Applications of Conductive Polymer Hydrogels for Supercapacitor, Solar Cell, and Energy Conversion
Abstract
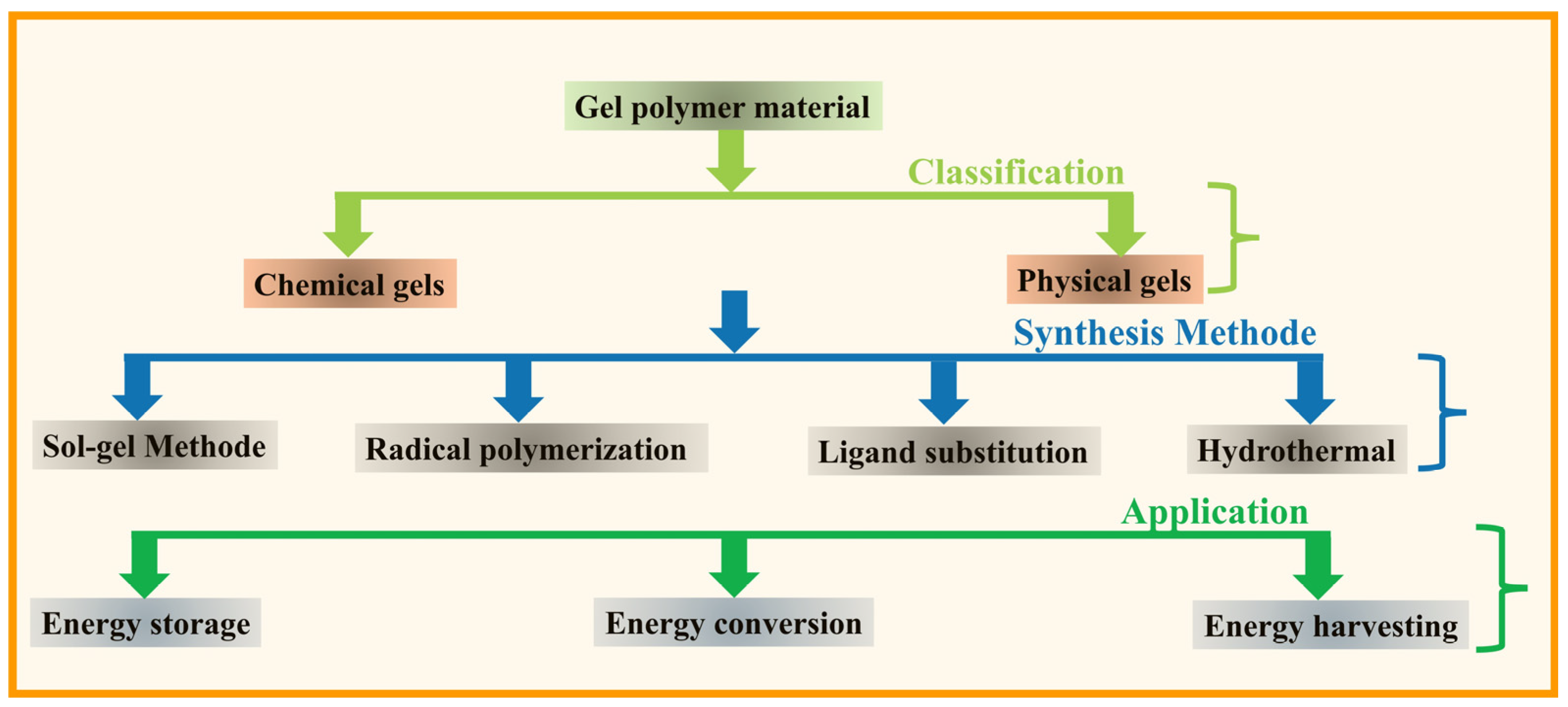
1. Introduction
2. Applications of Hydrogel-Derived Materials for Supercapacitors
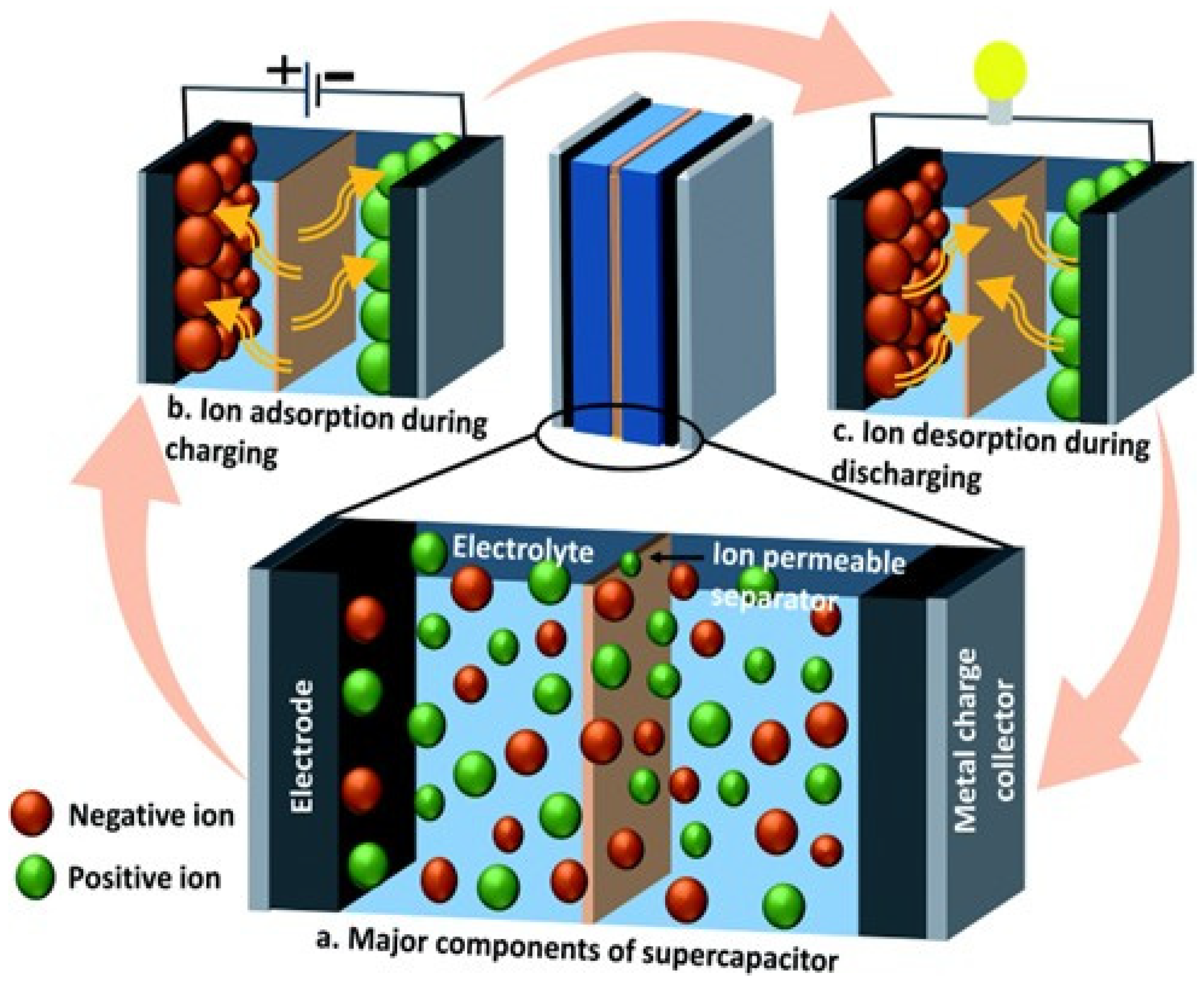
2.1. Hydrogels in Supercapacitors
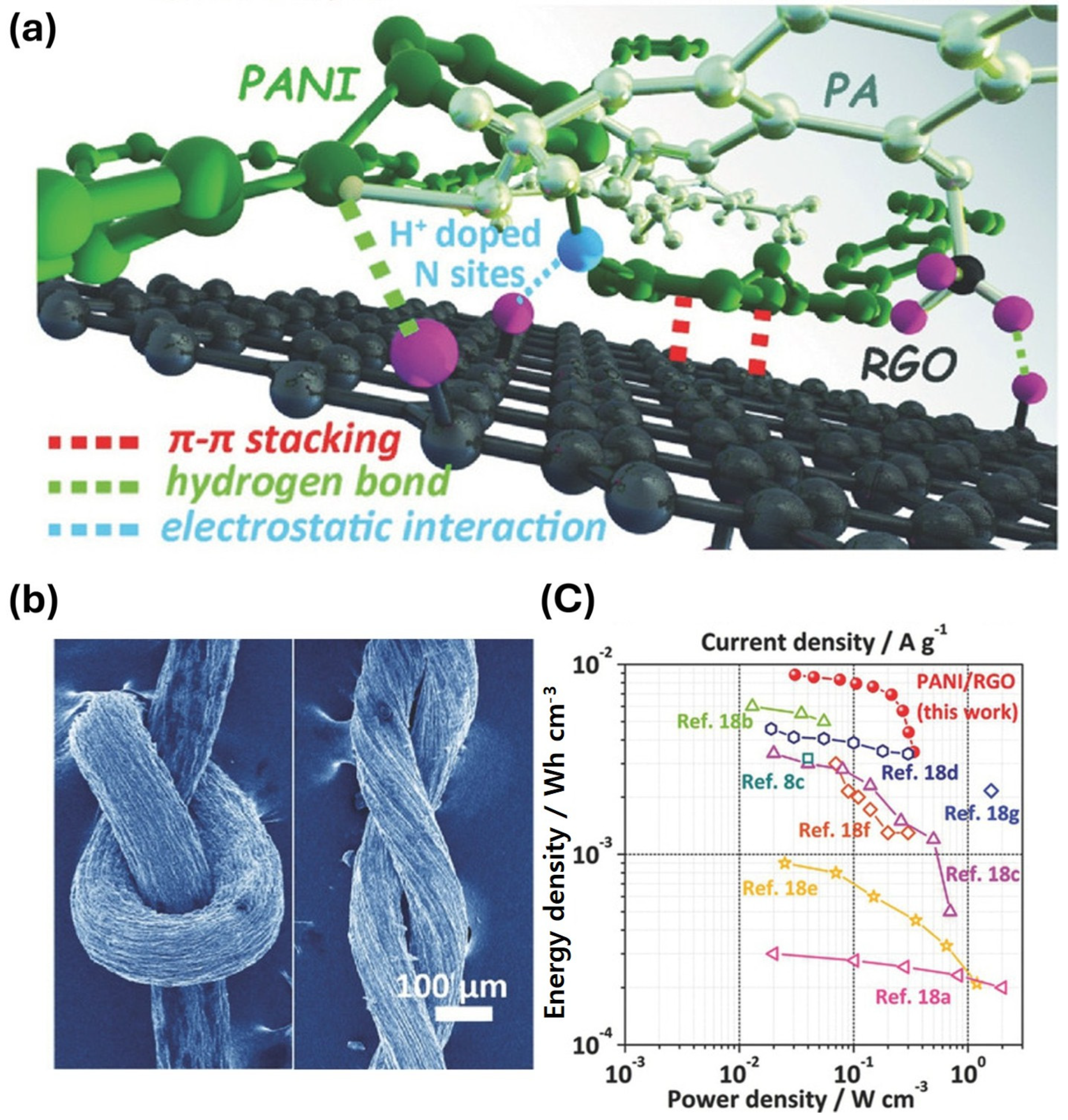
2.1.1. Hydrogel-Derived Electrodes
2.1.2. Hydrogel Electrolytes
2.1.3. Flexible Supercapacitors with Hydrogel Electrolytes
2.1.4. Hydrogel Electrolyte-Based Supercapacitors with Additional Functions
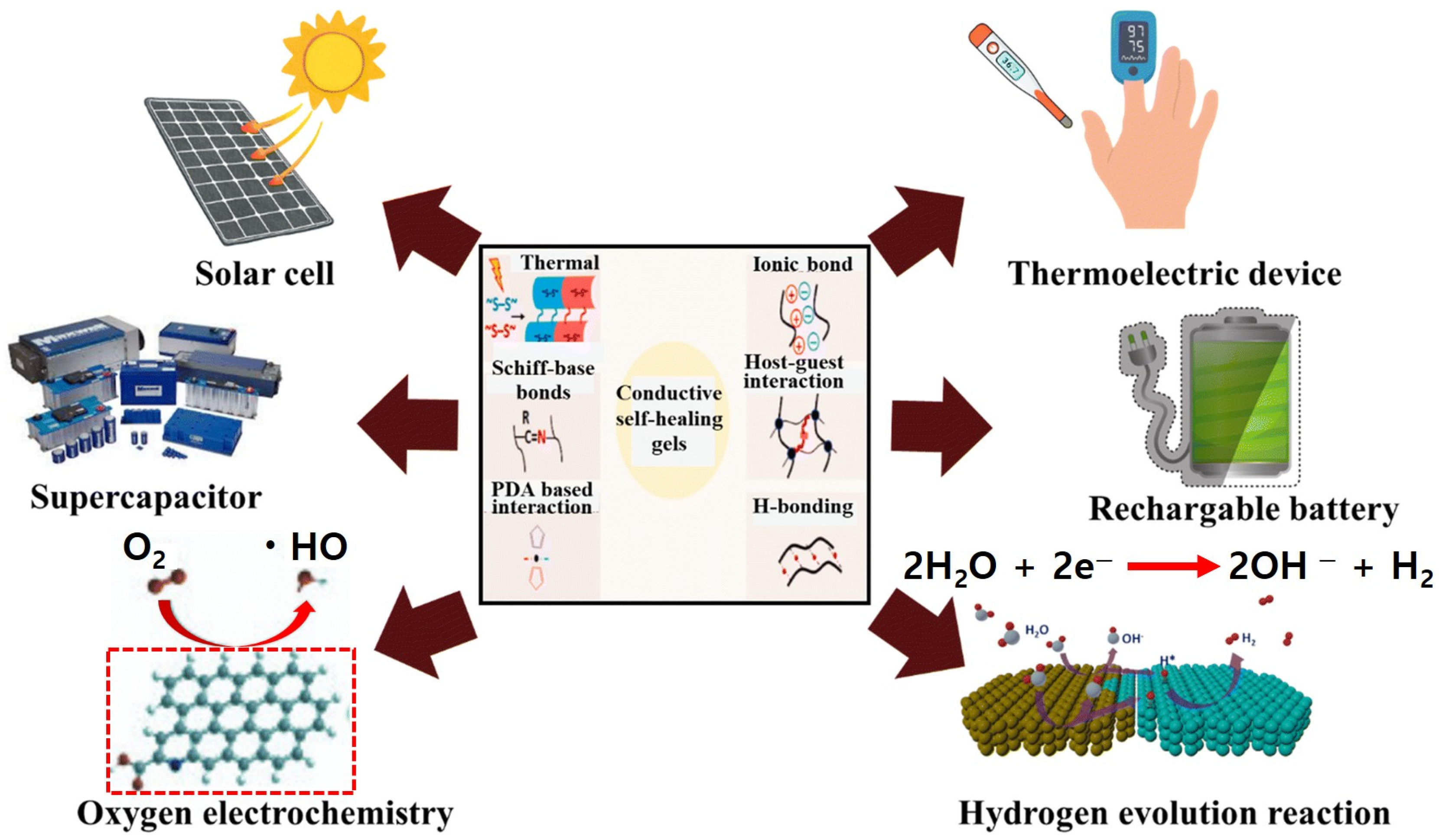
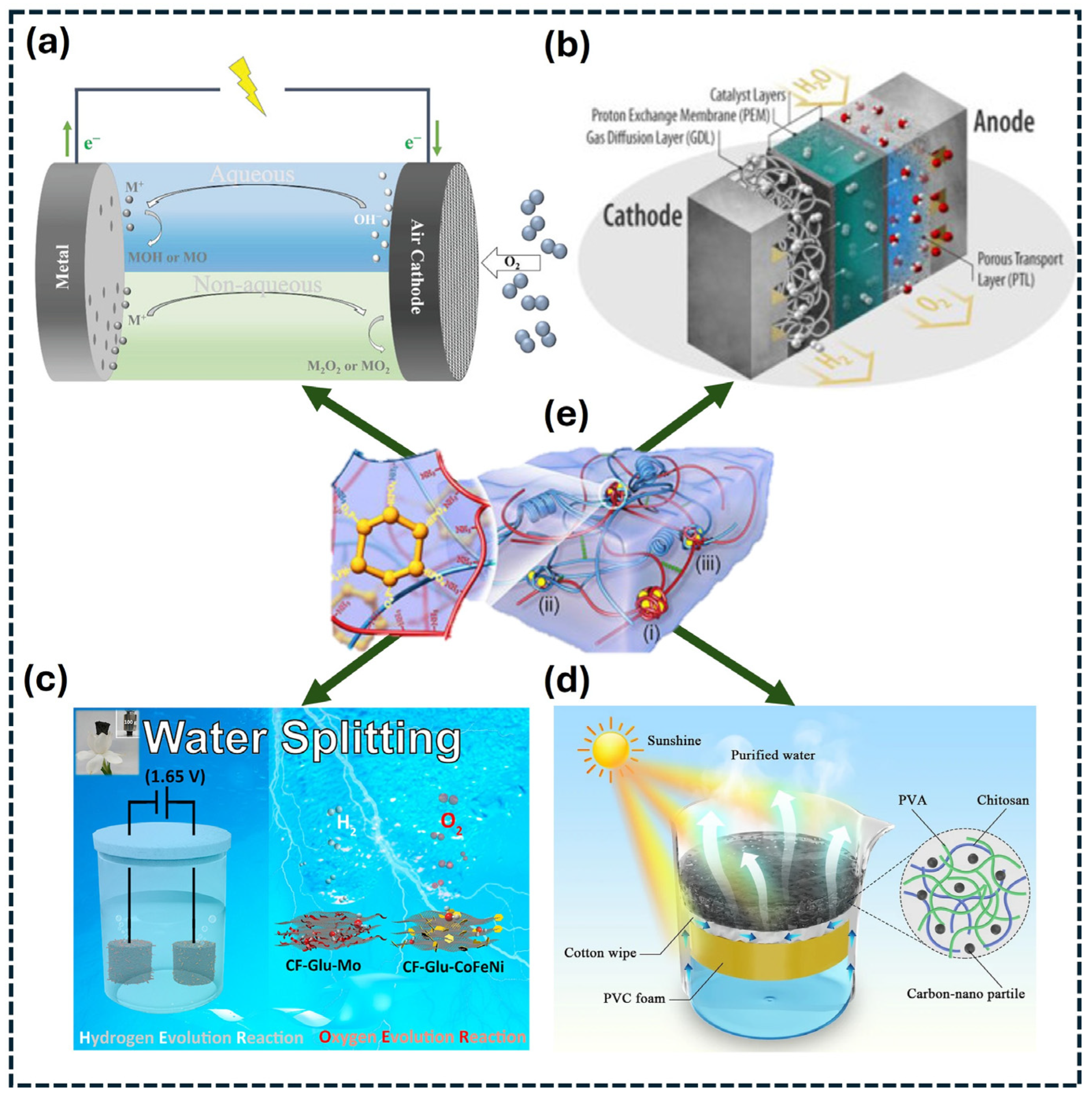
3. Applications of Hydrogel-Derived Materials for Energy Conversion Devices
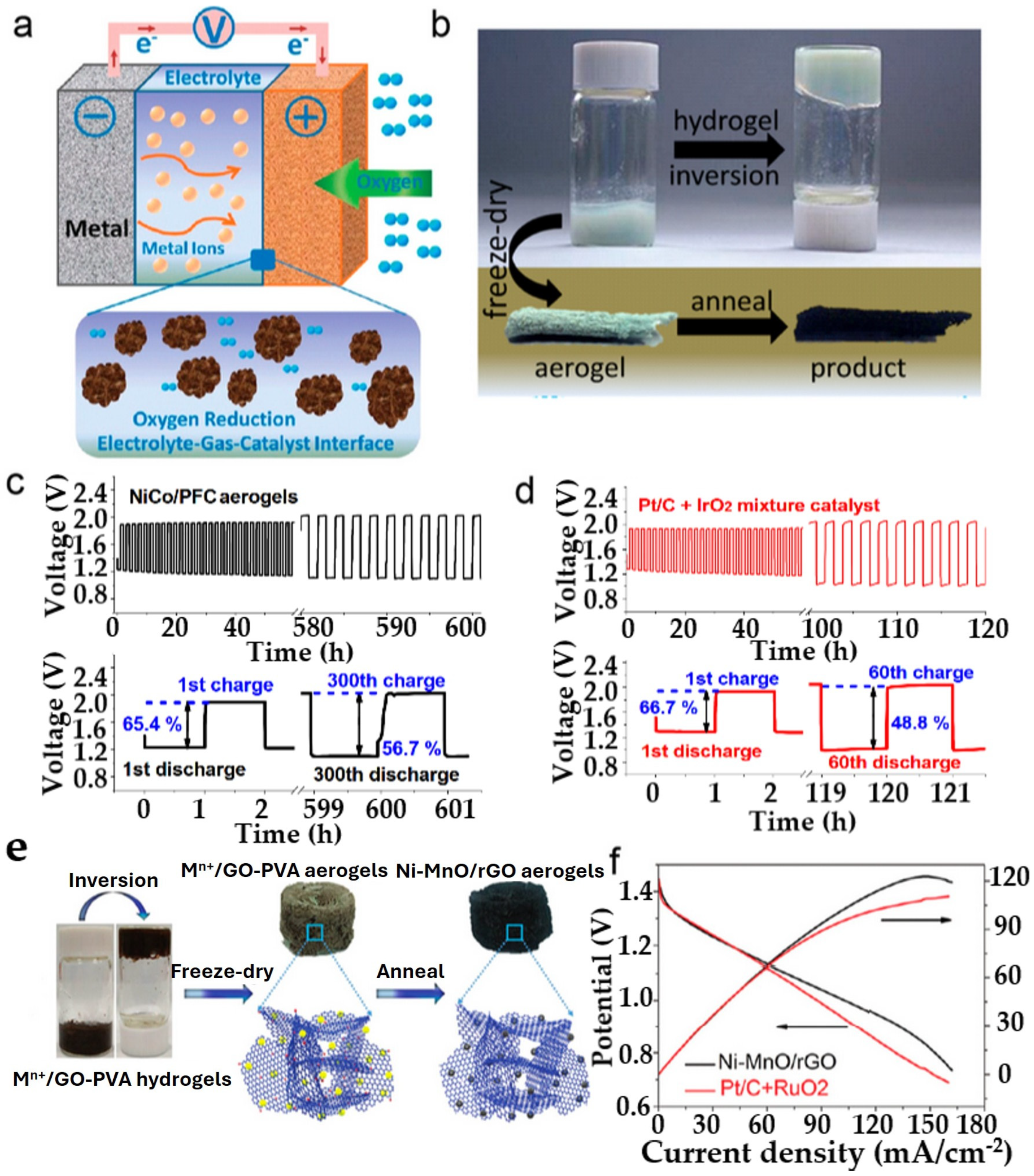
3.1. Metal–Air Batteries
3.2. Fuel Cells
3.3. Water-Splitting Electrolyzers
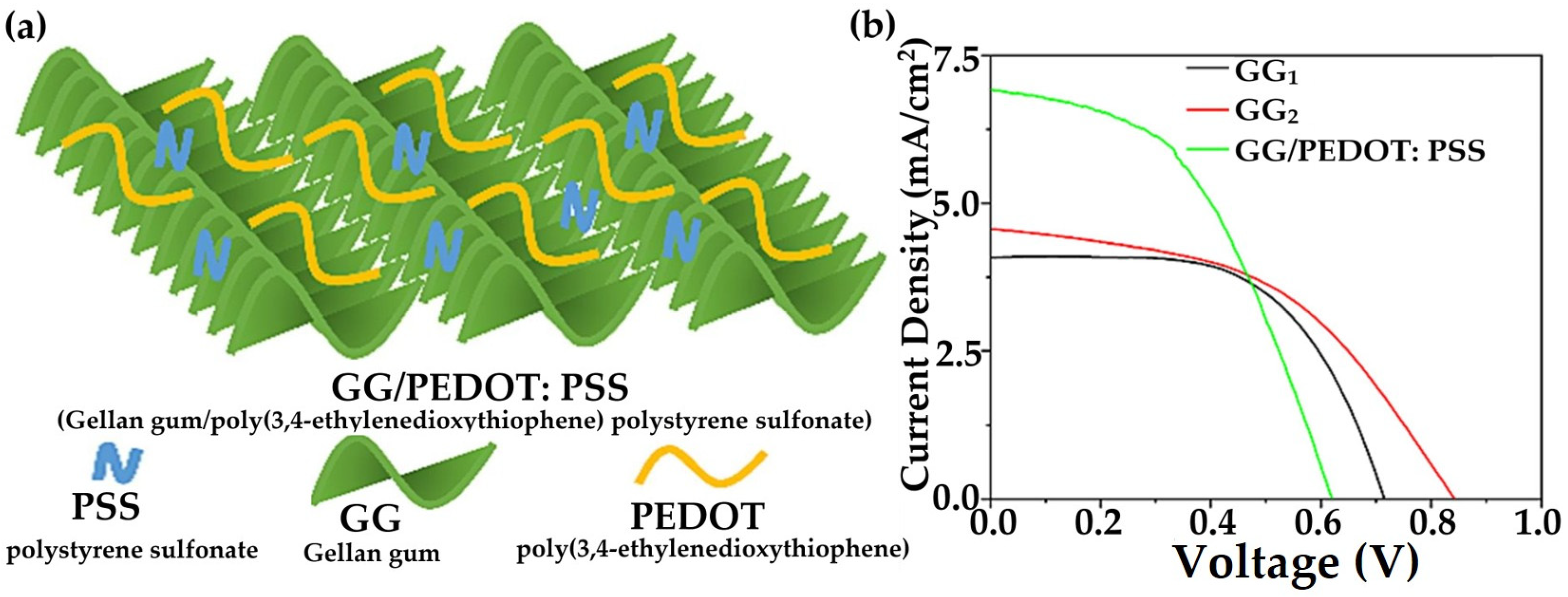
3.4. Hydrogels in Solar Cells
4. Challenges and Future Perspectives
- Strength and Durability: While hydrogels are known for their exceptional flexibility, they often lack sufficient mechanical strength and durability, especially under extreme conditions like high temperatures, humidity, or mechanical stress. This leads to performance decline, restricting their application in long-term uses such as wearable electronics and energy storage systems. To overcome these limitations, researchers are developing hybrid hydrogels that incorporate stronger materials, such as polymers or metallic nanoparticles, to enhance their mechanical properties. The integration of highly conductive materials like carbon nanotubes and graphene into hydrogel matrices has demonstrated potential for improving both mechanical strength and electrical conductivity. In addition, self-healing hydrogels, capable of autonomously repairing damage, present a promising solution to the durability issues associated with hydrogels. By incorporating dynamic covalent bonds or reversible cross-linking mechanisms, these hydrogels can restore their functionality after exposure to mechanical or environmental stress, which is essential for ensuring the long-term reliability of devices. These modifications aim to improve durability while maintaining flexibility, making hydrogels more suitable for demanding applications.
- Biodegradability and Environmental Concerns: Hydrogels are generally regarded as environmentally friendly, but the byproducts of certain synthetic hydrogels may pose long-term ecological risks. Ongoing research aims to create fully biodegradable hydrogels that can degrade safely in natural settings. These biodegradable hydrogels could minimize the environmental impact of synthetic hydrogels while maintaining their beneficial properties. Recently, biodegradable and biobased hydrogels, such as those derived from polysaccharides, have gained a lot of attention. Researchers are exploring natural polymers like cellulose and alginate to develop hydrogels that break down efficiently without releasing harmful residues.
- Ionic Conductivity: The ionic conductivity of hydrogels is generally lower than that of conventional solid-state electrolytes or metal-based conductors, despite being suitable for specific applications like supercapacitors. A major challenge is enhancing the ion transport within the hydrogel matrix while maintaining other critical properties such as biocompatibility and environmental sustainability. To address this challenge, researchers are exploring innovative strategies such as incorporating conductive nanoparticles, optimizing hydrogel composition, or designing hierarchical structures to enhance ion mobility. Balancing these improvements with sustainability and biocompatibility remains a critical focus for advancing hydrogel-based technologies.
- Manufacturing and Scalability: Scalability remains a major challenge for hydrogel-based devices, particularly in large-scale energy storage applications. Producing these systems on a large scale while ensuring uniformity and consistent performance is often difficult. Advancements in manufacturing techniques, such as 3D printing and automated assembly lines, could help improve scalability. Advances in 3D printing and other advanced fabrication techniques are facilitating precise control over hydrogel structures, enabling the development of hydrogels with customized properties for specific energy applications. This includes enhancing pore structures to improve ion transport and modifying polymer networks to enhance mechanical strength. Additionally, optimizing the hydrogel formulation to enhance its mechanical properties and stability can lead to more reliable large-scale production. Collaborations with materials scientists and engineers can also drive innovation in this sector to address these challenges effectively. Collaboration among experts in nanotechnology, organic electronics, and biomaterials is essential for advancing next-generation hydrogels for energy storage and conversion systems.
5. Conclusions
5.1. Summary of Key Points
5.2. Impact of Hydrogels on the Future of Energy Materials and Devices
5.3. Final Thoughts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenzo Lopez, M.; Kearns, V.R.; Patterson, E.A.; Curran, J.M. Passive Nanorheological Tool to Characterise Hydrogels. Nanoscale 2025, 17, 15338–15347. [Google Scholar] [CrossRef]
- Lloyd, E.C.; Dhakal, S.; Amini, S.; Alhasan, R.; Fratzl, P.; Tree, D.R.; Morozova, S.; Hickey, R.J. Porous Hierarchically Ordered Hydrogels Demonstrating Structurally Dependent Mechanical Properties. Nat. Commun. 2025, 16, 3792. [Google Scholar] [CrossRef]
- Zhang, C.W.; Si, M.; Chen, C.; He, P.; Fei, Z.; Xu, N.; He, X. Hierarchical Engineering for Biopolymer-Based Hydrogels with Tailored Property and Functionality. Adv. Mater. 2025, 37, 2414897. [Google Scholar] [CrossRef]
- Xue, B.; Han, X.; Zhu, H.; Li, Q.; Zhang, Y.; Bai, M.; Li, Y.; Li, Y.; Qin, M.; Nakajima, T.; et al. Hydrogels with Prestressed Tensegrity Structures. Nat. Commun. 2025, 16, 3637. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An Overview of Its Classifications, Properties, and Applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Raut, B.; Yun, S.; Kim, H.Y.; Nam, K.-W. A Comprehensive Review of Functional Gel Polymer Electrolytes and Applications in Lithium-Ion Battery. Gels 2024, 10, 563. [Google Scholar] [CrossRef]
- Luo, W.; Ren, L.; Hu, B.; Zhang, H.; Yang, Z.; Jin, L.; Zhang, D. Recent Development of Fibrous Hydrogels: Properties, Applications and Perspectives. Adv. Sci. 2025, 12, 2408657. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Han, D.; Bari, G.A.K.M.R.; Nam, K.-W. Electrochemical Storage Behavior of a High-Capacity Mg-Doped P2-Type Na2/3Fe1−yMnyO2 Cathode Material Synthesized by a Sol–Gel Method. Gels 2024, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Raut, B.; Ahmed, M.S.; Kim, H.-Y.; Rahman Khan, M.M.; Bari, G.A.K.M.R.; Islam, M.; Nam, K.-W. Battery-Type Transition Metal Oxides in Hybrid Supercapacitors: Synthesis and Applications. Batteries 2025, 11, 60. [Google Scholar] [CrossRef]
- Kamarulazam, F.; Bashir, S.; Pershaanaa, M.; Goh, Z.L.; Surender, G.; Elumalai, P.N.N.; Farhana, N.K.; Ramesh, S.; Ramesh, K. Stretchable, Self-Healable and Highly Conductive Natural-Rubber Hydrogel Electrolytes for Supercapacitors: Advanced Wearable Technology. J. Energy Storage 2023, 71, 108182. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.K.M.R.; Nam, K.-W. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef]
- Zafar, M.; Muhammad Imran, S.; Iqbal, I.; Azeem, M.; Chaudhary, S.; Ahmad, S.; Kim, W.Y. Graphene-Based Polymer Nanocomposites for Energy Applications: Recent Advancements and Future Prospects. Results Phys. 2024, 60, 107655. [Google Scholar] [CrossRef]
- Das, K.; Majumdar, D. Prospects of MXenes/Graphene Nanocomposites for Advanced Supercapacitor Applications. J. Electroanal. Chem. 2022, 905, 115973. [Google Scholar] [CrossRef]
- Passantino, J.M.; Wolfe, K.D.; Simon, K.T.; Cliffel, D.E.; Jennings, G.K. Photosystem I Enhances the Efficiency of a Natural, Gel-Based Dye-Sensitized Solar Cell. ACS Appl. Bio Mater. 2020, 3, 4465–4473. [Google Scholar] [CrossRef]
- Wang, Q.; Pang, J.; Yang, Y.; Zhu, X.; Ye, D.; Chen, R.; Liao, Q. Electricity Generation Property in Portable Direct Formate Fuel Cells (DFFCs) with Polyacrylamide Hydrogel. Chem. Eng. J. 2025, 517, 164331. [Google Scholar] [CrossRef]
- Torrejón-Guerrero, I.; Rodríguez-Gómez, A.; Granados-Fernández, R.; Andreu, C.M.; Rodrigo, M.A.; Vázquez, E.; Fernández-Marchante, C.M. Studying the Compatibility of Hydrogels as a Separator for Microbial Fuel Cells Developed by 3D-Printed Technology. Electrochim. Acta 2025, 536, 146683. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Alom, J.; Zaman, J.U.; Ban, S.; Veza, I.; Kalam, M.A.; Hessel, V.; Ahmed, M.B. Hydrogel-Derived Materials for Microbial Fuel Cell. J. Power Sources 2025, 625, 235688. [Google Scholar] [CrossRef]
- Nawab, S.; Sha, C.; Shah, S.B.; Keerio, H.A.; Zhu, Z.; Yong, Y.C. Trehalose-Powered Membraneless Enzymatic Fuel Cell Based on Flexible Alginate Composite Hydrogel Bioelectrodes. Process Biochem. 2025, 153, 18–25. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, Z.; Sun, N.; Ding, J.; Liu, S.; Zhang, S.; Lv, Z.; Xu, W.; Liu, G.; Liu, X. Advances in Flexible Hydrogels for Light-Thermal-Electricity Energy Conversion and Storage. J. Energy Storage 2023, 60, 106618. [Google Scholar] [CrossRef]
- Yu, J.; Gu, Y.; Ren, Y.; Lou, Q.; Ding, Y.; Qiu, Q.; Hu, C.; Ding, H.; Wu, D.; Mou, J.; et al. Characterization and Research Progress of Hydrogel Conductive Materials for Energy Storage Components. J. Energy Storage 2024, 101, 113813. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart Materials for Flexible Electronics and Devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Ahmed, M.S.; Yun, S.; Kim, H.-Y.; Nam, K.-W. Harnessing Radiation for Nanotechnology: A Comprehensive Review of Techniques, Innovations, and Application. Nanomaterials 2024, 14, 2051. [Google Scholar] [CrossRef]
- Khandaker, T.; Anik, M.A.A.M.; Nandi, A.; Islam, T.; Islam, M.M.; Hasan, M.K.; Dhar, P.K.; Latif, M.A.; Hossain, M.S. Recent Progress in Gel Catalysts: Boosting Efficiency for Sustainable Energy Applications. Catal. Sci. Technol. 2025, 15, 1357–1389. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W. Application of Hydrogel for Energy Storage and Conversion. Next Mater. 2023, 1, 100049. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Oh, J.; Choi, J. Molecular Mechanism of Ionic Conductivity Enhancement by Heterogeneous Interface in Composite Hydrogel Electrolytes. Batteries Supercaps 2025. [Google Scholar] [CrossRef]
- Du, Y.; Zheng, Y.; Liu, H.; Zhao, S.; Wang, X.; Yang, L. Moisture Harvesting by the Structure Regulation of Hygroscopic Hydrogel for Energy and Water Sustainability. Adv. Electron. Mater. 2025. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Jiang, X.; Zhang, Z.; Wang, Y.; Liu, H.; Li, Z.; Gane, P. Coupling of High Ion Transport Efficiency in Hydrogel Electrolytes and Interfacial Fusion for Performance Enhancement in All-Solid-State Paper-Based Self-Powered Electrochromic Devices with Low-Temperature Tolerance. J. Mater. Chem. A 2025, 13, 10060–10076. [Google Scholar] [CrossRef]
- Shuaibu, A.D.; Shah, S.S.; Alzahrani, A.S.; Aziz, M.A. Advancing Gel Polymer Electrolytes for Next-Generation High-Performance Solid-State Supercapacitors: A Comprehensive Review. J. Energy Storage 2025, 107, 114851. [Google Scholar] [CrossRef]
- Park, H.; Chung, J.; Yong, H.; Jung, J.; Jung, C. Roles of Gel Polymer Electrolytes for High-Power Activated Carbon Supercapacitors: Ion Reservoir and Binder-like Effects. RSC Adv. 2020, 10, 4690–4697. [Google Scholar] [CrossRef]
- Roy, B.K.; Tahmid, I.; Rashid, T.U. Chitosan-Based Materials for Supercapacitor Applications: A Review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Ding, Y.; Zhao, Y.; Li, Y.; Shi, Y.; Yu, G. Dopant-Enabled Supramolecular Approach for Controlled Synthesis of Nanostructured Conductive Polymer Hydrogels. Nano Lett. 2015, 15, 7736–7741. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem.-Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ren, J.; Li, M.; Zhang, M.; Li, Y.; Yang, W. Self-Healing, Self-Adhesive, Stretchable and Flexible Conductive Hydrogels for High-Performance Strain Sensors. Soft Matter 2023, 19, 5723–5736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, G.; Zhu, Y.; Liu, X.; Chen, Y.; Wu, H.; Wang, Y.; Shi, X.; Mao, C. 3D-Printable Self-Healing and Mechanically Reinforced Hydrogels with Host-Guest Non-Covalent Interactions Integrated into Covalently Linked Networks. Mater. Horizons 2019, 6, 733–742. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.; You, Z.; Li, W.; Chen, J.; Zheng, X.; Zheng, G.; Song, Z.; You, X.; Xu, Y. Ultrastrong and Tough Urushiol-Based Ionic Conductive Double Network Hydrogels as Flexible Strain Sensors. Polymers 2023, 15, 3219. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Chong, C.M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Liu, W. Hybrid Three-Dimensional Printing and Encapsulation Process for Cellulose Hydrogel Sensors. Int. J. Biol. Macromol. 2025, 302, 140571. [Google Scholar] [CrossRef]
- Ye, C.; Wei, C.; Liu, J.; Wong, T.H.; Liu, X.; Song, Z.; Wu, C.; Li, Z.; Lin, S. Mechano-Diffusion of Particles in Stretchable Hydrogels. Soft Matter 2025, 21, 2230–2241. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical Nanostructured Conducting Polymer Hydrogel with High Electrochemical Activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An Ultra-Sensitive Resistive Pressure Sensor Based on Hollow-Sphere Microstructure Induced Elasticity in Conducting Polymer Film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Peng, L.; Zhao, F.; Xiao, D.; Jin, Y.; Yu, G. Stretchable All-Gel-State Fiber-Shaped Supercapacitors Enabled by Macromolecularly Interconnected 3D Graphene/Nanostructured Conductive Polymer Hydrogels. Adv. Mater. 2018, 30, 1800124. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhao, D.; Han, S.; Meng, W.; Jin, X. Illite/Poly(Vinyl Alcohol) Hydrogel Electrolytes with High Mechanical Strength and Ionic Conductivity for Flexible Energy Storage Devices. Langmuir 2025, 41, 20127–20135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Gao, X.; Dong, B.; Sun, N.; Zheng, L. Poly(Ionic Liquid) Hydrogels Exhibiting Superior Mechanical and Electrochemical Properties as Flexible Electrolytes. J. Mater. Chem. A 2016, 4, 1112–1118. [Google Scholar] [CrossRef]
- Kwon, H.J.; Osada, Y.; Gong, J.P. Polyelectrolyte Gels-Fundamentals and Applications. Polym. J. 2006, 38, 1211–1219. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Lee, G.; Kim, D.; Kim, D.; Oh, S.; Yun, J.; Kim, J.; Lee, S.S.; Ha, J.S. Fabrication of a Stretchable and Patchable Array of High Performance Micro-Supercapacitors Using a Non-Aqueous Solvent Based Gel Electrolyte. Energy Environ. Sci. 2015, 8, 1764–1774. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Sang, M.; Wang, X.; Zuo, D.; Xu, J.; Zhang, H. A Supramolecular Hydrogel Electrolyte for High-Performance Supercapacitors. J. Energy Storage 2021, 33, 101931. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent Advances of Hydrogel Electrolytes in Flexible Energy Storage Devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Xu, W.; Chen, A. Application of Supramolecular Hydrogel in Supercapacitors: Opportunities and Challenges. Aggregate 2024, 5, e581. [Google Scholar] [CrossRef]
- Gu, Z.; Du, Y.; Yang, B.; Liu, X.; You, T.; Tian, W.; Tan, S.; Ji, J. GO Regulated Hydrogel Electrolytes with Superior Conducing and Antiswelling Ability for Flexible Supercapacitors. Ind. Eng. Chem. Res. 2024, 63, 7176–7183. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. A Powder Self-Healable Hydrogel Electrolyte for Flexible Hybrid Supercapacitors with High Energy Density and Sustainability. Small 2021, 17, 2006807. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-Based Hydrogel Electrolytes for Advanced Energy Storage/Conversion Devices: Properties, Applications, and Perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Kang, G.; Lu, N.; Li, L.; Wang, S.; Liu, S.; Yang, Y.; Luan, J.; Zhang, S.; Wang, G. Stretchable/Compressible Supercapacitors Based on High-Elasticity and Fatigue-Resistant Hydrogel Electrolyte Cross-Linked by Hydrophobic Nanospheres. Nano Lett. 2025, 25, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, R.; Ren, X.; Gao, G. A Wide Temperature-Tolerant Hydrogel Electrolyte Mediated by Phosphoric Acid towards Flexible Supercapacitors. Chem. Eng. J. 2021, 413, 127446. [Google Scholar] [CrossRef]
- Zang, L.; Liu, Q.; Qiu, J.; Yang, C.; Wei, C.; Liu, C.; Lao, L. Design and Fabrication of an All-Solid-State Polymer Supercapacitor with Highly Mechanical Flexibility Based on Polypyrrole Hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, Z.; Li, G.; Hu, T.; Zhang, C.; Wang, X. All-Solid-State Flexible Fiber-Based MXene Supercapacitors. Adv. Mater. Technol. 2017, 2, 1700143. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Yin, Q.; Wu, J.; Chen, P.; Zhang, G.; Liu, G.; Wu, C.; Xie, Y. A Zwitterionic Gel Electrolyte for Efficient Solid-State Supercapacitors. Nat. Commun. 2016, 7, 11782. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Li, N.; Yao, Y.; Liu, K.; Chen, T. Ultraflexible and Tailorable All-Solid-State Supercapacitors Using Polyacrylamide-Based Hydrogel Electrolyte with High Ionic Conductivity. Nanoscale 2017, 9, 18474–18481. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A Self-Healable and Highly Stretchable Supercapacitor Based on a Dual Crosslinked Polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef] [PubMed]
- Hu Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and Fabrication of Conductive Polymer Hydrogels and Their Applications in Flexible Supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, M.; Wang, G.; Bao, H.; Saha, P. A Facile Prestrain-Stick-Release Assembly of Stretchable Supercapacitors Based on Highly Stretchable and Sticky Hydrogel Electrolyte. J. Power Sources 2015, 284, 400–408. [Google Scholar] [CrossRef]
- Shi, Y.; Ha, H.; Al-Sudani, A.; Ellison, C.J.; Yu, G. Thermoplastic Elastomer-Enabled Smart Electrolyte for Thermoresponsive Self-Protection of Electrochemical Energy Storage Devices. Adv. Mater. 2016, 28, 7921–7928. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.G.; Kim, G.P.; Lee, M.; Song, H.D.; Yi, J. A Biodegradable Gel Electrolyte for Use in High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3503–3511. [Google Scholar] [CrossRef]
- Lee, G.; Kang, S.K.; Won, S.M.; Gutruf, P.; Jeong, Y.R.; Koo, J.; Lee, S.S.; Rogers, J.A.; Ha, J.S. Fully Biodegradable Microsupercapacitor for Power Storage in Transient Electronics. Adv. Energy Mater. 2017, 7, 1700157. [Google Scholar] [CrossRef]
- Tang, X.; Lui, Y.H.; Merhi, A.R.; Chen, B.; Ding, S.; Zhang, B.; Hu, S. Redox-Active Hydrogel Polymer Electrolytes with Different PH Values for Enhancing the Energy Density of the Hybrid Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 44429–44440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, G.; Zhan, Y.; Li, H.; Wang, Z.; Ma, L.; Wang, Y.; Niu, X.; Zhi, C. A Soft yet Device-Level Dynamically Super-Tough Supercapacitor Enabled by an Energy-Dissipative Dual-Crosslinked Hydrogel Electrolyte. Nano Energy 2019, 58, 732–742. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Liu, Z.; Tang, Z.; Yang, Q.; Zhao, Y.; Du, S.; Chen, Q.; Zhi, C. A Highly Elastic and Reversibly Stretchable All-Polymer Supercapacitor. Angew. Chem.-Int. Ed. 2019, 58, 15707–15711. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Liu, Y.; Lu, N.; Zhang, S.; Liu, F.; Wang, G. Mechanically Robust Hydrophobic Association Hydrogel Electrolyte with Efficient Ionic Transport for Flexible Supercapacitors. Chem. Eng. J. 2019, 374, 738–747. [Google Scholar] [CrossRef]
- Wei, J.; Wei, G.; Shang, Y.; Zhou, J.; Wu, C.; Wang, Q. Dissolution–Crystallization Transition within a Polymer Hydrogel for a Processable Ultratough Electrolyte. Adv. Mater. 2019, 31, 1900248. [Google Scholar] [CrossRef]
- Bashir, S.; Omar, F.S.; Hina, M.; Numan, A.; Iqbal, J.; Ramesh, S.; Ramesh, K. Synthesis and Characterization of Hybrid Poly (N, N-Dimethylacrylamide) Composite Hydrogel Electrolytes and Their Performance in Supercapacitor. Electrochim. Acta 2020, 332, 135438. [Google Scholar] [CrossRef]
- Yin, B.-S.; Zhang, S.-W.; Ke, K.; Wang, Z.-B. Advanced Deformable All-in-One Hydrogel Supercapacitor Based on Conducting Polymer: Toward Integrated Mechanical and Capacitive Performance. J. Alloys Compd. 2019, 805, 1044–1051. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Liu, J.; Zhang, J.; Ma, X.; Huang, Y. An Intrinsically Compressible and Stretchable All-in-One Configured Supercapacitor. Chem. Commun. 2018, 54, 6200–6203. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Biopolymer-Based Carboxylated Chitosan Hydrogel Film Crosslinked by HCl as Gel Polymer Electrolyte for All-Solid-Sate Supercapacitors. J. Power Sources 2019, 426, 47–54. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable Flexible Supercapacitors Based on All-Lignin-Based Hydrogel Electrolytes and Nanofiber Electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and Superior Healable Supercapacitors Based on a Double Cross-Linked Hydrogel Electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Li, D.; Xu, Z.; Ji, X.; Liu, L.; Gai, G.; Yang, J.; Wang, J. Deep Insight into Ionic Transport in Polyampholyte Gel Electrolytes towards High Performance Solid Supercapacitors. J. Mater. Chem. A 2019, 7, 16414–16424. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Jia, L.; Zhang, Q.; Xu, X. Stretchable and Self-Healing Integrated All-Gel-State Supercapacitors Enabled by a Notch-Insensitive Supramolecular Hydrogel Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 36028–36036. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Huang, J.; Liu, M. Low Temperature Tolerant Organohydrogel Electrolytes for Flexible Solid-state Supercapacitors. Adv. Energy Mater. 2018, 8, 1801967. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by Side Battery Technologies with Lithium-Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089. [Google Scholar] [CrossRef]
- Wrubel, J.A.; Milleville, C.; Klein, E.; Zack, J.; Park, A.M.; Bender, G. Estimating the Energy Requirement for Hydrogen Production in Proton Exchange Membrane Electrolysis Cells Using Rapid Operando Hydrogen Crossover Analysis. Int. J. Hydrogen Energy 2022, 47, 28244–28253. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, L.; Huang, Q.; Guo, Y.; Miao, T.; Hu, Y.; Qian, J.; Huang, S. Chitosan Hydrogel Derived Carbon Foam with Typical Transition-Metal Catalysts for Efficient Water Splitting. Carbon N. Y. 2021, 177, 160–170. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Tian, Y.; Caratenuto, A.; Chen, F.; Zheng, Y. Dome-Arrayed Chitosan/PVA Hydrogel-Based Solar Evaporator for Steam Generation. Sci. Rep. 2022, 12, 4403. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New Insights into Food Hydrogels with Reinforced Mechanical Properties: A Review on Innovative Strategies. Adv. Colloid Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–Air Batteries: From Oxygen Reduction Electrochemistry to Cathode Catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Davari, E.; Ivey, D.G. Bifunctional Electrocatalysts for Zn-Air Batteries. Sustain. Energy Fuels 2018, 2, 39–67. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xin, S.; Tang, Y.; Goodenough, J.B. Novel Hydrogel-Derived Bifunctional Oxygen Electrocatalyst for Rechargeable Air Cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef]
- Fu, G.; Yan, X.; Chen, Y.; Xu, L.; Sun, D.; Lee, J.M.; Tang, Y. Boosting Bifunctional Oxygen Electrocatalysis with 3D Graphene Aerogel-Supported Ni/MnO Particles. Adv. Mater. 2018, 30, 1704609. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel Cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Qiao, Z.; Hwang, S.; Li, X.; Wang, C.; Samarakoon, W.; Karakalos, S.; Li, D.; Chen, M.; He, Y.; Wang, M.; et al. 3D Porous Graphitic Nanocarbon for Enhancing the Performance and Durability of Pt Catalysts: A Balance between Graphitization and Hierarchical Porosity. Energy Environ. Sci. 2019, 12, 2830–2841. [Google Scholar] [CrossRef]
- Wang, J.; Pei, Z.; Liu, J.; Hu, M.; Feng, Y.; Wang, P.; Wang, H.; Nie, N.; Wang, Y.; Zhi, C.; et al. A High-Performance Flexible Direct Ethanol Fuel Cell with Drop-and-Play Function. Nano Energy 2019, 65, 104052. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct Atomic-Level Insight into the Active Sites of a High-Performance PGM-Free ORR Catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Li, B.; Ma, L.; Shi, L.; Xiong, Y.; Xu, H. Novel Iron/Cobalt-Containing Polypyrrole Hydrogel-Derived Trifunctional Electrocatalyst for Self-Powered Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1606497. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A Perspective toward Practical Lithium-Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Law, C.; Pathirana, S.C.; Li, X.; Anderson, A.Y.; Barnes, P.R.F.; Listorti, A.; Ghaddar, T.H.; ORegan, B.C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22, 4505–4509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, X.; Jiao, H.; Wu, M. Designing Highly Effective Mesoporous Carbon-Based Counter Electrodes for Liquid Electrolyte-Based and Quasi-Solid Dye-Sensitized Solar Cells. J. Electroanal. Chem. 2022, 908, 116104. [Google Scholar] [CrossRef]
- Ünlü, B.; Türk, S.; Özacar, M. Gellan Gum/PEDOT:PSS Gel Electrolyte and Application on Quasi-Solid Dye Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2024, 450, 115471. [Google Scholar] [CrossRef]
- Miranda, D.O.; Dorneles, M.F.; Oréfice, R.L. A Facile and Low-Cost Route for Producing a Flexible Hydrogel–PANI Electrolyte/Counter Electrode Applicable in Dye-Sensitized Solar Cells (DSSC). SN Appl. Sci. 2019, 1, 1598. [Google Scholar] [CrossRef]
- Khannam, M.; Boruah, R.; Dolui, S.K. An Efficient Quasi-Solid State Dye Sensitized Solar Cells Based on Graphene Oxide/Gelatin Gel Electrolyte with NiO Supported TiO2 Photoanode. J. Photochem. Photobiol. A Chem. 2017, 335, 248–258. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Q.; Qin, D.; Luo, Y.; Li, D.; Shen, Q.; Toyoda, T.; Meng, Q. Highly Efficient Quasi-Solid-State Quantum-Dot-Sensitized Solar Cell Based on Hydrogel Electrolytes. Electrochem. Commun. 2010, 12, 1776–1779. [Google Scholar] [CrossRef]
- Nath, B.C.; Gogoi, B.; Boruah, M.; Sharma, S.; Khannam, M.; Ahmed, G.A.; Dolui, S.K. High Performance Polyvinyl Alcohol/Multi Walled Carbon Nanotube/Polyaniline Hydrogel (PVA/MWCNT/PAni) Based Dye Sensitized Solar Cells. Electrochim. Acta 2014, 146, 106–111. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, L.; Yu, X.Y.; Qiu, K.Q.; Lü, X.Y.; Kuang, D.B.; Su, C.Y. Dextran Based Highly Conductive Hydrogel Polysulfide Electrolyte for Efficient Quasi-Solid-State Quantum Dot-Sensitized Solar Cells. Electrochim. Acta 2013, 92, 117–123. [Google Scholar] [CrossRef]
- Huo, Z.; Tao, L.; Wang, S.; Wei, J.; Zhu, J.; Dong, W.; Liu, F.; Chen, S.; Zhang, B.; Dai, S. A Novel Polysulfide Hydrogel Electrolyte Based on Low Molecular Mass Organogelator for Quasi-Solid-State Quantum Dot-Sensitized Solar Cells. J. Power Sources 2015, 284, 582–587. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Du, Z.; Pan, D.; Zhong, X. Graphene Hydrogel-Based Counter Electrode for High Efficiency Quantum Dot-Sensitized Solar Cells. J. Mater. Chem. A 2017, 5, 1614–1622. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Liu, N.; Zhao, Q. Synergy Effect of Carbon Nanotube and Graphene Hydrogel on Highly Efficient Quantum Dot Sensitized Solar Cells. Electrochim. Acta 2019, 327, 134937. [Google Scholar] [CrossRef]
- Mandal, P.; Manna, J.S.; Das, D.; Mitra, M.K. Energy Transfer Cascade in Bio-Inspired Chlorophyll-a/Polyacrylamide Hydrogel: Towards a New Class of Biomimetic Solar Cells. RSC Adv. 2016, 6, 90280–90289. [Google Scholar] [CrossRef]
- Ferrag, C.; Noroozifar, M.; Modarresi-Alam, A.R.; Kerman, K. Encapsulation of Poly(m-Aminobenzodioxol)-Fe3O4 superparamagnetic Nanorods and Iron (III) Thiocyanate Complex in Hydrogel toward Hybrid Solar Cells. J. Environ. Chem. Eng. 2021, 9, 105612. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric Hydrogels for Burn Wound Care: Advanced Skin Wound Dressings and Regenerative Templates. Burn. Trauma 2014, 2, 2321–3868. [Google Scholar] [CrossRef]
- Stupp, S.I.; LeBonheur, V.; Walker, K.; Li, L.-S.; Huggins, K.E.; Keser, M.; Amstutz, A. Supramolecular Materials: Self-Organized Nanostructures. Science 1997, 276, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, J.; Zeng, J.; Huang, S.; Hu, W.; Chen, J.; Li, M.; Wang, J.; Zhang, S. Enhanced Photocatalytic Activity of Sulfated Silica-Titania Composites Prepared by Impregnation Using Ammonium Persulfate Solution. Mater. Sci. Semicond. Process. 2014, 26, 62–68. [Google Scholar] [CrossRef]
- Ho, W.F.; Lim, K.M.; Yang, K.-L. In Situ Formation of Leak-Free Polyethylene Glycol (PEG) Membranes in Microfluidic Fuel Cells. Lab Chip 2016, 16, 4725–4731. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An Injectable Silk Sericin Hydrogel Promotes Cardiac Functional Recovery after Ischemic Myocardial Infarction. Acta Biomater. 2016, 41, 210–223. [Google Scholar] [CrossRef]
- Chen, Z.; To, J.W.F.; Wang, C.; Lu, Z.; Liu, N.; Chortos, A.; Pan, L.; Wei, F.; Cui, Y.; Bao, Z. A Three-Dimensionally Interconnected Carbon Nanotube-Conducting Polymer Hydrogel Network for High-Performance Flexible Battery Electrodes. Adv. Energy Mater. 2014, 4, 1400207. [Google Scholar] [CrossRef]
- Yang, X.; Trinh, H.M.; Agrahari, V.; Sheng, Y.; Pal, D.; Mitra, A.K. Nanoparticle-Based Topical Ophthalmic Gel Formulation for Sustained Release of Hydrocortisone Butyrate. Aaps Pharmscitech 2016, 17, 294–306. [Google Scholar] [CrossRef]
- Shang, W.; Zhu, J.; Liu, Y.; Kang, L.; Liu, S.; Huang, B.; Song, J.; Li, X.; Jiang, F.; Du, W. Establishing High-Performance Quasi-Solid Zn/I2 Batteries with Alginate-Based Hydrogel Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 24756–24764. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired Multifunctional Hybrid Hydrogel Promotes Wound Healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, X.; Dong, C. Dual Stimuli-responsive Supramolecular Polypeptide-based Hydrogel and Reverse Micellar Hydrogel Mediated by Host–Guest Chemistry. Adv. Funct. Mater. 2010, 20, 579–586. [Google Scholar] [CrossRef]
- Xiang, R.; Yang, B.; Yu, Q.; Wang, K.; Wang, C.; Wu, Y.; Tan, S. High-Alkali Poly (Sodium Acrylate) Hydrogels with High Conductivities and Soft Deformability for Flexible Zn-Air Batteries. Chem. Eng. J. 2024, 499, 155855. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Yang, R.; Chen, G. Thermal Conductivity Modeling of Periodic Two-Dimensional Nanocomposites. Phys. Rev. B 2004, 69, 195316. [Google Scholar] [CrossRef]
- Berggren, M.; Głowacki, E.D.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K. In Vivo Organic Bioelectronics for Neuromodulation. Chem. Rev. 2022, 122, 4826–4846. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, R.; Pani, B.; Sarkar, A.; Tripathi, M. Engineering the Future with Hydrogels: Advancements in Energy Storage Devices and Biomedical Technologies. New J. Chem 2024, 48, 10347. [Google Scholar] [CrossRef]
- Tavsanli, B.; Can, V.; Okay, O. Mechanically Strong Triple Network Hydrogels Based on Hyaluronan and Poly(N,N-Dimethylacrylamide). Soft Matter 2015, 11, 8517–8524. [Google Scholar] [CrossRef]
- Li, X.; Palazzolo, A. A Review of Flywheel Energy Storage Systems: State of the Art and Opportunities. J. Energy Storage 2022, 46, 103576. [Google Scholar] [CrossRef]
- Li, C.; Lou, X.; Shen, M.; Hu, X.; Guo, Z.; Wang, Y.; Hu, B.; Chen, Q. High Anodic Performance of Co 1,3,5-Benzenetricarboxylate Coordination Polymers for Li-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 15352–15360. [Google Scholar] [CrossRef]
- Wu, M.; Huang, N.; Lv, M.; Wang, F.; Ma, F.; Deng, Y.; Sun, P.; Zheng, Y.; Liu, W.; Ye, L. Organic-Inorganic Hybrid Hydrogel Electrolyte for High-Performance Quasi-Solid-State Zinc-Air Batteries. Front. Chem. Sci. Eng. 2025, 19, 15. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional Hydrogels for Next-Generation Batteries and Supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Park, W.; Zhao, Z.; Li, J.; Lim, C.K.; Wong, T.H.; Yiu, C.K.; Gao, Y.; Zhou, J. Stretchable Magnesium–Air Battery Based on Dual Ions Conducting Hydrogel for Intelligent Biomedical Applications. InfoMat 2023, 5, e12388. [Google Scholar] [CrossRef]
- Qu, S.; Liu, B.; Wu, J.; Zhao, Z.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Kirigami-Inspired Flexible and Stretchable Zinc–Air Battery Based on Metal-Coated Sponge Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 54833–54841. [Google Scholar] [CrossRef]
- Priya, A.S.; Kannan, K.; Henry, J.; Aepuru, R.; Shanmugaraj, K.; Pabba, D.P.; Sathish, M. Advancements in Hydrogel Materials for Next-Generation Energy Devices: Properties, Applications, and Future Prospects. Cellulose 2025, 32, 6307–6335. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in Natural Polymer-Based Hydrogels: Synthesis, Applications, and Future Directions in Biomedical and Environmental Fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Lei, W.; Suzuki, N.; Terashima, C.; Fujishima, A. Hydrogel Photocatalysts for Efficient Energy Conversion and Environmental Treatment. Front. Energy 2021, 15, 577–595. [Google Scholar] [CrossRef]
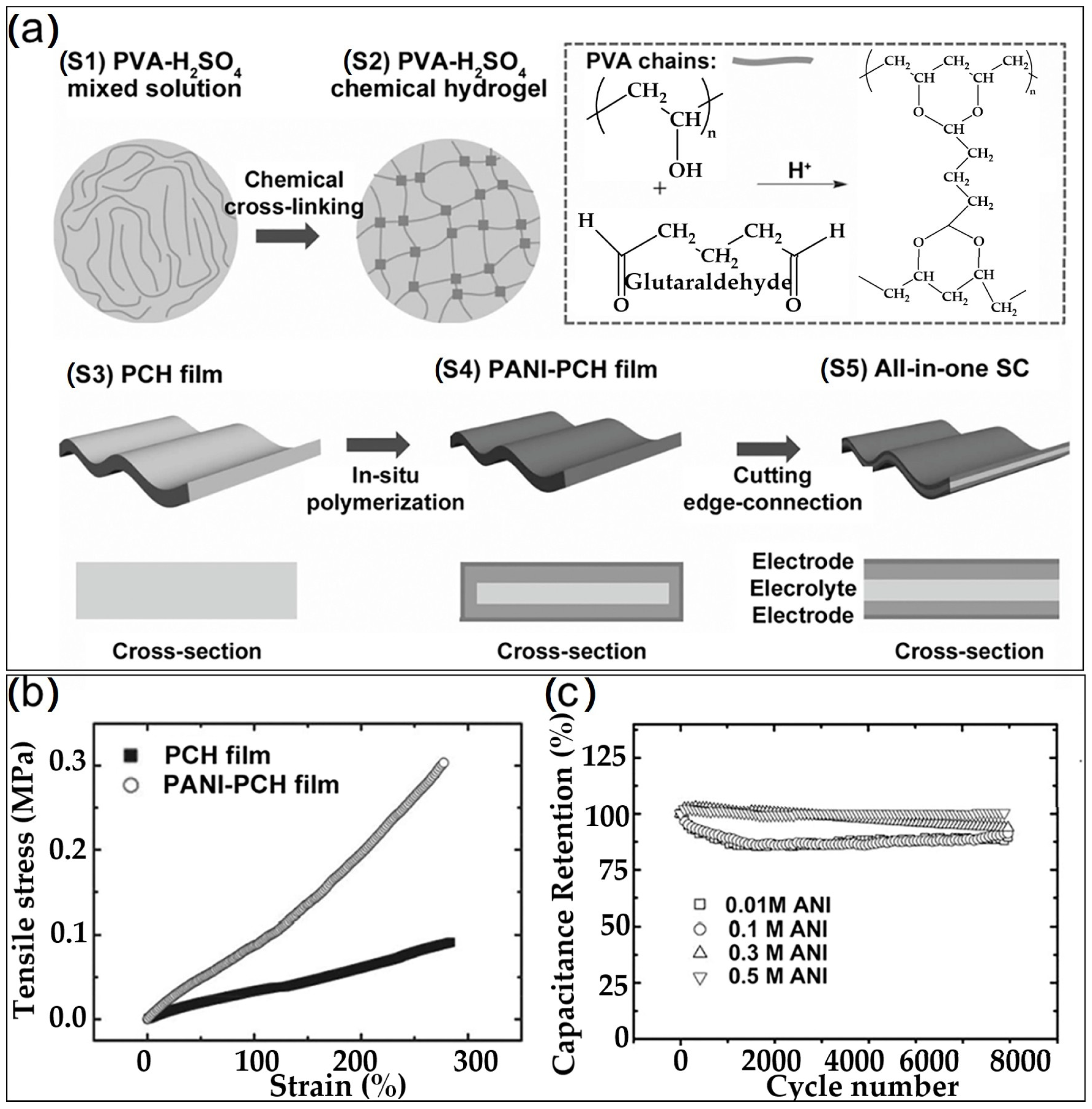
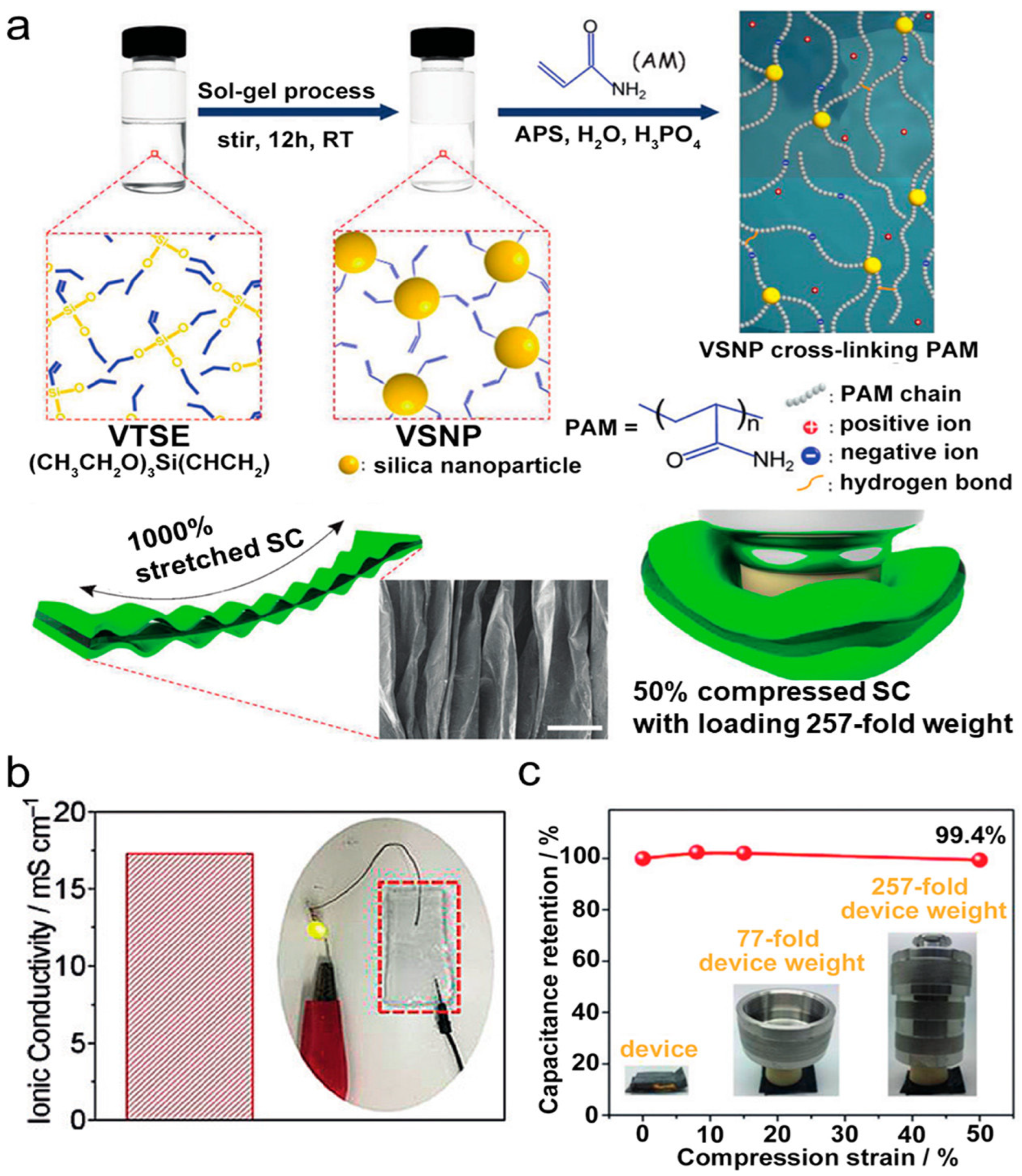
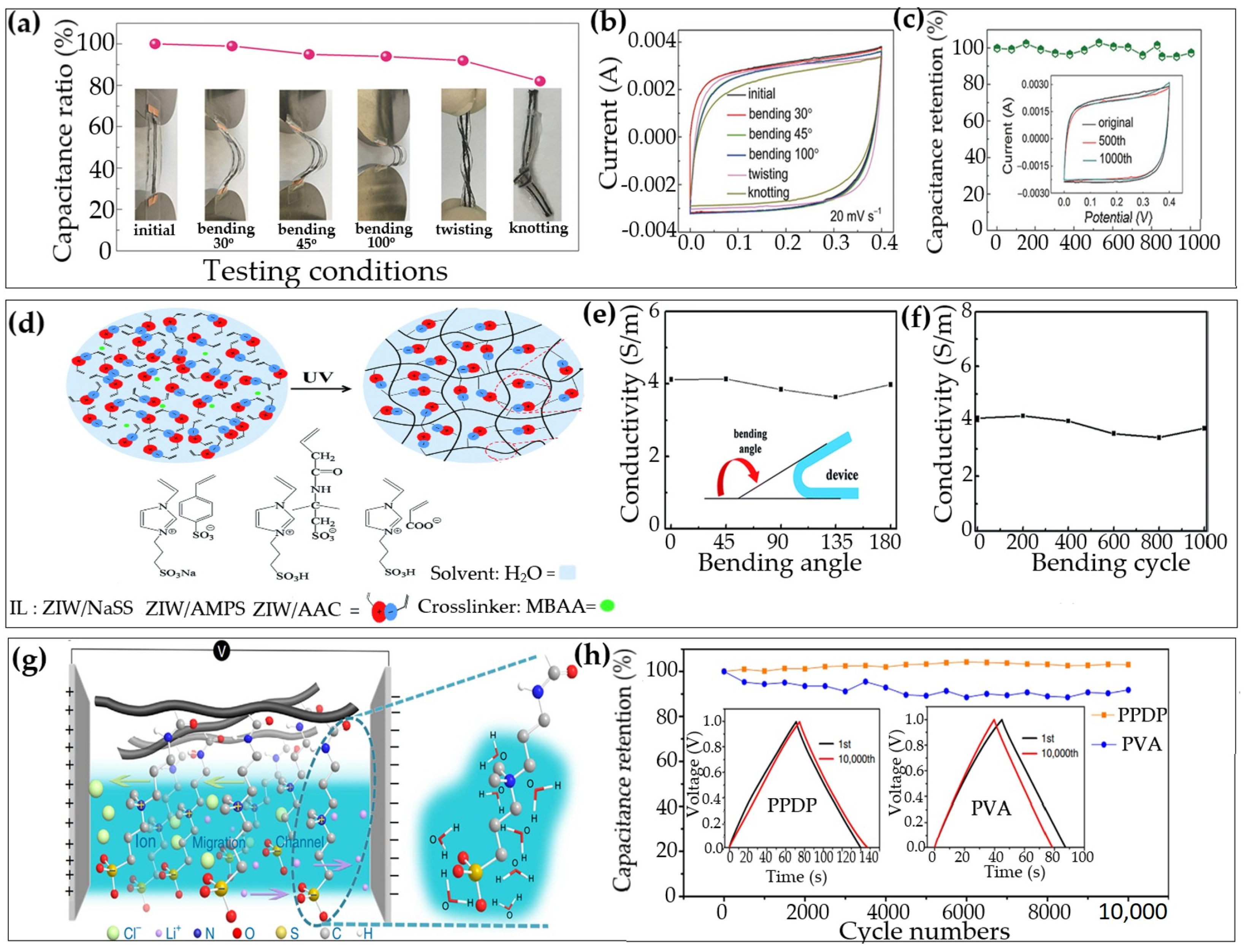
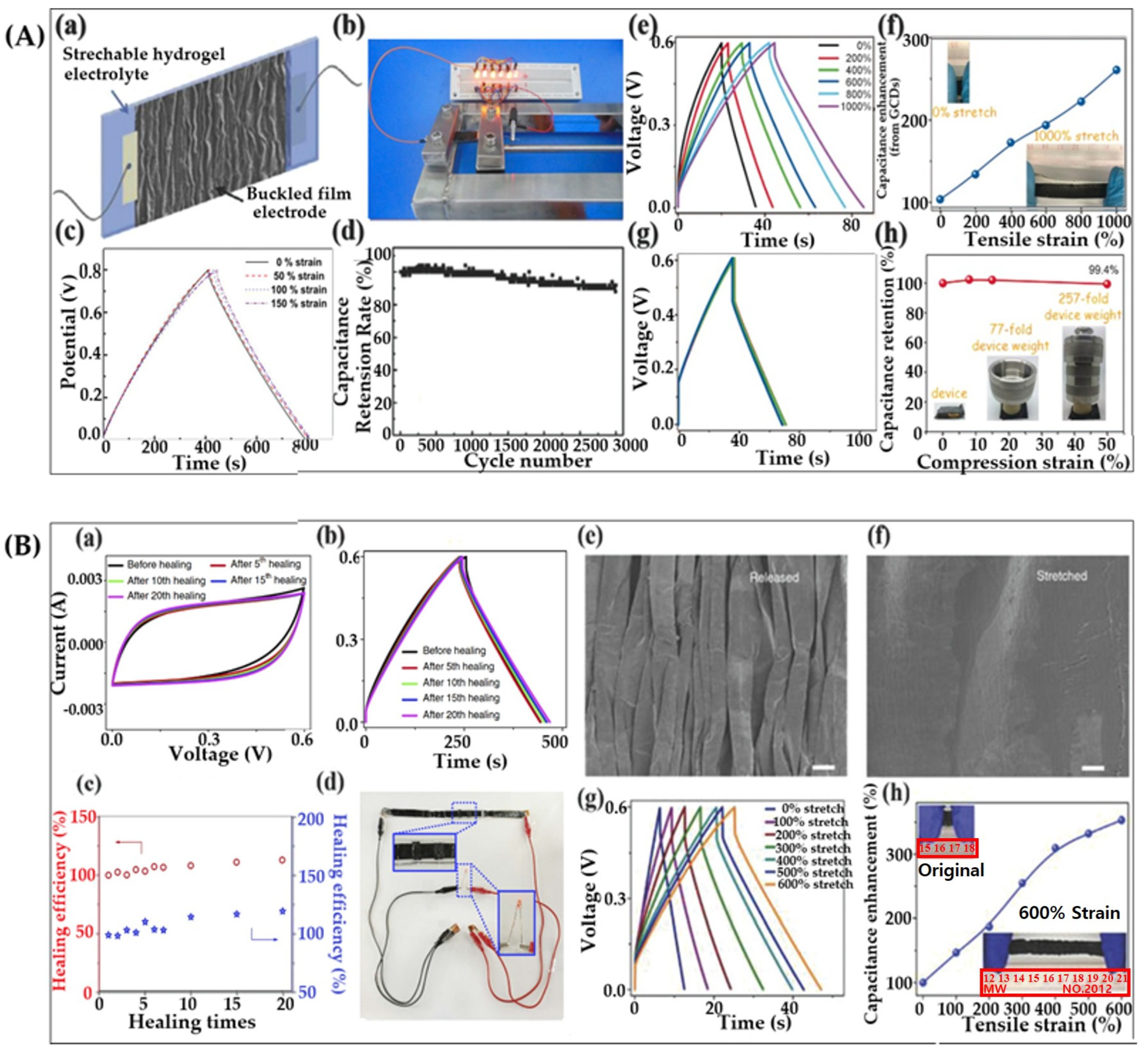



| No. | Electrode Material | Gel Polymer Electrolyte (GPE) | Ionic Conductivity | Capacitance (Specific/Areal) | Cycle Stability (Retention) | Ref. |
|---|---|---|---|---|---|---|
| 1 | PPy@CNT paper | Al-alginate/PAAm hydrogel | 29 mS/cm | 94.7 mF/cm2 @ 0.1 mA/cm2 | ~90% after 3000 cycles | [74] |
| 2 | Polypyrrole (PPy) | Agar/HPAAm double network | 2 S/m | 138.3 mF/cm2 @ 0.2 mA | — | [75] |
| 3 | Activated carbon | Hydrophobic GPE | 31.1 mS/cm | 130.3 F/g @ 0.5 A/g | ~97% after 5000 cycles @ 1 A/cm2 | [76] |
| 4 | Activated carbon | NaAc-infused crystal-type AAm gel | 0.85 S/m | 129.7 F/g @ 1.0 A/g | 86.6% after 100 cycles | [77] |
| 5 | Activated carbon | AG/AAm/LiCl GPE | 13 ± 0.8 mS/cm | 124.7 F/g @ 0.125 A/g | ~80% after 6000 cycles | [78] |
| 6 | Polypyrrole (PPy) | PVA/dilute H2SO4 | 0.05 S/cm | 58.8 F/g | 97% after 10,000 cycles @ 1 A/cm2 | [79] |
| 7 | CNT/PPy composite | PAA-based GPE | — | 0.22 mF/cm2 @ 5 mV/s | — | [80] |
| 8 | Activated carbon | Carboxylated chitosan | 8.69 10−2 S/cm | 45.9 F/g @ 0.5 A/g | — | [81] |
| 9 | Lignin/PAN electrospun nanofibers | Crosslinked lignin GPE | 10.35 mS/cm | 129.3 F/g @ 0.5 A/g | 95% after 10,000 cycles | [82] |
| 10 | CNT film | Poly(AMPS-co-DMAAm)/LAPONITE®/GO nanocomposite | 6.2 mS/cm | 180 mF/cm2 @ 0.5 mA | 91.88% after 10,000 cycles | [83] |
| 11 | Activated carbon | PAA-DAC GPE | ~8.2 mS/cm | 297 mF/cm2 @ 0.8 mA | 70% after 7000 cycles | [84] |
| 12 | PAD/H2SO4-PANI hydrogel | PAD/H2SO4 GPE | 57 mS/cm | 430 mF/cm2 @ 0.5 mA | — | [85] |
| 13 | CNT paper | PVA/H2O/EG/LiCl | 2.38 mS/cm @ −40 °C | [—] | 88.3% retention after 5000 cycles @ −20 °C | [86] |
| No. | Electrode Material | Gel Polymer Electrolyte System | Tensile Strength | Elastic Modulus | Maximum Strain | Capacitance Retention Under Deformation | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | PPy@CNT paper | Alginate/PAAm hydrogel | — | 429.8 kPa | — | 100% retention under 180° bending | [74] |
| 2 | Polypyrrole (PPy) | Agar/HPAm double-network | — | — | 100–3400% | Not reported | [75] |
| 3 | Activated carbon | Hydrophobic GPE | 431.9 kPa | — | 1150.2% | ~100% retention under 90° bending | [76] |
| 4 | Activated carbon | NaAc-infused acrylamide gel | — | 38.39 MPa | — | 105% retention at 350% strain | [77] |
| 5 | PPy | PVA/dilute H2SO4 | 56 kPa | — | 70–110% strain | 96% @ 70% strain, 90% @ 110% strain, 73% @ 180° twist | [79] |
| 6 | CNT/PPy composite | PAA-based GPE | ~16 kPa | — | 900% | ~100% @ 60% strain, ~10% @ 160° bending | [80] |
| 7 | Lignin/PAN nanofibers | Crosslinked lignin GPE | — | — | — | 90% @ 60° bending; >98% after repeated stretching (100–300%) | [82] |
| 8 | CNT film | Poly(AMPS-co-DMAAm)/LAPONITE®/GO nanocomposite | 34 kPa | — | 1173% | Not reported | [83] |
| 9 | PAD/H2SO4–PANI hydrogel | PAD/H2SO4 GPE | 1.8 MPa | — | 2900% | Not reported | [85] |
| 10 | CNT paper | PVA/H2O/EG/LiCl blend | — | — | 300% | 81.1% retention after 1000 cycles under 180° bending | [86] |
| S.N. | Type of Gel | Characteristics | Type of Solar Cell | Photoelectric Conversion Efficiency | Ref. |
|---|---|---|---|---|---|
| 01 | PANI Hybrid Hydrogel | Nanostructured, flexible, quasi-solid, UV-assisted synthesis, conductivity 0.003–0.02 S/cm | Dye-Sensitized Solar Cell (DSSC) using natural dye (Myrciaria cauliflora) | >2.0%, better than similar systems with the same dye | [110] |
| 02 | Graphene Oxide Hydrogel | Quasi-solid, GO forms conductive networks in gelatin, enhancing charge transport | DSSC with NiO@TiO2 photoanode | Up to 4.02% (with 5% NiO and 0.1% GO); 1.31% with TiO2 only | [111] |
| 03 | PAM–MBA Hydrogel | Chemically crosslinked, quasi-solid polysulfide electrolyte, 3D porous network, ionic conductivity 0.093 S/cm | CdS/CdSe QDSC | Up to 4.0%, improved stability and ion transport | [112] |
| 04 | PVA/MWCNT/PANI Hydrogel | In situ polymerization, enhanced charge transport via MWCNT network. | DSSC with I−/I3− gel electrolyte | Up to 2.18% (at 0.75% MWCNT content) | [113] |
| 05 | Dextran Hydrogel | Highly conductive, 15 wt% gelator, good pore penetration, fast electron transport, quasi-solid-state | CdS/CdSe QDSC | 3.23% (1 sun), up to 4.58% (0.12 sun) | [114] |
| 06 | 12-Hydroxystearic Acid Hydrogel | Low molecular mass organogelator, gel-liquid transition at 96 °C, enhanced stability, accelerated recombination | CdS/CdSe QDSC | 2.40% (1 sun), improved stability vs. liquid electrolyte | [115] |
| 07 | Graphene Hydrogel (GH) & GH–CuS Composite | 3D porous structure, high conductivity, enhanced catalytic activity, improved stability, low resistance | CdSeTe QDSC | 9.85% (GH), up to 10.71% (GH–CuS) | [116] |
| 08 | CNT–GH–CuS Composite Hydrogel | Ternary composite, high conductivity, low series resistance (~0.79 Ω), strong catalytic activity | Zn–Cu–In–Se QDSC | 14.02% (record efficiency) | [117] |
| 09 | Chlorophyll-a/PAM Hydrogel | Bio-inspired, exciton transport via ENAQT, synthesized via in situ and swelling methods, enhanced photocurrent in the swollen system | Bio-inspired photovoltaic assembly | 0.59%. | [118] |
| 10 | PAA-G-PABD-Fe3O4NRs Hydrogel | Nanocomposite hydrogel with Fe3O4 nanorods and [FeSCN]2+ complex, enhanced electron transfer, and high thermal stability | Hybrid Solar Cell (FTO|TiO2/Gel|Al) | 6.08% (2100% improvement over control) | [119] |
| No. | Hydrogel Material | Target Application | Device Type | Ref. |
|---|---|---|---|---|
| 1 | Polyacrylamide (PAM) | Supercapacitor | Energy storage | [120] |
| 2 | Poly(N-isopropylacrylamide) (PNIPAM) | Solar energy harvesting | Thermo-responsive solar systems | [121] |
| 3 | Poly(vinyl alcohol) (PVA) | Solar energy conversion | Photovoltaic cells | [122] |
| 4 | Poly(ethylene glycol) (PEG) | Fuel cell applications | Electrochemical systems | [123] |
| 5 | Chitosan-based hydrogel | Battery technology | Rechargeable energy systems | [124] |
| 6 | Poly(acrylic acid) (PAA) | Fuel cells | Proton exchange membranes | [125] |
| 7 | Poly(vinyl pyrrolidone) (PVP) | Solar cells | Photovoltaic devices | [126] |
| 8 | Alginate-based hydrogel | Battery systems | Lithium-ion batteries | [127] |
| 9 | Poly(ethylene oxide) (PEO) | Thermoelectric conversion | Waste heat recovery devices | [128] |
| 10 | Gelatin-based hydrogel | Supercapacitor | Energy storage | [129] |
| 11 | Poly(acrylamide-co-sodium acrylate) | Energy harvesting | Triboelectric nanogenerators | [130] |
| 12 | Poly(2-hydroxyethyl methacrylate) (PHEMA) | Battery systems | Lithium-ion batteries | [131] |
| 13 | Poly(ethylene glycol diacrylate) (PEGDA) | Solar energy | Dye-sensitized solar cells | [132] |
| 14 | Poly(N-vinylcaprolactam) (PVNCL) | Thermoelectric devices | Energy harvesting systems | [133] |
| 15 | Polythiophene | Supercapacitor | Energy storage | [134] |
| 16 | Polystyrenesulfonate | Fuel cells | Proton exchange membranes | [135] |
| 17 | Poly(2-isopropyl-2-oxazoline) (PiPOx) | Energy storage | Sodium-ion batteries | [136] |
| 18 | Poly(N,N-dimethylacrylamide) (PDMA) | Supercapacitor | Energy storage | [137] |
| 19 | Poly(itaconic acid) (PA) | Solar energy | Photovoltaic devices | [138] |
| 20 | PANa–PVP–TiO2(NH2) hybrid hydrogel | High ionic conductivity & flexibility | Quasi-solid-state Zn–air battery | [139] |
| 21 | Polyacrylamide (PAM)–Gelatin composite hydrogel | Enhanced ionic conductivity | Zn–air battery | [140] |
| 22 | Dual-ion conducting hydrogel (SDICH)—PVA-based with Na+ and Mg2+ ions | Intelligent biomedical electronics, wound healing | Fully stretchable Mg–air battery | [141] |
| 23 | In situ cross-linked polyacrylic acid (PAA)–KOH hydrogel | Flexible and stretchable energy storage | Kirigami-inspired Zn–air battery | [142] |
| 24 | Zirconium hydroxide hydrogel | Gas separation and conductivity enhancement | Alkaline water electrolyzer diaphragm | [143] |
| 25 | Natural polymer hydrogels (e.g., chitosan, alginate, lignin) | Water purification and environmental remediation | Hydrogel-based filtration systems | [144] |
| 26 | Hydrogel photocatalysts (e.g., TiO2, g-C3N4 embedded hydrogels) | Solar-driven water splitting and pollutant degradation | Photocatalytic water electrolyzer | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, S.C.; Ahmed, M.S.; Uddin, M.A.; Oh, Y.-C.; Park, J.; Nam, K.-W.; Islam, M. Applications of Conductive Polymer Hydrogels for Supercapacitor, Solar Cell, and Energy Conversion. Gels 2025, 11, 741. https://doi.org/10.3390/gels11090741
Sutradhar SC, Ahmed MS, Uddin MA, Oh Y-C, Park J, Nam K-W, Islam M. Applications of Conductive Polymer Hydrogels for Supercapacitor, Solar Cell, and Energy Conversion. Gels. 2025; 11(9):741. https://doi.org/10.3390/gels11090741
Chicago/Turabian StyleSutradhar, Sabuj Chandra, Md. Shahriar Ahmed, Mohammad Afsar Uddin, Ye-Chan Oh, Junwoo Park, Kyung-Wan Nam, and Mobinul Islam. 2025. "Applications of Conductive Polymer Hydrogels for Supercapacitor, Solar Cell, and Energy Conversion" Gels 11, no. 9: 741. https://doi.org/10.3390/gels11090741
APA StyleSutradhar, S. C., Ahmed, M. S., Uddin, M. A., Oh, Y.-C., Park, J., Nam, K.-W., & Islam, M. (2025). Applications of Conductive Polymer Hydrogels for Supercapacitor, Solar Cell, and Energy Conversion. Gels, 11(9), 741. https://doi.org/10.3390/gels11090741










