [G] | tgel [s] | G′ [Pa] | Fc [N] | τy [Pa] | η [Pa·s] at high = 102 s−1 | Shear Thinning | tR [s] | Note |
|---|---|---|---|---|---|---|---|---|---|
| Hemostatic Systems | 20–25 [47] 20 | 3–205 [39] 5–133 [46] 5.3–205 [47] 18 | 0–10,000 [39] 0–50 [46] 10,000 [47] 100–1000 | 5–15 | 10–50 | Low- Moderate (1–10) | Strong | 1–5 | Rapid gelation, bleeding control |
| Wound Dressings | 22–27 [44] 22 | 10–60 [44] < 180 | 10–10,000 [42] 10–900 [44] 10–10,000 [45] 81–236 | 5–20 | 5–40 | Low- Moderate (0.2–10) [44] 0.2–3 [45] 2–4 | Moderate- strong [42] 5000→0.4 [44] 103→0.2 [45] 3000→2 | 3–10 | Soft, spreadable adhesive gels |
| Tissue Engineering/Regenerative Scaffolds | 18–23 [56] 23 | 20–370 [49] 36–90 [50] 28–370 [57] 30–50 | 30–20,000 [48] 2493–6063 [49] 35–300 [50] 291–451 [56] 122–327 [57] 2000–20,000 [58] 30 | 10–35 | 20–500 [58] 500 | Low- Moderate (0.1–50) [48] 0.1–10 [50] 0.12–0.48 [51] 1–20 [57] 0.4 [58] 0.1–0.7 | Moderate- strong [48] 105→0.1 [50] 400→0.12 [51] 105→1 [57] 1000→0.4 [58] 40→0.1 | 5–20 | Mechanical stability for structural support |
| DDS | 23–30 [64] 27 | 10–200 [63] < 120 [64] < 35 [67] < 120 | 100–8500 [62] 3164–6646 [63] 7000 [64] 1500 [65] <8500 [66] 1500 | 5–25 | 5–80 | Tunable (1–200) [64] 20–40 (at 10 s−1) [66] 4 | Moderate [64] 15,000→20 [66] 500→4 | 10–900 [64] <900 | Complex Synthesis, Diffusion-dominated release |
| Reconstructive Materials | 20–27 [73] 20–27 | 10–150 [72] 30–45 [74] 118 | 200–35,000 [72] 35,000 [73] 10–1000 [76] 10,000–30,000 | 10–30 | 10–80 | 0.1–150 [72] 0.7 [75] 0.02 (at 103 s−1) | Moderate [72] 5.2→0.7 [75] 2→0.02 | 3–15 | Mechanical strength for structural Support, shape for defect, moderate stiffness to prevent tissue necrosis |
| Aesthetic Dermal Fillers (Fine line) | 28–32 [87] 31 | 5–120 | 50–200 | 8–20 | 5–20 | Moderate -high (10–50) | Moderate | 2–5 | Smooth spreading and natural contouring, controllable degradation for retouch procedures |
| Aesthetic Dermal Fillers (Deep line) | 22–27 [86] 25 | 5–150 [86] 84–134 | 100–40,000 [81] 100–400 [85] 1000 [86] 600–1500 [87] 30,000–40,000 | 0–35 [86] 0–10 [87] 1.2–1.4 | 20–120 | Moderate (0.2–200+) [81] 0.45 [84] 0.2 [85] 0.09–0.38 (at 103 s−1) | Moderate -strong [81] 40,000→0.45 [84] 200→0.2 [85] 10,000→0.09 | 2–10 | Mechanical strength for structural Support, shape fidelity, controllable degradation for retouch procedures |
| 3D-Printing Bioinks | 18–27 extrusion nozzle [93] 27 [94] 27 [95] 20 [99] 27 [102] 25 [105] 19–27 [106] 21 | < 1–300 [99] 20 [102] < 300 [106] 120–150 | 10–20,000 [93] 560–1060 [94] 1000 [95] 392–1046 [97] 200–1000 [105] 10–1000 [106] 7000–20,000 | 20–80 [106] 71 | 0.1–1000 [93] 0.1–100 [94] 1000 [106] 460–623 | High at low shear, Low at high shear [93] 1 [94] 1 [95] 1 [97] 0.1 (at 103 s−1) [105] 0.02 [106] 0.2 | Very strong [93] 10,000→1 [94] 5000→1 [95] 100→1 [97] 2000→0.1 [105] 42,000→0.02 [106] 200→0.2 | <1–3 | Immediate shapeability & recovery after extrusion, Tunable strength, controllable degradation |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.H. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Han, W.J.; Lee, J.H.; Lee, J.-K.; Choi, H.J. Remote-controllable, tough, ultrastretchable, and magneto-sensitive nanocomposite hydrogels with homogeneous nanoparticle dispersion as biomedical actuators, and their tuned structure, properties, and performances. Compos. Pt. B-Eng. 2022, 236, 109802. [Google Scholar] [CrossRef]
- Bonetti, L.; Borsacchi, S.; Soriente, A.; Boccali, A.; Calucci, L.; Raucci, M.G.; Altomare, L. Injectable in situ gelling methylcellulose-based hydrogels for bone tissue regeneration. J. Mater. Chem. B 2024, 12, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Chatterjee, K. Injectable and self-healing double network polysaccharide hydrogel as a minimally-invasive delivery platform. Carbohydr. Polym. 2022, 291, 119585. [Google Scholar] [CrossRef]
- Giang Phan, V.H.; Duong, H.T.T.; Thambi, T.; Nguyen, T.L.; Turabee, M.H.; Yin, Y.; Kim, S.H.; Kim, J.; Jeong, J.H.; Lee, D.S. Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo. Biomaterials 2019, 195, 100–110. [Google Scholar] [CrossRef]
- Song, L.; Qiu, H.; Chen, Z.; Wang, J.; Xu, Y.; Liu, Z.; Liu, S.; Wang, Z.; Zhu, X.; Zhang, K.; et al. Injectable hyaluronate/collagen hydrogel with enhanced safety and efficacy for facial rejuvenation. Collagen Leather 2024, 6, 21. [Google Scholar] [CrossRef]
- Wang, S.; Niu, Y.; Jia, P.; Liao, Z.; Guo, W.; Chaves, R.C.; Tran-Ba, K.H.; He, L.; Bai, H.; Sia, S.; et al. Alkaline activation of endogenous latent TGFbeta1 by an injectable hydrogel directs cell homing for in situ complex tissue regeneration. Bioact. Mater. 2022, 15, 316–329. [Google Scholar]
- Lee, H.; Jung, Y.; Lee, N.; Lee, I.; Lee, J.H. Nature-Derived Polysaccharide-Based Composite Hydrogels for Promoting Wound Healing. Int. J. Mol. Sci. 2023, 24, 16714. [Google Scholar] [CrossRef]
- Lee, I.; Lee, H.; Lee, N.; Jeong, Y.; Lee, J.H. Low-molecular-weight collagen/hyaluronic acid/alginate ternary hydrogels with various calcium ion concentrations and their tunable physiochemical and rheological properties. Macromol. Res. 2025, 33, 1439–1449. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef]
- Sarvepalli, S.; Pasika, S.R.; Vadarevu, S.; Bolla, S.; Bolla, P.K. A comprehensive review on injectable hydrogels for cell therapy. J. Drug Deliv. Sci. Technol. 2025, 105, 106648. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels-Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Ikura, R.; Park, J.; Osaki, M.; Yamaguchi, H.; Harada, A.; Takashima, Y. Design of self-healing and self-restoring materials utilizing reversible and movable crosslinks. NPG Asia Mater. 2022, 14, 10. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; He, Z.; Ji, L.; Zhang, W.; Tong, Y.; Luo, J.; Yu, D.; Zhang, Q.; Bi, Q. Advanced injectable hydrogels for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 954501. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, S.; Zhao, J.; Zhu, H.; Pan, X.; Zhao, B.; Sun, Z.; Li, N.; Hou, X. Development of injectable in situ hydrogels based on hyaluronic acid via Diels-Alder reaction for their antitumor activities studies. Int. J. Biol. Macromol. 2024, 262, 129642. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and Properties of Injectable Hydrogel for Tissue Filling. Pharmaceutics 2024, 16, 430. [Google Scholar] [CrossRef]
- Cheng, Q.; Ding, S.; Zheng, Y.; Wu, M.; Peng, Y.Y.; Diaz-Dussan, D.; Shi, Z.; Liu, Y.; Zeng, H.; Cui, Z.; et al. Dual Cross-Linked Hydrogels with Injectable, Self-Healing, and Antibacterial Properties Based on the Chemical and Physical Cross-Linking. Biomacromolecules 2021, 22, 1685–1694. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Xu, Y.; Fan, C.; Li, Z.A.; Zheng, L.; Luo, B.; Li, Z.P.; Lin, B.; Zha, Z.G.; et al. Collagen fibril-like injectable hydrogels from self-assembled nanoparticles for promoting wound healing. Bioact. Mater. 2024, 32, 149–163. [Google Scholar] [CrossRef]
- Yang, Y.; He, G.; Pan, Z.; Zhang, K.; Xian, Y.; Zhu, Z.; Hong, Y.; Zhang, C.; Wu, D. An Injectable Hydrogel with Ultrahigh Burst Pressure and Innate Antibacterial Activity for Emergency Hemostasis and Wound Repair. Adv. Mater. 2024, 36, e2404811. [Google Scholar] [CrossRef] [PubMed]
- Rothe, R.; Xu, Y.; Thomas, A.K.; Meister, S.; Zhang, Y.; Pietzsch, J.; Hauser, S. A modular, injectable, non-covalently assembled hydrogel system features widescale tunable degradability for controlled release and tissue integration. Biomaterials 2021, 269, 120637. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Bao, G.; He, Z.; Mohammadi, S.; Ravanbakhsh, H.; Lessard, L.; Li, J.; Mongeau, L. Injectable, Pore-Forming, Perfusable Double-Network Hydrogels Resilient to Extreme Biomechanical Stimulations. Adv. Sci. 2022, 9, e2102627. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Shi, Z.; Xu, Y.; Lian, Q.; Zhong, Q.; Liu, Q.; Chen, Y.; Pan, X.; Chen, R.; et al. Long-term antibacterial and biofilm dispersion activity of an injectable in situ crosslinked co-delivery hydrogel/microgel for treatment of implant infection. Chem. Eng. J. 2022, 433, 134451. [Google Scholar] [CrossRef]
- Gautam, R.; Pandey, S.; Ahmed, M.; Shukla, S.K.; Matai, I.; Shukla, J.; Soni, S. Silica-Coated Gold Nanorod-Incorporated Injectable Hydrogels for Photothermally-Modulated Localized Drug Delivery. ACS Appl. Nano Mater. 2025, 8, 15765–15779. [Google Scholar] [CrossRef]
- Calder, D.; Fathi, A.; Oveissi, F.; Maleknia, S.; Abrams, T.; Wang, Y.; Maitz, J.; Tsai, K.H.; Maitz, P.; Chrzanowski, W.; et al. Thermoresponsive and Injectable Hydrogel for Tissue Agnostic Regeneration. Adv. Healthc. Mater. 2022, 11, e2201714. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Beech, H.K.; Wang, S.; El-Zaatari, B.M.; Wang, X.; Sapir, L.; Ouchi, T.; Wang, Z.; Johnson, P.N.; Hu, Y.; et al. Molecular Characterization of Polymer Networks. Chem. Rev. 2021, 121, 5042–5092. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, K.; Liu, Q.; Eelkema, R. Self-Healing Injectable Polymer Hydrogel via Dynamic Thiol-Alkynone Double Addition Cross-Links. ACS Macro Lett. 2020, 9, 776–780. [Google Scholar]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Cui, T.; Wu, Y.; Ni, C.; Sun, Y.; Cheng, J. Rheology and texture analysis of gelatin/dialdehyde starch hydrogel carriers for curcumin controlled release. Carbohydr. Polym. 2022, 283, 119154. [Google Scholar] [CrossRef]
- Lopez Hernandez, H.; Souza, J.W.; Appel, E.A. A Quantitative Description for Designing the Extrudability of Shear-Thinning Physical Hydrogels. Macromol. Biosci. 2021, 21, e2000295. [Google Scholar]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Enoch, K.; S, R.C.; Somasundaram, A.A. Improved mechanical properties of Chitosan/PVA hydrogel—A detailed Rheological study. Surf. Interfaces 2023, 41, 103178. [Google Scholar] [CrossRef]
- Mo, C.; Zhang, W.; Zhu, K.; Du, Y.; Huang, W.; Wu, Y.; Song, J. Advances in Injectable Hydrogels Based on Diverse Gelation Methods for Biomedical Imaging. Small Methods 2024, 8, e2400076. [Google Scholar] [CrossRef]
- Moreira Filho, R.N.F.; de Oliveira, M.X.; Brito Soares, A.L.; Maciel Marques, L.S.; Chevallier, P.; Mantovani, D.; Andrade Feitosa, J.P.; Vieira, R.S. Impact of Crosslinking Degree on Chitosan and Oxidized Guar Gum-Based Injectable Hydrogels for Biomedical Applications. Adv. Mater. Technol. 2025, 10, 2400285. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Wang, F. Injectable fillers: Current status, physicochemical properties, function mechanism, and perspectives. RSC Adv. 2023, 13, 23841–23858. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.G.G.; Argenta, D.F.; Caon, T. The rheology of injectable hyaluronic acid hydrogels used as facial fillers: A review. Int. J. Biol. Macromol. 2024, 268, 131880. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Q.; Wang, S.; Wang, D.; Yip, R.C.S.; Xie, W.; Chen, H. Injectable Salecan/hyaluronic acid-based hydrogels with antibacterial, rapid self-healing, pH-responsive and controllable drug release capability for infected wound repair. Carbohydr. Polym. 2025, 347, 122750. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Hsiao, C.D.; Yeh, J.M.; Santiago, K.S. Innovation inspired by nature: Biocompatible self-healing injectable hydrogels based on modified-beta-chitin for wound healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr. Polym. 2022, 276, 118718. [Google Scholar] [PubMed]
- Yang, P.; Ju, Y.; Liu, X.; Li, Z.; Liu, H.; Yang, M.; Chen, X.; Lei, L.; Fang, B. Natural self-healing injectable hydrogels loaded with exosomes and berberine for infected wound healing. Mater. Today Bio 2023, 23, 100875. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (gamma-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Wang, R.; Chen, Q.; Yu, A.; Lu, A. Self-Healing, Injectable Hydrogel Dressing for Monitoring and Therapy of Diabetic Wound. Adv. Funct. Mater. 2024, 34, 2401209. [Google Scholar] [CrossRef]
- Shi, J.; Wang, D.; Wang, H.; Yang, X.; Gu, S.; Wang, Y.; Chen, Z.; Chen, Y.; Gao, J.; Yu, L.; et al. An injectable hemostatic PEG-based hydrogel with on-demand dissolution features for emergency care. Acta Biomater. 2022, 145, 106–121. [Google Scholar] [CrossRef]
- Fang, W.; Yang, L.; Chen, Y.; Hu, Q. Bioinspired multifunctional injectable hydrogel for hemostasis and infected wound management. Acta Biomater. 2023, 161, 50–66. [Google Scholar] [CrossRef]
- Shi, T.; Niu, D.; You, J.; Li, S.; Li, G.; Ren, K.; Yan, S.; Xu, G.; Yin, J. Injectable macro-porous chitosan/polyethylene glycol-silicotungstic acid double-network hydrogels based on “smashed gels recombination” strategy for cartilage tissue engineering. Int. J. Biol. Macromol. 2023, 233, 123541. [Google Scholar] [PubMed]
- Huang, L.; Wang, W.; Xian, Y.; Liu, L.; Fan, J.; Liu, H.; Zheng, Z.; Wu, D. Rapidly in situ forming an injectable Chitosan/PEG hydrogel for intervertebral disc repair. Mater. Today Bio 2023, 22, 100752. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, Y.K.; Lin, Y.T.; Lin, C.W.; Lan, G.Y.; Su, Y.C.; Hu, F.R.; Chang, K.H.; Chen, V.; Yeh, Y.C.; et al. Injectable, Antioxidative, and Tissue-Adhesive Nanocomposite Hydrogel as a Potential Treatment for Inner Retina Injuries. Adv. Sci. (Weinh) 2024, 11, e2308635. [Google Scholar]
- Atila, D.; Dalgic, A.D.; Krzeminska, A.; Pietrasik, J.; Gendaszewska-Darmach, E.; Bociaga, D.; Lipinska, M.; Laoutid, F.; Passion, J.; Kumaravel, V. Injectable Liposome-Loaded Hydrogel Formulations with Controlled Release of Curcumin and alpha-Tocopherol for Dental Tissue Engineering. Adv. Healthc. Mater. 2024, 13, e2400966. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Wang, C.; Shang, L. Living materials for regenerative medicine. Eng. Regen. 2021, 2, 96–104. [Google Scholar] [CrossRef]
- Wang, X.; Rivera-Bolanos, N.; Jiang, B.; Ameer, G.A. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv. Funct. Mater. 2019, 29, 1809009. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.; Yu, T.T.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Yi, Z.; Zhan, F.; Chen, Y.; Zhang, R.; Lin, H.; Zhao, L. An electroconductive hydrogel with injectable and self-healing properties accelerates peripheral nerve regeneration and motor functional recovery. Chem. Eng. J. 2023, 478, 147261. [Google Scholar] [CrossRef]
- Li, S.; Ke, Z.; Peng, X.; Fan, P.; Chao, J.; Wu, P.; Xiao, P.; Zhou, Y. Injectable and fast gelling hyaluronate hydrogels with rapid self-healing ability for spinal cord injury repair. Carbohydr. Polym. 2022, 298, 120081. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Bercea, M.; Rusu, A.G.; Simionescu, N.; Serban, A.M.; Bargan, A.; Nita, L.E.; Chiriac, A.P. Self-healing injectable hydrogels incorporating hyaluronic acid and phytic acid: Rheological insights and implications for regenerative medicine. Int. J. Biol. Macromol. 2024, 279, 135056. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Cui, S.; Jing, X.; Feng, Y.; Coseri, S. Rapid self-healing carboxymethyl chitosan/hyaluronic acid hydrogels with injectable ability for drug delivery. Carbohydr. Polym. 2024, 328, 121707. [Google Scholar] [CrossRef]
- Wei, P.S.; Chen, Y.J.; Lin, S.Y.; Chuang, K.H.; Sheu, M.T.; Ho, H.O. In situ subcutaneously injectable thermosensitive PEG-PLGA diblock and PLGA-PEG-PLGA triblock copolymer composite as sustained delivery of bispecific anti-CD3 scFv T-cell/anti-EGFR Fab Engager (BiTEE). Biomaterials 2021, 278, 121166. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Xu, Z.; Zhang, N.; Sharma, S.; Ramachandran, T.; Karthikeyan, A.; Thatoi, D.N.; Ismail, A.I. Injectable anticancer biodegradable hydrogel-based nanocomposites: Synergistic pH-responsive paclitaxel/beta-cyclodextrin nanocomplex delivery in polyvinyl alcohol hydrogel for targeted pancreatic ductal adenocarcinoma treatment. Int. J. Pharm. 2025, 677, 125514. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Cao, Y.; Hussain, Z.; Xu, M.; Liu, Y.; Ullah, I.; Lu, Z.; Osaka, A.; Lin, J.; et al. An Injectable Hydrogel Composing Anti-Inflammatory and Osteogenic Therapy toward Bone Erosions Microenvironment Remodeling in Rheumatoid Arthritis. Adv. Healthc. Mater. 2024, 13, e2304668. [Google Scholar]
- Qi, Z.; Zhang, T.; Kong, W.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Pan, S. A dual-drug enhanced injectable hydrogel incorporated with neural stem cells for combination therapy in spinal cord injury. Chem. Eng. J. 2022, 427, 130906. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xue, Y.; Jin, J.; Xu, Y.; Zeng, W.; Liu, J.; Xie, J. Injectable Hydrogel Delivery System with High Drug Loading for Prolonging Local Anesthesia. Adv. Sci. (Weinh) 2024, 11, e2309482. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, R.S.; Stiles, W.R.; Jo, M.; Zeng, L.; Rho, S.; Baek, Y.; Kim, J.; Kim, M.S.; Kang, H.; et al. Injectable Thermosensitive Hydrogels for a Sustained Release of Iron Nanochelators. Adv. Sci. (Weinh) 2022, 9, e2200872. [Google Scholar] [CrossRef]
- Kuwentrai, C.; Tang, W.; Lin, X.; Chi, T.; Liu, D.; Song, E.; Webber, M.J.; Huang, J.D.; Ye, Z. Injectable hydrogel-based drug formulation for enhancing tertiary lymphoid structure formation and cancer immunotherapy efficacy. J. Control. Release 2025, 384, 113897. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Liu, R.; Song, S.; Zheng, Z.F.; Zeng, Y.; Mei, Y.; Zhang, J.Y.; Duan, Q.; Lin, R.; et al. Sustained Endocytosis Inhibition via Locally-Injected Drug-Eluting Hydrogel Improves ADCC-Mediated Antibody Therapy in Colorectal Cancer. Adv. Sci. 2025, 12, e2407239. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable functional biomaterials for minimally invasive surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef]
- Van Belleghem, S.; Mahadik, B.; Snodderly, K.; Mote, Z.; Jiang, B.; Yu, J.R.; McLoughlin, S.; He, X.; Nam, A.J.; Fisher, J.P. Dual Extrusion Patterning Drives Tissue Development Aesthetics and Shape Retention in 3D Printed Nipple-Areola Constructs. Adv. Healthc. Mater. 2021, 10, 2101249. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Mo, Q.; Zheng, Y. Immediate prosthetic breast reconstruction after removal of the polyacrylamide hydrogel (PAAG) through a small areolar incision assisted with an endoscope. BMC Surg. 2022, 22, 332. [Google Scholar] [CrossRef]
- Cho, K.-H.; Uthaman, S.; Park, I.-K.; Cho, C.-S. Injectable biomaterials in plastic and reconstructive surgery: A review of the current status. Tissue Eng. Regen. Med. 2018, 15, 559–574. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Sharma, R.; Lin, H.; Appiani, S.; Cleland, J.L.; Yiu, S.C.; Kannan, R.M. OcuPair, a Novel Photo-crosslinkable PAMAM Dendrimer-Hyaluronic Acid Hydrogel Bandage/Bioadhesive for Corneal Injuries and Temporary Corneal Repair. Adv. Sci. 2025, 12, e2417731. [Google Scholar]
- Vashahi, F.; Martinez, M.R.; Dashtimoghadam, E.; Fahimipour, F.; Keith, A.N.; Bersenev, E.A.; Ivanov, D.A.; Zhulina, E.B.; Popryadukhin, P.; Matyjaszewski, K. Injectable bottlebrush hydrogels with tissue-mimetic mechanical properties. Sci. Adv. 2022, 8, eabm2469. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, L.; Wei, Y.; Tan, B.; Wu, Y.; Yi, C.; Liao, J. Photothermal hydrogel platform for prevention of post-surgical tumor recurrence and improving breast reconstruction. J. Nanobiotechnol. 2021, 19, 307. [Google Scholar]
- Wang, F.; Ning, A.; Sun, X.; Zhou, Y.; Deng, H.; Zhou, H.; Chen, S.; He, M.; Meng, Z.; Wang, Y.; et al. Fabrication of a transforming growth factor β1 functionalized silk sericin hydrogel through genetical engineering to repair alveolar bone defects in rabbit. Biomaterials 2025, 316, 122986. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, Q.; Liu, Q.; Lv, H.; Yuan, W.; Wang, Z.; Liu, Y.; Wu, J.; Zhao, L.; Wang, Y. Compliant, Tough, Fatigue-Resistant, and Biocompatible PHEMA-Based Hydrogels as a Breast Implant Material. ACS Omega 2025, 10, 35301–35308. [Google Scholar] [CrossRef]
- Cassuto, D.; Bellia, G.; Schiraldi, C. An overview of soft tissue fillers for cosmetic dermatology: From filling to regenerative medicine. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1857–1866. [Google Scholar]
- Corduff, N. Introducing aesthetic regenerative scaffolds: An immunological perspective. J. Cosmet. Dermatol. 2023, 22, 8–14. [Google Scholar]
- Hwang, Y.; Lee, J.S.; An, H.; Oh, H.; Sung, D.; Tae, G.; Choi, W.I. Hydroxyapatite-embedded levan composite hydrogel as an injectable dermal filler for considerable enhancement of biological efficacy. J. Ind. Eng. Chem. 2021, 104, 491–499. [Google Scholar] [CrossRef]
- Sun, L.; Sun, X.; Ruan, W.; Che, G.; Zhu, F.; Liu, C.; Wan, M. Mechanism of remodeling and local effects in vivo of a new injectable cosmetic filler. Sci. Rep. 2023, 13, 9599. [Google Scholar] [CrossRef]
- Perez, L.A.; Alonso, J.M.; Perez-Gonzalez, R.; Velasco, D.; Suarez-Cabrera, L.; de Aranda-Izuzquiza, G.; Saez-Martinez, V.; Hernandez, R. Injectable hyaluronic acid hydrogels via Michael addition as dermal fillers for skin regeneration applications. Biomater. Adv. 2025, 177, 214364. [Google Scholar] [CrossRef]
- Hong, B.M.; Hong, G.L.; Gwak, M.A.; Kim, K.H.; Jeong, J.E.; Jung, J.Y.; Park, S.A.; Park, W.H. Self-crosslinkable hyaluronate-based hydrogels as a soft tissue filler. Int. J. Biol. Macromol. 2021, 185, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, D.; Cigni, C.; Bellia, G.; Schiraldi, C. Restoring Adipose Tissue Homeostasis in Response to Aging: Initial Clinical Experience with Profhilo Structura®. Gels 2023, 9, 614. [Google Scholar] [CrossRef]
- Bai, L.; Guo, C.; Cao, S.; Zhang, X.; Li, X.; Fang, Q.; Zhang, Y.; Xu, G. A novel injectable Sericin/Nano-hydroxyapatite hydrogel as a dermal filler for soft tissue augmentation. Int. J. Biol. Macromol. 2025, 320, 145814. [Google Scholar] [CrossRef]
- Mei, R.; Zhu, H.; Bai, H.; Dong, W. An injectable, self-healing, and robust double-network composite hydrogel with incorporation of nano-lignin. Colloid Surf. A-Physicochem. Eng. Asp. 2025, 706, 135788. [Google Scholar] [CrossRef]
- Shen, C.; Zhou, X.; Jiang, J.; Dang, J.; Lin, T.; Hu, R.; Zhao, T.; Sun, D.; Zhang, M. Injectable amino-modified poly-L-lactic acid microspheres/hyaluronic acid-based hydrogel composites for soft tissue fillers. Biomater. Adv. 2025, 178, 214465. [Google Scholar] [CrossRef]
- You, D.G.; Jung, J.M.; Kim, C.H.; An, J.Y.; Bui, V.D.; Lee, J.; Um, W.; Jo, D.G.; Cho, Y.W.; Lee, D.S.; et al. Stem Cell-Derived Extracellular Vesicle-Bearing Injectable Hydrogel for Collagen Generation in Dermis. ACS Appl. Mater. Interfaces 2024, 16, 37698–37706. [Google Scholar] [CrossRef]
- Saravanou, S.F.; Ioannidis, K.; Dimopoulos, A.; Paxinou, A.; Kounelaki, F.; Varsami, S.M.; Tsitsilianis, C.; Papantoniou, I.; Pasparakis, G. Dually crosslinked injectable alginate-based graft copolymer thermoresponsive hydrogels as 3D printing bioinks for cell spheroid growth and release. Carbohydr. Polym. 2023, 312, 120790. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Z.; Xiong, J.; Xia, J.; Wang, Y.; Yang, L.; Liang, Y. 3D bioprinted scaffolds of polysaccharide hydrogels in osteochondral and cartilage tissue engineering. Des. Monomers Polym. 2023, 26, 258–272. [Google Scholar] [CrossRef]
- Jahani Kadousaraei, M.; Yamada, S.; Aydin, M.S.; Rashad, A.; Cabeza, N.M.; Mohamed-Ahmed, S.; Gjerde, C.G.; Malkoch, M.; Mustafa, K. Bioprinting of mesenchymal stem cells in low concentration gelatin methacryloyl/alginate blends without ionic crosslinking of alginate. Sci. Rep. 2025, 15, 6609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.; Wang, Y.; Wu, T.; Liang, Y.; Deng, K.; Luan, G.; Chen, Y.; Huang, Z.; Yue, K. Direct 3D Bioprinting of Tough and Antifatigue Cell-Laden Constructs Enabled by a Self-Healing Hydrogel Bioink. Biomacromolecules 2023, 24, 2549–2562. [Google Scholar] [CrossRef]
- Habib, M.A.; Khoda, B. Rheological analysis of bio-ink for 3D bio-printing processes. J. Manuf. Process 2022, 76, 708–718. [Google Scholar] [CrossRef]
- Jongprasitkul, H.; Parihar, V.S.; Turunen, S.; Kellomaki, M. pH-Responsive Gallol-Functionalized Hyaluronic Acid-Based Tissue Adhesive Hydrogels for Injection and Three-Dimensional Bioprinting. ACS Appl. Mater. Interfaces 2023, 15, 33972–33984. [Google Scholar] [CrossRef]
- Hull, S.M.; Lou, J.; Lindsay, C.D.; Navarro, R.S.; Cai, B.; Brunel, L.G.; Westerfield, A.D.; Xia, Y.; Heilshorn, S.C. 3D bioprinting of dynamic hydrogel bioinks enabled by small molecule modulators. Sci. Adv. 2023, 9, eade7880. [Google Scholar] [CrossRef]
- Ravi, S.; Chokkakula, L.P.P.; Giri, P.S.; Korra, G.; Dey, S.R.; Rath, S.N. 3D Bioprintable Hypoxia-Mimicking PEG-Based Nano Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2023, 15, 19921–19936. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Stevic, M.; Buanz, A.; Uddin, M.J.; Tamburic, S. Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review. Cosmetics 2022, 9, 115. [Google Scholar] [CrossRef]
- Manousi, E.; Chatzitaki, A.-T.; Vakirlis, E.; Karavasili, C.; Fatouros, D.G. Development and in vivo evaluation of 3D printed hydrogel patches for personalized cosmetic use based on skin type. J. Drug Deliv. Sci. Technol. 2024, 92, 105306. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef]
- Graca, A.; Tonioli, V.; Martins, A.M.; Ribeiro, H.M.; Marto, J. 3D-printed hydrogel patch for controlled topical release: Gelatin/tannic acid formulation meets additive manufacturing. Eur. J. Pharm. Biopharm. 2025, 214, 114802. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Xiang, S.; Jiang, C.; Wu, W.; Ruan, S.; Du, Q.; Chen, T.; Xue, Y.; Chen, H.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. AAPS PharmSciTech 2020, 21, 159. [Google Scholar] [CrossRef]
- Kamlow, M.-A.; Vadodaria, S.; Gholamipour-Shirazi, A.; Spyropoulos, F.; Mills, T. 3D printing of edible hydrogels containing thiamine and their comparison to cast gels. Food Hydrocoll. 2021, 116, 106550. [Google Scholar] [CrossRef]
- Kong, Y.; Lin, S.; Chen, S.; Jing, L.; Liu, Z.; Wu, M.; Yu, X.; Fu, C.; Wang, J.; Huang, D. Pumpkin seed protein-based hydrogel as gelatin mimics and edible inks in 3D-Printed food. Food Hydrocoll. 2025, 162, 111014. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, K.; Tian, H.; Liu, X.; Liu, G.; Hu, Z.; Zhao, L. Analysis on the printability and rheological characteristics of bigel inks: Potential in 3D food printing. Food Hydrocoll. 2022, 129, 107675. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Y.; Zhang, X.; Yang, G.; Jin, W.; Peng, D.; Huang, Q. Correlation between 3D printability and rheological properties of biopolymer fluid: A case study of alginate-based hydrogels. J. Food Eng. 2024, 370, 111970. [Google Scholar] [CrossRef]
- Montoya, J.; Medina, J.; Molina, A.; Gutiérrez, J.; Rodríguez, B.; Marín, R. Impact of viscoelastic and structural properties from starch-mango and starch-arabinoxylans hydrocolloids in 3D food printing. Addit. Manuf. 2021, 39, 101891. [Google Scholar] [CrossRef]
- Tian, H.; Wang, K.; Lan, H.; Wang, Y.; Hu, Z.; Zhao, L. Effect of hybrid gelator systems of beeswax-carrageenan-xanthan on rheological properties and printability of litchi inks for 3D food printing. Food Hydrocoll. 2021, 113, 106482. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lee, H.; Jeong, Y.; Lee, N.; Lee, I.; Lee, J.H. Recent Advances in Injectable Hydrogels for Biomedical and Aesthetic Applications: Focus on Rheological Characteristics. Gels 2026, 12, 11. https://doi.org/10.3390/gels12010011
Lee H, Jeong Y, Lee N, Lee I, Lee JH. Recent Advances in Injectable Hydrogels for Biomedical and Aesthetic Applications: Focus on Rheological Characteristics. Gels. 2026; 12(1):11. https://doi.org/10.3390/gels12010011
Chicago/Turabian StyleLee, Hyerin, Yujin Jeong, Nayeon Lee, Inhye Lee, and Jin Hyun Lee. 2026. "Recent Advances in Injectable Hydrogels for Biomedical and Aesthetic Applications: Focus on Rheological Characteristics" Gels 12, no. 1: 11. https://doi.org/10.3390/gels12010011
APA StyleLee, H., Jeong, Y., Lee, N., Lee, I., & Lee, J. H. (2026). Recent Advances in Injectable Hydrogels for Biomedical and Aesthetic Applications: Focus on Rheological Characteristics. Gels, 12(1), 11. https://doi.org/10.3390/gels12010011





