Amphiphilic Thermoresponsive Triblock PLA-PEG-PLA and Diblock mPEG-PLA Copolymers for Controlled Deferoxamine Delivery
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Copolymers
2.2. Glass Transition and Crystallization
2.2.1. XRD
2.2.2. DSC
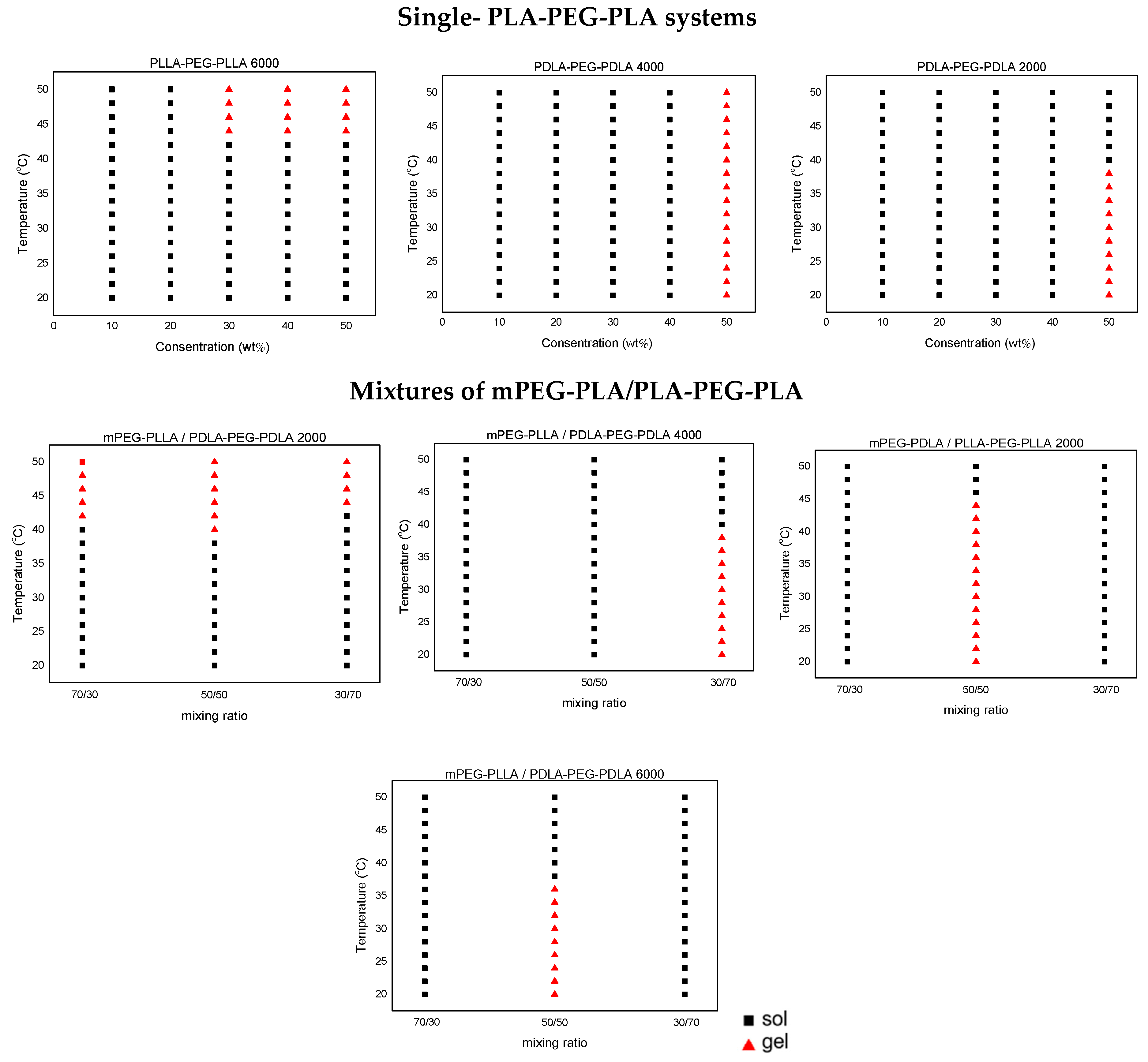
2.3. Evaluation of Thermoresponsive Behavior and In Situ Gelation
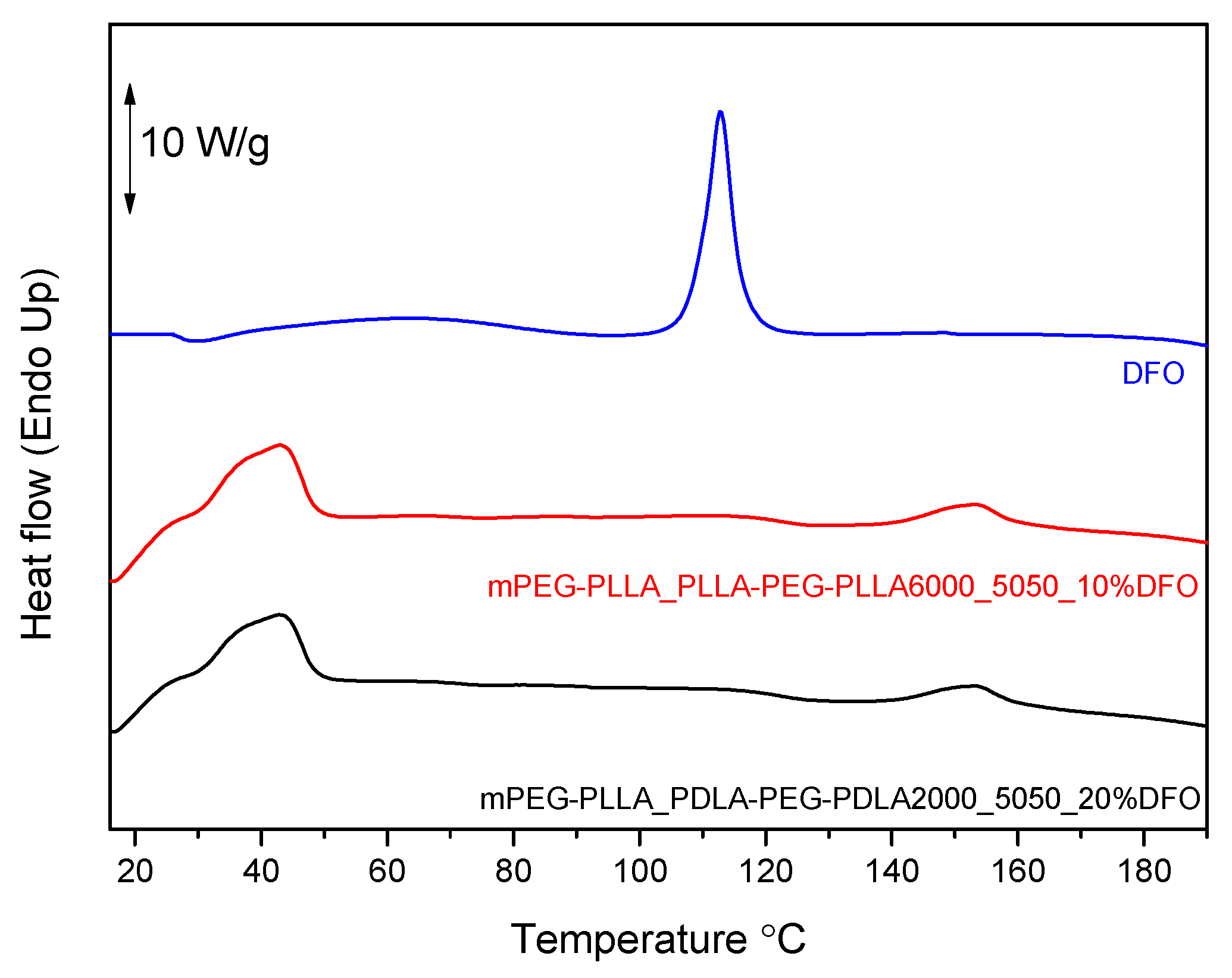
2.4. DFO-Loaded Hydrogels
2.4.1. Characterization of DFO-Loaded Hydrogels
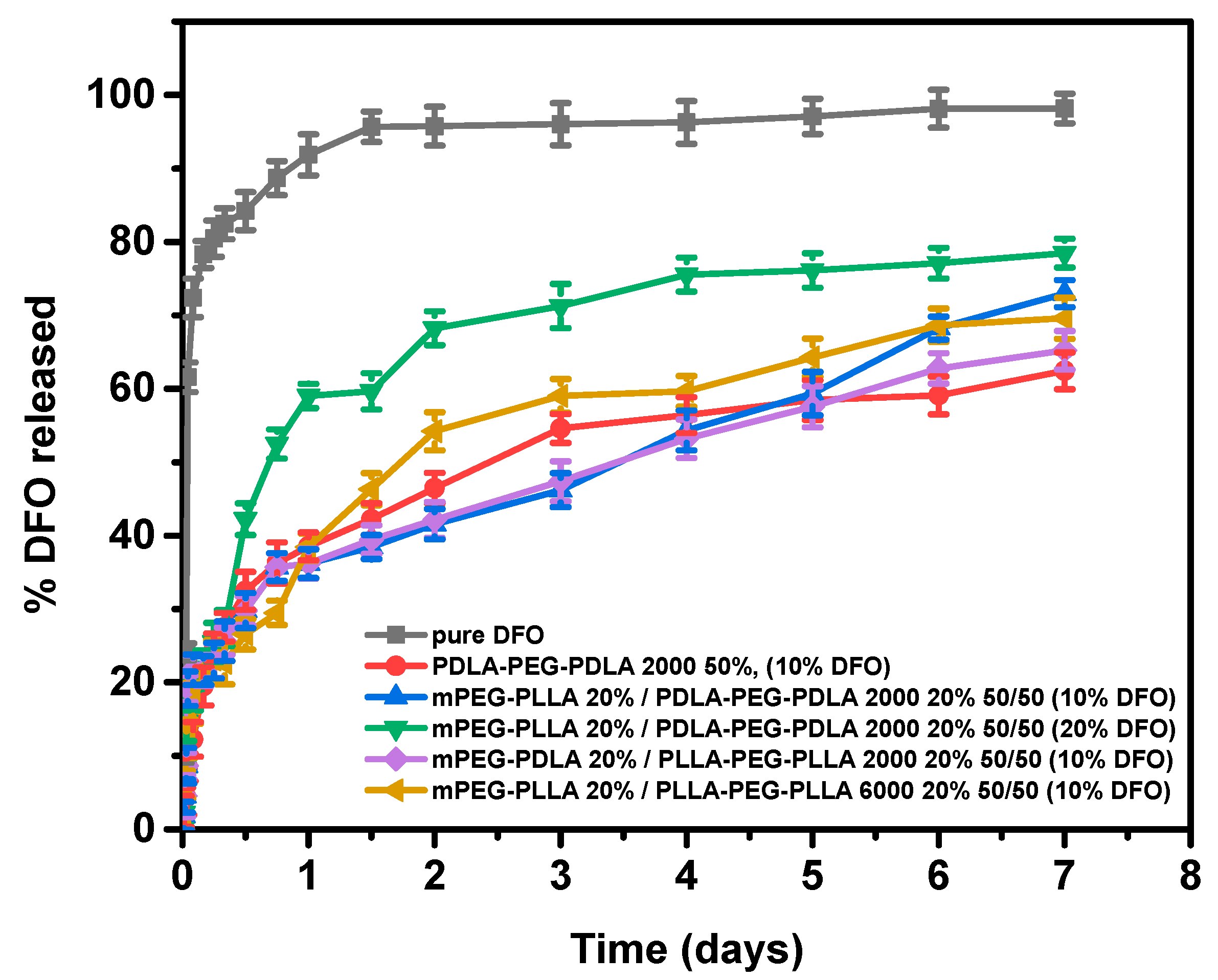
2.4.2. In Vitro Drug Release
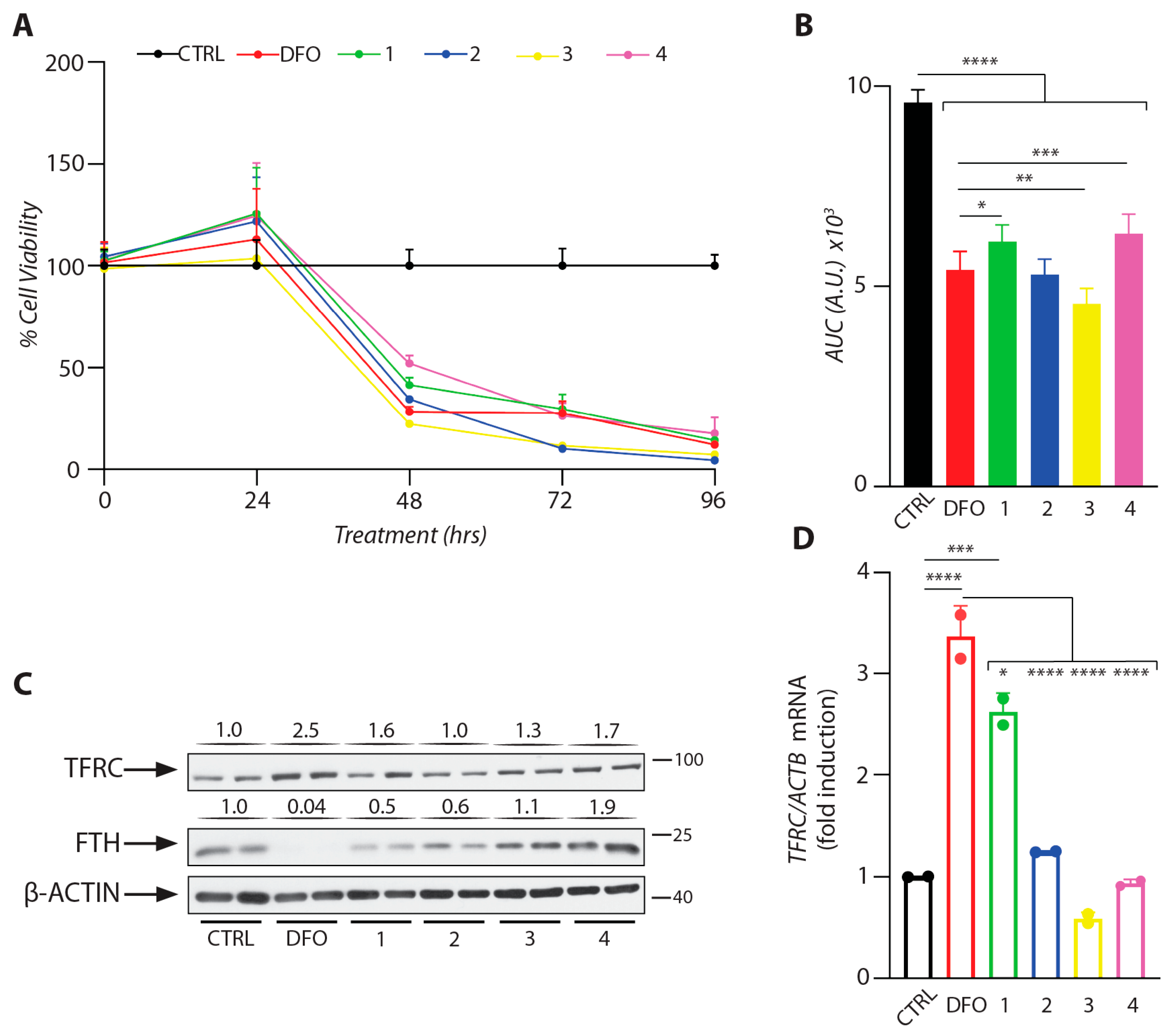
2.4.3. Biological Activity of DFO-Loaded Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PLA-PEG-PLA Triblock and mPEG-PLA Diblock Copolymers
4.3. Characterization of the Synthesized Copolymers
4.3.1. Nuclear Magnetic Resonance (1H NMR)
4.3.2. Fourier-Transform Infrared Spectroscopy (ATR–FTIR)
4.3.3. Gel Permeation Chromatography (GPC)
4.3.4. Differential Scanning Calorimetry (DSC)
4.3.5. X-Ray Diffraction (XRD)
4.4. In Situ Gelation and Investigation of Thermoresponsive Behavior
4.5. High-Pressure Liquid Chromatography (HPLC), Quantitative Analysis, and Drug Loading
4.6. In Vitro Evaluation of Cell Viability
4.6.1. Cell Culture
4.6.2. Quantitative Real-Time PCR (qRT-PCR)
4.6.3. Western Blot
4.6.4. Crystal Violet Cell Viability Assay
4.6.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLA | Poly(Lactic Acid) |
| PEG | Polyethylene Glycol |
| DFO | Deferoxamine |
| DSC | Differential Scanning Calorimetry |
| XRD | X-Ray Diffraction |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
References
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Brittenham, G.M.; Griffith, P.M.; Nienhuis, A.W.; McLaren, C.E.; Young, N.S.; Tucker, E.E.; Allen, C.J.; Farrell, D.E.; Harris, J.W. Efficacy of Deferoxamine in Preventing Complications of Iron Overload in Patients with Thalassemia Major. N. Engl. J. Med. 1994, 331, 567–573. [Google Scholar] [CrossRef]
- Velasquez, J.; Wray, A.A. Deferoxamine; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Saito, N.; Murakami, N.; Takahashi, J.; Horiuchi, H.; Ota, H.; Kato, H.; Okada, T.; Nozaki, K.; Takaoka, K. Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv. Drug Deliv. Rev. 2005, 57, 1037–1048. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Bikiaris, N.D.; Xanthopoulou, E.; Ioannidis, R.O.; Bikiaris, D.N. A decade of innovation: Synthesis, properties and applications of PLA copolymers. Prog. Polym. Sci. 2025, 167, 101991. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties-from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging uses of PLA–PEG copolymer in cancer drug delivery. 3 Biotech 2022, 12, 41. [Google Scholar] [CrossRef]
- Yuan, Y.; Raheja, K.; Milbrandt, N.B.; Beilharz, S.; Tene, S.; Oshabaheebwa, S.; Gurkan, U.A.; Samia, A.C.S.; Karayilan, M. Thermoresponsive polymers with LCST transition: Synthesis, characterization, and their impact on biomedical frontiers. RSC Appl. Polym. 2023, 1, 158–189. [Google Scholar] [CrossRef]
- Mao, H.; Pan, P.; Shan, G.; Bao, Y. In situ formation and gelation mechanism of thermoresponsive Stereocomplexed hydrogels upon mixing diblock and triblock poly(lactic acid)/poly(ethylene glycol) copolymers. J. Phys. Chem. B 2015, 119, 6471–6480. [Google Scholar] [CrossRef] [PubMed]
- Abebe, D.G.; Fujiwara, T. Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLA prepared via hybrid micelles of pre-mixed copolymers with different PEG lengths. Biomacromolecules 2012, 13, 1828–1836. [Google Scholar] [CrossRef]
- Movaffagh, J.; Hadizadeh, F.; Khodaverdi, E.; Khalili, B.; Shiadeh, S.N.R.; Kamali, H.; Oroojalian, F. Preparation and in vitro evaluation of injectable formulations of levothyroxine sodium using in situ forming hydrogel temperature-responsive systems based on PLA-PEG-PLA and PLGA-PEG-PLGA triblock copolymers. Iran. J. Basic Med. Sci. 2022, 25, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Lippens, E.; Swennen, I.; Gironès, J.; Declercq, H.; Vertenten, G.; Vlaminck, L.; Gasthuys, F.; Schacht, E.; Cornelissen, R. Cell survival and proliferation after encapsulation in a chemically modified Pluronic® F127 hydrogel. J. Biomater. Appl. 2013, 27, 828–839. [Google Scholar] [CrossRef]
- Javali, M.A.; Vandana, K.L. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian Soc. Periodontol. 2012, 16, 43–48. [Google Scholar] [CrossRef]
- Xu, G.; Chen, S.; Yan, X.; Yang, C.; Chen, Z. Synthesis and Hydrophilic Performance of Poly(Lactic Acid)-Poly(Ethylene Glycol) Block Copolymers. Am. J. Anal. Chem. 2016, 7, 299–305. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. PLA-PEG-PLA copolymer-based polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs: Preparation and evaluation with atorvastatin and lisinopril. Drug Dev. Ind. Pharm. 2014, 40, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Sringam, J.; Kajornprai, T.; Trongsatitkul, T.; Suppakarn, N. Shape Memory Performance and Microstructural Evolution in PLA/PEG Blends: Role of Plasticizer Content and Molecular Weight. Polymers 2025, 17, 225. [Google Scholar] [CrossRef]
- Feng, L.; Ding, J.; Hu, H.; Lv, Z.; Zhang, Y.; Xu, B.; Quan, J.; Hao, S.; Fan, H.; Hang, Z. Preparation and Characterization of Bio-Based PLA/PEG/g-C3N4 Low-Temperature Composite Phase Change Energy Storage Materials. Polymers 2023, 15, 2872. [Google Scholar] [CrossRef]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Confined crystallization, melting behavior and morphology in PEG-b-PLA diblock copolymers: Amorphous versus crystalline PLA. J. Polym. Sci. 2020, 58, 455–465. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Klonos, P.A.; Christodoulou, E.; Barmpalexis, P.; Kyritsis, A. Plasticization Effects of PEG of Low Molar Fraction and Molar Mass on the Molecular Dynamics and Crystallization of PLA-b-PEG-b-PLA Triblock Copolymers Envisaged for Medical Applications. J. Phys. Chem. B 2025, 129, 3514–3528. [Google Scholar] [CrossRef]
- Klonos, P.A.; Evangelopoulou, A.; Terzopoulou, Z.; Zamboulis, A.; Valera, M.Á.; Mangas, A.; Kyritsis, A.; Bikiaris, D.N. Revisiting Non-Conventional Crystallinity-Induced Effects on Molecular Mobility in Sustainable Diblock Copolymers of Poly(propylene adipate) and Polylactide. Molecules 2022, 27, 7449. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Klonos, P.A.; Kyritsis, A.; Barmpalexis, P. Structural and thermodynamical investigation in triblock copolymers of polylactide and poly(ethylene glycol), PLA-b-PEG-b-PLA, envisaged for medical applications. Mater. Today Commun. 2023, 38, 107799. [Google Scholar] [CrossRef]
- Furushima, Y.; Schick, C.; Toda, A. Crystallization; recrystallization, and melting of polymer crystals on heating and cooling examined with fast scanning calorimetry. Polym. Cryst. 2018, 1, e10005. [Google Scholar] [CrossRef]
- Klonos, P.A.; Terzopoulou, Z.; Zamboulis, A.; Valera, M.Á.; Mangas, A.; Kyritsis, A.; Pissis, P.; Bikiaris, D.N. Direct and indirect effects on molecular mobility in renewable polylactide-poly(propylene adipate) block copolymers as studied via dielectric spectroscopy and calorimetry. Soft Matter 2022, 18, 3725–3737. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Physical crosslinking of hydrogels: The potential of dynamic and reversible bonds in burn care. Coord. Chem. Rev. 2025, 542, 216868. [Google Scholar] [CrossRef]
- Prasser, Q.; Fuhs, T.; Torger, B.; Neubert, R.; Brendler, E.; Vogt, C.; Mertens, F.; Plamper, F.A. Nonequilibrium Colloids: Temperature-Induced Bouquet Formation of Flower-like Micelles as a Time-Domain-Shifting Macromolecular Heat Alert. ACS Appl. Mater. Interfaces 2023, 15, 57950–57959. [Google Scholar] [CrossRef]
- Shibata, M.; Terashima, T.; Koga, T. Micellar aggregation and thermogelation of amphiphilic random copolymers in water hierarchically dependent on chain length. Eur. Polym. J. 2022, 168, 111091. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules 1999, 32, 7064–7069. [Google Scholar] [CrossRef]
- Umemura, M.; Kim, J.-H.; Aoyama, H.; Hoshino, Y.; Fukumura, H.; Nakakaji, R.; Sato, I.; Ohtake, M.; Akimoto, T.; Narikawa, M.; et al. The iron chelating agent, deferoxamine detoxifies Fe(Salen)-induced cytotoxicity. J. Pharmacol. Sci. 2017, 134, 203–210. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, C.; Gong, K.; Li, H.; Song, H.; Zhang, Y.; Ding, D.; Liu, J.; Guo, J.; Fang, L. Mussel-inspired quaternary composite hydrogels with high strength and high tissue adhesion for transdermal drug delivery: Synergistic hydrogen bonding and drug release mechanism. Chem. Eng. J. 2023, 465, 142942. [Google Scholar] [CrossRef]
- El-Gizawy, S.A.; Nouh, A.; Saber, S.; Kira, A.Y. Deferoxamine-loaded transfersomes accelerates healing of pressure ulcers in streptozotocin-induced diabetic rats. J. Drug Deliv. Sci. Technol. 2020, 58, 101732. [Google Scholar] [CrossRef]
- Xu, L.; Guan, R.; Yu, B.; Li, Y.; Liu, H.; Jiang, Y. Fluorene methoxycarbonyl-PEG-deferoxamine conjugates ‘hitchhike’ with albumin in situ for iron overload therapy. Int. J. Pharm. 2022, 625, 122136. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Kołbuk, D.; Ciechomska, M.; Jeznach, O.; Sajkiewicz, P. Effect of crystallinity and related surface properties on gene expression of primary fibroblasts. RSC Adv. 2022, 12, 4016–4028. [Google Scholar] [CrossRef]
- Bajbouj, K.; Shafarin, J.; Hamad, M. High-dose deferoxamine treatment disrupts intracellular iron homeostasis, reduces growth, and induces apoptosis in metastatic and nonmetastatic breast cancer cell lines. Technol. Cancer Res. Treat. 2018, 17, 1533033818764470. [Google Scholar] [CrossRef]
- Saletta, F.; Rahmanto, Y.S.; Siafakas, A.R.; Richardson, D.R. Cellular iron depletion and the mechanisms involved in the iron-dependent regulation of the growth arrest and DNA damage family of genes. J. Biol. Chem. 2011, 286, 35396–35406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Suh, J.M.; Sohn, Y.S.; Bae, Y.H.; Kim, S.W.; Jeong, B. Thermogelling poly(caprolactone-6-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules 2005, 38, 5260–5265. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, 343–346. [Google Scholar] [CrossRef]
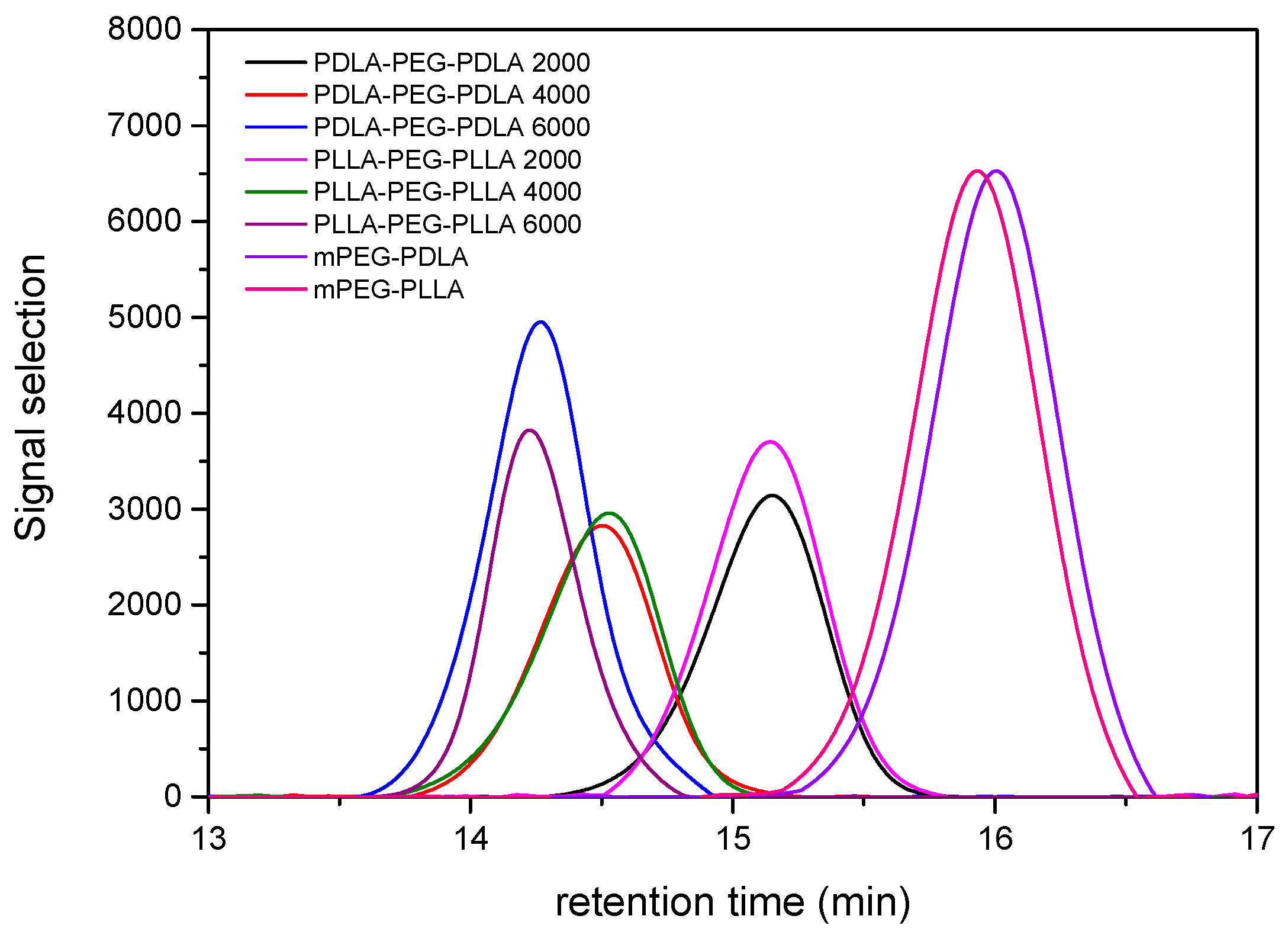
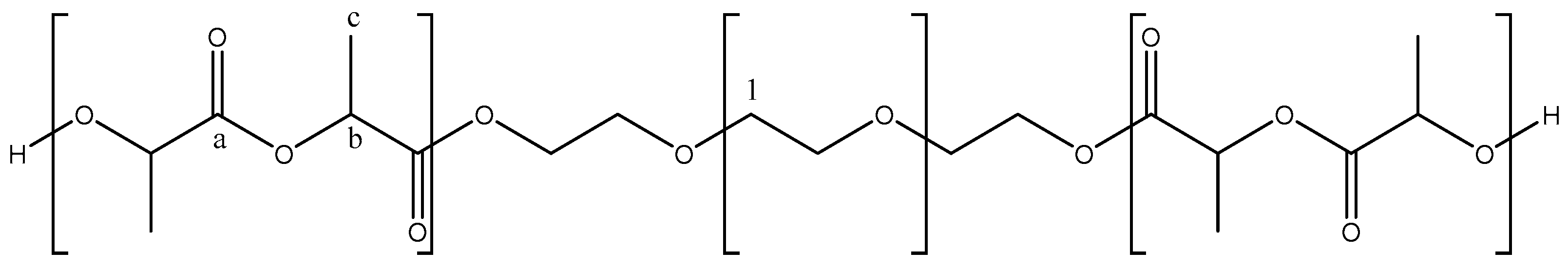
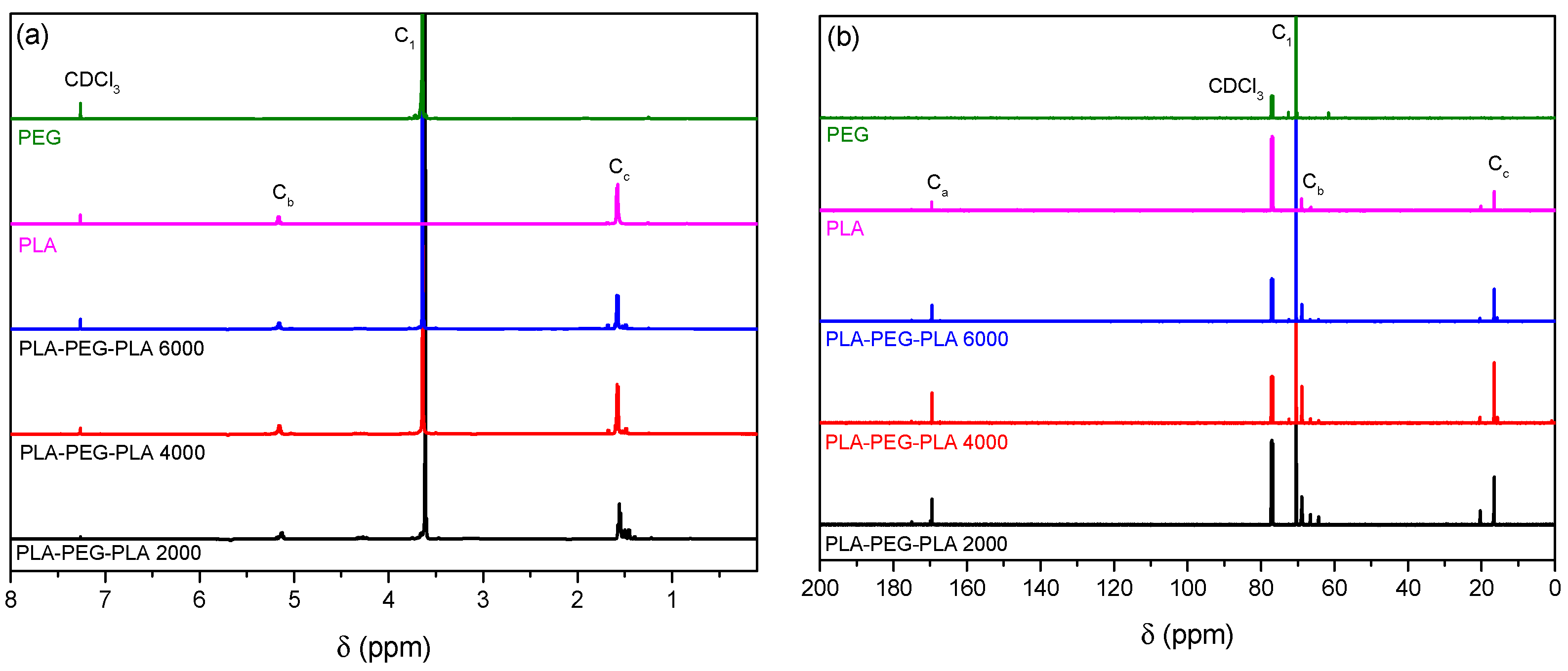

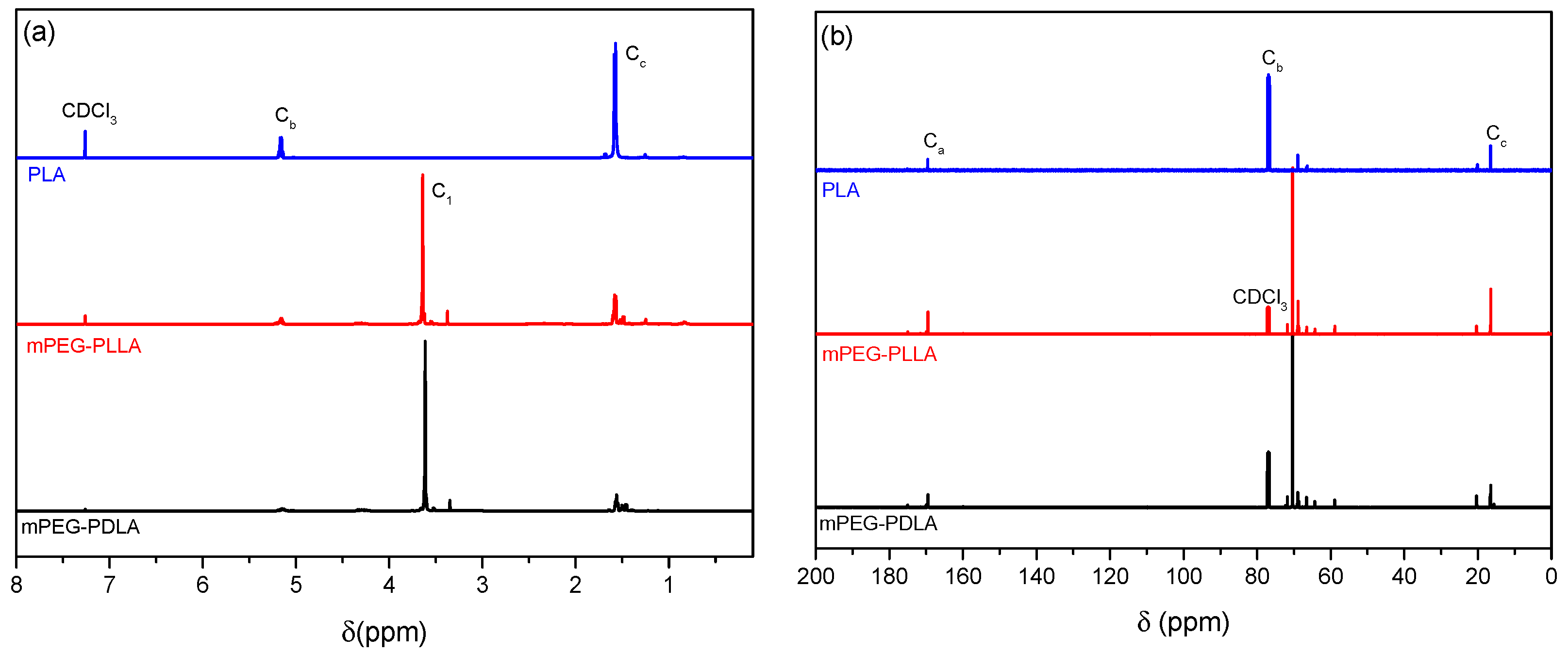
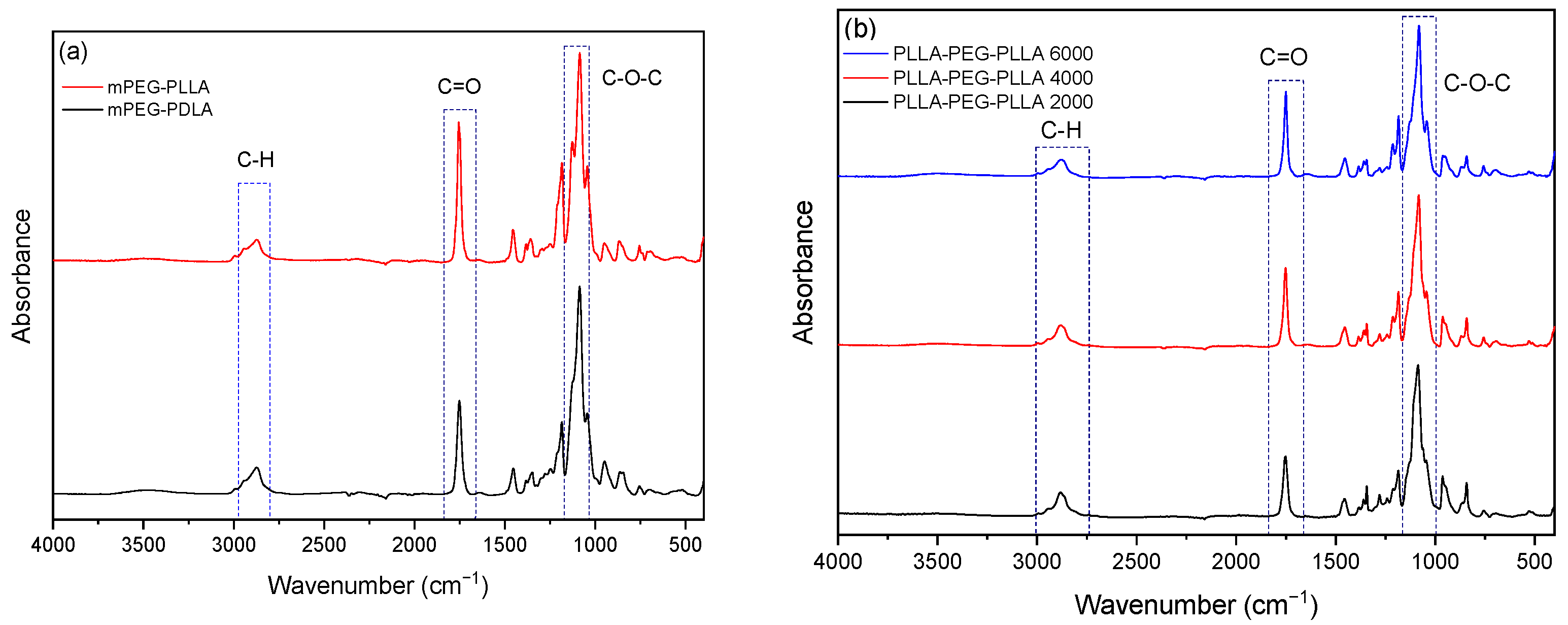
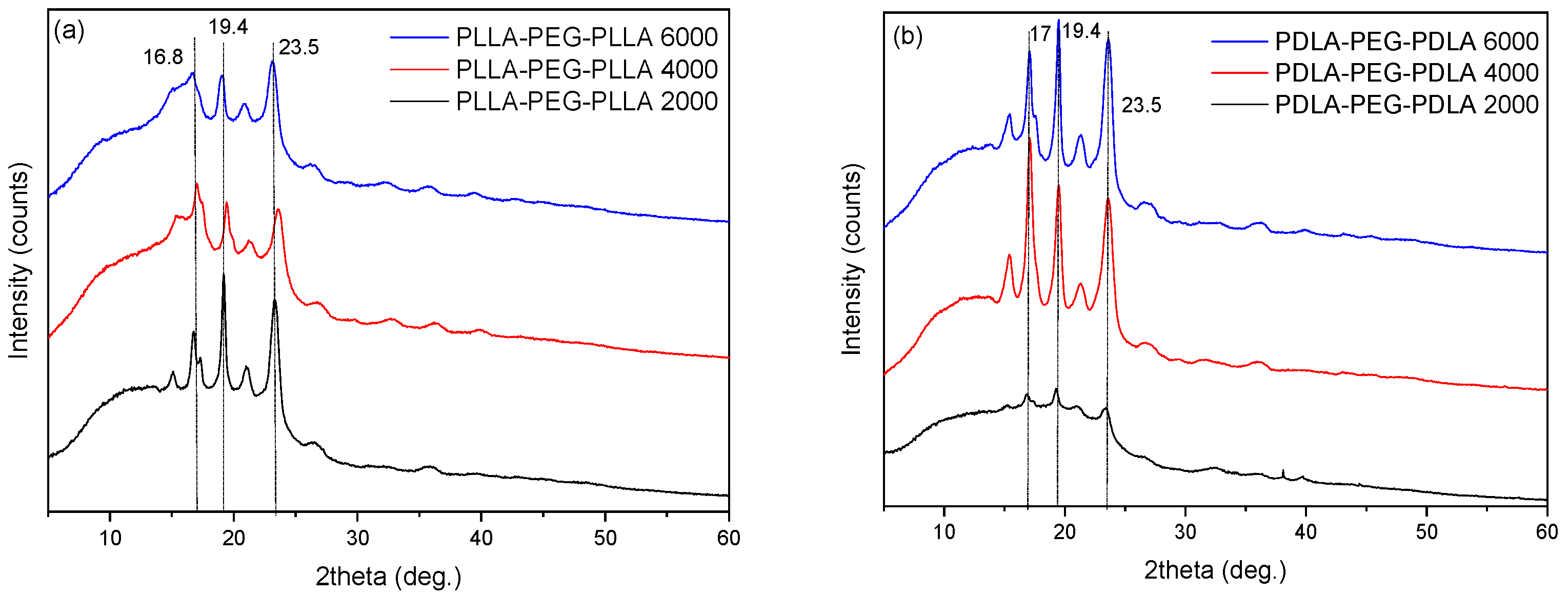
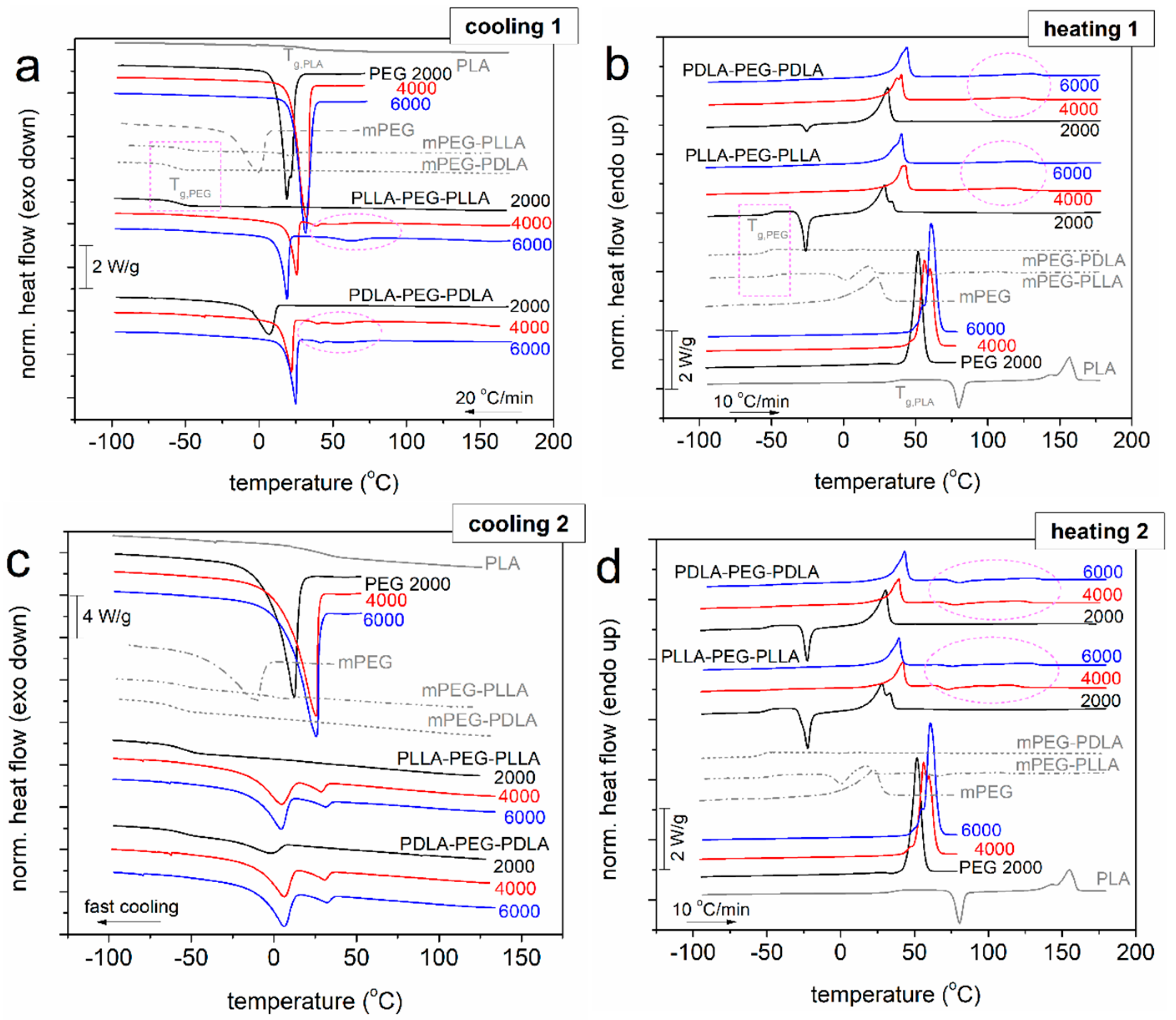
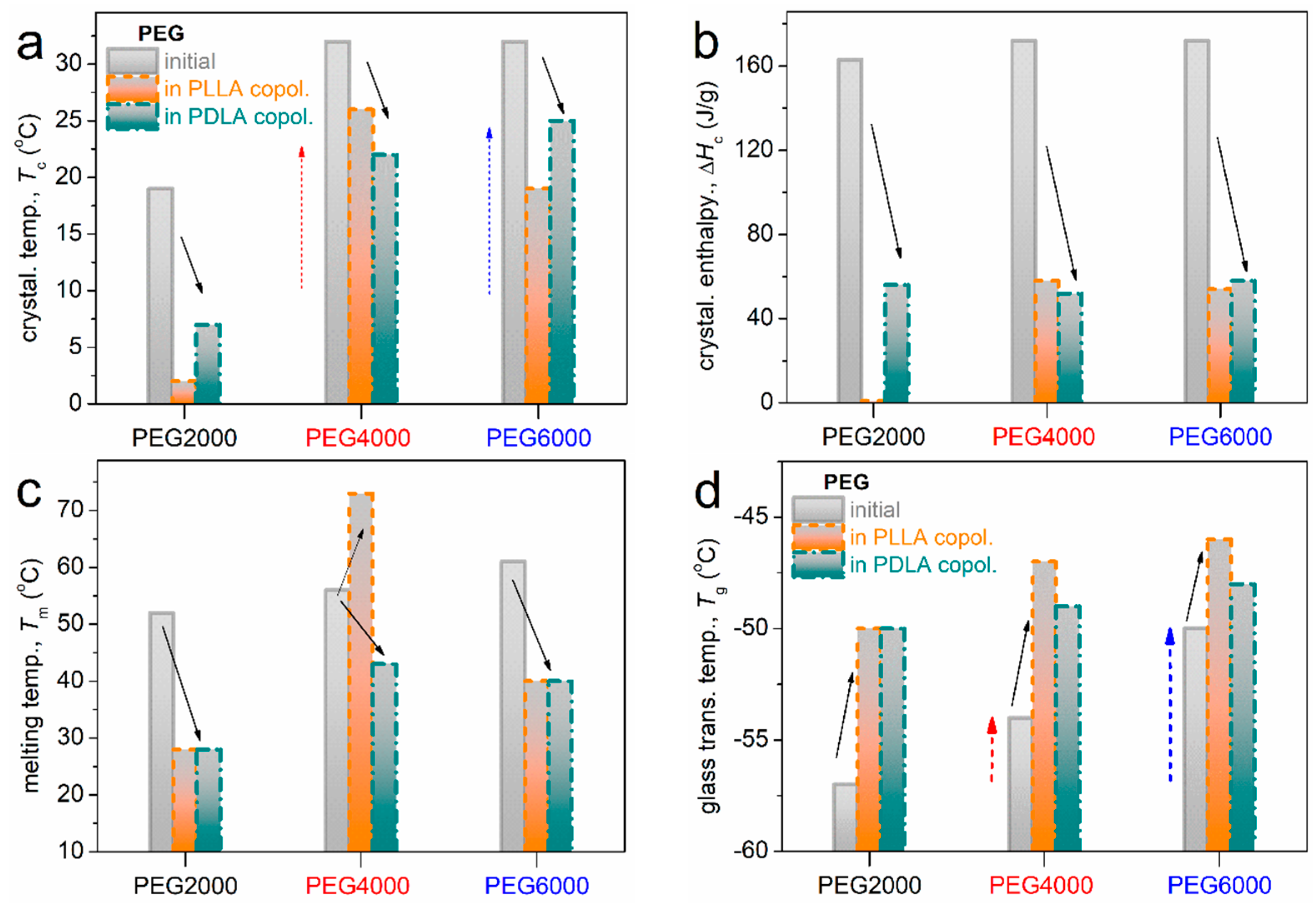

| Block Copolymer | Mn (g/mol) | PDI |
|---|---|---|
| PDLA-PEG-PDLA 2000 | 6251 | 1.06 |
| PDLA-PEG-PDLA 4000 | 13,374 | 1.05 |
| PDLA-PEG-PDLA 6000 | 17,343 | 1.04 |
| PLLA-PEG-PLLA 2000 | 6266 | 1.05 |
| PLLA-PEG-PLLA 4000 | 12,736 | 1.05 |
| PLLA-PEG-PLLA 6000 | 17,817 | 1.03 |
| mPEG-PDLA | 2209 | 1.08 |
| mPEG-PLLA | 2530 | 1.10 |
| Formulation | Sample | DFO (mg) | PBS (% w/v) | Mass Ratio |
|---|---|---|---|---|
| 1 | mPEG-PLLA 750/PDLA-PEG-PDLA 2000 | 2 | 20% | 50/50 |
| 20% | ||||
| 2 | mPEG-PDLA 750/PLLA-PEG-PLLA 2000 | 2 | 20% | 50/50 |
| 20% | ||||
| 3 | mPEG-PLLA 750/PLLA-PEG-PLLA 2000 | 2 | 20% | 50/50 |
| 20% | ||||
| 4 | PDLA-PEG-PDLA 2000 | 2 | 50% | - |
| PLLA-PEG-PLLA/PBS (% w/w) | |||||
|---|---|---|---|---|---|
| PLLA-PEG-PLLA (1000) | 10% | 20% | 30% | 40% | 50% |
| PLLA-PEG-PLLA (1500) | 10% | 20% | 30% | 40% | 50% |
| PLLA-PEG-PLLA (2000) | 10% | 20% | 30% | 40% | 50% |
| PLLA-PEG-PLLA (4000) | 10% | 20% | 30% | 40% | 50% |
| PLLA-PEG-PLLA (6000) | 10% | 20% | 30% | 40% | 50% |
| PDLA-PEG-PDLA (2000) | 10% | 20% | 30% | 40% | 50% |
| PDLA-PEG-PDLA (4000) | 10% | 20% | 30% | 40% | 50% |
| PDLA-PEG-PDLA (6000) | 10% | 20% | 30% | 40% | 50% |
| mPEG-PLLA | 10% | 20% | 30% | 40% | 50% |
| mPEG-PDLA | 10% | 20% | 30% | 40% | 50% |
| Polymer Blends (Mass Ratio 70:30, 50:50, and 30:70) |
|---|
| mPEG-PLLA/PDLA-PEG-PDLA (2000) |
| mPEG-PLLA/PDLA-PEG-PDLA (4000) |
| mPEG-PLLA/PDLA-PEG-PDLA (6000) |
| mPEG-PDLA/PLLA-PEG-PLLA (2000) |
| mPEG-PDLA/PLLA-PEG-PLLA (4000) |
| mPEG-PDLA/PLLA-PEG-PLLA (6000) |
| mPEG-PLLA/PLLA-PEG-PLLA (6000) |
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| TFRC | GCAAGTAGATGGCGATAACAG | GACGATCACAGCAATAGTCCC |
| ACTB | AGGATGCAGAAGGAGATCACT | GGGTGTAACGCAACTAAGTCATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikiaris, N.D.; Malini, E.; Christodoulou, E.; Klonos, P.A.; Kyritsis, A.; Galaris, A.; Pantopoulos, K. Amphiphilic Thermoresponsive Triblock PLA-PEG-PLA and Diblock mPEG-PLA Copolymers for Controlled Deferoxamine Delivery. Gels 2025, 11, 742. https://doi.org/10.3390/gels11090742
Bikiaris ND, Malini E, Christodoulou E, Klonos PA, Kyritsis A, Galaris A, Pantopoulos K. Amphiphilic Thermoresponsive Triblock PLA-PEG-PLA and Diblock mPEG-PLA Copolymers for Controlled Deferoxamine Delivery. Gels. 2025; 11(9):742. https://doi.org/10.3390/gels11090742
Chicago/Turabian StyleBikiaris, Nikolaos D., Ermioni Malini, Evi Christodoulou, Panagiotis A. Klonos, Apostolos Kyritsis, Apostolos Galaris, and Kostas Pantopoulos. 2025. "Amphiphilic Thermoresponsive Triblock PLA-PEG-PLA and Diblock mPEG-PLA Copolymers for Controlled Deferoxamine Delivery" Gels 11, no. 9: 742. https://doi.org/10.3390/gels11090742
APA StyleBikiaris, N. D., Malini, E., Christodoulou, E., Klonos, P. A., Kyritsis, A., Galaris, A., & Pantopoulos, K. (2025). Amphiphilic Thermoresponsive Triblock PLA-PEG-PLA and Diblock mPEG-PLA Copolymers for Controlled Deferoxamine Delivery. Gels, 11(9), 742. https://doi.org/10.3390/gels11090742










