Hydrogel Versus Alternative Vehicles for (Trans)dermal Delivery of Propranolol Hydrochloride—In Vitro and Ex Vivo Studies
Abstract
1. Introduction
2. Results and Discussion
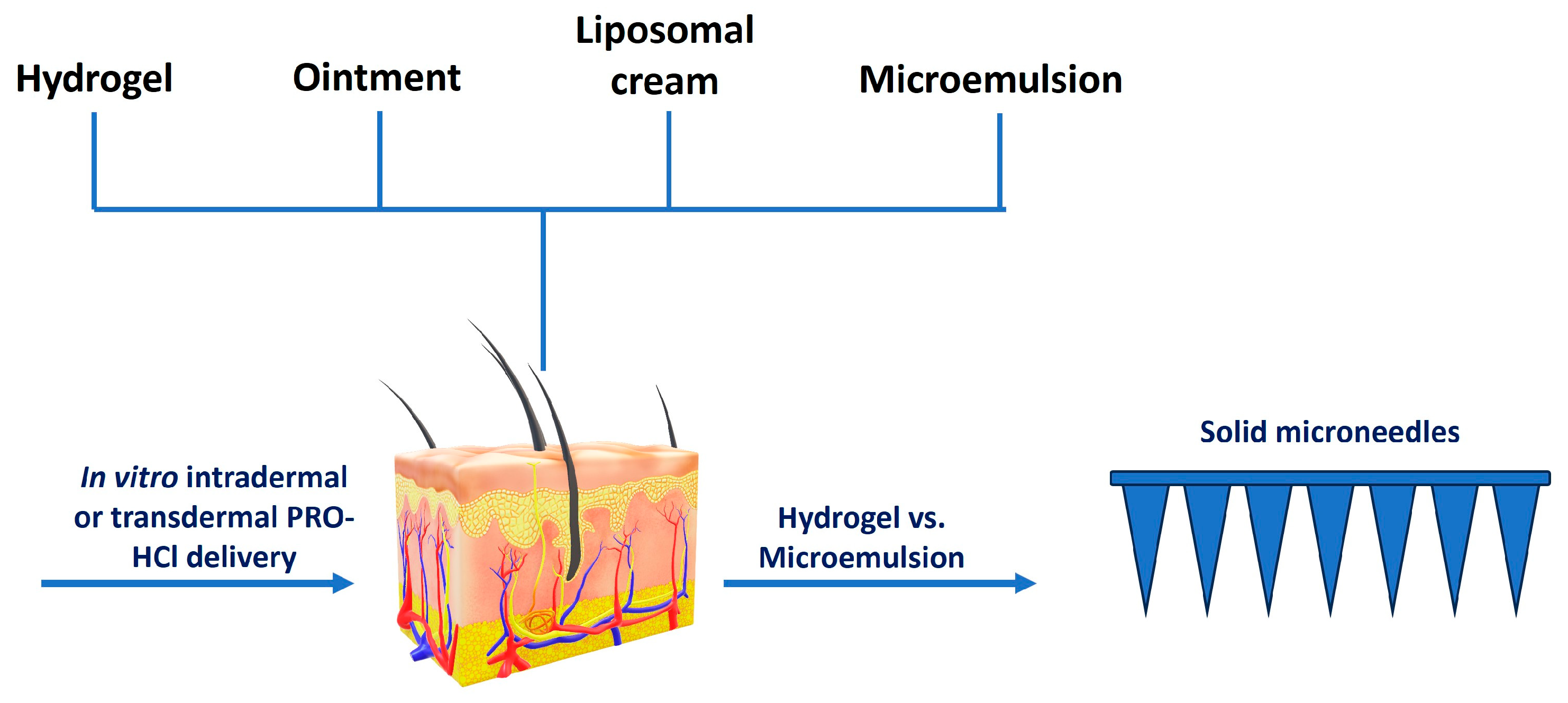
2.1. Selection of Formulations
2.2. Formulations Characterization
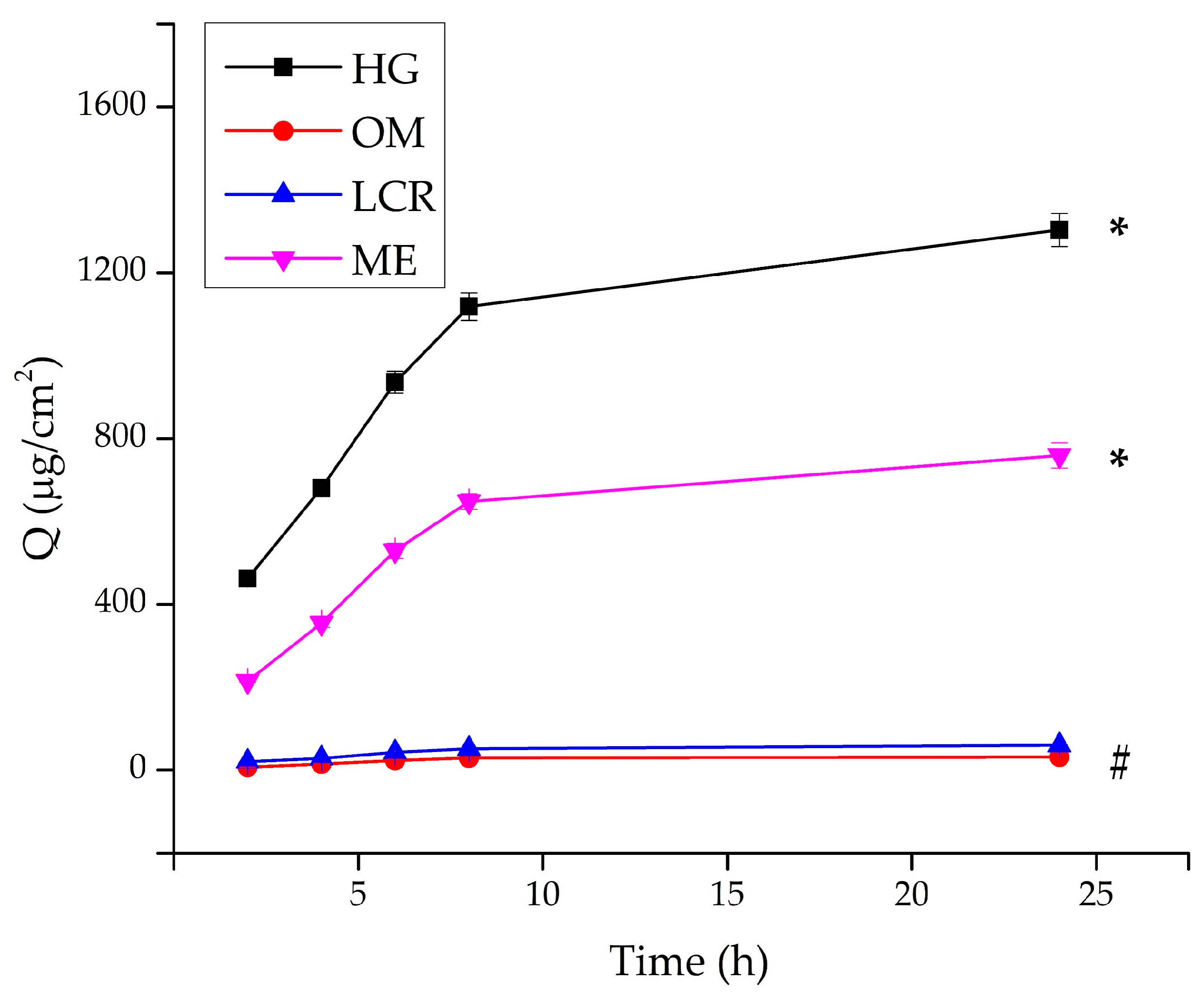
2.3. In Vitro Drug Release Studies
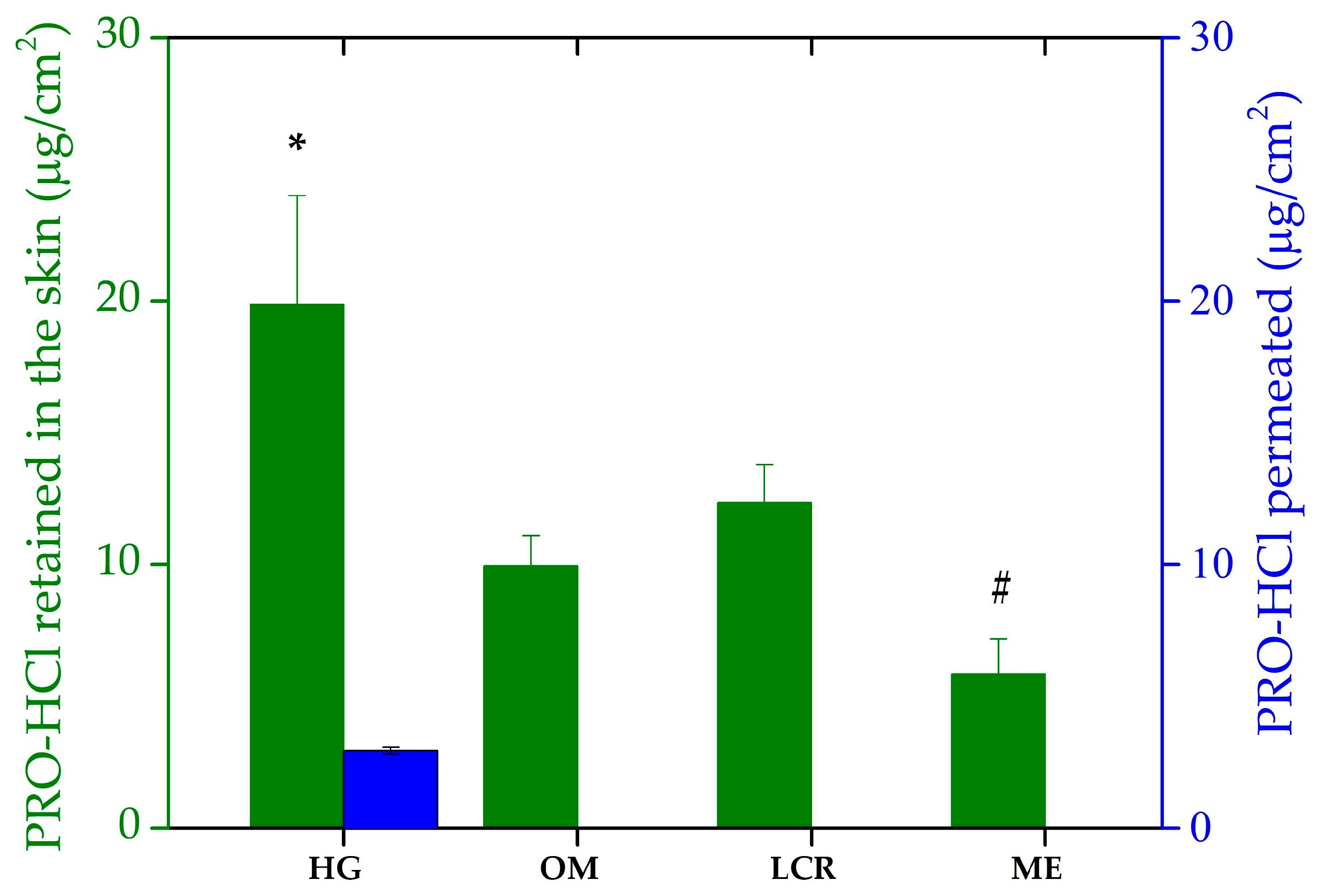
2.4. Ex Vivo Drug Penetration/Permeation Studies
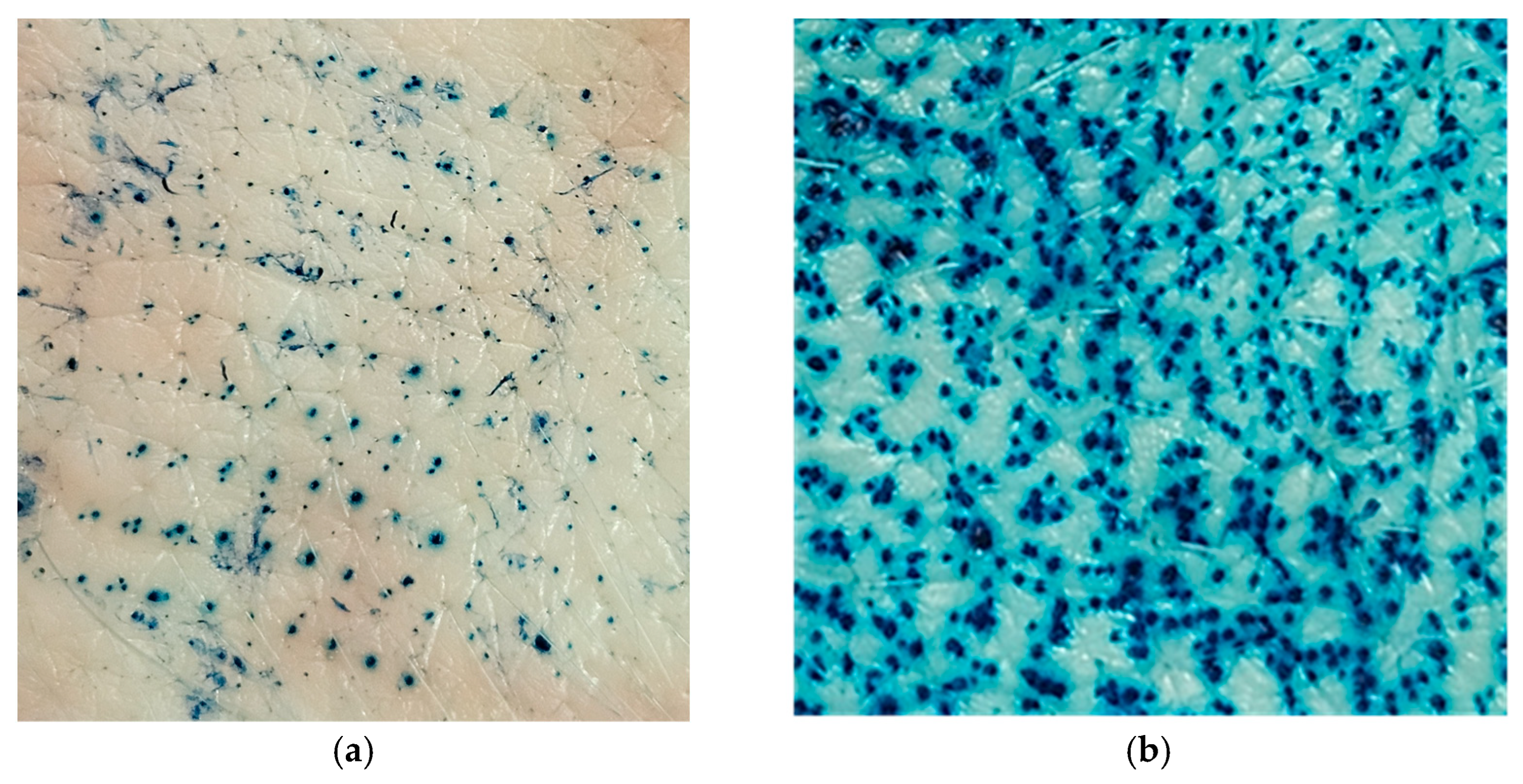
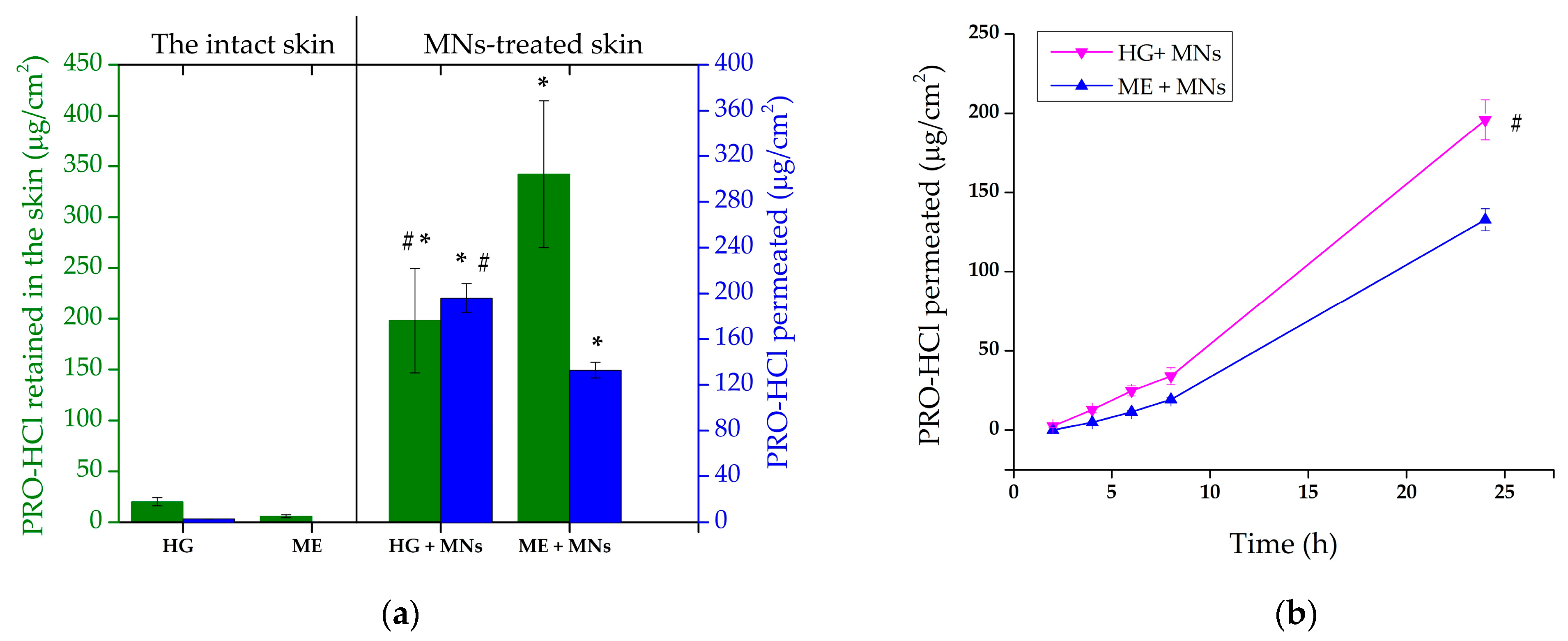
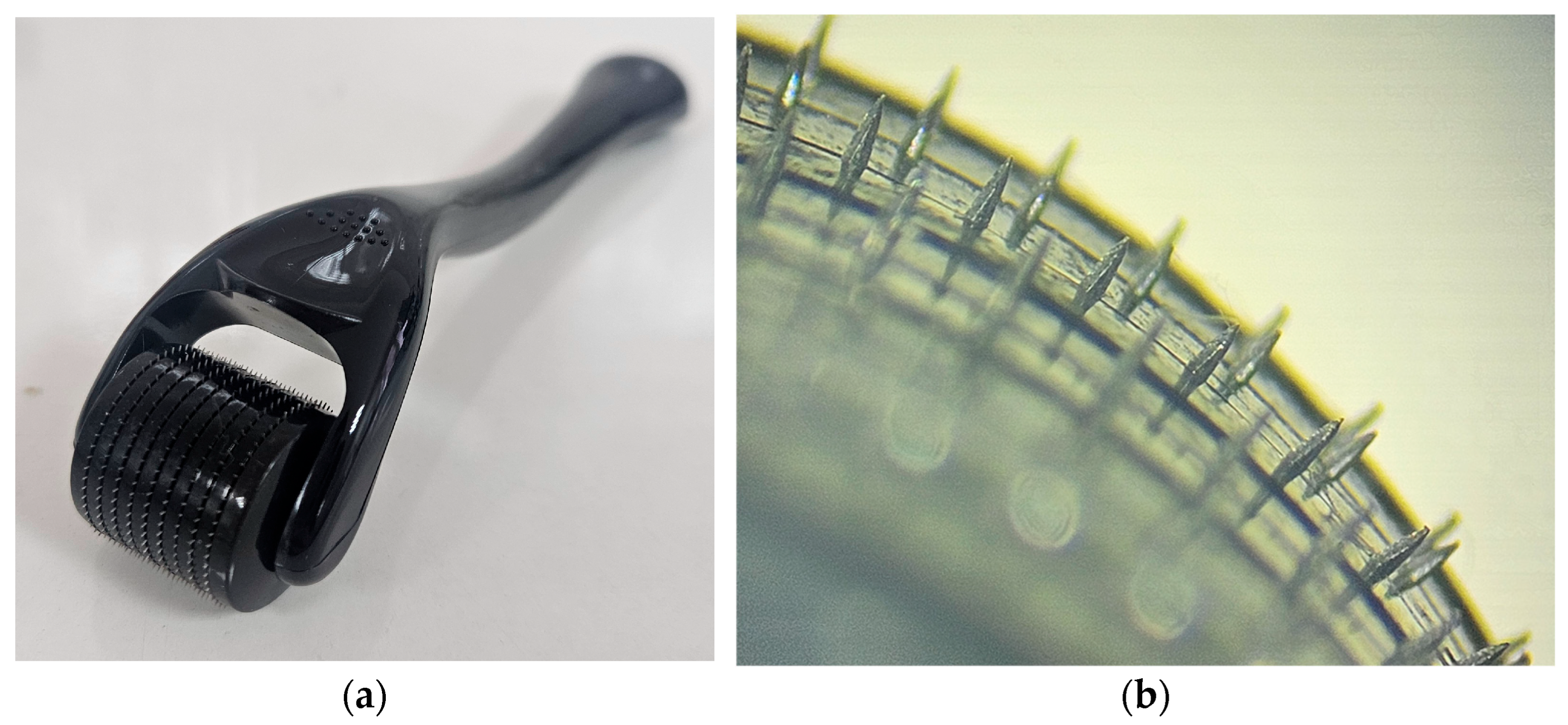
2.5. Ex Vivo MNs-Assisted Drug Penetration/Permeation Studies
3. Conclusions
4. Materials and Methods
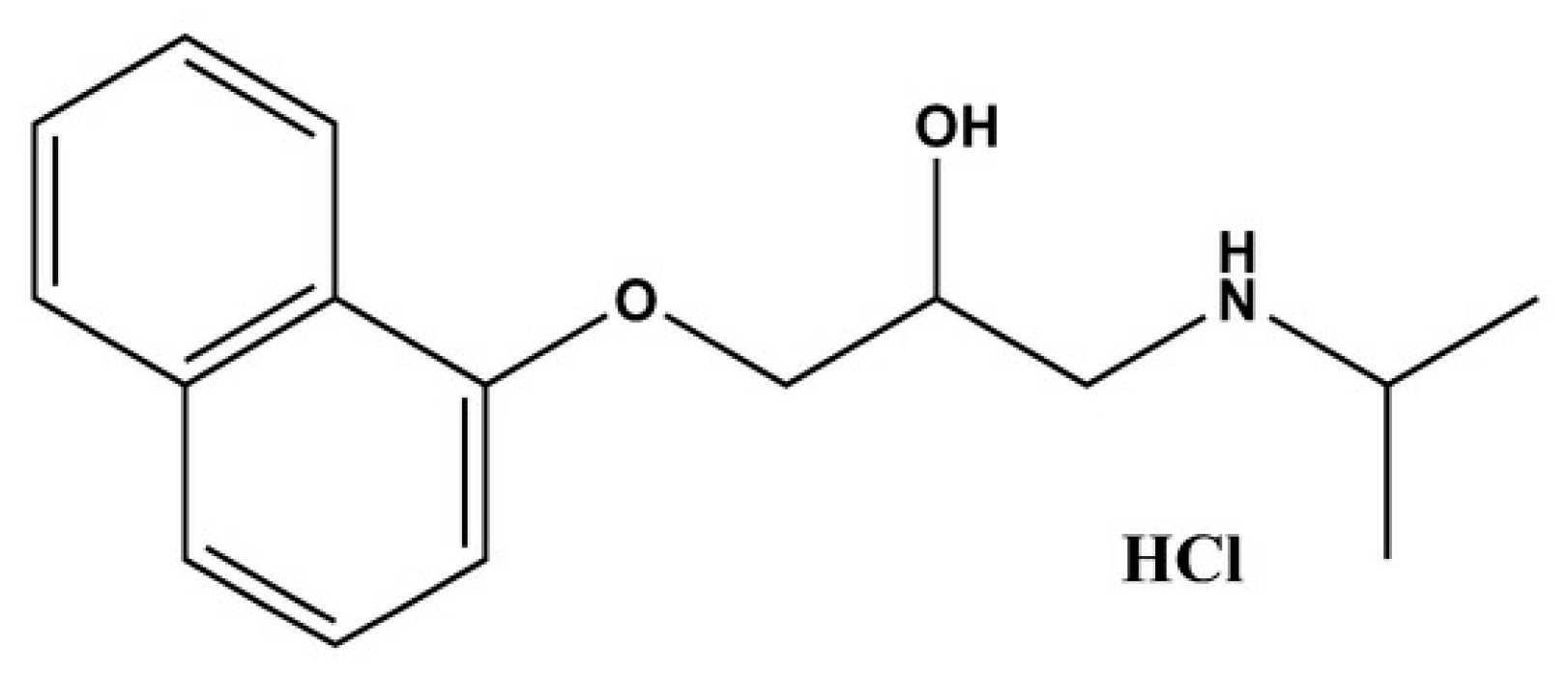
4.1. Materials
4.2. Preparation of Formulations
4.3. Characterization of the Investigated Formulations
4.4. In Vitro Drug Release Studies
4.5. Ex Vivo Drug Penetration/Permeation Studies
4.6. Ex Vivo Drug Penetration/Permeation Studies on MNs Preterated Skin
4.7. HPLC Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRO-HCl | Propranolol hydrochloride |
| SC | The stratum corneum |
| OM | Ointment |
| LCR | Liposomal cream |
| HG | Hydrogel |
| ME | Microemulsion |
| MNs | Microneedles |
References
- Al-Kassas, R.; Wen, J.; Cheng, A.E.-M.; Kim, A.M.-J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- Abla, K.K.; Domiati, S.; El Majzoub, R.; Mehanna, M.M. Propranolol-loaded limonene-based microemulsion thermo-responsive mucoadhesive nasal nanogel: Design, in vitro assessment, ex vivo permeation, and brain biodistribution. Gels 2023, 9, 491. [Google Scholar] [CrossRef]
- Abdullah, T.; Al-Kinani, K. Propranolol nanoemulgel: Preparation, in-vitro and ex-vivo characterization for a potential local hemangioma therapy. Pharmacia 2024, 71, 1–12. [Google Scholar] [CrossRef]
- Kashiwagura, Y.; Hakamata, A.; Shirai, M.; Endoh, A.; Tanaka, S.; Inui, N.; Watanabe, H.; Namiki, N.; Uchida, S. Topical formulations of propranolol for infantile hemangiomas: Characteristics of formulations and three cases of infants administered topical propranolol cream. Chem. Pharm. Bull. 2022, 70, 277–282. [Google Scholar] [CrossRef]
- Nagata, E.; Kashiwagura, Y.; Okada, E.; Tanaka, S.; Sano, S.; Nishida, M.; Hayano, S.; Iwashima, S.; Hakamata, A.; Odagiri, K. Efficacy and safety of propranolol cream in infantile hemangioma: A prospective pilot study. J. Pharmacol. Sci. 2022, 149, 60–65. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and evaluation of dissolving microneedles for enhanced dermal delivery of propranolol hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Meng, S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin. Drug Deliv. 2019, 16, 847–867. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Pescina, S.; Santi, P. The influence of formulation and excipients on propranolol skin permeation and retention. BioMed Res. Int. 2018, 2018, 1281673. [Google Scholar] [CrossRef]
- Moghimipour, E.; Tajmal, A.; Kaydan, H.H.; Salimi, A. Analysing the Effect of Permeation Enhancers on the In Vitro Skin Permeability of Propranolol: A Mechanistic Study. J. Res. Pharm. 2023, 27, 67–73. [Google Scholar] [CrossRef]
- Lacassia, C.; Cutrignelli, A.; la Forgia, F.M.; Fontana, S.; Lopalco, A.; Denora, N.; Lopedota, A.A. Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma. Pharmaceutics 2025, 17, 83. [Google Scholar] [CrossRef]
- Liu, S.; Deng, T.; Cheng, H.; Lu, J.; Wu, J. Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases. Pharmaceutics 2025, 17, 746. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin barrier function: The interplay of physical, chemical, and immunologic properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and challenges in the treatment of skin diseases with the transdermal drug delivery system. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef]
- Kelchen, M.N.; Brogden, N.K. In Vitro Skin Retention and Drug Permeation through Intact and Microneedle Pretreated Skin after Application of Propranolol Loaded Microemulsions. Pharm. Res. 2018, 35, 228. [Google Scholar] [CrossRef]
- Atkinson, J.P.; Maibach, H.I.; Dragicevic, N. Targets in Dermal and Transdermal Delivery and Classification of Penetration Enhancement Methods. In Percutaneous Penetration Enhancers—Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 93–108. [Google Scholar]
- Padula, C.; Nicoli, S.; Pescina, S.; Santi, P. Thin polymeric films for the topical delivery of propranolol. Colloids Surf. B Biointerfaces 2019, 174, 582–586. [Google Scholar] [CrossRef]
- Venkatesan, J.; Liu, W.; Madry, H.; Cucchiarini, M. Alginate hydrogel-guided rAAV-mediated FGF-2 and TGF-β delivery and overexpression stimulates the biological activities of human meniscal fibrochondrocytes for meniscus repair. Eur. Cell Mater. 2024, 47, 1–14. [Google Scholar] [CrossRef]
- Labie, H.; Blanzat, M. Hydrogels for dermal and transdermal drug delivery. Biomater. Sci. 2023, 11, 4073–4093. [Google Scholar] [CrossRef]
- Cerchiara, T.; Bigucci, F.; Luppi, B. Hydrogel Vehicles for Hydrophilic Compounds. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 285–297. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Luo, J.; Cui, Y.; Chen, J.; Zeng, B.; Deng, Z.; Shao, L. “Double-sided protector” Janus hydrogels for skin and mucosal wound repair: Applications, mechanisms, and prospects. J. Nanobiotechnol. 2025, 23, 387. [Google Scholar] [CrossRef]
- Cirulli, A.; Borgheti-Cardoso, L.N.; Torras, N.; Martínez Fraiz, E. Mimicking human skin constructs using norbornene-pullulan-based hydrogels. Int. J. Bioprinting 2024, 10, 222–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, C.; Wang, Z.; Lan, F.; Wan, L.; Li, A.; Zheng, P.; Zhu, W.; Pan, Q. A spatial confinement biological heterogeneous cascade nanozyme composite hydrogel combined with nitric oxide gas therapy for enhanced treatment of psoriasis and diabetic wound. Chem. Eng. J. 2025, 507, 160629. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Bourdon, F.; Lecoeur, M.; Leconte, L.; Ultré, V.; Kouach, M.; Odou, P.; Vaccher, C.; Foulon, C. Evaluation of Pentravan®, Pentravan® Plus, Phytobase®, Lipovan® and Pluronic Lecithin Organogel for the transdermal administration of antiemetic drugs to treat chemotherapy-induced nausea and vomiting at the hospital. Int. J. Pharm. 2016, 515, 774–787. [Google Scholar] [CrossRef]
- Lehman, P.A.; Raney, S.G. In Vitro percutaneous absorption of ketoprofen and testosterone: Comparison of pluronic lecithin organogel vs. pentravan cream. Int. J. Pharm. Compd. 2012, 16, 248–252. [Google Scholar]
- Kirilov, P.; Ducrotté-Tassel, A.; Salvi, J.-P.; Perrot, S.; Haftek, M.; Boulieu, R.; Pirot, F. Ex-Vivo percutaneous absorption of enrofloxacin: Comparison of LMOG organogel vs. pentravan cream. Int. J. Pharm. 2016, 498, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef]
- de Campos Araújo, L.M.P.; Thomazine, J.A.; Lopez, R.F.V. Development of microemulsions to topically deliver 5-aminolevulinic acid in photodynamic therapy. Eur. J. Pharm. Biopharm. 2010, 75, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Naoui, W.; Bolzinger, M.A.; Fenet, B.; Pelletier, J.; Valour, J.P.; Kalfat, R.; Chevalier, Y. Microemulsion microstructure influences the skin delivery of an hydrophilic drug. Pharm. Res. 2011, 28, 1683–1695. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Iglesias-Vilas, G.; Blanco-Méndez, J.; López-Quintela, M.A.; Marty, J.-P.; Guy, R.H. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur. J. Pharm. Biopharm. 1997, 43, 37–42. [Google Scholar] [CrossRef]
- Bubić Pajić, N.; Vucen, S.; Ilić, T.; O’Mahony, C.; Dobričić, V.; Savić, S. Comparative efficacy evaluation of different penetration enhancement strategies for dermal delivery of poorly soluble drugs—A case with sertaconazole nitrate. Eur. J. Pharm. Sci. 2021, 164, 105895. [Google Scholar] [CrossRef]
- Ilic, T.; Pantelic, I.; Lunter, D.; Djordjevic, S.; Markovic, B.; Rankovic, D.; Daniels, R.; Savic, S. Critical quality attributes, in vitro release and correlated in vitro skin permeation-in vivo tape stripping collective data for demonstrating therapeutic (non)equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef]
- Casiraghi, A.; Musazzi, U.M.; Rocco, P.; Franze, S.; Minghetti, P. Topical Treatment of Infantile Haemangiomas: A Comparative Study on the Selection of a Semi-Solid Vehicle. Skin Pharmacol. Physiol. 2016, 29, 210–219. [Google Scholar] [CrossRef]
- Cvjeticanin, M.; Zorica, D.; Krstonosic, V.; Hadnadjev, M.; Stojanac, I.; Ramic, B.; Drobac, M.; Petrovic, L.; Atanackovic, T. The Influence of Temperature on Rheological Properties of Three Root Canal Sealers. Mater. Plast. 2022, 59, 174–182. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3. [Google Scholar]
- Shah, V.P.; Yacobi, A.; Radulescu, F.S.; Miron, D.S.; Lane, M.E. A science based approach to topical drug classification system (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef]
- Nemati, E.; Mokhtarzadeh, A.; Panahi-Azar, V.; Mohammadi, A.; Hamishehkar, H.; Mesgari-Abbasi, M.; Ezzati Nazhad Dolatabadi, J.; de la Guardia, M. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS PharmSciTech 2019, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Amaral, M.; González-Mira, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of Risperidone: Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef]
- Wolska, E.; Szymańska, M. Comparison of the in vitro drug release methods for the selection of test conditions to characterize solid lipid microparticles. Pharmaceutics 2023, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Bubic Pajic, N.; Nikolic, I.; Mitsou, E.; Papadimitriou, V.; Xenakis, A.; Randjelovic, D.; Dobricic, V.; Smitran, A.; Cekic, N.; Calija, B.; et al. Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: Phase behavior study and microstructure influence on drug biopharamaceutical properties. J. Mol. Liq. 2018, 272, 746–758. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Nowak, A.; Klebeko, J.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N. Assessment of the effect of structural modification of ibuprofen on the penetration of ibuprofen from Pentravan®(semisolid) formulation using human skin and a transdermal diffusion test model. Materials 2021, 14, 6808. [Google Scholar] [CrossRef]
- Bayer, I.S. Controlled drug release from nanoengineered polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Aubock, J.; Wolzt, M.; Valenta, C. In vitro vs. In vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Quality of Transdermal Patches; EMA/CHMP/QWP/608924/2014; Committee for Medicinal Products for Human Use: London, UK, 2014. [Google Scholar]
- Xiong, R.; Ling, G.; Zhang, Y.; Guan, J.; Zhang, P. Nucleic acid delivery by ionizable nanocarriers for brain disease treatment. Brain-X 2023, 1, e7. [Google Scholar] [CrossRef]
- Kretsos, K.; Nitsche, J.M.; Kasting, G.B. Distributed diffusion–clearance model for transient drug distribution within the skin. J. Pharm. Sci. 2004, 93, 2820–2835. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.; Brogden, N.K.; Stinchcomb, A.L. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin. Drug Deliv. 2010, 7, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.; Stinchcomb, A.L. Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride. Pharm. Res. 2011, 28, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zu, Q.; Yu, Y.; Bi, X.; Zhang, R.; Di, L. Microneedle-Assisted Percutaneous Delivery of a Tetramethylpyrazine-Loaded Microemulsion. Molecules 2017, 22, 2022. [Google Scholar] [CrossRef]
- Mojeiko, G.; de Brito, M.; Salata, G.C.; Lopes, L.B. Combination of microneedles and microemulsions to increase celecoxib topical delivery for potential application in chemoprevention of breast cancer. Int. J. Pharm. 2019, 560, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Peterchristoper, G.V. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Ghaemi, B.; Amani, A. Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: Permeation, antibacterial and safety assessments. Eur. J. Pharm. Sci. 2018, 120, 142–151. [Google Scholar] [CrossRef]
- Zhou, W.; He, S.; Yang, Y.; Jian, D.; Chen, X.; Ding, J. Formulation, characterization and clinical evaluation of propranolol hydrochloride gel for transdermal treatment of superficial infantile hemangioma. Drug Dev. Ind. Pharm. 2015, 41, 1109–1119. [Google Scholar] [CrossRef]
- Hoppel, M.; Ettl, H.; Holper, E.; Valenta, C. Influence of the composition of monoacyl phosphatidylcholine based microemulsions on the dermal delivery of flufenamic acid. Int. J. Pharm. 2014, 475, 156–162. [Google Scholar] [CrossRef]
- Sahoo, S.; Pani, N.R.; Sahoo, S.K. Effect of microemulsion in topical sertaconazole hydrogel: In vitro and in vivo study. Drug Deliv. 2016, 23, 338–345. [Google Scholar] [CrossRef]
- Bubić Pajić, N.; Ilić, T.; Nikolić, I.; Dobričić, V.; Pantelić, I.; Savić, S. Alkyl polyglucoside-based adapalene-loaded microemulsions for targeted dermal delivery: Structure, stability and comparative biopharmaceutical characterization with a conventional dosage form. J. Drug Deliv. Sci. Technol. 2019, 54, 101245. [Google Scholar] [CrossRef]
- USP39-NF34; United States Pharmacopeia 39—National Formulary 34, Chapter 3: Topical and Transdermal Drug Products—Product Quality Tests. United States Pharmacopeial Convention: Rockville, MD, USA, 2016.
- USP37-NF32; United States Pharmacopeia 37—National Formulary 32, Chapter 1724: Semisolid Drug Products—Performance Tests. United States Pharmacopeial Convention: Rockville, MD, USA, 2014.
- Omolu, A.; Bailly, M.; Day, R.M. Assessment of solid microneedle rollers to enhance transmembrane delivery of doxycycline and inhibition of MMP activity. Drug Deliv. 2017, 24, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Dhayalamurthi, S.; Anandan, P.; Saravanan, D. Development and validation of a dissolution method for novel fixed dose combination of etodolac and propranolol hydrochloride tablets by RP-HPLC. Acta Chromatogr. 2011, 23, 551–566. [Google Scholar] [CrossRef]
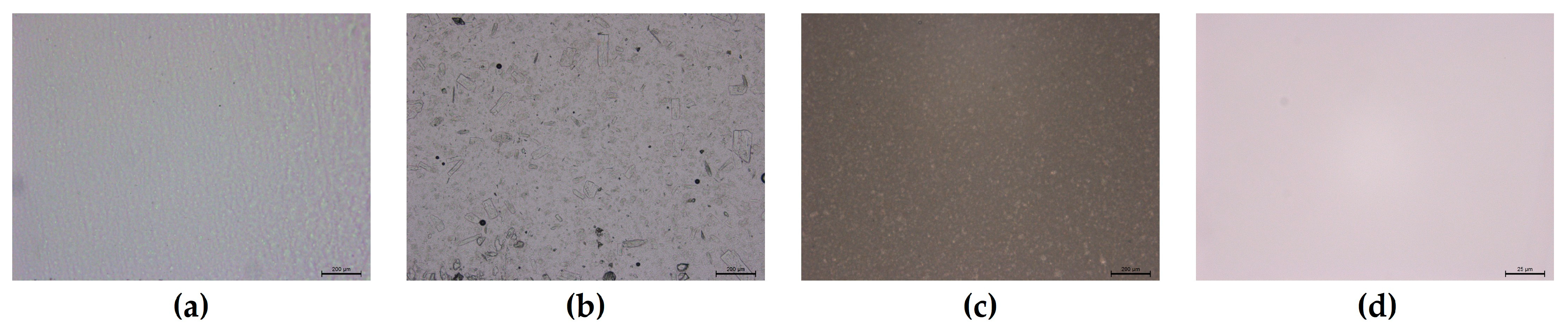
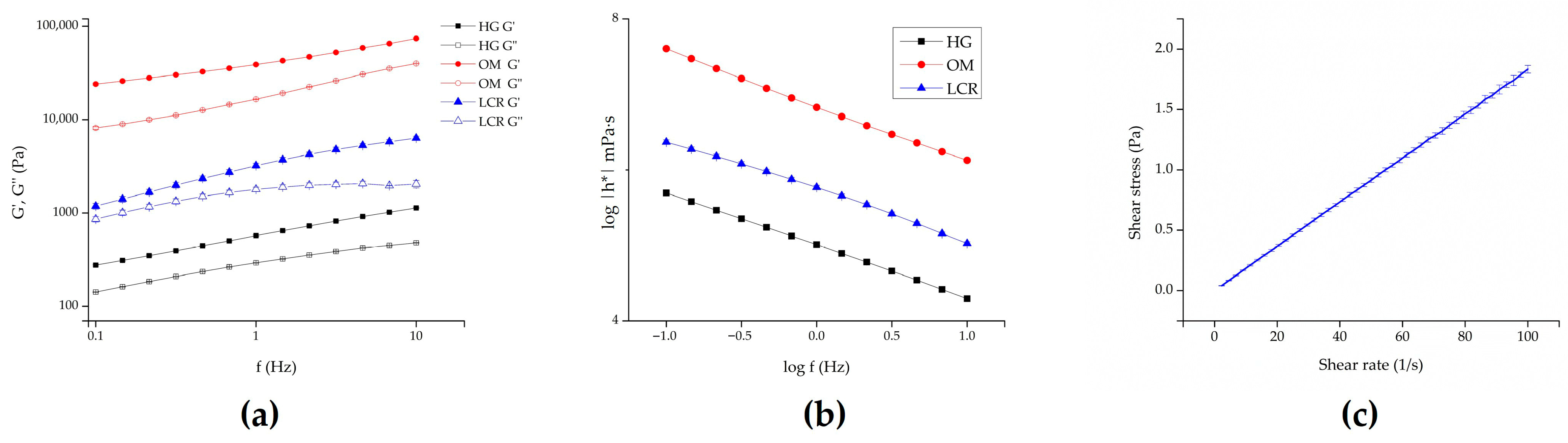
| Formulation Code | pH (Mean ± SD, n = 3) | Drug Content (%) (Mean ± SD, n = 3) | Drug Content (%) (Mean ± SD, n = 3) After 1 Month |
|---|---|---|---|
| HG | 7.08 ± 0.02 | 97.76 ± 3.12 | 95.96 ± 2.78 |
| OM | 6.19 ± 0.11 | 86.69 ± 1.84 | 84.17 ± 1.45 |
| LCR | 5.19 ± 0.07 | 95.27 ± 1.21 | 96.42 ± 4.36 |
| ME | 5.44 ± 0.04 | 100.24 ± 2.48 | 98.69 ± 1.93 |
| Formulation Code | Release Rate (µg/cm2h−1/2) | Q24h (µg/cm2) | PRO-HCl Released (%) | Kinetic Model (r2) |
|---|---|---|---|---|
| HG | 492.97 ± 12.81 a | 2396.86 ± 59.80 a | 48.18 ± 1.20 a | Korsmeyer-Peppas (0.998) |
| OM | 10.68 ± 1.02 b | 50.41 ± 3.87 b | 1.01 ± 0.08 b | Higuchi (0.9722) |
| LCR | 22.08 ± 0.26 b | 106.53 ± 0.76 b | 2.14 ± 0.01 b | Higuchi (0.9996) |
| ME | 300.40 ± 7.77 a | 1395.47 ± 33.99 a | 28.05 ± 0.68 a | Higuchi (0.9995) |
| Formulation Code | PRO-HCl Penetrated (Intact Skin) (µg/cm2) | PRO-HCl Penetrated (MN-Treated Skin) (µg/cm2) | Enhancement Ratio | Permeation Rate (MN-Treated Skin) (µg/cm2h) | PRO-HCl Permeated (MN-Treated Skin) (µg/cm2) |
|---|---|---|---|---|---|
| HG | 19.87 ± 4.12 a | 198.19 ± 51.41 a | 8.7 | 9.07 ± 3.39 | 195.85 ± 12.77 a |
| ME | 5.85 ± 1.34 | 342.34 ± 72.36 | 60.3 | 6.30 ± 1.98 | 132.76 ± 6.88 |
| Ingredients | Formulation Code | |||
|---|---|---|---|---|
| HG | HG | LCR | ME | |
| Carmellose sodium 600 | 5.0 | - | - | - |
| Glycerol, 85% | 10.0 | - | - | - |
| Cholesterol | - | 5.0 | - | - |
| Lanolin | - | 15.0 | - | - |
| Liquid paraffine | - | 15.0 | - | - |
| Petrolatum | - | 65.0 | - | - |
| Pentravan® | - | - | up to 100.0 | - |
| CapryolTM 90 | - | - | - | 8.0 |
| Polysorbate 80 | - | - | - | 16.0 |
| Ethanol | - | - | - | 16.0 |
| Double-distilled water | 85.0 | - | q.s. | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bubić Pajić, N.; Kaurin, M.; Klepić, A.; Knežević Ratković, D.; Stojmenovski, A.; Krstonošić, V.; Škrbic, R. Hydrogel Versus Alternative Vehicles for (Trans)dermal Delivery of Propranolol Hydrochloride—In Vitro and Ex Vivo Studies. Gels 2026, 12, 10. https://doi.org/10.3390/gels12010010
Bubić Pajić N, Kaurin M, Klepić A, Knežević Ratković D, Stojmenovski A, Krstonošić V, Škrbic R. Hydrogel Versus Alternative Vehicles for (Trans)dermal Delivery of Propranolol Hydrochloride—In Vitro and Ex Vivo Studies. Gels. 2026; 12(1):10. https://doi.org/10.3390/gels12010010
Chicago/Turabian StyleBubić Pajić, Nataša, Milica Kaurin, Adrijana Klepić, Darija Knežević Ratković, Aneta Stojmenovski, Veljko Krstonošić, and Ranko Škrbic. 2026. "Hydrogel Versus Alternative Vehicles for (Trans)dermal Delivery of Propranolol Hydrochloride—In Vitro and Ex Vivo Studies" Gels 12, no. 1: 10. https://doi.org/10.3390/gels12010010
APA StyleBubić Pajić, N., Kaurin, M., Klepić, A., Knežević Ratković, D., Stojmenovski, A., Krstonošić, V., & Škrbic, R. (2026). Hydrogel Versus Alternative Vehicles for (Trans)dermal Delivery of Propranolol Hydrochloride—In Vitro and Ex Vivo Studies. Gels, 12(1), 10. https://doi.org/10.3390/gels12010010







