In Situ Forming Poloxamer-Based Thermo-Sensitive Hydrogels for Ocular Application: A Focus on the Derivatives 407 and 188
Abstract
1. Introduction
2. Approaches to Ophthalmic Treatment
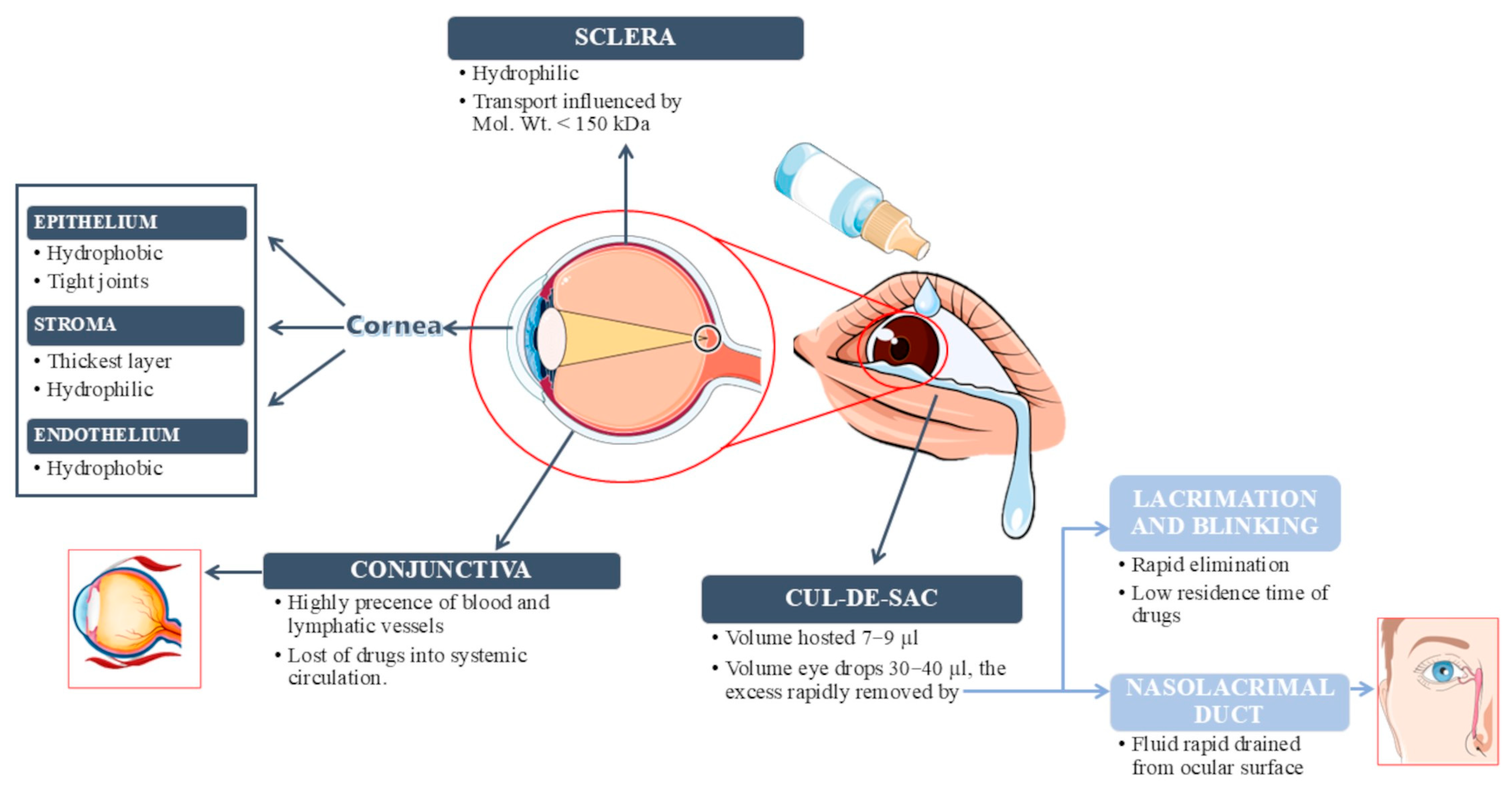
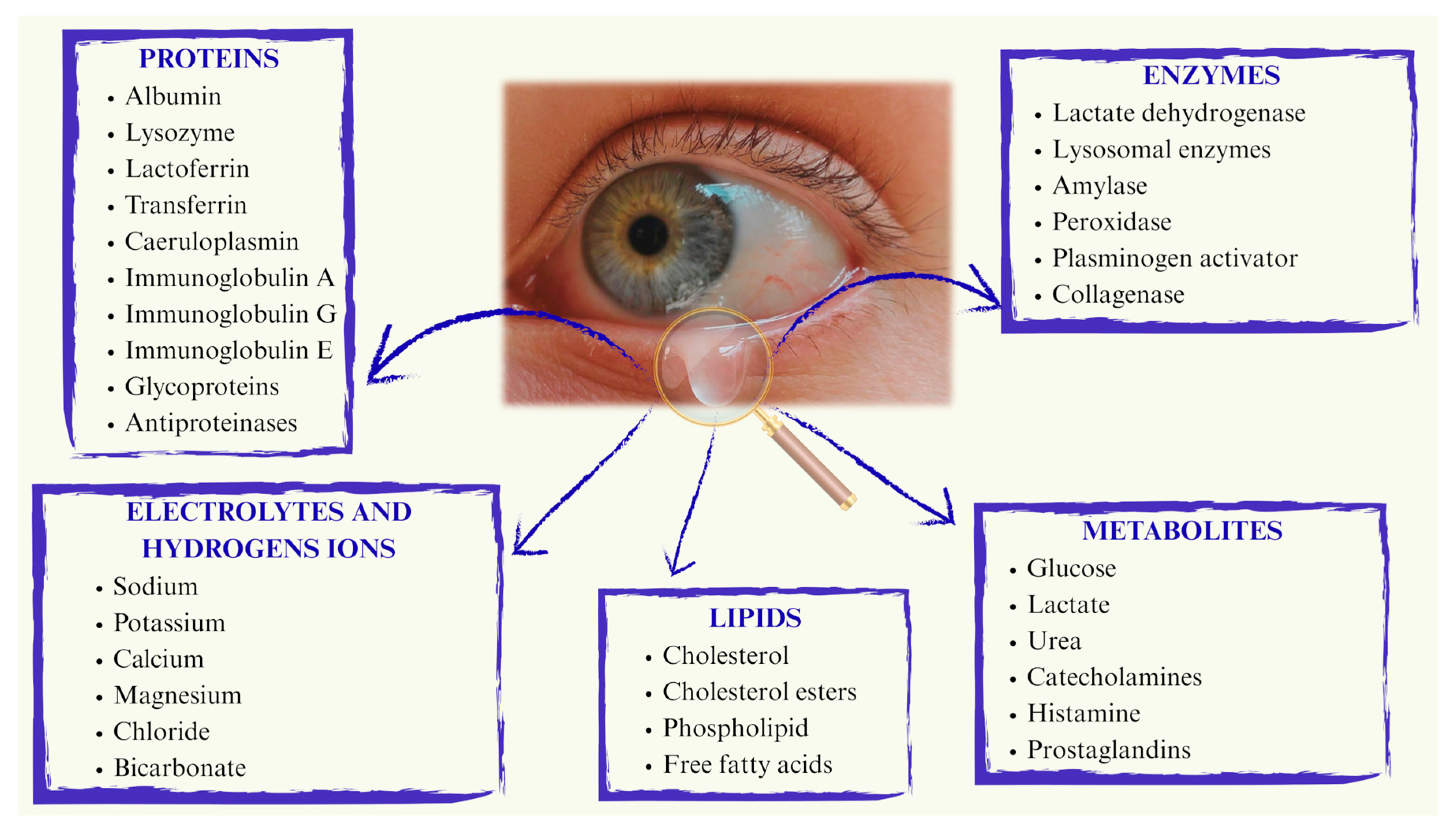
2.1. Physiological Limitations Affecting Therapy
2.2. Ocular Drug Delivery Systems
- Patients can self-administer the treatment, leading to improved compliance;
- Enhanced stability of the drug encapsulated within the formulation;
- Reduced drug elimination due to increased residence time on the ocular or intraocular surface or in the conjunctival sac, as well as improved absorption through the corneal cells;
- The ability to modulate drug release, reducing the frequency of instillations and minimizing drug loss from the precorneal area;
- The potential for polymer manipulation to achieve specific tissue targeting [36].
3. Hydrogels
3.1. General Properties of Hydrogels
- Post-loading: this involves the entrapment of the drug molecule within the established three-dimensional network of the hydrogel matrix;
- In situ loading: in this approach, the drug forms interactions with the solution or the polymer before the hydrogel structure is created [46].
3.2. In Situ Hydrogels
3.3. Thermo-Sensitive In Situ Hydrogels
4. Poloxamers
4.1. General Properties of Poloxamers
4.2. Poloxamer 407 Hydrogels for the Treatment of Ocular Diseases
4.3. Poloxamer 188 Hydrogels for the Treatment of Ocular Diseases
- at concentrations lower than the CMC and at temperatures below the CMT, the polymer exists in solution as single unimers above CMC and CMT individual molecules aggregate to form micellar structures;
- as the temperature increases further, the micellar structures alter their microstructure, forming a denser network due to a reduction in the hydrophilicity of the PPO residues compared to the PEO chains. Additionally, P188 solutions become soft solids at high temperatures. Therefore, a reversible liquid-to-solid transition occurs, representing the shift from a viscoelastic liquid to a soft solid [117].
5. Characterization and Analysis of Thermosensitive In Situ Hydrogels for Ocular Applications
| Parameter to Be Evaluated | Objective and Method of Analysis | Ideal Key Outcome | References |
|---|---|---|---|
| Appearance and Clarity | Visual inspection for clarity, color uniformity and absence of particles to prevent vision blurring. | Clear, uniform formulation with no particles. | [134] |
| Sterility | Evaluation of the absence of microbial contamination. Sterilization methods include autoclaving and filtration. The European Pharmacopoeia test involves incubating the sample for 14 days in specific media. | The formulation remains sterile with no physical or chemical alterations. | [138,139,142] |
| Osmolarity | Measurement of osmolarity using a cryoscopic osmometer. The goal is to prevent irritation. | Osmolarity similar to that of natural tears (approximately 310 mOsm/kg), with a recommended upper limit of 340 mOsm/kg. | [143,144] |
| pH | pH measurement to prevent irritation and side effects. | Ideal pH range between 6.5 and 7.6, with an optimal pH of 7.2. | [145] |
| Sol–Gel Transition | Evaluation of the temperature at which the transition from liquid to gel occurs, often using the tube inversion method. | Transition at ocular surface temperature after instillation. | [72,148] |
| Gelling Capacity | Evaluation of the time required for gel formation and dissolution. | Rapid gel formation and prolonged dissolution over several hours, for extended contact time. | [151] |
| Viscosity and Rheology | Measurement of viscosity and rheological properties (G′ and G″ moduli) with a rheometer to determine drug residence time. | The formulation should be a free-flowing liquid (G″ > G′) at room temperature for easy application and transform into a gel (G′ > G″) at physiological temperature to withstand blinking forces. | [152] |
| Physical Stability and Drug Release | Evaluation of the release profile of the active compound(s) and physical stability over time. | Prolonged drug release and long-term stability of the formulation. | [155,156] |
| Ocular Irritation | Evaluation of irritation potential. The Draize test on albino rabbits is the traditional method. | Low or no irritation potential. | [30] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research Progress of In-Situ Gelling Ophthalmic Drug Delivery System. Asian J. Pharm. Sci. 2019, 14, 102342. [Google Scholar] [CrossRef]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to Prolong the Residence Time of Drug Delivery Systems on Ocular Surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Smith, J.; Brown, L.; Zhang, Y. Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure–Permeability Relationships. Gels 2025, 11, 123–138. [Google Scholar]
- Awwad, S.; Mohamed Ahmed, A.H.A.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of Pharmacology in the Eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Assiri, A.A.; Glover, K.; Mishra, D.; Waite, D.; Vora, L.K.; Thakur, R.R.S. Block Copolymer Micelles as Ocular Drug Delivery Systems. Drug Discov. Today 2024, 29, 104098. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Kang, Y.; Wang, M. Glutathione-Responsive Polymeric Micelles Formed by a Biodegradable Amphiphilic Triblock Copolymer for Anticancer Drug Delivery and Controlled Release. ACS Biomater. Sci. Eng. 2015, 1, 585–592. [Google Scholar] [CrossRef]
- Rahmanian, M.; Oroojalian, F.; Kesharwani, P.; Sahebkar, A. Current Trends in Triblock Copolymer-Based Multifunctional Nanotheranostics for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2024, 99, 105985. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and Lipid-Based Nanocarriers for Ocular Drug Delivery: Current Status and Future Perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef]
- Sha, X.; Chan, L.; Fan, X.; Guo, P.; Chen, T.; Liu, L.; Zhong, J. Thermosensitive Tri-Block Polymer Nanoparticle-Hydrogel Composites as Payloads of Natamycin for Antifungal Therapy Against Fusarium Solani. Int. J. Nanomed. 2022, 17, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Chirio, D.; Peira, E.; Brunella, V.; Jadhav, S.A.; Abellán Rubio, E.; Chindamo, G.; Gallarate, M. Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach. Gels 2019, 9, 884. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as 800 a Promising Drug Delivery Carrier for Topical Ocular Therapy; An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Jakka, D.; Sandoval, M.A.; Kulkarni, V.R.; Bao, Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics 2024, 16, 1325. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Karkan, S.; Amiri Ramsheh, N.; Arkaban, H.; Narooie-Noori, F.; Sargazi, S.; Mirinejad, S.; Roostaee, M.; Sargazi, S.; Barani, M.; Malahat Shadman, S.; et al. Nanosuspensions in Ophthalmology: Overcoming Challenges and Enhancing Drug Delivery for Eye Diseases. Int. J. Pharm. 2024, 658, 124226. [Google Scholar] [CrossRef]
- Mustafa, S. Ophthalmology: Navigating Ocular Barriers with Advanced Nanocarriers. Ger. J. Pharm. Biomater. 2024, 3, 34–58. [Google Scholar] [CrossRef]
- Hatami-Marbini, H. Effect of Hydration on Viscoelastic Tensile Properties of Sclera. Vision 2025, 9, 1. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Guidi, L.; Cascone, M.G.; Rosellini, E. Light-Responsive Polymeric Nanoparticles for Retinal Drug Delivery: Design Cues, Challenges and Future Perspectives. Heliyon 2024, 10, e26616. [Google Scholar] [CrossRef]
- Wu, K.; Gore, A.; Graham, R.; Meller, R. Solubilization of Cyclosporine in Topical Ophthalmic Formulations: Preformulation Risk Assessment on a New Solid Form. J. Pharm. Sci. 2019, 108, 3233–3239. [Google Scholar] [CrossRef]
- Johnson, T.V.; Gupta, P.K.; Vudathala, D.K.; Blair, I.A.; Tanna, A.P. Thermal Stability of Bimatoprost, Latanoprost, and Travoprost Under Simulated Daily Use. J. Ocul. Pharmacol. Ther. 2011, 27, 51–59. [Google Scholar] [CrossRef]
- Shah, A.M.; Galor, A. Impact of Ocular Surface Temperature on Tear Characteristics: Current Insights. Clin. Optom. 2021, 13, 51–62. [Google Scholar] [CrossRef]
- Destruel, P.L.; Zeng, N.; Maury, M.; Mignet, N.; Boudy, V. In Vitro and in Vivo Evaluation of in Situ Gelling Systems for Sustained Topical Ophthalmic Delivery: State of the Art and Beyond. Drug Discov. Today 2017, 22, 638–651. [Google Scholar] [CrossRef]
- Recchioni, A.; Mocciardini, E.; Ponzini, E.; Tavazzi, S. Viscoelastic Properties of the Human Tear Film. Exp Eye Res 2022, 219, 109083. [Google Scholar] [CrossRef]
- Han, J.; Shu, H.; Zhang, L.; Huang, S. Latest Advances in Hydrogel Therapy for Ocular Diseases. Polymer 2024, 306, 127207. [Google Scholar] [CrossRef]
- Wang, T.J.; Rethi, L.; Ku, M.Y.; Nguyen, H.T.; Chuang, A.E.Y. A Review on Revolutionizing Ophthalmic Therapy: Unveiling the Potential of Chitosan, Hyaluronic Acid, Cellulose, Cyclodextrin, and Poloxamer in Eye Disease Treatments. Int. J. Biol. Macromol. 2024, 273, 132700. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Sakthivel, M.; Nischal, K.K.; Fedorchak, M.V. Drug Delivery Systems and Novel Formulations to Improve Treatment of Rare Corneal Disease. Drug Discov. Today 2019, 24, 1564–1574. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Qi, Q.; Wei, Y.; Zhang, X.; Guan, J.; Mao, S. Challenges and Strategies for Ocular Posterior Diseases Therapy via Non-Invasive Advanced Drug Delivery. J. Control. Release 2023, 361, 191–211. [Google Scholar] [CrossRef]
- Bisen, A.C.; Biswas, A.; Dubey, A.; Sanap, S.N.; Agrawal, S.; Yadav, K.S.; Singh, V.; Rawat, P.; Sagar, S.; Mugale, M.N.; et al. A Review on Polymers in Ocular Drug Delivery Systems. MedComm-Biomater. Appl. 2024, 3, e77. [Google Scholar] [CrossRef]
- Manjeri, A.; George, S.D. Hydrogel-Embedded Polydimethylsiloxane Contact Lens for Ocular Drug Delivery. ACS Appl. Bio. Mater. 2024, 7, 7324–7331. [Google Scholar] [CrossRef]
- Račić, A.; Krajišnik, D. Biopolymers in Mucoadhesive Eye Drops for Treatment of Dry Eye and Allergic Conditions: Application and Perspectives. Pharmaceutics 2023, 15, 470. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82. [Google Scholar] [CrossRef]
- Lai, J.Y.; Luo, L.J.; Nguyen, D.D. Multifunctional Glutathione-Dependent Hydrogel Eye Drops with Enhanced Drug Bioavailability for Glaucoma Therapy. Chem. Eng. J. 2020, 402, 126190. [Google Scholar] [CrossRef]
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and Characterization of Timolol Maleate-Loaded Quaternized Chitosan-Based Thermosensitive Hydrogel: A Transparent Topical Ocular Delivery System for the Treatment of Glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Emerging Strategies Targeting Genes and Cells in Glaucoma. Vision Res. 2025, 227, 108533. [Google Scholar] [CrossRef]
- El-Feky, Y.A.; Fares, A.R.; Zayed, G.; El-Telbany, R.F.A.; Ahmed, K.A.; El-Telbany, D.F.A. Repurposing of Nifedipine Loaded in Situ Ophthalmic Gel as a Novel Approach for Glaucoma Treatment. Biomed. Pharmacother. 2021, 142, 112008. [Google Scholar] [CrossRef]
- Torkashvand, A.; Izadian, A.; Hajrasouliha, A. Advances in Ophthalmic Therapeutic Delivery: A Comprehensive Overview of Present and Future Directions. Surv. Ophthalmol. 2024, 69, 967–983. [Google Scholar] [CrossRef]
- Haldon, R.A.; Leeb, B.E. Structure and Permeability of Porous Films of Poly(Hydroxy Ethyl Methacrylate). Br. Polym. J. 1972, 4, 491–501. [Google Scholar] [CrossRef]
- Fan, X.; Chen, Z.; Sun, H.; Zeng, S.; Liu, R.; Tian, Y. Polyelectrolyte-Based Conductive Hydrogels: From Theory to Applications. Soft Sci. 2022, 2, 10. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, C.; Xiong, Y.; Ma, S.; Sun, C.; Xu, W. A Review of Recent Advances in Drug Loading, Mathematical Modeling and Applications of Hydrogel Drug Delivery Systems. J. Mater. Sci. 2024, 59, 15077–15116. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Fu, J.; Panhuis, M.I.H. Hydrogel Properties and Applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef]
- Kabir, H.; Mahdavi, S.S.; Abdekhodaie, M.J.; Rafii, A.B.; Merati, M. Development of an In-Situ Forming Collagen-Based Hydrogel as a Regenerative Bioadhesive for Corneal Perforations. Int. J. Biol. Macromol. 2024, 278, 134761. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Niu, J.; Zhang, Y.; Wang, M.; Shen, Y.; Chen, X.; Mao, Y.; Li, Q. Keratin Formed Bioadhesive Ophthalmic Gel for the Bacterial Conjunctivitis Treatment. AAPS PharmSciTech 2024, 25, 77. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Sáez Martínez, V.; Martínez de Cestafe, N.; Alonso, J.M.; Olavarrieta, C.; Ucelay López de Heredia, M.; Benito Cid, S.; Pérez González, R. Effectiveness Evaluation of Hyaluronic Acid-Based Commercial Eye Drops to Treat Ophthalmic Dry Eye Disease. Carbohydr. Polym. Technol. Appl. 2024, 8, 100577. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Fung, M.P.; Chen, Y.Q.; Chiu, Y.C. Development of Mucoadhesive Methacrylic Anhydride-Modified Hydroxypropyl Methylcellulose Hydrogels for Topical Ocular Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105450. [Google Scholar] [CrossRef]
- Garg, A.; Agrawal, R.; Singh Chauhan, C.; Deshmukh, R. In-Situ Gel: A Smart Carrier for Drug Delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Cassano, R.; Di Gioia, M.L.; Trombino, S. Gel-Based Materials for Ophthalmic Drug Delivery. Gels 2021, 7, 130. [Google Scholar] [CrossRef]
- Kanwar, N.; Sinha, V.R. In Situ Forming Depot as Sustained-Release Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 93–136. [Google Scholar] [CrossRef]
- Das, B.; Chattopadhyay, D.; Rana, D. The Gamut of Perspectives, Challenges, and Recent Trends for: In Situ Hydrogels: A Smart Ophthalmic Drug Delivery Vehicle. Biomater. Sci. 2020, 8, 4665–4691. [Google Scholar] [CrossRef]
- Dahma, Z.; Álvarez-Álvarez, C.; de la Torre-Iglesias, P.M. A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs. Colloids Interfaces 2025, 9, 17. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2018, 6, 7. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ban, J.; Mo, Z.; Zhang, Y.; An, P.; Liu, L.; Xie, Q.; Du, Y.; Xie, B.; Zhan, X.; et al. A Potential Nanoparticle-Loaded in Situ Gel for Enhanced and Sustained Ophthalmic Delivery of Dexamethasone. Nanotechnology 2018, 29, 425101. [Google Scholar] [CrossRef]
- Mehrazin, M.; Asefnejad, A.; Mousavi, S.R.; Naeimi, F.; Khonakdar, H.A. Investigating the Rheological Behavior of Poloxamer–Chitosan Thermogel for in Situ Drug Delivery of Doxorubicin in Breast Cancer Treatment: Designed by Response Surface Method (RSM). Polym. Bull. 2024, 81, 15899–15930. [Google Scholar] [CrossRef]
- Rad, I.; Esmaeili, E.; Jahromi, B.B. Application of Thermo-Responsive Polymers as Smart Biomaterials in Wound Dressing. Polym. Bull. 2024, 81, 11399–11420. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 202008123. [Google Scholar] [CrossRef]
- Yan, D.; Ouyang, W.; Lin, J.; Liu, Z. Smart Coating by Thermo-Sensitive Pluronic F-127 for Enhanced Corneal Healing via Delivery of Biological Macromolecule Progranulin. Int. J. Biol. Macromol. 2023, 253, 127586. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ahmadi, Z.; Saeb, M.R.; Mozafari, M. Poloxamer-Based Stimuli-Responsive Biomaterials. Mater. Today Proc. 2018, 5, 15516–15523. [Google Scholar] [CrossRef]
- Villapiano, F.; Silvestri, T.; Lo Gatto, C.; Aleo, D.; Campani, V.; Graziano, S.F.; Giancola, C.; D’Aria, F.; De Rosa, G.; Biondi, M.; et al. Thermosensitive In Situ Gelling Poloxamers/Hyaluronic Acid Gels for Hydrocortisone Ocular Delivery. Gels 2024, 10, 193. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Hill, A.G.; Sharma, M. Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect. Gels 2022, 8, 484. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Santhan, S. Mixed Micellar Gel of Poloxamer Mixture for Improved Solubilization of Poorly Water-Soluble Ibuprofen and Use as Thermosensitive In Situ Gel. Pharmaceutics 2024, 16, 1055. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Ermenlieva, N.; Tsibranska, S.; Tcholakova, S.; Rangelov, S.; Momekova, D. Development and Evaluation of Doxycycline Niosomal Thermoresponsive in Situ Gel for Ophthalmic Delivery. Int. J. Pharm. 2020, 591, 120010. [Google Scholar] [CrossRef]
- Liu, X.; Pei, J.; Li, J.; Zhu, H.; Zheng, X.; Zhang, X.; Ruan, B.; Chen, L. Recent Advances in Resveratrol Derivatives: Structural Modifications and Biological Activities. Molecules 2025, 30, 958. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sánchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic®/Casein Micelles for Ophthalmic Delivery of Resveratrol: In Vitro, Ex Vivo, and in Vivo Tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New Highlights of Resveratrol: A Review of Properties against Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sangitra, S.N.; Pujala, R.K. Temperature-Dependent Yield Stress and Wall Slip Behaviour of Thermoresponsive Pluronic F127 Hydrogels. RSC Adv. 2024, 14, 23772–23784. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers 2021, 13, 3038. [Google Scholar] [CrossRef]
- Padaga, S.G.; Ch, S.; Paul, M.; Wable, B.D.; Ghosh, B.; Biswas, S. Chitosan Oligosaccharide/Pluronic F127 Micelles Exhibiting Anti-Biofilm Effect to Treat Bacterial Keratitis. Carbohydr. Polym. 2024, 330, 121818. [Google Scholar] [CrossRef]
- El-Shahed, S.A.; Hassan, D.H.; El-Nabarawi, M.A.; El-Setouhy, D.A.; Abdellatif, M.M. Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis. Polymers 2024, 16, 2240. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Parmar, M.B.; Shetty, K.H.; Patel, A.R.; Desai, B.V.; Vyas, B.A.; Desai, D.T.; Kalaiselvan, P.; Masoudi, S.; Shah, D.O.; et al. Role of Micelle Dynamics in Enhancing Cyclosporine Uptake in Hyaluronic Acid-Contact Lenses for Improved Critical Lens Properties in Dry Eye Management. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 688, 133550. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A.; Fakhari, A. Thermogelling Properties of Purified Poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Castañeda Ruiz, A.J.; Shetab Boushehri, M.A.; Phan, T.; Carle, S.; Garidel, P.; Buske, J.; Lamprecht, A. Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates. Pharmaceutics 2022, 14, 2575. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-Responsive In Situ Gelling System for Nose-to-Brain Drug Delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Singh, T.R.R. Poloxamer-Based in Situ Gelling Thermoresponsive Systems for Ocular Drug Delivery Applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shemkai, J.C.; Zhang, Q.; Qiao, F.; Yu, N.; Zhao, J.; Zuo, W.; Yang, J. Fabrication of an Alginate-Poloxamer 407 Thermosensitive Carboxymethyl Chitosan Stabilized Nanosuspension Hydrogel for Enhanced Ocular Delivery of Clobetasol Propionate in Uveitis Management. Int. J. Biol. Macromol. 2025, 319, 145356. [Google Scholar] [CrossRef]
- Li, X.; Park, E.; Hyun, K.; Oktavia, L.; Kwak, M. Rheological Analysis of Core-Stabilized Pluronic F127 by Semi-Interpenetrating Network (SIPN) in Aqueous Solution. J. Rheol. 2018, 62, 107–120. [Google Scholar] [CrossRef]
- Dewan, M.; Adhikari, A.; Jana, R.; Chattopadhyay, D. Development, Evaluation and Recent Progress of Ocular in Situ Gelling Drug Delivery Vehicle Based on Poloxamer 407. J. Drug Deliv. Sci. Technol. 2023, 88, 104885. [Google Scholar] [CrossRef]
- Bao, L.; Qiao, F.; Yu, N.; Zhao, Q.; Zuo, W.; Yang, J. Thermo-Responsive in Situ Gel of Fluticasone Propionate Nanosuspension Modified with Carboxymethyl Chitosan for Enhanced Blepharitis Therapy. Int. J. Biol. Macromol. 2025, 309, 143028. [Google Scholar] [CrossRef]
- Ou, L.; Wu, Z.; Hu, X.; Huang, J.; Yi, Z.; Gong, Z.; Li, H.; Peng, K.; Shu, C.; Koole, L.H. A Tissue-Adhesive F127 Hydrogel Delivers Antioxidative Copper-Selenide Nanoparticles for the Treatment of Dry Eye Disease. Acta Biomater. 2024, 175, 353–368. [Google Scholar] [CrossRef]
- Puente-Iglesias, M.; Cuartero-Martínez, A.; Touriño-Peralba, R.; Rodríguez-Ares, M.T.; Giráldez, M.J.; Yebra-Pimentel, E.; García-Quintanilla, L.; García-Otero, X.; González-Barcia, M.; Zarra-Ferro, I.; et al. Clinical Effectiveness, Safety, and Compliance of Two Compounded Formulations of Tacrolimus Eye Drops: An Open-Label, Sequential Prospective Study. Int. J. Mol. Sci. 2024, 25, 9847. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Gu, J.; Yuan, J.; Chen, J. Tacrolimus in the Treatment of Ocular Diseases. BioDrugs 2011, 25, 89–103. [Google Scholar] [CrossRef]
- Modi, D.; Mohammad; Warsi, M.H.; Garg, V.; Bhatia, M.; Kesharwani, P.; Jain, G.K. Formulation Development, Optimization, and in Vitro Assessment of Thermoresponsive Ophthalmic Pluronic F127-Chitosan in Situ Tacrolimus Gel. J. Biomater. Sci. Polym. Ed. 2021, 32, 1678–1702. [Google Scholar] [CrossRef]
- Khan, J.; Khan, A.; Khan, A.; Khan, D.; Shaikh, A.H.; Baig, M.S. In Situ Gel: A Promising Ocular Drug Delivery System. Int. J. Adv. Life Sci. Res. 2024, 7, 65–77. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In Situ Ophthalmic Gel Forming Systems of Poloxamer 407 and Hydroxypropyl Methyl Cellulose Mixtures for Sustained Ocular Delivery of Chloramphenicole: Optimization Study by Factorial Design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Huang, C.; Peng, L.; Xu, X.; Lu, Y.; Wang, X.; Lan, Z.; Chen, J.; Zhou, Y. Preparation and Characteristics of a Thermosensitive in Situ Gel Loaded with Chitosan Nanoparticles for Optimal Ocular Delivery of Chloramphenicol. J. Drug Deliv. Sci. Technol. 2023, 89, 104962. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, P.; Li, J. Elucidation of Colloid Performances of Thermosensitive In Situ–Forming Ophthalmic Gel Formed by Poloxamer 407 for Loading Drugs. J. Pharm. Sci. 2020, 109, 1703–1713. [Google Scholar] [CrossRef]
- Paul, S.; Majumdar, S.; Chakraborty, M.; Mukherjee, S.; Sarkar, N.; Prajapati, B.; Ali, N.; AlAsmari, A.F.; Nishat, S. In-Situ Gel Bases Ocular Delivery System of Ganciclovir, in-Vivo and in-Vitro Investigation. BMC Pharmacol. Toxicol. 2025, 26, 102. [Google Scholar] [CrossRef] [PubMed]
- Chandler, H.L.; Moradi, S.; Green, S.W.; Chen, P.; Madden, C.; Zhang, L.; Zhang, Z.; Park, K.H.; Ma, J.; Zhu, H.; et al. Development of an Ophthalmic Hydrogel to Deliver MG53 and Promote Corneal Wound Healing. Pharmaceutics 2025, 17, 526. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, Y.K. Wound Healing by Enhancing Cell Proliferation: A Thermoreversible Formulation Containing Raloxifene. Cell Tissue Bank 2025, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Kumar, S.; Kunwar, A.; Nath, S.; Sarma, H.D.; Tripathi, A.; Verma, G.; Chaudhari, D.P.; Aswal, V.K.; Melo, J.S. Structural and Therapeutic Properties of Curcumin Solubilized Pluronic F127 Micellar Solutions and Hydrogels. J. Mol. Liq. 2020, 314, 113591. [Google Scholar] [CrossRef]
- Faris Taufeq, F.Y.; Habideen, N.H.; Rao, L.N.; Podder, P.K.; Katas, H. Potential Hemostatic and Wound Healing Effects of Thermoresponsive Wound Dressing Gel Loaded with Lignosus Rhinocerotis and Punica Granatum Extracts. Gels 2023, 9, 48. [Google Scholar] [CrossRef]
- Qu, S.; Zheng, S.; Muhammad, S.; Huang, L.; Guo, B. An Exploration of the Ocular Mysteries Linking Nanoparticles to the Patho-Therapeutic Effects against Keratitis. J. Nanobiotechnol. 2025, 23, 184. [Google Scholar] [CrossRef] [PubMed]
- Muszanska, A.K.; Busscher, H.J.; Herrmann, A.; Van der Mei, H.C.; Norde, W. Pluronic-Lysozyme Conjugates as Anti-Adhesive and Antibacterial Bifunctional Polymers for Surface Coating. Biomaterials 2011, 32, 6333–6341. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Makky, S.; Teba, H.E.; Agwa, M.M.; Abd ElAziz, M.M.; Awad, R.; Hassan, Y.Y.; Abdelsattar, A.S.; Connerton, I.F.; El-Shibiny, A. Potential of VB_Pa_ZCPS1 Phage Embedded in Situ Gelling Formulations as an Ocular Delivery System to Attenuate Pseudomonas Aeruginosa Keratitis in a Rabbit Model. J. Control. Release 2025, 380, 52–70. [Google Scholar] [CrossRef]
- Marandi, M.F.; Safary, A.; Abdolahinia, E.D.; Fathi, M.; Barar, J.; Omidi, Y. Poloxamer407/Gellan Gum-Based in Situ Hydrogels for Ocular Co-Delivery of Antibiotics and Corticosteroids. Eur. J. Pharm. Biopharm. 2025, 213, 114739. [Google Scholar] [CrossRef]
- Li, D.X.; Han, M.J.; Balakrishnan, P.; Yan, Y.D.; Oh, D.H.; Joe, J.H.; Seo, Y.; Kim, J.O.; Park, S.M.; Yong, C.S.; et al. Enhanced Oral Bioavailability of Flurbiprofen by Combined Use of Micelle Solution and Inclusion Compound. Arch. Pharm. Res. 2010, 33, 95–101. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Jhawat, V.; Desu, P.K.; Gandhi, S.; Nakkala, R.K.; Singh, S. Formulation and Evaluation of Thermosensitive Flurbiprofen in Situ Nano Gel for the Ocular Delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 1584–1597. [Google Scholar] [CrossRef]
- Wang, J.; Viola, M.; Migliorini, C.; Paoletti, L.; Arpicco, S.; Di Meo, C.; Matricardi, P. Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review. Pharmaceutics 2023, 15, 2508. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Noh, H. Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers. Gels 2023, 9, 718. [Google Scholar] [CrossRef]
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Hosny, K.M.; Rizg, W.Y.; Eshmawi, B.A.; Badr, M.Y.; Safhi, A.Y.; Murshid, S.S.A. Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection. Gels 2022, 8, 241. [Google Scholar] [CrossRef]
- Morsi, N.; Ghorab, D.; Refai, H.; Teba, H. Ketoroloac Tromethamine Loaded Nanodispersion Incorporated into Thermosensitive in Situ Gel for Prolonged Ocular Delivery. Int. J. Pharm. 2016, 506, 57–67. [Google Scholar] [CrossRef]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and Mucoadhesive in Situ Ocular Gel for Effective Local Delivery and Antifungal Activity of Itraconazole Nanocrystal in the Treatment of Fungal Keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Di Spirito, N.A.; Grizzuti, N.; Casalegno, M.; Castiglione, F.; Pasquino, R. Phase Transitions of Aqueous Solutions of Pluronic F68 in the Presence of Diclofenac Sodium. Int. J. Pharm. 2023, 644, 123353. [Google Scholar] [CrossRef] [PubMed]
- Inyang, E.; Abhyankar, V.; Chen, B.; Cho, M. Modulation of in Vitro Brain Endothelium by Mechanical Trauma: Structural and Functional Restoration by Poloxamer 188. Sci. Rep. 2020, 10, 3054. [Google Scholar] [CrossRef]
- Anosov, A.A.; Borisova, E.D.; Konstantinov, O.O.; Smirnova, E.Y.; Korepanova, E.A.; Kazamanov, V.A.; Derunets, A.S. The Changes in the Conductance of Bilayer Lipid Membranes Caused by Pluronics L61 and F68: Similarities and Differences. Russ. J. Electrochem. 2024, 60, 339–347. [Google Scholar] [CrossRef]
- Jiao, J. Polyoxyethylated Nonionic Surfactants and Their Applications in Topical Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Darusman, F.; Rusdiana, T.; Sopyan, I.; Rahma, H.; Hanifa, M. Recent Progress in Pharmaceutical Excipients as P-Glycoprotein Inhibitors for Potential Improvement of Oral Drug Bioavailability: A Comprehensive Overview. Pharmacia 2025, 72, e140734. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Amato, G.; Carbone, C.; Diaz-Rodriguez, P.; Musumeci, T.; Concheiro, A.; Alvarez-Lorenzo, C.; Puglisi, G. Micelle-Nanogel Platform for Ferulic Acid Ocular Delivery. Int. J. Pharm. 2020, 576, 118986. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Z.Y.; Wang, T.J.; Drew, V.J.; Tseng, C.L.; Fang, H.W.; Lin, F.H. Herbal Supplement in a Buffer for Dry Eye Syndrome Treatment. Int. J. Mol. Sci. 2017, 18, 1697. [Google Scholar] [CrossRef]
- Sheshala, R.; Wai, N.Z.; Said, I.D.; Ashraf, K.; Lim, S.M.; Ramasamy, K.; Zeeshan, F. Poloxamer and Chitosan-Based In Situ Gels Loaded with Orthosiphon Stamineus Benth. Extracts Containing Rosmarinic Acid for the Treatment of Ocular Infections. Turk. J. Pharm. Sci. 2022, 19, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Andonova, V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals 2023, 16, 474. [Google Scholar] [CrossRef]
- Niamprem, P.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Nile Red-Loaded Nanostructured Lipid Carriers (NLCs) across the Porcine Cornea. Colloids Surf. B Biointerfaces 2019, 176, 371–378. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; Li, K.; Hong, W.; Lv, Q.; Li, Y.; Xie, S.; Han, J.; Tian, B. Cell Penetrating Peptide TAT-Functionalized Liposomes for Efficient Ophthalmic Delivery of Flurbiprofen: Penetration and Its Underlying Mechanism, Retention, Anti-Inflammation and Biocompatibility. Int. J. Pharm. 2021, 598, 120405. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Feng, J.; Zeng, T.; Guo, Q.; Zhang, Z.; Ding, C.; Tian, B.; Sai, S. Flurbiprofen Loaded Thermosensitive Nanohydrogel for Ophthalmic Anti-Inflammatory Therapy. J. Drug Deliv. Sci. Technol. 2022, 70, 103253. [Google Scholar] [CrossRef]
- Adısanoğlu, P.; Özgüney, I. Development and Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations Containing Flurbiprofen Solid Dispersions. Gels 2024, 10, 267. [Google Scholar] [CrossRef]
- Hamed, R.; Abu Kwiak, A.D.; Al-Adhami, Y.; Hammad, A.M.; Obaidat, R.; Abusara, O.H.; Huwaij, R.A. Microemulsions as Lipid Nanosystems Loaded into Thermoresponsive In Situ Microgels for Local Ocular Delivery of Prednisolone. Pharmaceutics 2022, 14, 1975. [Google Scholar] [CrossRef]
- Chaudhari, P.; Naik, R.; Sruthi Mallela, L.; Roy, S.; Birangal, S.; Ghate, V.; Balladka Kunhanna, S.; Lewis, S.A. A Supramolecular Thermosensitive Gel of Ketoconazole for Ocular Applications: In Silico, in Vitro, and Ex Vivo Studies. Int. J. Pharm. 2022, 613, 121409. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, A.; Qin, Y.; Chen, X.; Feng, X.; He, G.; Zhu, X.; Xiao, Y.; Yu, X.; Zhong, T.; et al. Preparation of a Thermosensitive and Antibacterial in Situ Gel Using Poloxamer-Quaternized Chitosan for Sustained Ocular Delivery of Levofloxacin Hydrochloride. Int. J. Biol. Macromol. 2024, 283, 137479. [Google Scholar] [CrossRef]
- Cai, M.; Zhong, H.; Wang, X.; Li, L.; Zhou, X.; Wang, Y.; Hua, X.; Guo, S.; Yuan, X. Pathology-Inspired Collagen-Binding Thermosensitive Micelle Drops Enable Prolonged and Efficient Treatment of Fungal Keratitis. Bioact. Mater. 2025, 50, 396–413. [Google Scholar] [CrossRef]
- Alsheikh, R.; Haimhoffer, Á.; Nemes, D.; Ujhelyi, Z.; Fehér, P.; Józsa, L.; Vasvári, G.; Pető, Á.; Kósa, D.; Nagy, L.; et al. Formulation of Thermo-Sensitive In Situ Gels Loaded with Dual Spectrum Antibiotics of Azithromycin and Ofloxacin. Polymers 2024, 16, 2954. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Giraldo-Gomez, D.M.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D.; González-Del Carmen, M.; González-Torres, M.; Cortés, H.; Leyva-Gómez, G. Insights into Terminal Sterilization Processes of Nanoparticles for Biomedical Applications. Molecules 2021, 26, 2068. [Google Scholar] [CrossRef]
- Gohil, U.; Chandarana, C.; Prajapati, P.; Prajapati, B. Polymeric Nanoparticles in Ophthalmology: A Comprehensive Review of Therapeutic Applications. Bionanoscience 2025, 15, 151. [Google Scholar] [CrossRef]
- Pišlová, M.; Kolářová, K.; Vokatá, B.; Brož, A.; Ulbrich, P.; Bačáková, L.; Kolská, Z.; Švorčík, V. A New Way to Prepare Gold Nanoparticles by Sputtering—Sterilization, Stability and Other Properties. Mater. Sci. Eng. C 2020, 115, 111087. [Google Scholar] [CrossRef] [PubMed]
- Balu, A.; Johnson, T.; Sundara, R.; Seetharaman, S. Optimization and Evaluation of Temperature Trig-Gered in Situ Gel Formulation Using Design of Experiments (DoE) and HET-CAM Test. J. Nanomed. 2020, 3, 1031. [Google Scholar]
- USP31/NF26; The United States Pharmacopeial Convention. Port City Press: Pikesville, MD, USA, 2007.
- Winter, C.; Springer, A.; Descamps, J.-L.; Hoefinghoff, J.; Mohammad-Sadegh, S.; Paudel, A.; Stankovic-Brandl, M. Unraveling the Effects of Filtration, Process Interruptions, and Post-Process Agitation on Protein Aggregation. AAPS PharmSciTech 2025, 26, 85. [Google Scholar] [CrossRef]
- Na, J.; Suh, D.; Cho, Y.H.; Baek, Y. Comparative Evaluation of the Performance of Sterile Filters for Bioburden Protection and Final Fill in Biopharmaceutical Processes. Membranes 2022, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Ordoyo-Pascual, J.; Ruiz-Alonso, S.; Gallego, I.; Saenz-del-Burgo, L.; Pedraz, J.L. Effects of Beta and Gamma Radiation Sterilization on Growth Factor-Loaded Nanoparticles: An Innovative Approach for Osteoarticular Disorders Treatment. Drug Deliv. Transl. Res. 2025, 15, 3716–3736. [Google Scholar] [CrossRef]
- Cruz-Cazarim, E.L.C.; Cazarim, M.S.; Ogunjimi, A.T.; Petrilli, R.; Rocha, E.M.; Lopez, R.F.V. Prospective Insulin-Based Ophthalmic Delivery Systems for the Treatment of Dry Eye Syndrome and Corneal Injuries. Eur. J. Pharm. Biopharm. 2019, 140, 1–10. [Google Scholar] [CrossRef]
- Acharya, A.; Goudanavar, P.; Chitti, R.; Dinnimath, B.M. Preparation of Gellan Gum and Chitosan Based In-Situ Gel of Timolol Maleate for Ophthalmic Drug Delivery and Evaluation of Physicochemical Properties and Drug Release Profile. Acta Sci. Pharm. Sci. 2019, 3, 68–78. [Google Scholar]
- Sanchez Armengol, E.; Grassiri, B.; Piras, A.M.; Zambito, Y.; Fabiano, A.; Laffleur, F. Ocular Antibacterial Chitosan-Maleic Acid Hydrogels: In Vitro and in Vivo Studies for a Promising Approach with Enhanced Mucoadhesion. Int. J. Biol. Macromol. 2024, 254, 127939. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Falcão, M.A.; Amaral, V.A.; Contarato, J.L.; Gelfuso, G.M.; Cunha-Filho, M.; Gratieri, T. Hydrogel-Based Hybrid Membrane Enhances in Vitro Ophthalmic Drug Evaluation in the OphthalMimic Device. Methods 2024, 230, 21–31. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Falcão, M.A.; Alves Amaral, V.; Contarato, J.L.A.; Barbalho, A.M.; Kaori Diógenes, G.; Mariana Gomes Silva, M.; Carvalho de Barros do Vale Rochelle, B.; Gelfuso, G.M.; Cunha-Filho, M.; et al. OphthalMimic: A New Alternative Apparatus without Animal Tissue for the Evaluation of Topical Ophthalmic Drug Products. Methods 2024, 228, 1–11. [Google Scholar] [CrossRef]
- Mi, B.; Mu, J.; Ding, X.; Guo, S.; Hua, X. Responsive Microneedles for Diagnostic and Therapeutic Applications of Ocular Diseases. Small Methods 2025, 9, e2402048. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Zhang, Y.; Tian, J.; Ling, G.; Zhang, P. Construction of a Thermosensitive Gel Based on Hydroxypropyl-β-Cyclodextrin/Meloxicam Inclusion Complexes for Improving Meloxicam Solubility and Prolonging Drug Retention Time in the Cornea. Drug Deliv. Transl. Res. 2025, 15, 3185–3198. [Google Scholar] [CrossRef] [PubMed]
- Destruel, P.L.; Zeng, N.; Seguin, J.; Douat, S.; Rosa, F.; Brignole-Baudouin, F.; Dufaÿ, S.; Dufaÿ-Wojcicki, A.; Maury, M.; Mignet, N.; et al. Novel in Situ Gelling Ophthalmic Drug Delivery System Based on Gellan Gum and Hydroxyethylcellulose: Innovative Rheological Characterization, in Vitro and in Vivo Evidence of a Sustained Precorneal Retention Time. Int. J. Pharm. 2020, 574, 118734. [Google Scholar] [CrossRef] [PubMed]
- Elmotasem, H.; Awad, G.E.A. A Stepwise Optimization Strategy to Formulate in Situ Gelling Formulations Comprising Fluconazole-Hydroxypropyl-Beta-Cyclodextrin Complex Loaded Niosomal Vesicles and Eudragit Nanoparticles for Enhanced Antifungal Activity and Prolonged Ocular Delivery. Asian J. Pharm. Sci. 2020, 15, 617–636. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for in Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.O.; Ferreira-Nunes, R.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. In Situ Gelling Microemulsion for Topical Ocular Delivery of Moxifloxacin and Betamethasone. J. Mol. Liq. 2022, 360, 119559. [Google Scholar] [CrossRef]
- Sathe, P.; Kailasam, V.; Nagarjuna, V.; Sharma, H.; Velpandian, T.; Garg, P.; Nirmal, J. Nanomicelles Empower Natamycin in Treating Fungal Keratitis: An in Vitro, Ex Vivo and in Vivo Study. Int. J. Pharm. 2024, 656, 124118. [Google Scholar] [CrossRef]
- Chomchalao, P.; Saelim, N.; Lamlertthon, S.; Sisopa, P.; Tiyaboonchai, W. Mucoadhesive Hybrid System of Silk Fibroin Nanoparticles and Thermosensitive In Situ Hydrogel for Amphotericin B Delivery: A Potential Option for Fungal Keratitis Treatment. Polymers 2024, 16, 148. [Google Scholar] [CrossRef]
- Kumbhar, S.T.; Salunke, M.A.; Mane, P.T.; Wakure, B.S. The Development of an Innovative Ophthalmic In-Situ Gel Containing Posaconazole. Int. J. Drug Deliv. Technol. 2024, 14, 913–918. [Google Scholar] [CrossRef]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in Ophthalmic Applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef]
- Ronderos, V.; Bollag, W.B. Therapeutic Benefits of Glycerol in Dry Eye Disease. Front. Med. 2024, 11, 1531670. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Bonacci, S.; Iriti, G.; Procopio, A.; Fresta, M.; Cosco, D. SCLAREIN (SCLAREol Contained in ZeIN) Nanoparticles: Development and Characterization of an Innovative Natural Nanoformulation. Int. J. Biol. Macromol. 2021, 193, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, L.M.; Garidel, P. Hydrogel Formulations for Biologicals: Current Spotlight from a Commercial Perspective. Ther. Deliv. 2018, 9, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Gagliardi, A.; Farhan, A.; Voci, S.; Costa, N.; Bulotta, S.; Mollace, V.; Palma, E.; Majid, M.; Cosco, D. Poloxamer 407-Based Hydrogels Containing Rutin Increase the In Vitro and In Vivo Wound Healing Phenomena. ACS Appl. Bio. Mater. 2025, 8, 1972–1983. [Google Scholar] [CrossRef]

| Source | Composition | Crosslinking | Configuration | Ionic Charge | Property | Response |
|---|---|---|---|---|---|---|
| Natural | Homopolymer | Physical | Amorphous | Non-ionic | Conventional | Physical stimuli |
| Synthetic | Copolymer | Chemical | Crystalline | Anionic | Intelligent | Chemical stimuli |
| Semi-synthetic | Semi-IPN | Semi-crystalline | Cationic | |||
| IPN | Ampholytic |
| Type and Amount of Poloxamer Used | Other Components | Type of Hydrogel | Type of Embedded Carrier | Active Compound | Applications | References |
|---|---|---|---|---|---|---|
| P407 15% w/w | HPMC 1.5% w/w | E | Niosomes | Doxycycline | Antibacterial activity | [72] |
| P407 8.16% w/v | HPMC 0.77% w/v | G | - | Chloramphenicol | Antimicrobial activity | [95] |
| P407 | HPMC | G | - | Berberine | Antitumor activity; Antibiotic property; Antioxidant; Anti-inflammatory effects; Gastroenteric discomfort; Diabetes in clinic | [97] |
| P407 17.5–22.5% w/v | Chitosan | G | - | Tacrolimus | Allograft corneal rejection; Mooren’s ulcer; Allergic conjunctivitis; Immunogenic inflammatory ocular surface diseases; Posterior uveitis; Posterior blepharitis | [93] |
| P407 P188 | HPMC K4M | G | - | Nifedipine | Glaucoma | [40] |
| P407 15–20% w/v P188 0–7.50% w/v | HPMC 0.5–1.5% w/v | E | Nanocrystals | Itraconazole | Fungal infections | [115] |
| P407 14% w/w | Carbopol 934 0.3% w/w | E, N | HPMC nanoparticles | Flurbiprofen | Prevention of miosis throughout ocular surgery; Postoperative ocular inflammation | [109] |
| P7: P407 21% w/v P188 4% w/v; P8: P407 21% w/v P188 5% w/v | F5: chitosan 1.5% w/v β-glycerophosphate 45% w/v | G | - | Orthosiphon Stamineus Benth | Antimicrobial activity | [124] |
| F2: P407 15% w/w HA 0.2% w/w F4: P407 10% w/w HA 0.4% w/w | - | E | Cubosomes | Voriconazole | Fungal infections | [113] |
| P407 27.36% w/v P188 6.22% w/v | - | E, N | Nanostructured lipid carriers | Flurbiprofen | Anti-inflammatory therapy | [128] |
| P407 10% and 12% w/w P407 12% w/w and P188 1–10% w/w | - | E, M | Lipid nanosystems oil-in-water (O/W) microemulsion | Prednisolone | Ocular inflammatory disease | [130] |
| P407 20% w/w P188 5% w/w | Carbopol 0.2% w/w | G | Ketoconazole complexed with sulfobutylether-β-cyclodextrin | Fungal infections | [131] | |
| P407 20% wt | - | G | - | Progranulin | Anti-inflammatory action; Regeneration and re-epithelialization of corneal tissue | [66] |
| P407 20.5% w/v P188 5.0% w/v | - | E | Chitosan nanoparticles | Chloramphenicol | Antimicrobial activity | [96] |
| Aldehyde-functionalized P407 (AF127 different concentrations) | - | G | Polyvinylpyrrolidone nanoparticles | Se | Antioxidant; Dry eye disease | [90] |
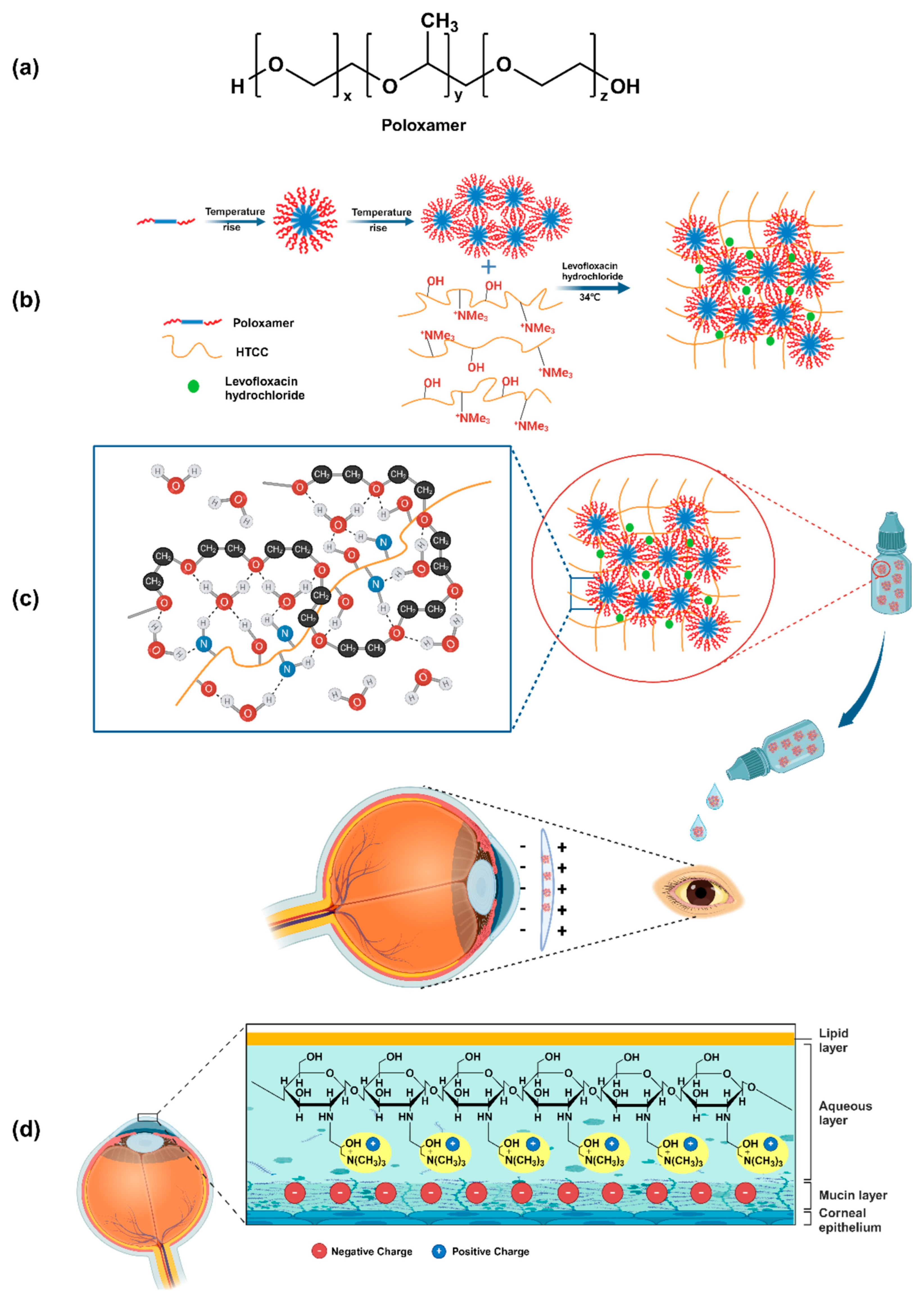
| P407 P188 | N-(2-hydroxy-3-trimethylammonium) propyl chitosan chloride | M | - | Levofloxacin hydrochloride | Fungal keratitis | [132] |
| P407 | Alginate | E, N | Carboxymethyl chitosan nanosuspension | Clobetasol propionate | Uveitis treatment | [86] |
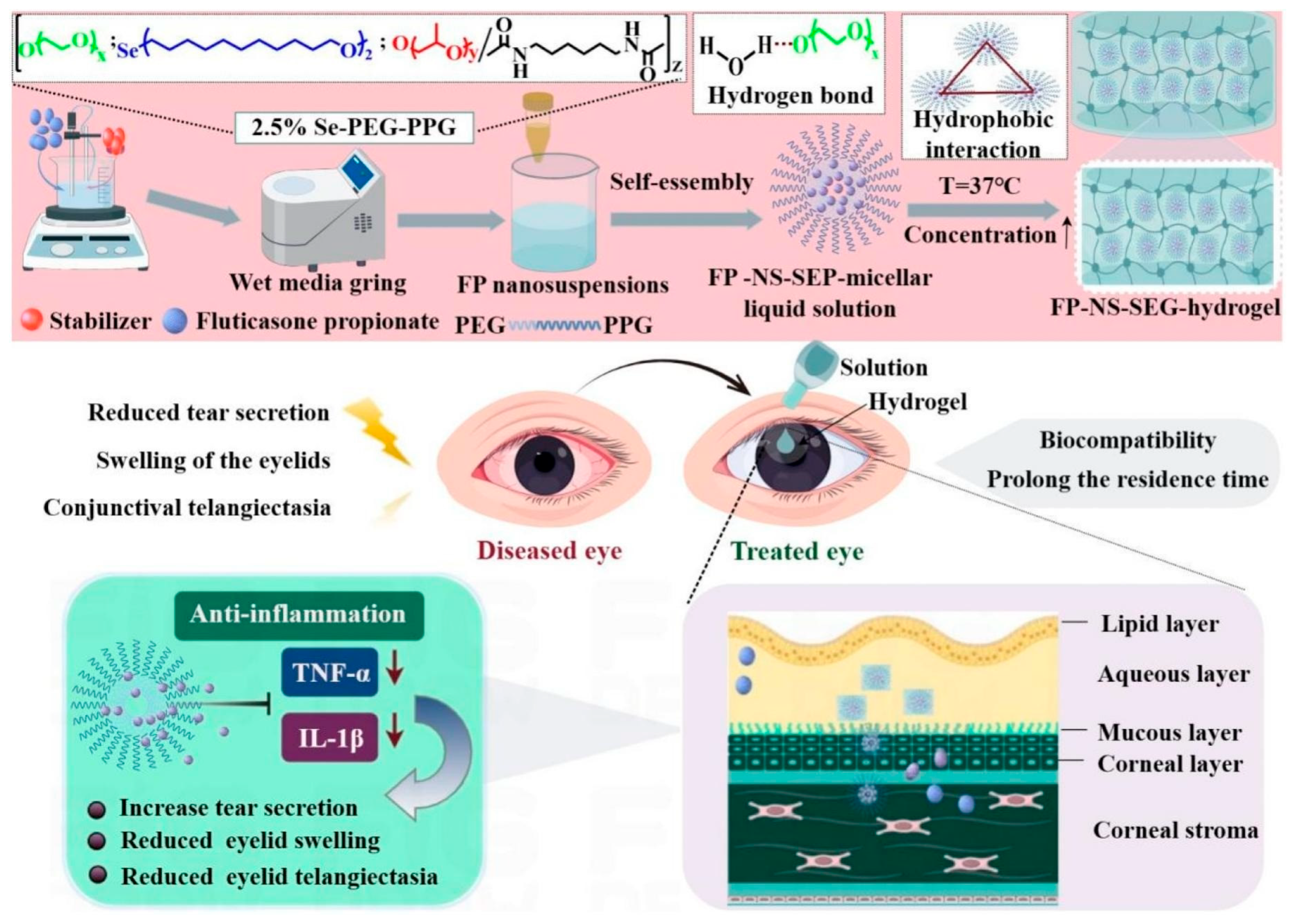
| P407-based gel (SEP) | Selenol | M | Nanosuspension | Fluticasone propionate | Blepharitis | [89] |
| P407 15% w/v | HPMC E-50 LV 1% w/v | G | - | Ganciclovir | Cytomegalovirus retinitis; Herpetic keratitis | [98] |
| P407 18.0 w/v% P188 5.0 w/v% | HPMC (1.0 w/v%) | G | - | (rh)MG53 protein | Promote corneal healing | [99] |
| P407 14% | HPMC K4M 1.5% | E | Bacteriophage | phage vB_Pa_ZCPS1 | Pseudomonas aeruginosa keratitis | [105] |
| P407 16.5, 17, and 18% w/v | Gellan Gum 0.1% w/v | E, M | Chitosan nanoparticles | Dexamethasone Polymyxin B sulfate Neomycin sulfate | Inflammation and infection | [106] |
| P407 P188 | Tannic Acid | G | - | Amphotericin B | Corneal ulcers | [133] |
| P407 10–20% w/v | Alginate | G | - | Raloxifene | Wound healing | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, E.; Giuliano, E.; Gagliardi, A.; Gaetano, V.; Frisina, M.; Verdiglione, M.; Cosco, D. In Situ Forming Poloxamer-Based Thermo-Sensitive Hydrogels for Ocular Application: A Focus on the Derivatives 407 and 188. Gels 2025, 11, 752. https://doi.org/10.3390/gels11090752
Longo E, Giuliano E, Gagliardi A, Gaetano V, Frisina M, Verdiglione M, Cosco D. In Situ Forming Poloxamer-Based Thermo-Sensitive Hydrogels for Ocular Application: A Focus on the Derivatives 407 and 188. Gels. 2025; 11(9):752. https://doi.org/10.3390/gels11090752
Chicago/Turabian StyleLongo, Emanuela, Elena Giuliano, Agnese Gagliardi, Valeria Gaetano, Marialaura Frisina, Mario Verdiglione, and Donato Cosco. 2025. "In Situ Forming Poloxamer-Based Thermo-Sensitive Hydrogels for Ocular Application: A Focus on the Derivatives 407 and 188" Gels 11, no. 9: 752. https://doi.org/10.3390/gels11090752
APA StyleLongo, E., Giuliano, E., Gagliardi, A., Gaetano, V., Frisina, M., Verdiglione, M., & Cosco, D. (2025). In Situ Forming Poloxamer-Based Thermo-Sensitive Hydrogels for Ocular Application: A Focus on the Derivatives 407 and 188. Gels, 11(9), 752. https://doi.org/10.3390/gels11090752









