Deep Learning-Enabled Flexible PVA/CNPs Hydrogel Film Sensor for Abdominal Respiration Monitoring
Abstract
1. Introduction
2. Results and Discussion
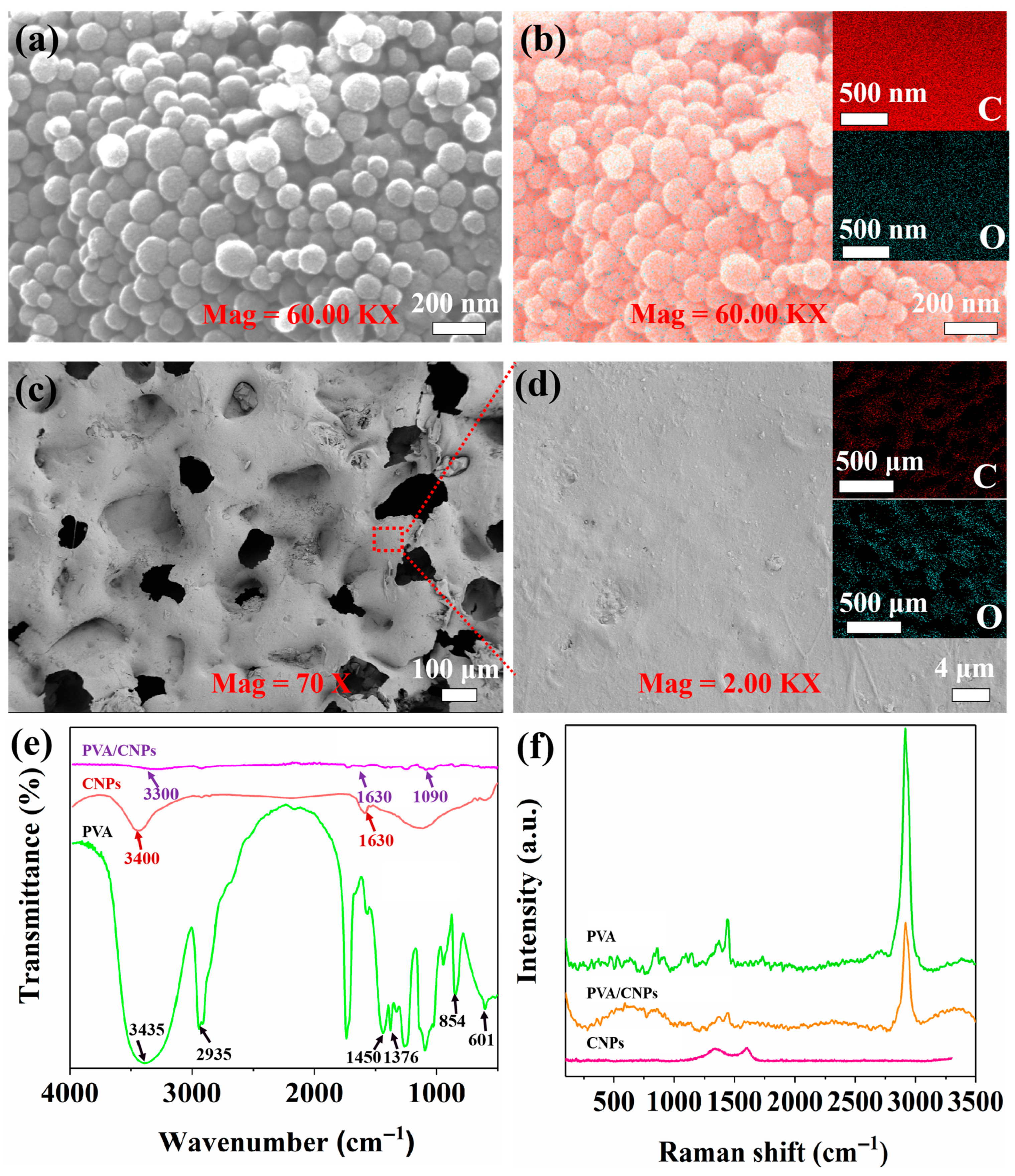
2.1. Structure Characterisation
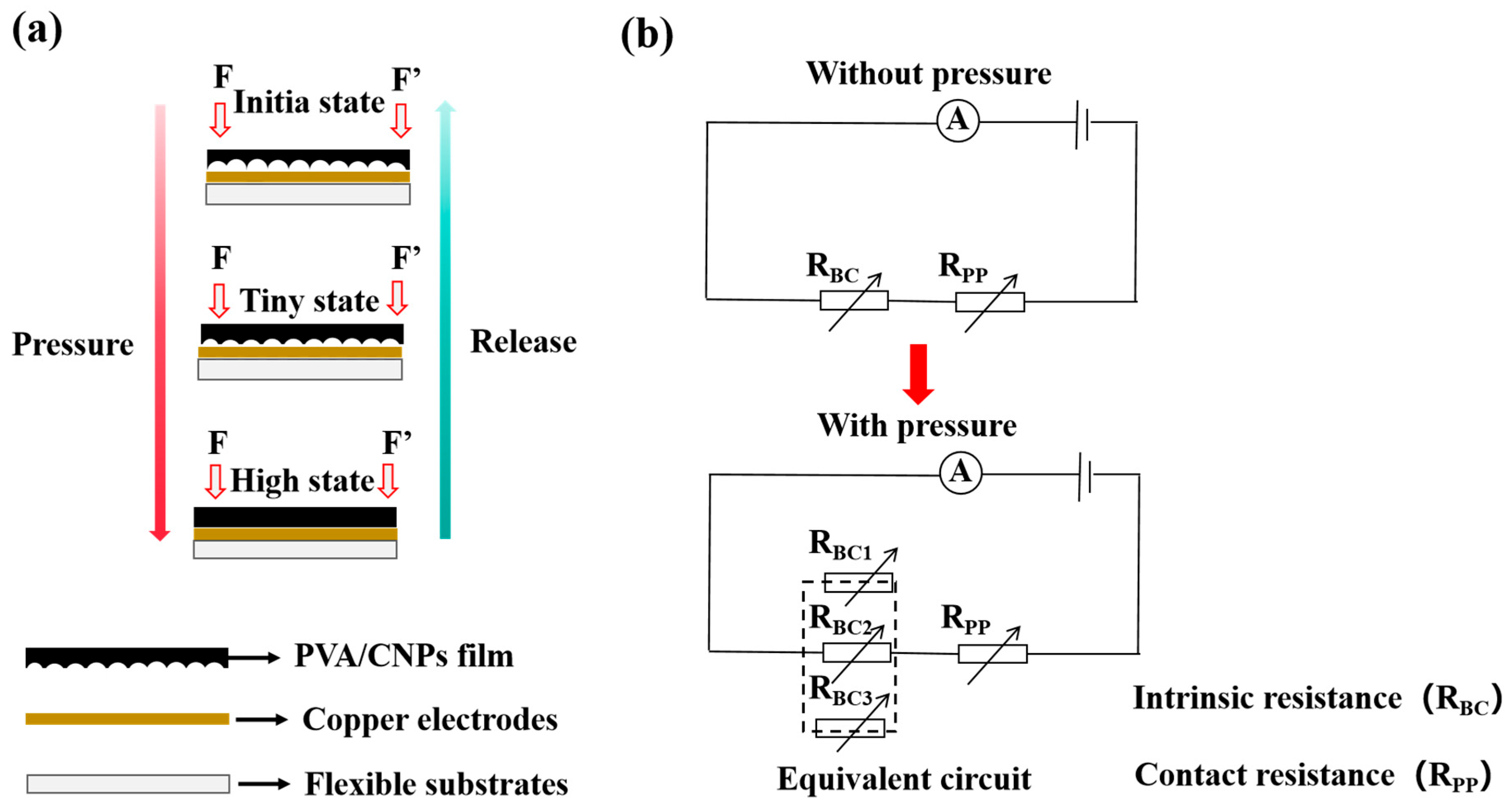
2.2. Sensing Properties
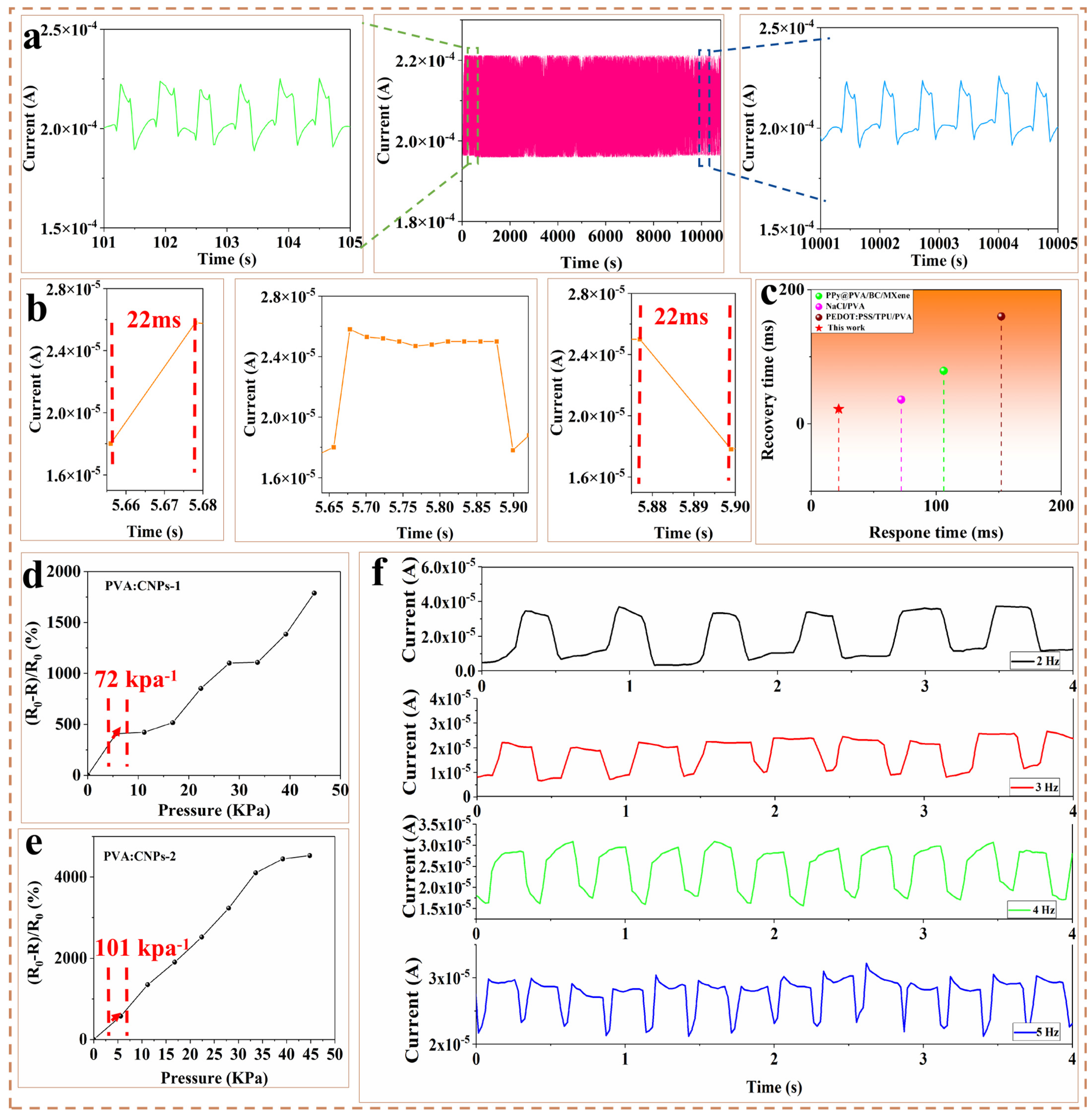
| Sensing Material | Sensitivity (kPa−1) | Cyclic Durability | Response/Recovery Time (ms) | Ref. |
|---|---|---|---|---|
| Ionic-liquid/TPU complex | 40.64 (0–1 kPa) 0.28 (1000–4000 kPa) | 6000 | 41/58 | [48] |
| Carbomer/rGo composite | 3.1 (0–13 kPa) 0.22 (13–400 kPa) | 10,000 | 14.9/22.4 | [49] |
| Graphene coated micro-patterned PDMS | 18.94 (0–40 kPa) | ~5500 | 284/102 | [50] |
| Interlink PDMS/CNT network | 0.15 (0–47 kPa) 0.08 (47–214 kPa) | 10,000 | 6/6 | [44] |
| Patterned Ecoflex/rGO composite | 4.68 (0–140 kPa) 11.09 (140–200 kPa) | 1000 | N/A | [51] |
| Sandpaper templated MXene/PDMS | 3.94 (0–7.5 kPa) 0.0564 (27–119 kPa) | 7500 | 70/84 | [52] |
| Graphene coated PDMS | 3.54 (0–100 kPa) 18.87 (100–140 kPa) | >10,000 | 125/129 | [53] |
| ZnO/MXene/PU fibrous membrane | 35.8 (0.2–35 kPa) 6.1(35–120 kPa) 1.2 (120–260 kPa) | 10,000 | 100/60 | [54] |
| Micro-structured PDMS coated with CNTs/ PPNWFs | 6.31 (0–50 kPa) 3.07 (50–800 kPa) | 10,000 | 72/88 | [55] |
| Hemispherical graded micro-structured PDMS | 1.357 (0–5 kPa) 0.077 (5–50 kPa) | 6000 | 50/60 | [56] |
| PEDOT: PSS coated PDMS | 2.32 (0–100 kPa) | 3000 | 240/100 | [57] |
| PVA/CNPs | 101 (0–44.8 kPa) | 20,000 | 22/22 | This work |
2.3. Pressure Identification of PVA/CNPs Hydrogel Film Sensor
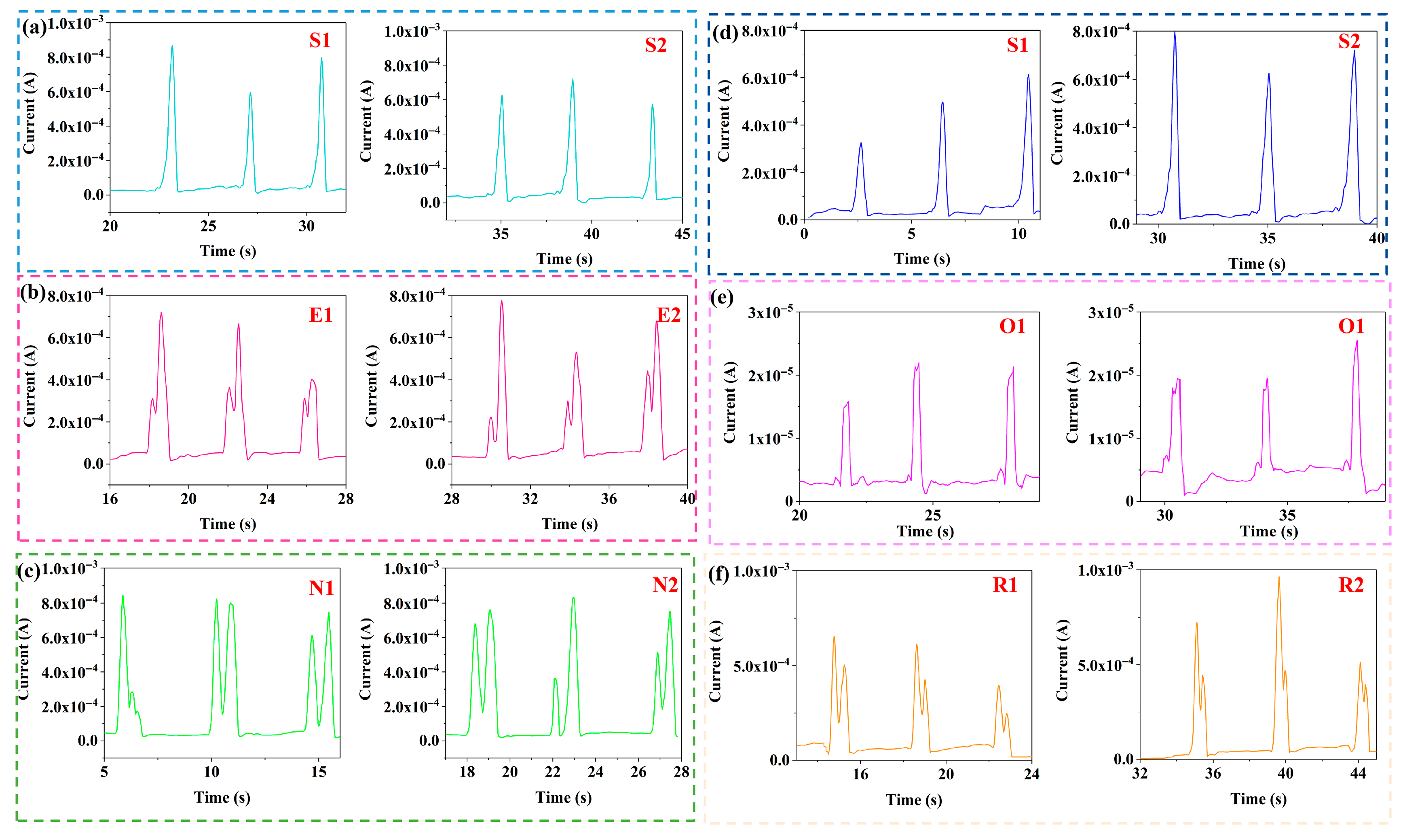
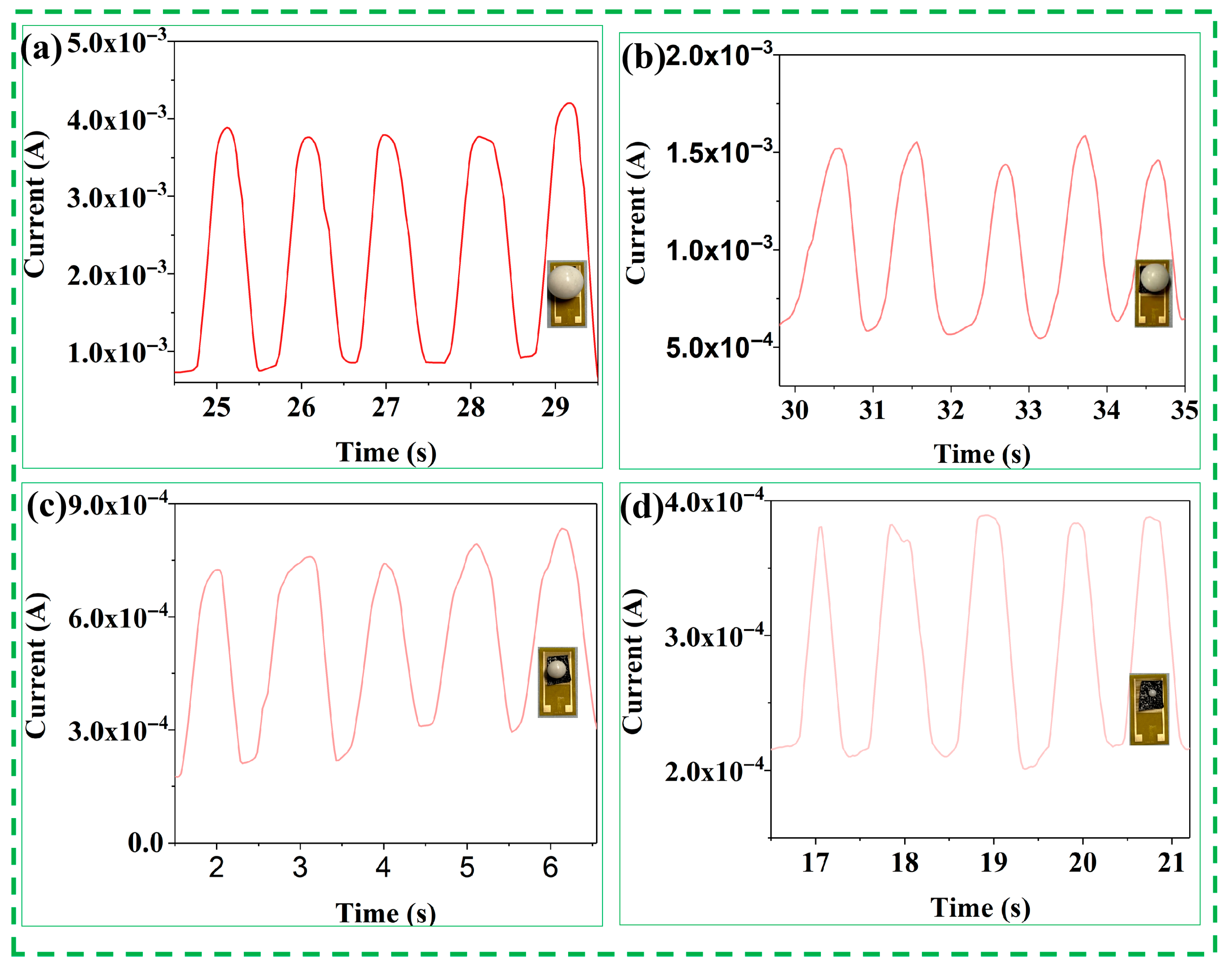
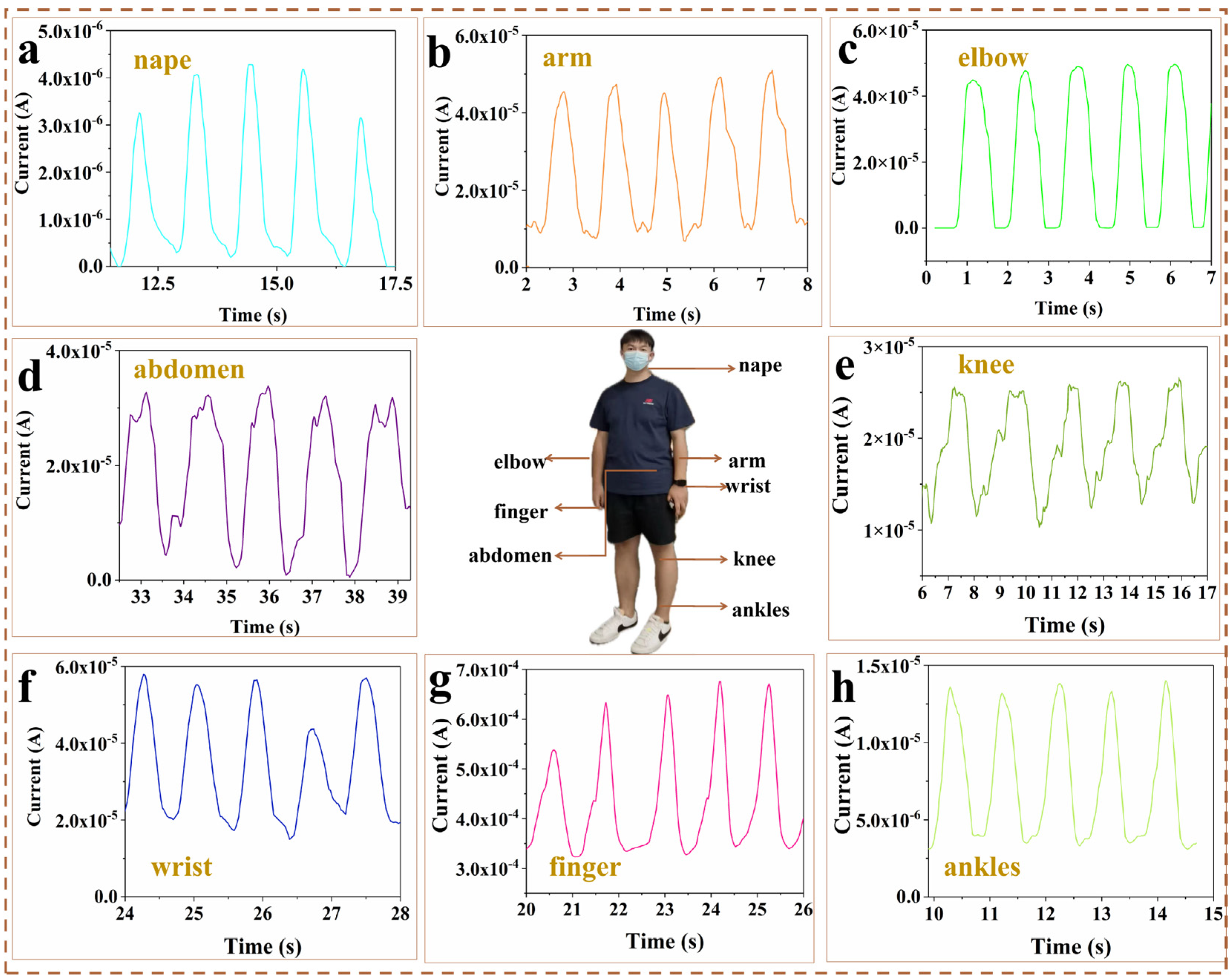
2.4. Applications for Human Activity Monitoring
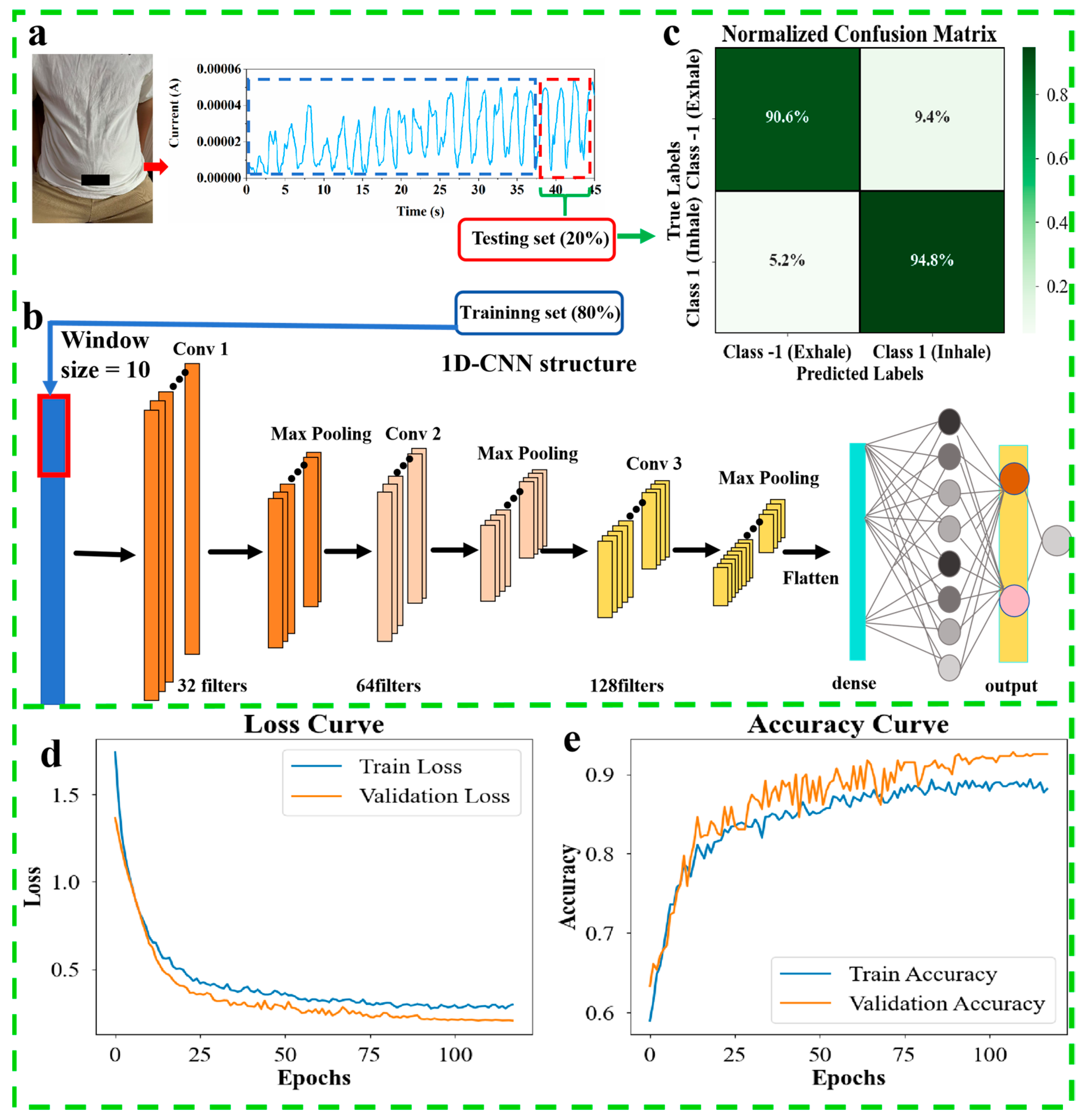
2.5. Deep Learning Analysis of Breathing Phase Classification Using a Single PVA/CNPs Hydrogel Film Sensor
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CNPs
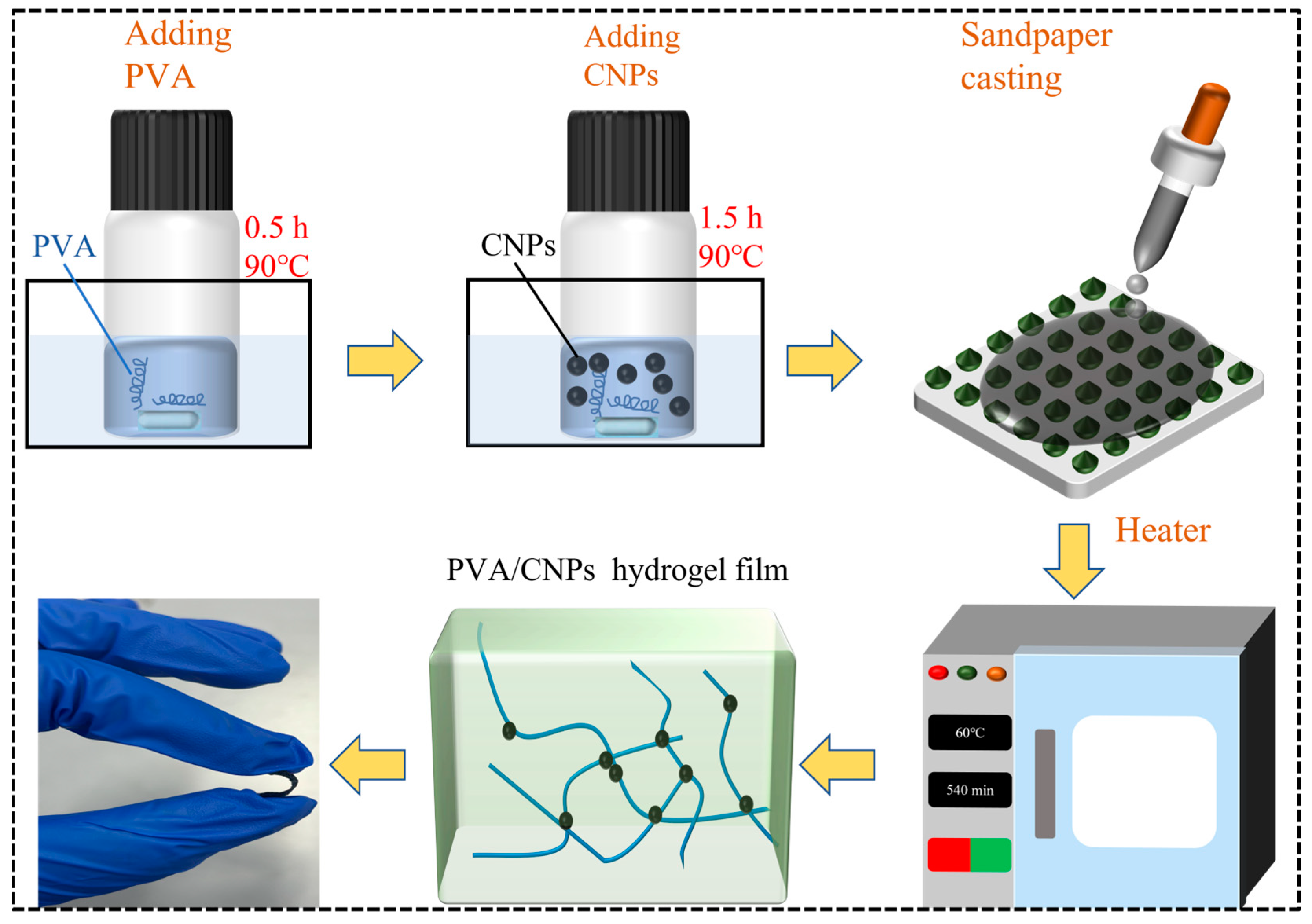
4.3. Fabrication of PVA/CNPs Hydrogel Film Sensor
4.4. CNPs Morphology Characterisation
4.5. Characterisation
4.6. 1D-CNN Model
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | polyvinyl alcohol |
| 1D-CNN | one-dimensional convolutional neural networks |
| CNPs | carbon nanospheres |
| CI | cuttlefish ink |
References
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Abe, M.; Muraki, K.; Tachibana, S.; Wang, Y.F.; Hong, J.; Tokito, S. Microporous induced fully printed pressure sensor for wearable soft robotics machine interfaces. Adv. Intell. Syst. 2020, 2, 2000179. [Google Scholar] [CrossRef]
- Rangarajan, S.l; Kidane, A.; Qian, G.; Rajko, S.; Birchfield, D. The design of a pressure sensing floor for movement-based human computer interaction. In Smart Sensing and Context; Springer: Berlin/Heidelberg, Germany, 2007; pp. 46–61. [Google Scholar]
- Kim, S.; Amjadi, M.; Lee, T.I.; Jeong, Y.; Kwon, D.; Kim, M.S.; Park, I. Wearable, ultrawide-range, and bending-insensitive pressure sensor based on carbon nanotube network-coated porous elastomer sponges for human interface and healthcare devices. ACS Appl. Mater. Interfaces 2019, 11, 23639–23648. [Google Scholar]
- Annese, V.F.; De Venuto, D.; Martin, C.; Cumming, D.R. Biodegradable pressure sensor for health-care. In Proceedings of the 21st IEEE International Conference on Electronics, Circuits and Systems (ICECS), Marseille, France, 7–10 December 2014; pp. 598–601. [Google Scholar]
- Pierre Claver, U.; Zhao, G. Recent progress in flexible pressure sensors based electronic skin. Adv. Eng. Mater. Adv. Eng. Mater. 2021, 23, 2001187. [Google Scholar]
- Nguyen, T.; Dinh, T.; Phan, H.P.; Pham, T.A.; Dau, V.T.; Nguyen, N.T.; Dao, D.V. Advances in ultrasensitive piezoresistive sensors: From conventional to flexible and stretchable applications. Mater. Horiz. 2021, 8, 2123–2150. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Z.; Chandrasekaran, S.; Liu, Y.; Wu, M. Self-healing, antibacterial, and conductive double network hydrogel for strain sensors. Carbohydr. Polym. 2023, 303, 120468. [Google Scholar] [CrossRef]
- Gu, J.; Huang, J.; Chen, G.; Hou, L.; Zhang, J.; Zhang, X.; Liu, H. Multifunctional poly (vinyl alcohol) nanocomposite organohydrogel for flexible strain and temperature sensor. ACS Appl. Mater. Interfaces 2020, 12, 40815–40827. [Google Scholar] [CrossRef]
- Zhu, B.; Niu, Z.; Wang, H.; Leow, W.R.; Li, Y.; Zheng, L.; Chen, X. Microstructured graphene arrays for highly sensitive flexible tactile sensors. Small 2014, 10, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-healable multifunctional electronic tattoos based on silk and graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar]
- Cheng, R.; Zeng, J.; Wang, B.; Li, J.; Cheng, Z.; Xu, J.; Chen, K. Ultralight, flexible and conductive silver nanowire/nanofibrillated cellulose aerogel for multifunctional strain sensor. Chem. Eng. J. 2021, 424, 130565. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Wen, Z.; Cheng, P.; Zheng, H.; Shao, H.; Lee, S.T. Liquid-metal-based super-stretchable and structure-designable triboelectric nanogenerator for wearable electronics. ACS Nano 2018, 12, 2027–2034. [Google Scholar] [CrossRef]
- Chen, T.; Yang, G.; Li, Y.; Li, Z.; Ma, L.; Yang, S.; Wang, J. Temperature-adaptable pressure sensors based on MXene-coated GO hierarchical aerogels with superb detection capability. Carbon 2022, 200, 47–55. [Google Scholar] [CrossRef]
- Han, F.; Luo, J.; Pan, R.; Wu, J.; Guo, J.; Wang, Y.; Zhang, Q. Vanadium dioxide nanosheets supported on carbonized cotton fabric as bifunctional textiles for flexible pressure sensors and zinc-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 41577–41587. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Yao, Y.; Ye, Y.; Zhang, C.; Xu, J.; Zhong, D.; Xia, L. Flexible strain sensor based on carbonized corn stalk with three-dimensional network. Ind. Crops Prod. 2025, 225, 120496. [Google Scholar] [CrossRef]
- Karthikeyan, N.; Vijayalakhmi, K.A. Low temperature plasma exposed activated peanut shell carbon with Al-doped CdO air cathode is employed in Al-air batteries and supercapacitors. J. Power Sources 2025, 632, 236358. [Google Scholar] [CrossRef]
- Heydari, M.; Moheb, A.; Ghiaci, M.; Masoomi, M. Effect of cross-linking time on the thermal and mechanical properties and pervaporation performance of poly(vinyl alcohol) membrane cross-linked with fumaric acid used for dehydration of isopropanol. J. Appl. Polym. Sci. 2013, 128, 1640–1651. [Google Scholar] [CrossRef]
- Hashim, H.; Abdallh, M.; Yousif, E. Studying the influence of cobalt chloride on the optical properties of poly (vinyl alcohol) films. J. Al-Nahrain Univ. 2012, 15, 40–45. [Google Scholar]
- Fernandes, D.M.; Andrade, J.L.; Lima, M.K.; Silva, M.F.; Andrade, L.H.C.; Lima, S.M.; Pineda, E.G. Thermal and photochemical effects on the structure, morphology, thermal and optical properties of PVA/Ni0.04Zn0.96O and PVA/Fe0.03Zn0.97O nanocomposite films. Polym. Degr. Stab. 2013, 98, 1862–1868. [Google Scholar] [CrossRef]
- Sreeja, S.; Sreedhanya, S.; Smijesh, N.; Philip, R.; Muneera, C.I. Organic dye impregnated poly(vinyl alcohol) nanocomposite as an efficient optical limiter: Structure, morphology and photophysical properties. J. Mater. Chem. C 2013, 1, 3851–3861. [Google Scholar] [CrossRef]
- Sreedhar, S.; Illyaskutty, N.; Sreedhanya, S.; Philip, R.; Muneera, C.I. An organic dyepolymer (phenol red-poly(vinyl alcohol)) composite architecture towards tunableoptical and -saturable absorption characteristics. J. Appl. Phys. 2016, 119, 193106. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, M.P.; Park, J.; Lee, Y.; Ghosh, S.K.; Kim, J.; Ko, H. Binary Spiky/spherical nanoparticle films with hierarchical micro/nanostructures for high-performance flexible pressure sensors. ACS Appl. Mater. Interfaces 2020, 12, 58403–58411. [Google Scholar]
- Tee, B.C.K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable flexible pressure sensors using microstructured elastomer geometries for intuitive electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Li, T.; Luo, H.; Qin, L.; Wang, X.; Xiong, Z.; Ding, H.; Zhang, T. Flexible capacitive tactile sensor based on micropatterned dielectric layer. Small 2016, 12, 5042–5048. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, C.; Yang, L.; Wu, J.; Li, J. Preparation of graphene/polydimethylsiloxane flexible resistive pressure sensors based on direct ink writing 3D printing. Sens. Actuators A Phys. 2025, 382, 116148. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Wang, W.; Zhao, N.; He, P.; Liu, J.; Yang, B. Flexible pressure sensors with combined spraying and self-diffusion of carbon nanotubes. ACS Appl. Mater. Interfaces 2022, 14, 38409–38420. [Google Scholar] [CrossRef]
- Song, K.; Zhao, R.; Wang, Z.L.; Yang, Y. Conjuncted pyro-piezoelectric effect for self-powered simultaneous temperature and pressure sensing. Adv. Mater. 2019, 31, 1902831. [Google Scholar] [CrossRef]
- Guo, H.; Pu, X.; Chen, J.; Meng, Y.; Yeh, M.H.; Liu, G.; Wang, Z.L. A highly sensitive, self-powered triboelectric auditory sensor for social robotics and hearing aids. Sci. Robot. 2018, 3, eaat2516. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, Y.; Feng, Z.; Sun, C.; Fan, W.; Zhou, Z.; Yang, J. Triboelectric vibration sensor for a human-machine interface built on ubiquitous surfaces. Nano Energy 2019, 59, 689–696. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Zhang, Z.; Shi, Q.; He, T.; Liu, H.; Lee, C. Haptic-feedback smart glove as a creative human-machine interface (HMI) for virtual/augmented reality applications. Sci. Adv. 2020, 6, eaaz8693. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Hao, S.; Peng, X.; Hu, L. Deep learning for sensor-based activity recognition: A survey. Pattern Recognit. Lett. 2019, 119, 3–11. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Zhu, M.; He, T.; Lee, C. Technologies toward next generation human machine interfaces: From machine learning enhanced tactile sensing to neuromorphic sensory systems. Appl. Phys. Rev. 2020, 7, 031305. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Zahrai, S.M.; Bitaraf, M. An integrated deep neural network model combining 1D CNN and LSTM for structural health monitoring utilizing multisensor time-series data, Struct. Health Monit. 2025, 24, 447–465. [Google Scholar]
- Shi, Q.; Zhang, Z.; He, T.; Sun, Z.; Wang, B.; Feng, Y.; Lee, C. Deep learning enabled smart mats as a scalable floor monitoring system. Nat. Commun. 2020, 11, 4609. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, W.; Ye, Z.; Dong, Y.; Wan, L.; Zhang, Z.; Wang, Z. In Situ graft-on fibrous composites and nanostructure interlocking facilitate highly stable wearable sensors for sids prevention. Adv. Fiber Mater. 2024, 6, 825–840. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, L.; Huang, J.; Li, S.; Chen, Z.; Zhang, W.; Lai, Y. Rational designedmicrostructure pressure sensors with highly sensitive and wide detection range performance. J. Mater. Sci. Technol. 2022, 130, 184–192. [Google Scholar]
- Bai, N.; Wang, L.; Wang, Q.; Deng, J.; Wang, Y.; Lu, P.; Guo, C.F. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat. Commun. 2020, 11, 209. [Google Scholar] [CrossRef]
- Taspika, M.; Permatasari, F.A.; Nuryadin, B.W.; Mayangsari, T.R.; Aimon, A.H.; Iskandar, F. Simultaneous ultraviolet and first near-infrared window absorption of luminescent carbon dots/PVA composite film. RSC Adv. 2019, 9, 7375–7381. [Google Scholar]
- Yang, W.; Han, X.; Yin, C.; Zhang, X.; Peng, Q.; Yi, C. Biomass carbon nanosphere-based piezoresistive flexible pressure sensors for motion capture and health monitoring. Compos. Commun. 2024, 51, 102041. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels synthesis, biological and physicochemical properties and silver release kinetics. Compos. Part B Eng. 2018, 154, 75–185. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.X.; Li, Z.M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, X.; Li, G.; Wang, L.; Bai, N.; Guo, C.F. Highly stable flexible pressure sensors with a quasi-homogeneous composition and interlinked interfaces. Nat. Commun. 2022, 13, 1317. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Y.; Fan, L.; Ye, D.; Xu, W.; Xu, J. Ultralight PPy@PVA/BC/MXene composite aerogels for high-performance, supercapacitor eltrodes and pressure sensors. Appl. Surf. Sci. 2023, 624, 157138. [Google Scholar] [CrossRef]
- Luo, F.; Chen, B.; Ran, X.; Ouyang, W.; Yao, Y.; Shang, L. Wearable and self-powered triboelectric sensors based on NaCl/PVA hydrogel for driver multidimensional information monitoring. Nano Energy 2023, 118, 109035. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Wang, H.; Yu, B.; Qian, L.; Zhao, Z.J. Starfish-inspired ultrasensitive piezoresistive pressure sensor with an ultra-wide detection range for healthcare and intelligent production. Chem. Eng. J. 2024, 497, 154953. [Google Scholar]
- Hyun, J.E.; Lim, T.; Kim, S.H.; Lee, J.H. Wearable ion gel based pressure sensor with high sensitivity and ultra-wide sensing range for human motion detection. Chem. Eng. J. 2024, 484, 149464. [Google Scholar] [CrossRef]
- Cao, K.; Wu, M.; Bai, J.; Wen, Z.; Zhang, J.; Wang, T.; Jiang, L. Beyond skin pressure sensing: 3D printed laminated graphene pressure sensing material combines extremely low detection limits with wide detection range. Adv. Funct. Mater. 2022, 32, 2202360. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; He, J.; Huo, Z.; Zhou, R.; Han, X.; Zhai, J. Localizing strain via micro-cage structure for stretchable pressure sensor arrays with ultralow spatial crosstalk. Nat. Commun. 2023, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qiao, Y.; Guo, R.; Naveed, S.; Hirtz, T.; Li, X.; Ren, T.L. Triode-mimicking graphene pressure sensor with positive resistance variation for physiology and motion monitoring. ACS Nano 2020, 14, 10104–10114. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Li, Z.; Zheng, T.; Wang, J.; Zhao, Z.J.; Zhao, L.; Jiang, Z. Highly-sensitive, broad-range, and highly-dynamic MXene pressure sensors with multi-level nano-microstructures for healthcare and soft robots applications. Chem. Eng. J. 2024, 485, 149750. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, C.; Xie, H.; Niu, S.; Han, Z.; Ren, L. A continuous pressure positioning sensor with flexible multilayer structures based on a combinatorial bionic strategy. Adv. Funct. Mater. 2024, 34, 2314479. [Google Scholar] [CrossRef]
- Lei, P.; Bao, Y.; Zhang, W.; Gao, L.; Zhu, X.; Xu, J.; Ma, J. Synergy of ZnO nanowire arrays and electrospun membrane gradient wrinkles in piezoresistive materials for wide-sensing range and high-sensitivity flexible pressure sensor. Adv. Fiber Mater. 2024, 6, 414–429. [Google Scholar] [CrossRef]
- Cui, X.; Jiang, Y.; Hu, L.; Cao, M.; Xie, H.; Zhang, X.; Zhu, Y. Synergistically microstructured flexible pressure sensors with high sensitivity and ultrawide linear range for full-range human physiological monitoring. Adv. Mater. Technol. 2023, 8, 2200609. [Google Scholar] [CrossRef]
- Su, S.; Zhang, X.; Dang, D.; Wang, Z.; Tong, Z. A high-performance flexible capacitive pressure sensor with 3-d printed hemispherical graded microstructures. IEEE Sens. J. 2024, 24, 5966–5975. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Liu, Y.; Yang, C.; Liu, W.; Qi, M.; Zhang, D. PDMS film-based flexible pressure sensor array with surface protruding structure for human motion detection and wrist posture recognition. ACS Appl. Mater. Interfaces 2024, 16, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.T.; Le, H.A.T.; Ra, Y.; Weldemhret, T.G.; Kim, H.; Choi, K.; Park, Y.T. Recovered graphene-hydrogel nanocomposites for multi-modal human. Compos. Part B Eng. 2025, 291, 111997. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Z.; Pan, X.; Ye, W.; Bermak, A. Mixture gases classification based on multi-label one-dimensional deep convolutional neural network. IEEE Access 2019, 7, 12630–12637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Zhang, X.; Shu, Z.; Yin, C.; Liu, B. Deep Learning-Enabled Flexible PVA/CNPs Hydrogel Film Sensor for Abdominal Respiration Monitoring. Gels 2025, 11, 743. https://doi.org/10.3390/gels11090743
Peng C, Zhang X, Shu Z, Yin C, Liu B. Deep Learning-Enabled Flexible PVA/CNPs Hydrogel Film Sensor for Abdominal Respiration Monitoring. Gels. 2025; 11(9):743. https://doi.org/10.3390/gels11090743
Chicago/Turabian StylePeng, Chengcheng, Xinjiang Zhang, Ziyan Shu, Cailiu Yin, and Baorong Liu. 2025. "Deep Learning-Enabled Flexible PVA/CNPs Hydrogel Film Sensor for Abdominal Respiration Monitoring" Gels 11, no. 9: 743. https://doi.org/10.3390/gels11090743
APA StylePeng, C., Zhang, X., Shu, Z., Yin, C., & Liu, B. (2025). Deep Learning-Enabled Flexible PVA/CNPs Hydrogel Film Sensor for Abdominal Respiration Monitoring. Gels, 11(9), 743. https://doi.org/10.3390/gels11090743





