Nano Gel/Hydrogel-Based Components for Battery Technology: An Overview
Abstract
1. Introduction
| Hydrogel | Preparation Method | Application Fields | Performance/Efficiency | References |
|---|---|---|---|---|
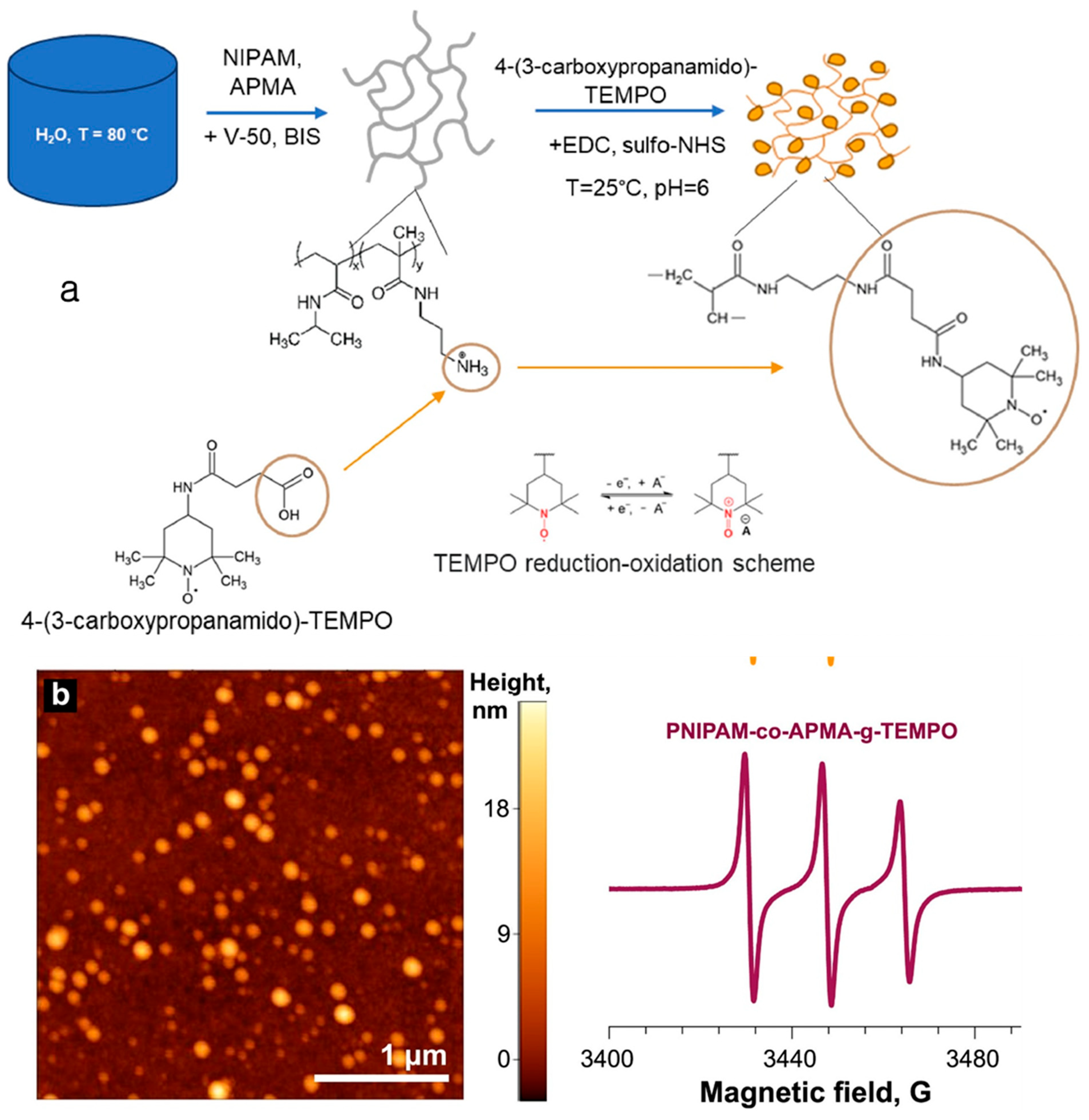
| TEMPO-grafted PNIPAM-co-APMA nanogels | A two-step synthetic method under mild aqueous conditions. | Redox flow battery electrolytes | Nanogels facilitate electron transfer between the electrode and nanogels in solution | [16] |
| Acetonitrile-based gel | A multi-step preparation process | Dye-sensitized solar cells | The largest η value of 4.17% | [25] |
| Imidazolium ionic liquid-based nanoparticle-integrated gel | Multi-step preparation method | Electrolyte of lithium-ion battery | Exhibits ionic conductivity of 3.2 × 10−3 S cm−1 at ambient temperature | [26] |
| 3D graphene/PANI composite hydrogel | Free radical photopolymerization (UV-curing) | Electrode of Zn-ion hybrid cell | Hydrogel electrode exhibits a large capacity of 154 mA h g−1 | [21] |
| Hemoglobin-poly (acrylic acid) nanogel | Multi-step preparation method | Bioelectrodes | Can retain >95% electroactivity after storing for 14 days | [20] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposite | Solution-cast technique | Sodium–sulfur batteries | Highest ion is 4.1 × 10−3 S cm−1 at room temperature | [27] |
| Copoly (phthalazinone biphenyl ether sulfone nanofiber–gel | A multi-step process involves electrospinning, infiltration, and in situ polymerization | Quasi-solid batteries, specifically lithium metal batteries | Ionic conductivity is 1.36 mS cm−1 | [28] |
| Poly (vinylidene fluoride)-hexafluoropropylene nanosheet gel polymer electrolyte (GPE) | A multi-step preparation method involving precise hydrothermal synthesis | As electrolytes in lithium batteries | Batteries assembled with the Nano-GPE of show 128 mAhg−1 discharge capacities | [29] |
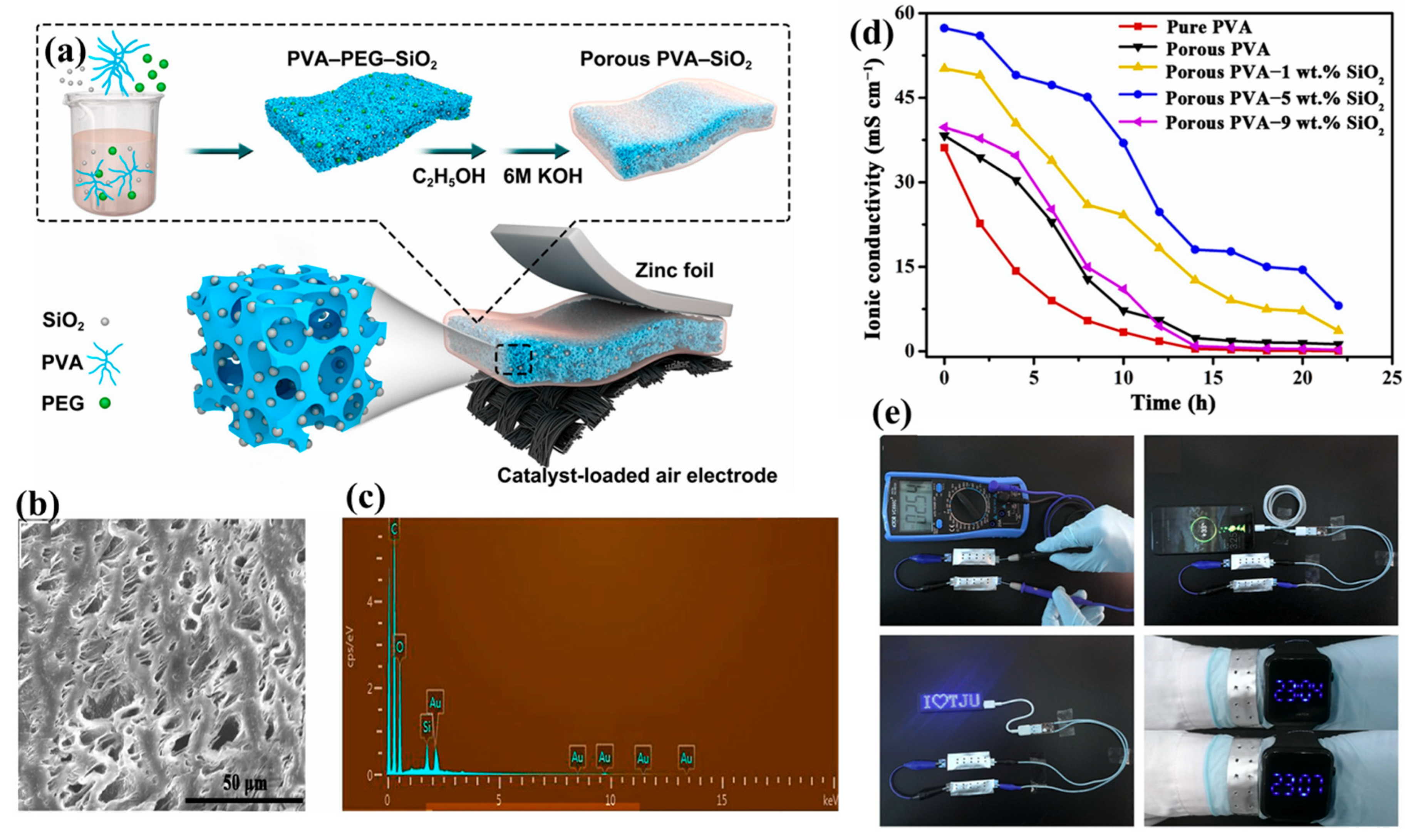
| Porous poly (vinyl alcohol) (PVA)-based nanocomposite gel | A phase-inversion method (a selective dissolution mechanism) | Electrolyte of zinc–air batteries | Shows ionic conductivity of 57.3 mS cm−1 | [30] |
| Poly (vinylidene fluoride-co-hexafluoropropylene) based gel electrolyte | Multi step preparation method (ionic liquid-mediated nano-composite polymer gel electrolyte) | As electrolyte in rechargeable battery | Specific discharge capacity is 138 mAh/g | [31] |
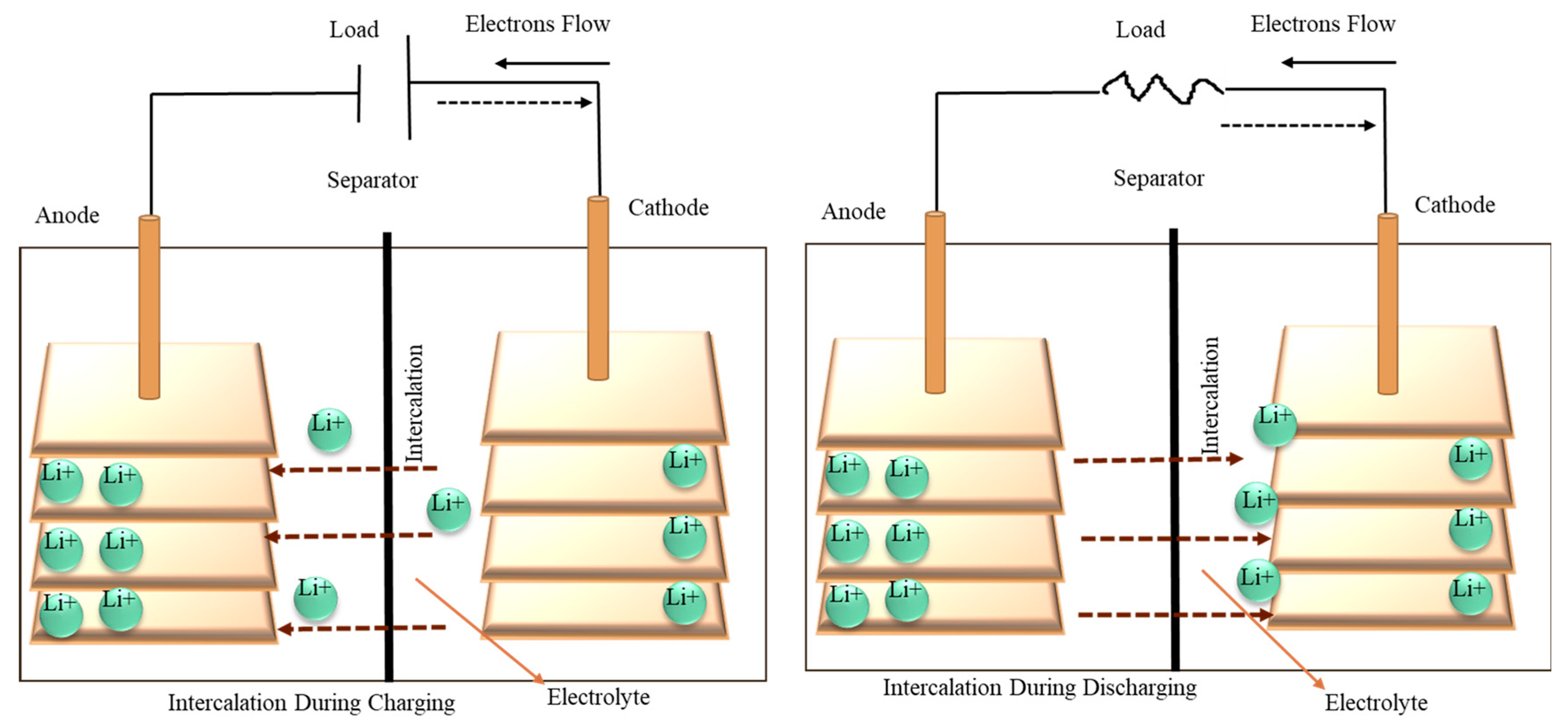
2. Battery Technology
3. Nanogel Based Components of Battery
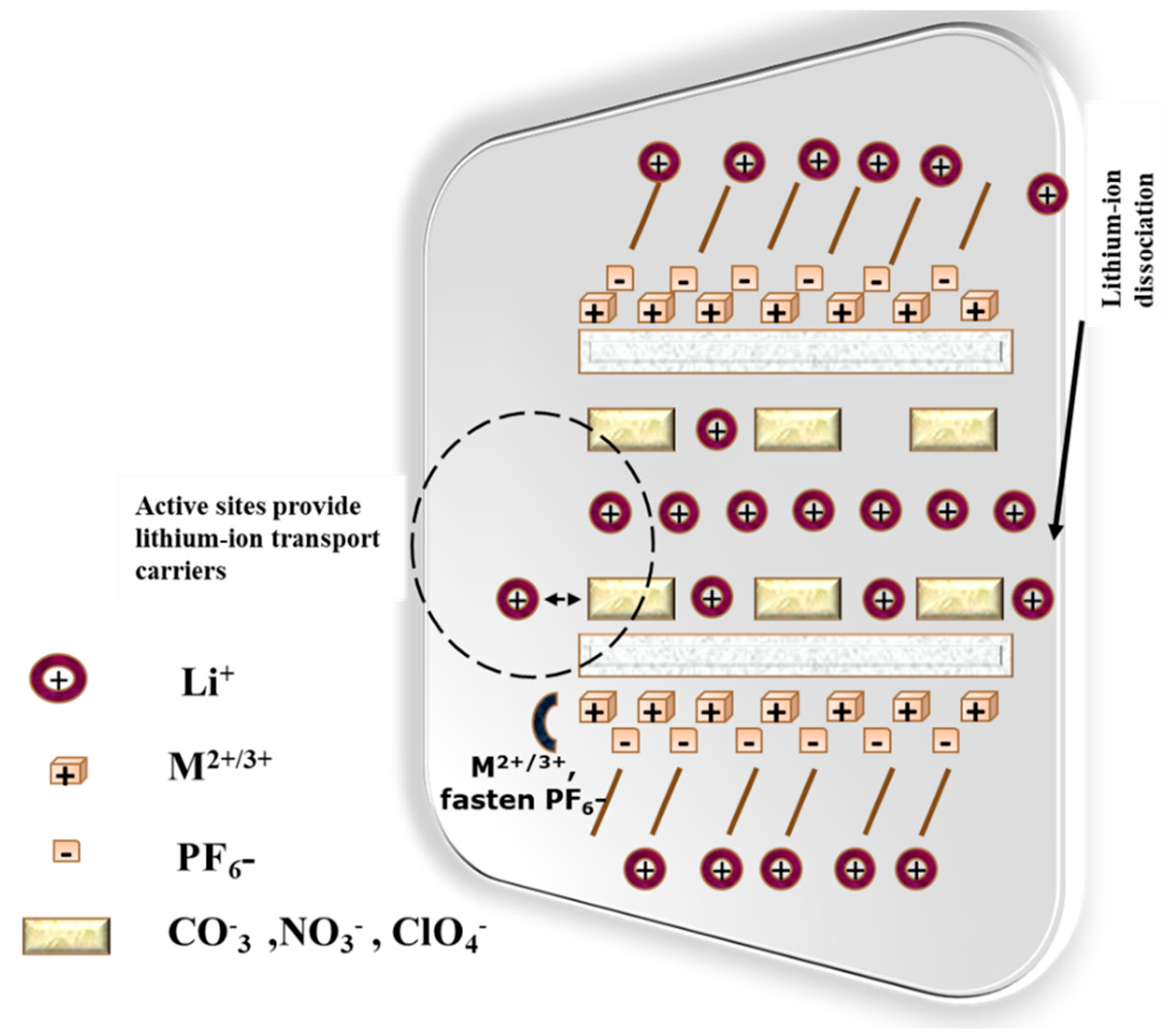
3.1. Nanogel-Based Electrolytes for Battery
3.2. Nanogel-Based Electrodes for Battery
3.3. Nanogel-Based Gelators in Battery
3.4. Nanogel-Based Membranes for Battery
4. Scope of Improvement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the Concept of Polymer Gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Haque, S.N.; Bhuyan, M.M.; Jeong, J.-H. Radiation-Induced Hydrogel for Water Treatment. Gels 2024, 10, 375. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Islam, M.; Jeong, J.-H. The Preparation and Characterization of N,N-Dimethyl Acrylamide-Diallyl Maleate Gel/Hydrogel in a Non-Aqueous Solution. Gels 2023, 9, 598. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Jophous, M.; Jeong, J.H. Synthesis and Characterization of Gamma Radiation-Induced (3-Acrylamidopropyl) Trimethylammonium Chloride-Acrylic Acid Functional Superabsorbent Hydrogel. Polym. Bull. 2022, 80, 8651–8664. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Perale, G.; Rossi, F. Nanogel Functionalization: A Versatile Approach to Meet the Challenges of Drug and Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541. [Google Scholar] [CrossRef]
- Manafi, P.; Nazockdast, H.; Karimi, M.; Sadighi, M.; Magagnin, L. Microstructural Development and Rheological Study of a Nanocomposite Gel Polymer Electrolyte Based on Functionalized Graphene for Dye-Sensitized Solar Cells. Polymers 2020, 12, 1443. [Google Scholar] [CrossRef]
- Hasan, N.; Bhuyan, M.M.; Jeong, J.-H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers 2024, 16, 2030. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Wang, S.H.; Wu, F.G. Nanogels: Synthesis, Properties, and Recent Biomedical Applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef]
- Suo, G.; Cheng, Y.; Mu, R.; Hou, X.; Yang, Y.; Ye, X.; Zhang, L. Bimetallic MnMoO4 Nanostructures on Carbon Fibers as Flexible Cathode for High Performance Zinc-Ion Batteries. J. Colloid Interface Sci. 2023, 641, 981–989. [Google Scholar] [CrossRef]
- Suo, G.; Musab Ahmed, S.; Cheng, Y.; Zhang, J.; Li, Z.; Hou, X.; Yang, Y.; Ye, X.; Feng, L.; Zhang, L.; et al. Heterostructured CoS2/CuCo2S4@N-Doped Carbon Hollow Sphere for Potassium-Ion Batteries. J. Colloid Interface Sci. 2022, 608, 275–283. [Google Scholar] [CrossRef]
- Vittal, R.; Kim, K.J.; Gomathi, H.; Yegnaraman, V. CTAB-Promoted Prussian Blue-Modified Electrode and Its Cation Transport Characteristics for K+, Na+, Li+ and NH 4+ Ions. J. Phys. Chem. B 2008, 112, 1149–1156. [Google Scholar] [CrossRef]
- Paudel, A.; Crum, A.N.; Wang, Y. A Full Metal-Free Flexible Ammonium-Ion Battery with Biodegradable Hydrogel Electrolyte. J. Mater. Chem. A 2024, 12, 11975–11985. [Google Scholar] [CrossRef]
- Kimura, K.; Marangon, V.; Fukuda, T.; Suzuki, M.; Soontornnon, N.; Tominaga, Y.; Hassoun, J. TEMPO-Oxidized Cellulose Nanofiber Hydrogel Electrolyte for Rechargeable Zn-Ion Batteries. Chem. Commun. 2024, 60, 13698–13701. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Inozemtseva, A.I.; Nazarov, M.A.; Nikolenko, A.D.; Zhvanskaya, E.S.; Kiselyova, O.I.; Motyakin, M.V.; Kutyakov, S.V.; Pakhomov, A.A.; Itkis, D.M.; et al. Nanoarchitectonics and Electrochemical Properties of Redox-Active Nanogels for Redox Flow Battery Electrolytes. Electrochim. Acta 2024, 475, 143534. [Google Scholar] [CrossRef]
- Ye, T.; Wang, J.; Jiao, Y.; Li, L.; He, E.; Wang, L.; Li, Y.; Yun, Y.; Li, D.; Lu, J.; et al. A Tissue-Like Soft All-Hydrogel Battery. Adv. Mater. 2022, 34, 2105120. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, G.A.; Korsah, M.; Jeyasundar, P.G.S.A.; Ahmed, M.; Lau, S.Y.; Danquah, M.K. Nanotechnology-Based Lithium-Ion Battery Energy Storage Systems. Sustainability 2024, 16, 9231. [Google Scholar] [CrossRef]
- Ghimire, A.; Zore, O.; Thilakarathne, V.; Briand, V.; Lenehan, P.; Lei, Y.; Kasi, R.; Kumar, C. “Stable-on-the-Table” Biosensors: Hemoglobin-Poly (Acrylic Acid) Nanogel BioElectrodes with High Thermal Stability and Enhanced Electroactivity. Sensors 2015, 15, 23868–23885. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.; Minella, C.B.; Meligrana, G.; Mulas, G.; Bodoardo, S.; Bongiovanni, R.; Penazzi, N. Development of Gel-Polymer Electrolytes and Nano-Structured Electrodes for Li-Ion Polymer Batteries. J. Appl. Electrochem. 2008, 38, 985–992. [Google Scholar] [CrossRef]
- Adroher-Benítez, I.; Martín-Molina, A.; Ahualli, S.; Quesada-Pérez, M.; Odriozola, G.; Moncho-Jordá, A. Competition between Excluded-Volume and Electrostatic Interactions for Nanogel Swelling: Effects of the Counterion Valence and Nanogel Charge. Phys. Chem. Chem. Phys. 2017, 19, 6838–6848. [Google Scholar] [CrossRef] [PubMed]
- Shokrieh, A.; Lu, R.; Zhang, B.; Sharma, B.P.; Wei, Z. A Buffering PVDF-HFP-Based Gel Polymer Electrolyte for Stable and Flexible Lithium Batteries. Nanoscale 2024, 16, 17954–17963. [Google Scholar] [CrossRef]
- Mesgarnejad, A.; Karma, A. Vulnerable Window of Yield Strength for Swelling-Driven Fracture of Phase-Transforming Battery Materials. npj Comput. Mater. 2020, 6, 58. [Google Scholar] [CrossRef]
- Chang, W.C.; Sie, S.Y.; Yu, W.C.; Lin, L.Y.; Yu, Y.J. Preparation of Nano-Composite Gel Electrolytes with Metal Oxide Additives for Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 212, 333–342. [Google Scholar] [CrossRef]
- Li, M.; Liao, Y.; Liu, Q.; Xu, J.; Sun, P.; Shi, H.; Li, W. Application of the Imidazolium Ionic Liquid Based Nano-Particle Decorated Gel Polymer Electrolyte for High Safety Lithium Ion Battery. Electrochim. Acta 2018, 284, 188–201. [Google Scholar] [CrossRef]
- Kumar, D.; Suleman, M.; Hashmi, S.A. Studies on Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Based Gel Electrolyte Nanocomposite for Sodium-Sulfur Batteries. Solid State Ion. 2011, 202, 45–53. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.; Wang, Z.; Yang, E.; Jiang, W.; Zhang, S.; Jian, X.; Hu, F. A Nano Fiber–Gel Composite Electrolyte with High Li+ Transference Number for Application in Quasi-Solid Batteries. eScience 2023, 3, 100090. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Zeng, L.; Huang, X.; Lv, W.; Wang, Q. The Nanofiber Gel Electrolytes with Ultra-High Ionic Conductivity Regulated by Different Acid Radical Ions for Lithium Batteries. Chem. Eng. J. 2024, 496, 154252. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous Nanocomposite Gel Polymer Electrolyte with High Ionic Conductivity and Superior Electrolyte Retention Capability for Long-Cycle-Life Flexible Zinc–Air Batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Kataria, S.; Verma, Y.L.; Gupta, H.; Singh, S.K.; Srivastava, N.; Dhar, R.; Singh, R.K. Ionic Liquid Mediated Nano-Composite Polymer Gel Electrolyte for Rechargeable Battery Application. Polym. Technol. Mater. 2020, 59, 952–958. [Google Scholar] [CrossRef]
- Heth, C.L. Energy on Demand: A Brief History of the Development of the Battery. Substantia 2019, 3, 73–82. [Google Scholar] [CrossRef]
- U.S. EIA. Monthly Energy Review. Mon. Energy Rev. 2025, 35, 1–278. Available online: https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf (accessed on 29 August 2025).
- Peljo, P.; Villevieille, C.; Girault, H.H. The Redox Aspects of Lithium-Ion Batteries. Energy Environ. Sci. 2024, 18, 1658–1672. [Google Scholar] [CrossRef]
- Oldham, K.; Myland, J. Fundamentals of Electrochemical Science; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323139639. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 1119334055. [Google Scholar]
- Barak, M. Electrochemical Power Sources: Primary and Secondary Batteries; IET: Stevenage, UK, 1980; ISBN 0906048265. [Google Scholar]
- Adams, S.; Appetechi, G.B.; Avdeev, M.V.; Balbuena, P.B.; Bardt, H.; Berendes, E.; Blatov, V.A.; Bobrikov, I.; Bulusheva, L.G.; Canepa, P. Electrochemical Storage Materials: From Crystallography to Manufacturing Technology; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; ISBN 3110493985. [Google Scholar]
- Hellström, S. The Discovery of Static Electricity and Its Manifestation. In ESD—Scourge Electron.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–20. [Google Scholar] [CrossRef]
- Albornoz, M.; Rivera, M.; Wheeler, P.; Ramírez, R. High Pulsed Voltage Alkaline Electrolysis for Water Splitting. Sensors 2023, 23, 3820. [Google Scholar] [CrossRef] [PubMed]
- Van Troostwijk, A.P.; Deiman, J.R. Sur Une Manière de Décomposer l’Eau En Air Inflammable et En Air Vital. Obs. Phys. 1789, 35, 369–378. [Google Scholar]
- Snelders, H.A.M. The Amsterdam experiment on the analysis and synthesis of water (1789). Ambix 1979, 26, 116–133. [Google Scholar] [CrossRef]
- Hoang, A.L.; Balakrishnan, S.; Hodges, A.; Tsekouras, G.; Al-Musawi, A.; Wagner, K.; Lee, C.Y.; Swiegers, G.F.; Wallace, G.G. High-Performing Catalysts for Energy-Efficient Commercial Alkaline Water Electrolysis. Sustain. Energy Fuels 2022, 7, 31–60. [Google Scholar] [CrossRef]
- Coutts, A. William Cruickshank of Woolwich. Ann. Sci. 1959, 15, 121–133. [Google Scholar] [CrossRef]
- Wilkinson, C., XXXVI. Description of an Improved Galvanic Trough. Philos. Mag. 1807, 29, 243–244. [Google Scholar] [CrossRef][Green Version]
- Phogat, P.; Dey, S.; Wan, M. Powering the Sustainable Future: A Review of Emerging Battery Technologies and Their Environmental Impact. RSC Sustain. 2025, 3, 3266–3306. [Google Scholar] [CrossRef]
- Schumacher, E.A.; Heise, G.W. The Alkaline Cell with Copper Oxide or Air Depolarization (1902–1952). J. Electrochem. Soc. 1952, 99, 191C–196C. [Google Scholar] [CrossRef]
- Rasmussen, S.C. History of Energy Technologies and Lessons for the Future. An Int. J. Hist. 2019, 3. [Google Scholar]
- Planté, G. The Storage of Electrical Energy: And Researches in the Effects Created by Currents Combining Quantity with High Tension; Kessinger Pub: Whittaker, MI, USA, 1887; ISBN 0598385576. [Google Scholar]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Jungner, E.W. Anordning Vid Elektroder for Elektriska Accumulatorer. Swedish Patent 8558, 27 May 1897. [Google Scholar]
- Jungner, E.W. Sätt Att På Elektrolytisk Väg Förstora Ytan Af Sådana Metaller, Hvilkas Syreföreningar Äro Kemiskt Olösliga i Alkaliska Lösningar. Swedish Patent 15567, 4 October 1901. [Google Scholar]
- Milewska, A.; Świerczek, K.; Tobola, J.; Boudoire, F.; Hu, Y.; Bora, D.K.; Mun, B.S.; Braun, A.; Molenda, J. The Nature of the Nonmetal-Metal Transition in LixCoO 2 Oxide. Solid State Ion. 2014, 263, 110–118. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2. Solid State Ion. 1981, 3–4, 171–174. [Google Scholar] [CrossRef]
- Whittingham, M.S. The Role of Ternary Phases in Cathode Reactions. J. Electrochem. Soc. 1976, 123, 315–320. [Google Scholar] [CrossRef]
- Massé, R.C.; Liu, C.; Li, Y.; Mai, L.; Cao, G. Energy Storage through Intercalation Reactions: Electrodes for Rechargeable Batteries. Natl. Sci. Rev. 2017, 4, 26–53. [Google Scholar] [CrossRef]
- Armand, M.B. Intercalation Electrodes. In Materials for Advanced Batteries; Springer: Berlin/Heidelberg, Germany, 1980; pp. 145–161. [Google Scholar]
- Park, J.; Xu, Z.-L.; Kang, K. Solvated Ion Intercalation in Graphite: Sodium and Beyond. Front. Chem. 2020, 8, 432. [Google Scholar] [CrossRef]
- BOBBY/UPS Battery Center Ltd. How Does Intercalation Work in Batteries? BOBBY/UPS Battery Center Ltd.: Tonawanda, NY, USA, 2014. [Google Scholar]
- Manfo, T.A.; Şahin, M.E. Intercalation Reaction in Lithium-Ion Battery: Effect on Cell Characteristics. Int. J. Mater. Eng. Technol. 2023, 6, 70–78. [Google Scholar]
- Garche, J. Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 0323958222. [Google Scholar]
- Fransson, L.M.L.; Vaughey, J.T.; Edström, K.; Thackeray, M.M. Structural Transformations in Intermetallic Electrodes for Lithium Batteries. J. Electrochem. Soc. 2003, 150, A86. [Google Scholar] [CrossRef]
- Timmons, A.; Dahn, J.R. In Situ Optical Observations of Particle Motion in Alloy Negative Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2006, 153, A1206. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, M.; Ren, J.; Wei, W.; Nai, J. Polymer Additives in Liquid Electrolyte for Advanced Lithium Batteries. Nanoscale 2025, 17, 11275–11292. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Dodda, J.M.; Deshmukh, K.; Bezuidenhout, D.; Yeh, Y.-C. Hydrogels: Definition, History, Classifications, Formation, Constitutive Characteristics, and Applications. In Multicomponent Hydrogels; The Royal Society of Chemistry: London, UK, 2023; pp. 1–25. ISBN 9781839167270. [Google Scholar]
- Choi, S.H.; Kim, I.D.; Hong, J.M.; Park, K.H.; Oh, S.G. Effect of the Dispersibility of BaTiO3 Nanoparticles in BaTiO3/Polyimide Composites on the Dielectric Properties. Mater. Lett. 2007, 61, 2478–2481. [Google Scholar] [CrossRef]
- Mahmood, A.; Ijaz, H.; Muhammad Sarfraz, R.; Zafar, N.; Zaman, M.; Azam, M. Polymeric Hydrogels and Nanogels: Classification, Development and Pharmaceutical Applications. In Hydrogels and Nanogels-Applications in Medicine; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Hussain AL-Mayahy, M.; Imad Hameed, H. Hydrogels and Nanogels as a Promising Carrier for Drug Delivery. In Hydrogels and Nanogels-Applications in Medicine; IntechOpen: London, UK, 2023; pp. 1–17. [Google Scholar] [CrossRef]
- Priya, A.S.; Kannan, K.; Henry, J.; Aepuru, R.; Shanmugaraj, K.; Pabba, D.P.; Sathish, M. Advancements in Hydrogel Materials for Next-Generation Energy Devices: Properties, Applications, and Future Prospects. Cellulose 2025, 32, 6307–6335. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Ünlü, B.; Türk, S.; Özacar, M. Gellan Gum/PEDOT:PSS Gel Electrolyte and Application on Quasi-Solid Dye Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2024, 450, 115471. [Google Scholar] [CrossRef]
- Jacob, M.M.E.; Hackett, E.; Giannelis, E.P. From Nanocomposite to Nanogel Polymer Electrolytes. J. Mater. Chem. 2003, 13, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhang, L.; Zong, C.; Xu, A.; Zhang, Y.; Geng, B.; Zhang, S. Enhanced Breakdown Strength and Suppressed Dielectric Loss of Polymer Nanocomposites with BaTiO3 Fillers Modified by Fluoropolymer. RSC Adv. 2020, 10, 7065–7072. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Feng, W.; Zhen, Y.; Zhao, P.; Li, L.; Cai, Z. The Effects of the Size and Content of BaTiO 3 Nanoparticles on Solid Polymer Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Solid State Electrochem. 2019, 23, 749–758. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Zhong, C.; Deng, Y.; Zhao, N.; Hu, W. NiCo 2 S 4 Nanocrystals Anchored on Nitrogen-Doped Carbon Nanotubes as a Highly Efficient Bifunctional Electrocatalyst for Rechargeable Zinc-Air Batteries. Nano Energy 2017, 31, 541–550. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Gao, D.; Miao, S.; Wang, G.; Bao, X. High-Density Iron Nanoparticles Encapsulated within Nitrogen-Doped Carbon Nanoshell as Efficient Oxygen Electrocatalyst for Zinc–Air Battery. Nano Energy 2015, 13, 387–396. [Google Scholar] [CrossRef]
- Liu, B.; Qu, S.; Kou, Y.; Liu, Z.; Chen, X.; Wu, Y.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. In Situ Electrodeposition of Cobalt Sulfide Nanosheet Arrays on Carbon Cloth as a Highly Efficient Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. ACS Appl. Mater. Interfaces 2018, 10, 30433–30440. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent Advances in Zinc–Air Batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Mastella, P.; Todaro, B.; Luin, S. Nanogels: Recent Advances in Synthesis and Biomedical Applications. Nanomaterials 2024, 14, 1300. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A Review of Electrode Materials for Electrochemical Supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Abbey, C.; Joos, G. Supercapacitor Energy Storage for Wind Energy Applications. IEEE Trans. Ind. Appl. 2007, 43, 769–776. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Jin, R.; Ma, Y.; Sun, Y.; Li, H.; Wang, Q.; Chen, G. Manganese Cobalt Oxide (MnCo2O4) Hollow Spheres as High Capacity Anode Materials for Lithium-Ion Batteries. Energy Technol. 2017, 5, 293–299. [Google Scholar] [CrossRef]
- Jin, R.; Jiang, H.; Sun, Y.; Ma, Y.; Li, H.; Chen, G. Fabrication of NiFe2O4/C Hollow Spheres Constructed by Mesoporous Nanospheres for High-Performance Lithium-Ion Batteries. Chem. Eng. J. 2016, 303, 501–510. [Google Scholar] [CrossRef]
- Madej, E.; Espig, M.; Baumann, R.R.; Schuhmann, W.; La Mantia, F. Optimization of Primary Printed Batteries Based on Zn/MnO2. J. Power Sources 2014, 261, 356–362. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Zhu, Q.; Tan, G.; Li, L. Self-Regulative Nanogelator Solid Electrolyte: A New Option to Improve the Safety of Lithium Battery. Adv. Sci. 2016, 3, 1500306. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.T.; Lakshmanan, B.; Mathias, M.F. Electrochemistry and the Future of the Automobile. J. Phys. Chem. Lett. 2010, 1, 2204–2219. [Google Scholar] [CrossRef]
- Wu, F.; Tan, G.; Chen, R.; Li, L.; Xiang, J.; Zheng, Y. Novel Solid-State Li/LiFePO 4 Battery Configuration with a Ternary Nanocomposite Electrolyte for Practical Applications. Adv. Mater. 2011, 23, 5081–5085. [Google Scholar] [CrossRef]
- Song, Y.; Sheng, L.; Wang, L.; Xu, H.; He, X. From Separator to Membrane: Separators Can Function More in Lithium Ion Batteries. Electrochem. Commun. 2021, 124, 106948. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y. Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators. Polymers 2023, 15, 3690. [Google Scholar] [CrossRef]
- Chae, W.; Kim, B.; Ryoo, W.S.; Earmme, T. A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-Ion Polymer Batteries. Polymers 2023, 15, 803. [Google Scholar] [CrossRef]
- Park, S.; Choi, S.; Lim, J.; Seo, J.P.; Lee, H.; Kim, S.; Roh, Y.; Rhee, J.; Lee, Y.M. Highly Thermally Conductive Ceramic-Coated Separators with Aluminum Nitride for Mitigating Thermal Runaway in Lithium-Ion Batteries. Chem. Eng. J. 2025, 513, 162732. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Huang, Z.; Wang, Y.; Li, Y. Advancements in Glass Fiber Separator Technology for Lithium-Sulfur Batteries: The Role of Transport, Material Properties, and Modifications. ACS Omega 2025, 10, 3228–3261. [Google Scholar] [CrossRef]
- Joia, R.; Modaqeq, T.; Mohammadi, M.H. Principles and Requirements of Battery Membranes: Ensuring Efficiency and Safety in Energy Storage. Eur. J. Theor. Appl. Sci. 2024, 2, 493–505. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D. Composite Membrane Containing Titania Nanofibers for Battery Separators Used in Lithium-Ion Batteries. Membranes 2023, 13, 499. [Google Scholar] [CrossRef]
- Lu, L.; Gu, J.; Wei, X.; Wei, Z.; Zhao, Y. Nanofibrous Composite Separator for Lithium-Ion Batteries with High Safety. ACS Appl. Nano Mater. 2025, 8, 3915–3926. [Google Scholar] [CrossRef]
- Schadeck, U.; Kyrgyzbaev, K.; Zettl, H.; Gerdes, T.; Moos, R. Flexible, Heat-Resistant, and Flame-Retardant Glass Fiber Nonwoven/Glass Platelet Composite Separator for Lithium-Ion Batteries. Energies 2018, 11, 999. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Shang, Y.; Wang, L.; He, X.; Li, J.; Zhao, P.; Wang, Y. Nanocomposite Polymer Membrane Derived from Nano TiO 2 -PMMA and Glass Fiber Nonwoven: High Thermal Endurance and Cycle Stability in Lithium Ion Battery Applications. J. Mater. Chem. A 2015, 3, 17697–17703. [Google Scholar] [CrossRef]
- Slesarenko, N.A.; Chernyak, A.V.; Khatmullina, K.G.; Baymuratova, G.R.; Yudina, A.V.; Tulibaeva, G.Z.; Shestakov, A.F.; Volkov, V.I.; Yarmolenko, O. V Nanocomposite Polymer Gel Electrolyte Based on TiO2 Nanoparticles for Lithium Batteries. Membranes 2023, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Heasman, P.; Rostami, J.; Benselfelt, T.; Linares, M.; Li, H.; Iakunkov, A.; Sellman, F.; Östmans, R.; Hamedi, M.M.; et al. Dynamic Networks of Cellulose Nanofibrils Enable Highly Conductive and Strong Polymer Gel Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2212806. [Google Scholar] [CrossRef]
- Peng, J.; Lu, D.; Wu, S.; Yang, N.; Cui, Y.; Ma, Z.; Liu, M.; Shi, Y.; Sun, Y.; Niu, J.; et al. Lithium Superionic Conductive Nanofiber-Reinforcing High-Performance Polymer Electrolytes for Solid-State Batteries. J. Am. Chem. Soc. 2024, 146, 11897–11905. [Google Scholar] [CrossRef]
- Kim, S.; Jung, H.K.; Handayani, P.L.; Kim, T.; Jung, B.M.; Choi, U.H. Fast Li + Transport via Silica Network-Driven Nanochannels in Ionomer-in-Framework for Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2210916. [Google Scholar] [CrossRef]
- Rane, N.; Choudhary, S.; Rane, J. Enhancing Lithium-Ion Battery Performance with Emerging Electrolyte Materials for Sustainable Energy Storage Solutions: A Comprehensive Review and Prospects. SSRN Electron. J. 2023, 4643648. [Google Scholar] [CrossRef]
- Matsuda, Y.; Nakazawa, S.; Tanaka, M.; Kawakami, H. Polymer Composite Electrolytes Membrane Consisted of Polyacrylonitrile Nanofibers Containing Lithium Salts: Improved Ion Conductive Characteristics and All-Solid-State Battery Performance. Macromol. Chem. Phys. 2025, 226, 2400196. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Yu, L.; Qi, L.; Oh, K.-S.; Hu, P.; Lee, S.-Y.; Chen, C. Gel Polymer Electrolytes for Rechargeable Batteries toward Wide-Temperature Applications. Chem. Soc. Rev. 2024, 53, 5291–5337. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, Flexible, Solid Polymer Composite Electrolyte Enabled with Aligned Nanoporous Host for Lithium Batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H. Deposition of Functional Organic and Inorganic Layer on the Cathode for the Improved Electrochemical Performance of Li-s Battery. Korean Chem. Eng. Res. 2017, 55, 483–489. [Google Scholar] [CrossRef]
- Hwang, K.; Sohn, H.; Yoon, S. Mesostructured Niobium-Doped Titanium Oxide-Carbon (Nb-TiO2-C) Composite as an Anode for High-Performance Lithium-Ion Batteries. J. Power Sources 2018, 378, 225–234. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, N.-G.; Ryu, K.S.; Chang, S.H. Characteristics of PVdF-HFP/TiO2 Composite Membrane Electrolytes Prepared by Phase Inversion and Conventional Casting Methods. Electrochim. Acta 2006, 51, 5636–5644. [Google Scholar] [CrossRef]
- Jeong, Y.; Seok, D.; Lee, S.; Shin, W.H.; Sohn, H. Polymer/Inorganic Nanohybrid Membrane on Lithium Metal Electrode: Effective Control of Surficial Growth of Lithium Layer and Its Improved Electrochemical Performance. Membr. J. 2020, 30, 30–37. [Google Scholar] [CrossRef]
- Gwon, H.; Park, K.; Chung, S.C.; Kim, R.H.; Kang, J.K.; Ji, S.M.; Kim, N.J.; Lee, S.; Ku, J.H.; Do, E.C.; et al. A Safe and Sustainable Bacterial Cellulose Nanofiber Separator for Lithium Rechargeable Batteries. Proc. Natl. Acad. Sci. USA 2019, 116, 19288–19293. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, Z.; Ho, H.M.K.; Gan, Y.X.; Panariello, L.; Gkogkos, G.; Gavriilidis, A.; Craig, D.Q.M. Microfluidic Synthesis of Protein-Loaded Nanogels in a Coaxial Flow Reactor Using a Design of Experiments Approach. Nanoscale Adv. 2021, 3, 2039–2055. [Google Scholar] [CrossRef]
- Pandey, T.; Sharma, A.; Sandhu, A.; Pandey, V. Controlled Polymerization in Microfluidics: Advancement in Nanogel Synthesis. SPE Polym. 2024, 5, 113–126. [Google Scholar] [CrossRef]


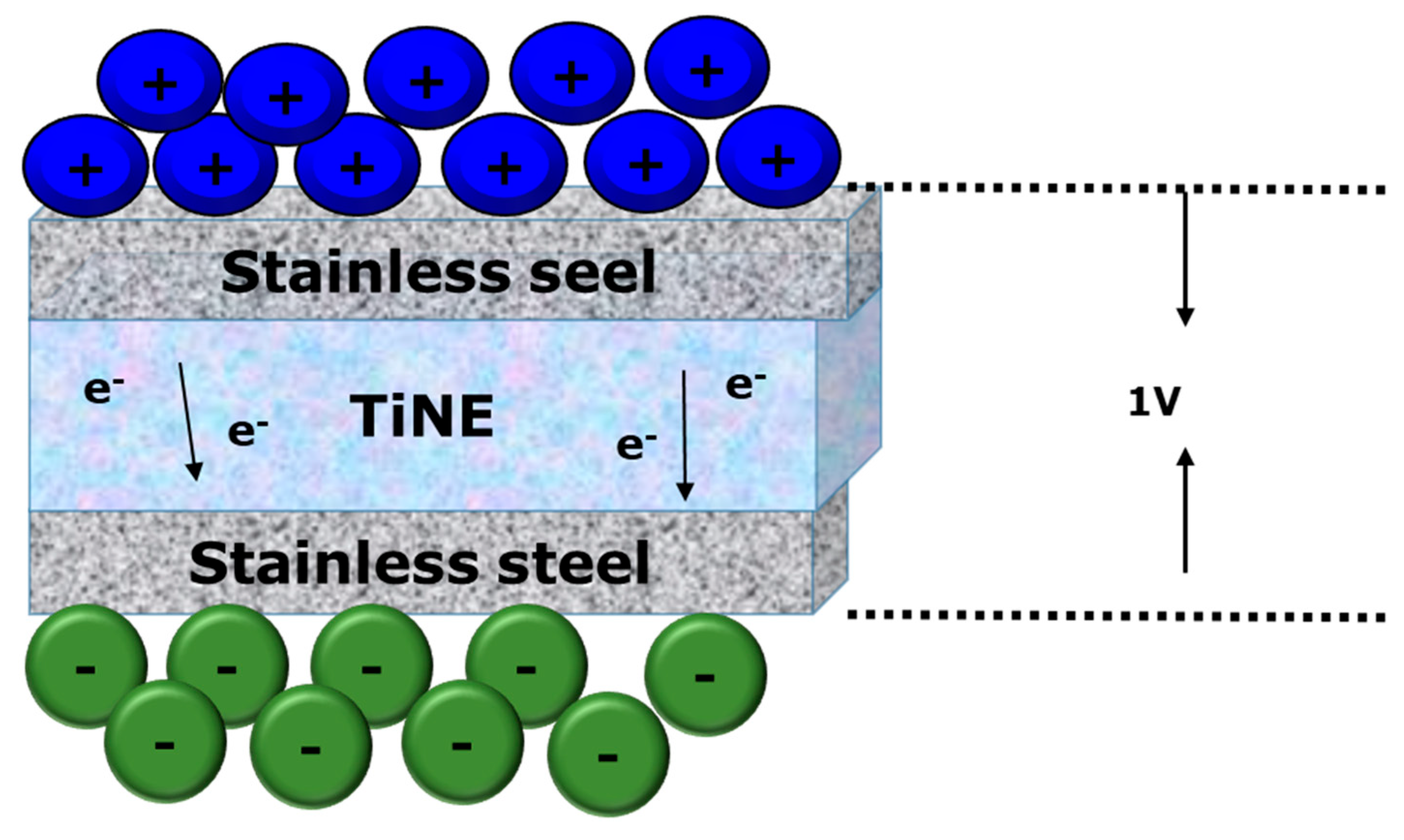
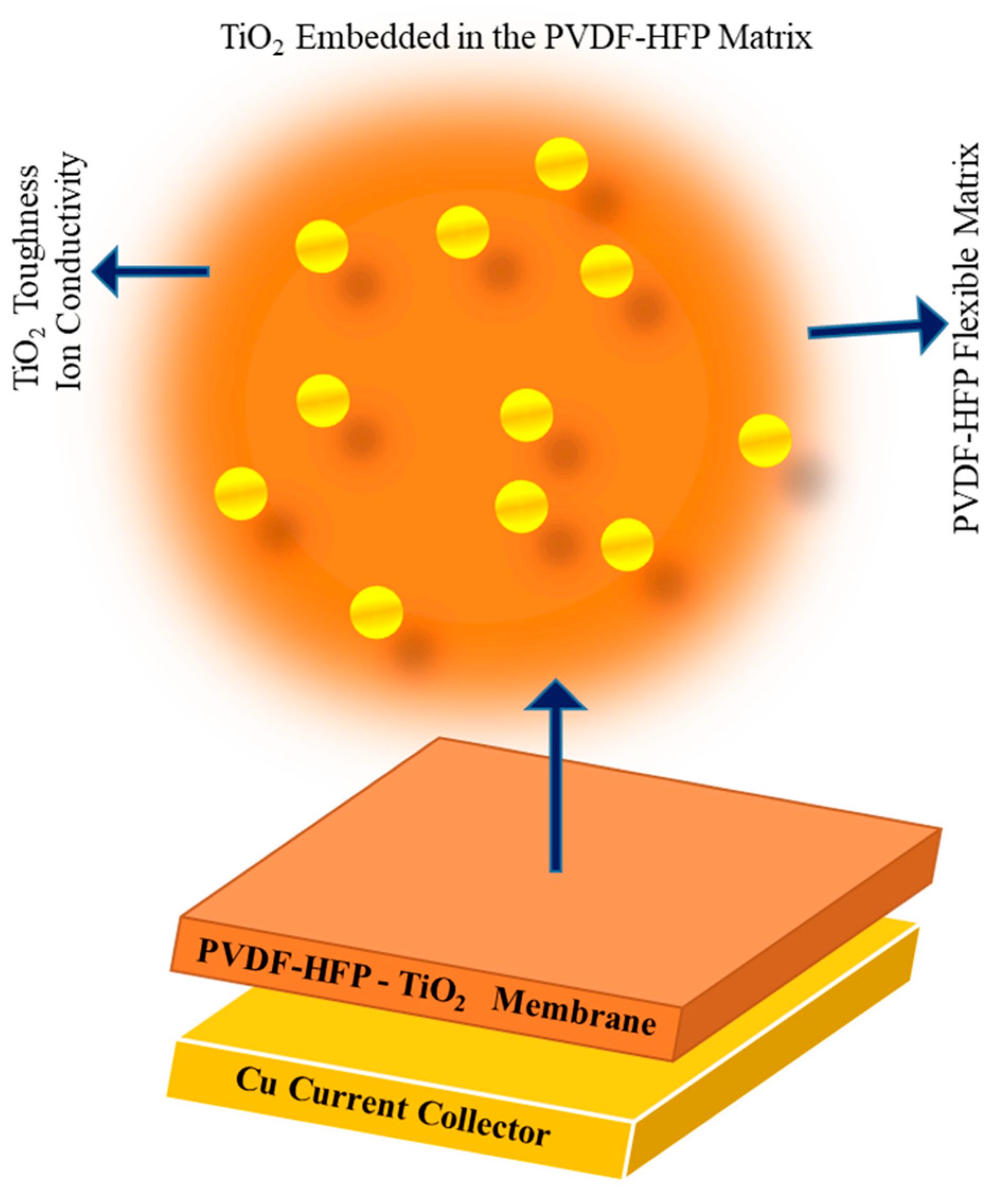
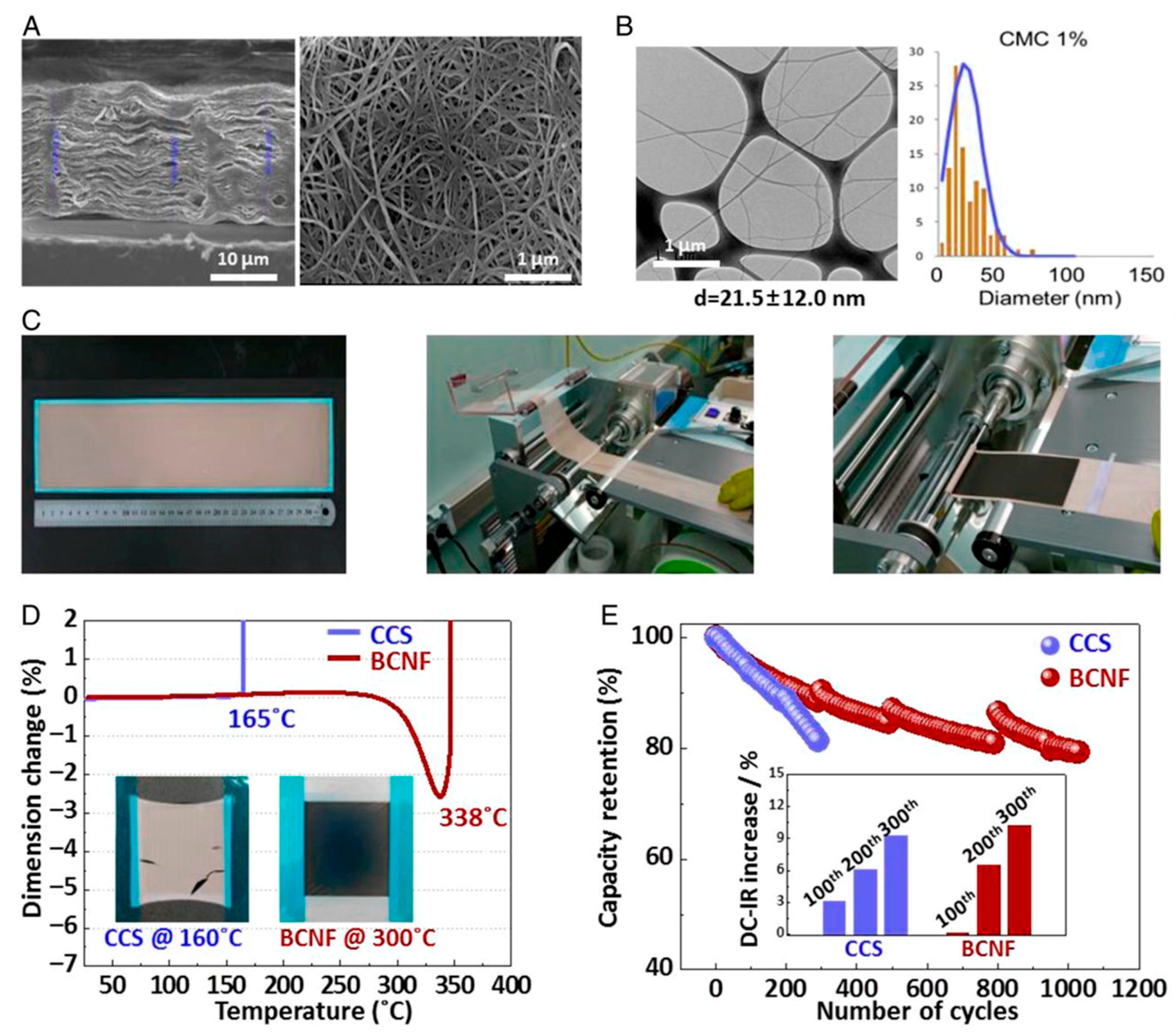
| Feature | Conventional Hydrogels | Nanogels | References |
|---|---|---|---|
| Structural difference | Bulk gel networks of natural or synthetic linear polymer chains that are macroscopic (mm–cm size). Network is crosslinked either physically (H-bonding, ionic, hydrophobic) or chemically (covalent). Greater capacity to retain water due to its larger mesh size. | Particles at the nanoscale, usually between 20 and 200 nm, are scattered across medium. Network of crosslinked polymers restricted to nanoscale regions. Chemical crosslinking is frequently used, however for fine control, self-assembly or microfluidic synthesis are alternative options. High surface-to-volume ratio; smaller mesh size. | [66,67] |
| Physical and Chemical Properties | Tissue-like, soft qualities. ballooning in bulk; a delayed reaction to stimuli. | Nanoscale particles contain water. The tiny size and large surface area causes rapid swelling and deswelling. very adjustable in terms of temperature, redox, pH, and enzyme triggers. | [68] |
| Functional Difference | Drugs scattered over the bulk gel; mostly released via diffusion. Serve as a reservoir or scaffold. Tissue scaffolds, contact lenses, and wound treatments. | The release of drugs enclosed in nanogel particles may be targeted and stimulated. Systemic circulation, tailored delivery (such as tumor targeting), and injectable. Serves in intracellular imaging, gene therapy, targeted drug delivery, and nanomedicine. | [65], |
| Advantages and Limitations | High water retention, biocompatibility, ease of synthesis, and suitability for large-scale uses. Poor mechanical strength, slow reaction time, and restricted ability to aim. | High surface area, injectable, focused delivery, and adjustable response. More intricate production, problems with stability in vivo, and possible immune system clearance. | [65,69] |
| Application in Battery | For flexible or stretchy batteries, their bulk structure offers strong mechanical support. Ionic conductivity is facilitated by high-water content, particularly in aqueous battery systems. | They improve charge/discharge rates by facilitating quicker ion diffusion due to their large surface area and porosity. Their tiny size and dispersibility make them easy to include into composite electrodes or electrolytes. | [70] |
| Separator Name | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Polymer-Based Separator | It is widely used in lithium ion batteries due to their chemical stability, light weight, flexibility, and cost-effectiveness. | Compared to the other types, it has limited thermal stability and mechanical strength. | [92,93] |
| Ceramic Coated Separator | Mechanical strength and thermal efficiency are higher, which increases safety and lowers the risk of thermal runaway. | Potential brittleness and production cost are higher. | [94] |
| Glass Fiber Separator | It has improved ionic conductivity because of good chemical stability and high porosity. | For higher stress applications, it is unsuitable because of its fragility and low durability. | [95] |
| Nano-woven Fabric Separator | It is often used in lead–acid batteries due to its better mechanical strength and flexibility. | Compared to a polymer-based separator, it has low ionic conductivity. | [96,97] |
| Composite Separator | It combines the advantages of several materials to provide improved durability and performance. | The manufacturing process is complex and has a higher cost. | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuyan, M.M.; Lee, K. Nano Gel/Hydrogel-Based Components for Battery Technology: An Overview. Gels 2025, 11, 762. https://doi.org/10.3390/gels11090762
Bhuyan MM, Lee K. Nano Gel/Hydrogel-Based Components for Battery Technology: An Overview. Gels. 2025; 11(9):762. https://doi.org/10.3390/gels11090762
Chicago/Turabian StyleBhuyan, Md Murshed, and Kyungjun Lee. 2025. "Nano Gel/Hydrogel-Based Components for Battery Technology: An Overview" Gels 11, no. 9: 762. https://doi.org/10.3390/gels11090762
APA StyleBhuyan, M. M., & Lee, K. (2025). Nano Gel/Hydrogel-Based Components for Battery Technology: An Overview. Gels, 11(9), 762. https://doi.org/10.3390/gels11090762








