Emerging Biomedical Applications of Sustainable Cellulose Nanocrystal-Incorporated Hydrogels: A Scoping Review
Abstract
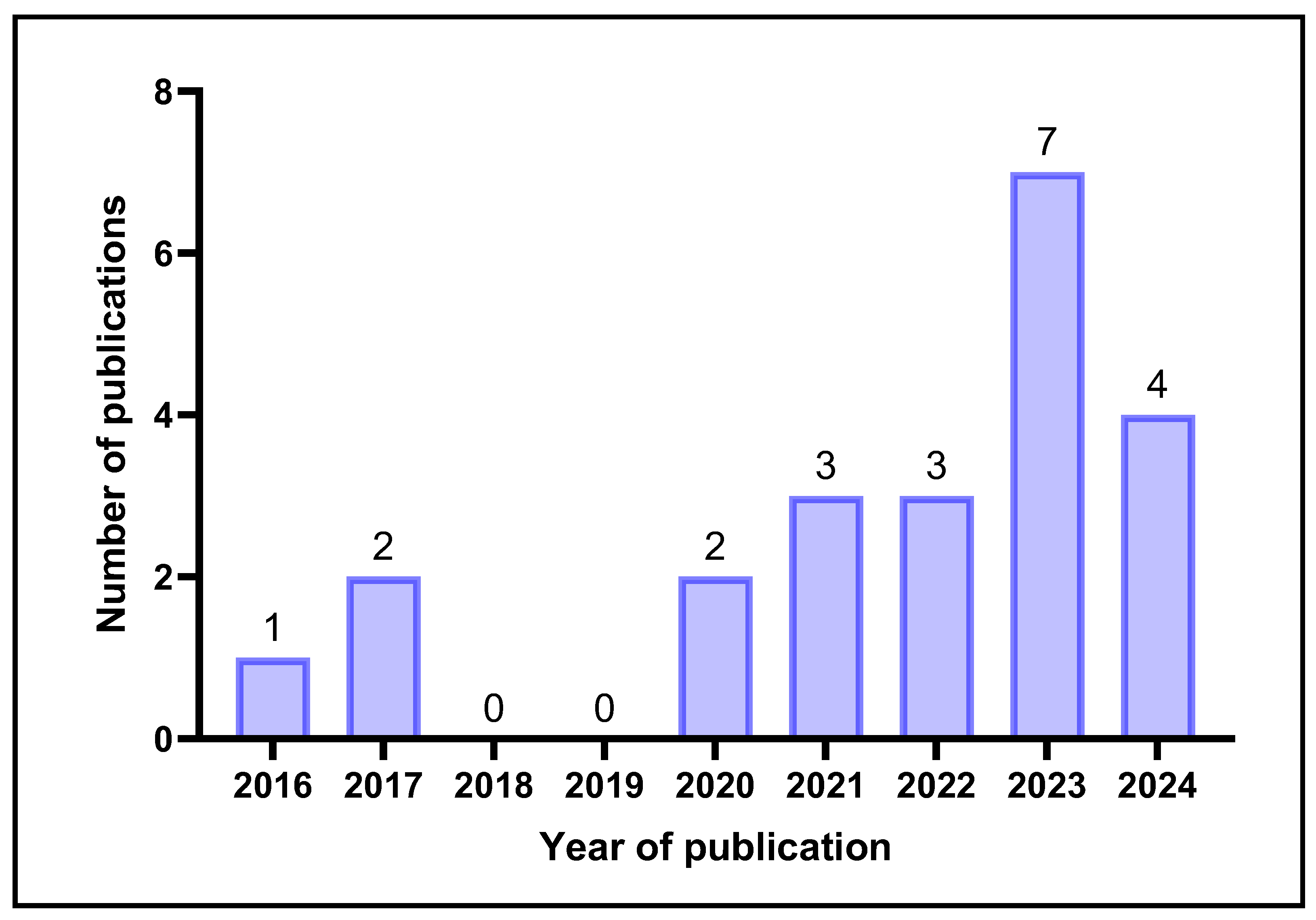
1. Introduction
2. Results and Discussion
2.1. CNC Sources, Extraction, and Modifications
2.2. Biomedical Applications
2.2.1. Tissue Engineering
Bone Repair and Regeneration
Cartilage Repair
2.2.2. Wound Healing and Repair
2.2.3. Medical Implants and Sensors
Cardiac Valve Regeneration
Extravascular Stent
Autologous Fat Grafting
Glucose Level Sensor
2.2.4. Drug Loading, Retention, and Release
Anti-Inflammatory Drug Release
Antimicrobial Drug Release
Antitumour Drug Delivery
Soft Robot for Controlled Drug Delivery
pH-Controlled Drug Delivery in the Gut
3. Conclusions
4. Methodology
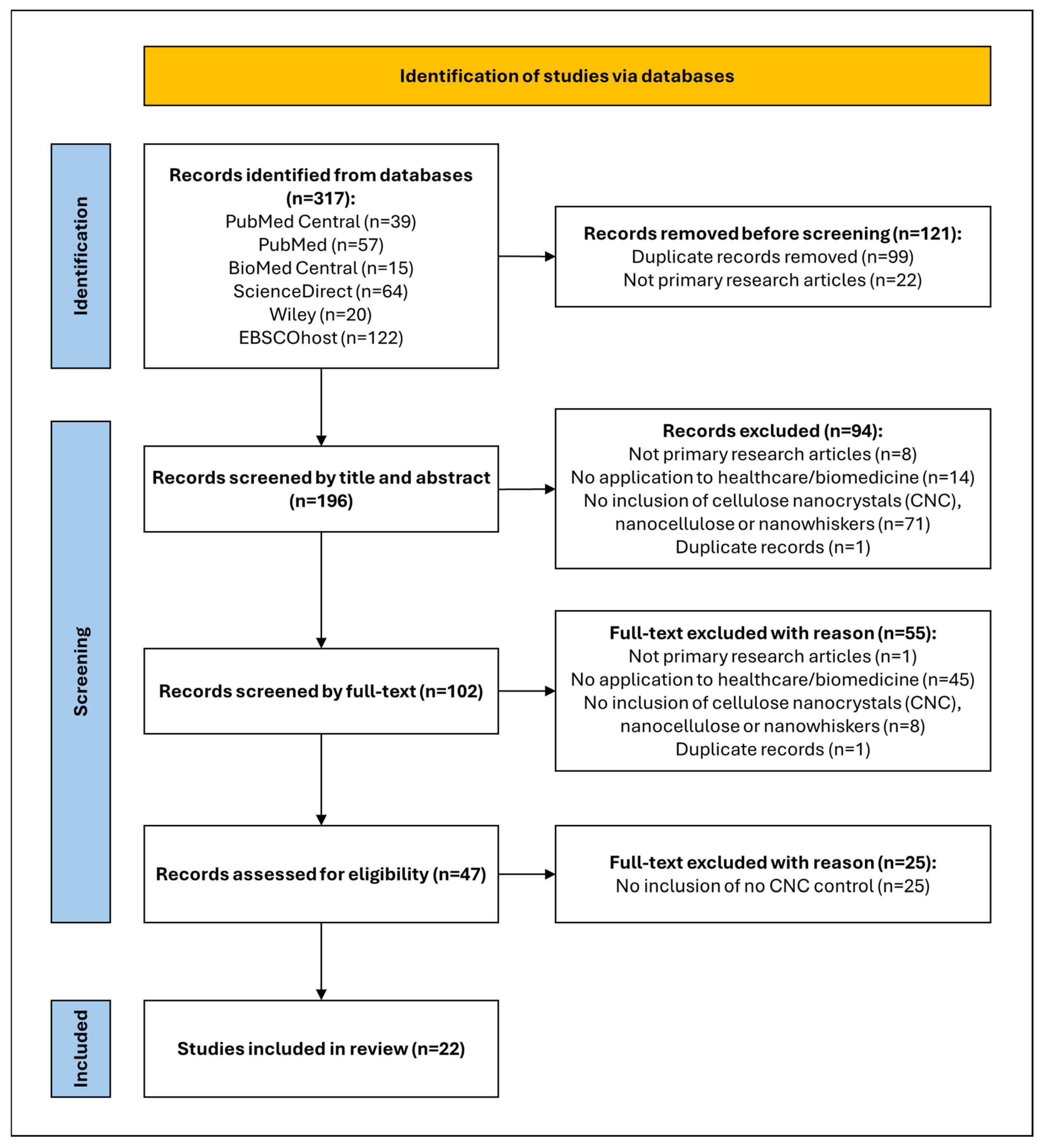
4.1. Data Sources and Searches
4.2. Inclusion and Exclusion Criteria
- Open access and/or open archive availability of the full-text article
- Publication type is a journal article
- Primary research study design
- Applications to healthcare or biomedicine
- Utilisation of cellulose nanocrystals or nanocellulose, or nanowhiskers
- Utilisation of appropriate controls, including negative (hydrogel with no CNC incorporation) and positive controls
4.3. Study Selection and Quality Assurance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvaraj, S.; Chauhan, A.; Dutta, V.; Verma, R.; Rao, S.K.; Radhakrishnan, A.; Ghotekar, S. A state-of-the-art review on plant-derived cellulose-based green hydrogels and their multifunctional role in advanced biomedical applications. Int. J. Biol. Macromol. 2024, 265, 130991. [Google Scholar] [CrossRef]
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable nanomaterials for biomedical applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Balakrishnan, P.; Chandradhara, D.; Poovathankandy, D.; Thomas, S. General scenarios of cellulose and its use in the biomedical field. Mater. Today Chem. 2019, 13, 59–78. [Google Scholar] [CrossRef]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.D.; Chuah, C.H. A review of recent advances of cellulose-based intelligent-responsive hydrogels as vehicles for controllable drug delivery system. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef] [PubMed]
- Kassie, B.B.; Daget, T.M.; Tassew, D.F. Synthesis, functionalization, and commercial application of cellulose-based nanomaterials. Int. J. Biol. Macromol. 2024, 278, 134990. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-based nanocomposites for sustainable applications: A review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Carnero Canales, C.S.; Primo, L.M.D.G.; Colturato, V.M.M.; Polinário, G.; Di Filippo, L.D.; Duarte, J.L.; Chorilli, M.; da Silva Barud, H.; Pavan, F.R. Cellulose from bacteria as a delivery system for improved treatment of infectious diseases: A review of updates and prospects. Int. J. Biol. Macromol. 2024, 277, 133831. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Nizamuddin, S.; Xu, J.; Vancov, T.; Chen, C. Cellulose nanocrystals from agriculture and forestry biomass: Synthesis methods, characterization and industrial applications. Environ. Sci. Pollut. Res. 2024, 31, 58745–58778. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the potential biological impact of cellulose nanocrystals. Fibers 2016, 4, 21. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D printing of hydrogel polysaccharides for biomedical applications: A review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Nanoparticle decorated cellulose nanocrystals (CNC) composites for energy, catalysis, and biomedical applications. Adv. Funct. Mater. 2025, 35, 2412869. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, B.; Hu, K.; Yuan, B.; Sun, X.; Song, X.; Tang, Z.; Lin, H.; Zhu, X.; Zheng, Y.; et al. Evidence-based biomaterials research. Bioact. Mater. 2022, 15, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The application status of nanoscale cellulose-based hydrogels in tissue engineering and regenerative biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Dagnino, E.P.; Ehman, N.; Area, M.C. Recent advances in cellulose nanocrystal production from green methods. Processes 2025, 13, 790. [Google Scholar] [CrossRef]
- Sugiura, K.; Saito, M.; Sawada, T.; Tanaka, H.; Serizawa, T. Cellodextrin phosphorylase-catalyzed single-process production and superior mechanical properties of organic–inorganic hybrid hydrogels composed of surface-carboxylated synthetic nanocelluloses and hydroxyapatite. ACS Sustain. Chem. Eng. 2022, 10, 13484–13494. [Google Scholar] [CrossRef]
- Min, K.; Tae, G. Cellular infiltration in an injectable sulfated cellulose nanocrystal hydrogel and efficient angiogenesis by VEGF loading. Biomater. Res. 2023, 27, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, W.; Koppolu, R.; van Bochove, B.; Seppälä, J.; Hupa, L.; Willför, S.; Xu, C.; Wang, X. Injectable thiol-ene hydrogel of galactoglucomannan and cellulose nanocrystals in delivery of therapeutic inorganic ions with embedded bioactive glass nanoparticles. Carbohydr. Polym. 2022, 276, 118780. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Wang, W.; Bai, L.; Chen, H.; Yang, L.; Yin, K.; Yang, H.; Wei, D. Functionalized cellulose nanocrystals construct self-healing nanocomposite hydrogel-based sweat sensors for glucose detection. Ind. Crops Prod. 2024, 216, 118728. [Google Scholar] [CrossRef]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved mechanical properties and sustained release behavior of cationic cellulose nanocrystals reinforced cationic cellulose injectable hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef]
- Chen, T.; Yao, T.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhu, S.; Wang, Z. An injectable hydrogel for simultaneous photothermal therapy and photodynamic therapy with ultrahigh efficiency based on carbon dots and modified cellulose nanocrystals. Adv. Funct. Mater. 2021, 31, 2106079. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-laden nanocellulose/chitosan-based bioinks for 3D bioprinting and enhanced osteogenic cell differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Wan Ishak, W.H.; Rosli, N.A.; Ahmad, I.; Ramli, S.; Mohd Amin, M.C.I. Drug delivery and in vitro biocompatibility studies of gelatin-nanocellulose smart hydrogels cross-linked with gamma radiation. J. Mater. Res. Technol. 2021, 15, 7145–7157. [Google Scholar] [CrossRef]
- Wu, T.; Liu, H.; Wang, H.; Bu, Y.; Liu, J.; Chen, X.; Yan, H.; Lin, Q. Fabrication of alginate/sericin/cellulose nanocrystals interpenetrating network composite hydrogels with enhanced physicochemical properties and biological activity. J. Appl. Polym. Sci. 2024, 141, e55052. [Google Scholar] [CrossRef]
- Ferrante, M.; Álvarez, V.A.; Narain, R.; Ounkaew, A.; González, J.S. Enhancing the properties of chitosan–pectin hydrogels with cellulose nanowhiskers for potential applications in wound dressings. Macromol. Chem. Phys. 2024, 225, 2400088. [Google Scholar] [CrossRef]
- González, K.; Guaresti, O.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Gabilondo, N. The role of cellulose nanocrystals in biocompatible starch-based clicked nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 143, 265–272. [Google Scholar] [CrossRef]
- Okamoto, T.; Patil, A.J.; Nissinen, T.; Mann, S. Self-assembly of colloidal nanocomposite hydrogels using 1D cellulose nanocrystals and 2D exfoliated organoclay layers. Gels 2017, 3, 11. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Valencia, S.; Rivera, E.; Segura, T.; Burillo, G. Reinforcement of acrylamide hydrogels with cellulose nanocrystals using gamma radiation for antibiotic drug delivery. Gels 2023, 9, 602. [Google Scholar] [CrossRef]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J. Biomed. Mater. Res. Part A 2022, 110, 76–91. [Google Scholar] [CrossRef]
- Nasseri, R.; Bouzari, N.; Huang, J.; Golzar, H.; Jankhani, S.; Tang, X.S.; Mekonnen, T.H.; Aghakhani, A.; Shahsavan, H. Programmable nanocomposites of cellulose nanocrystals and zwitterionic hydrogels for soft robotics. Nat. Commun. 2023, 14, 6108. [Google Scholar] [CrossRef]
- Marquis, M.; Musino, D.; Gemin, V.; Kolypczuk, L.; Passerini, D.; Capron, I. Alginate microgels encapsulation strategy of silver nanoparticles active against Candida albicans. Carbohydr. Polym. Technol. Appl. 2023, 6, 100405. [Google Scholar] [CrossRef]
- Read, S.A.; Go, C.S.; Ferreira, M.J.S.; Ligorio, C.; Kimber, S.J.; Dumanli, A.G.; Domingos, M.A.N. Nanocrystalline cellulose as a versatile engineering material for extrusion-based bioprinting. Pharmaceutics 2023, 15, 2432. [Google Scholar] [CrossRef] [PubMed]
- de Ávila Gonçalves, S.; da Fonsêca, J.H.L.; d’Ávila, M.A.; Vieira, R.P. Synthesis of thermally and pH-responsive poly(2-(dimethylamino)ethyl methacrylate)-based hydrogel reinforced with cellulose nanocrystals for sustained drug release. Int. J. Biol. Macromol. 2024, 277, 134168. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Li, Q.; Dai, C.; Li, X.; Kong, X.; Fan, Y.; Yin, H.; Ge, J. A novel nanocellulose-gelatin-AS-IV external stent resists EndMT by activating autophagy to prevent restenosis of grafts. Bioact. Mater. 2023, 22, 466–481. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; Maturavongsadit, P.; Hingtgen, S.D.; Benhabbour, S.R. Injectable pH thermo-responsive hydrogel scaffold for tumoricidal neural stem cell therapy for glioblastoma multiforme. Pharmaceutics 2022, 14, 2243. [Google Scholar] [CrossRef]
- King, J.L.; Shrivastava, R.; Shah, P.D.; Maturavongsadit, P.; Benhabbour, S.R. Injectable pH and thermo-responsive hydrogel scaffold with enhanced osteogenic differentiation of preosteoblasts for bone regeneration. Pharmaceutics 2023, 15, 2270. [Google Scholar] [CrossRef]
- Oskarsdotter, K.; Nordgård, C.T.; Apelgren, P.; Säljö, K.; Solbu, A.A.; Eliasson, E.; Sämfors, S.; Sætrang, H.E.M.; Asdahl, L.C.; Thompson, E.M.; et al. Injectable in situ crosslinking hydrogel for autologous fat grafting. Gels 2023, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Paravyan, G.; Shrivastava, R.; Benhabbour, S.R. Thermo-/pH-responsive chitosan-cellulose nanocrystals based hydrogel with tunable mechanical properties for tissue regeneration applications. Materialia 2020, 12, 100681. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, Z.; Zou, X.; You, X.; Cai, Z.; Huang, J. Advancements in tissue engineering for articular cartilage regeneration. Heliyon 2024, 10, e25400. [Google Scholar] [CrossRef]
- Miao, K.; Zhou, Y.; He, X.; Xu, Y.; Zhang, X.; Zhao, H.; Zhou, X.; Gu, Q.; Yang, H.; Liu, X.; et al. Microenvironment-responsive bilayer hydrogel microspheres with gelatin-shell for osteoarthritis treatment. Int. J. Biol. Macromol. 2024, 261, 129862. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Rakoczy, R.; Nowak, A.; Konopacki, M.; Klebeko, J.; Świątek, E.; Janus, E.; Duchnik, W.; Wenelska, K.; Kucharski, Ł.; et al. Transdermal delivery systems for ibuprofen and ibuprofen modified with amino acids alkyl esters based on bacterial cellulose. Int. J. Mol. Sci. 2021, 22, 6252. [Google Scholar] [CrossRef]
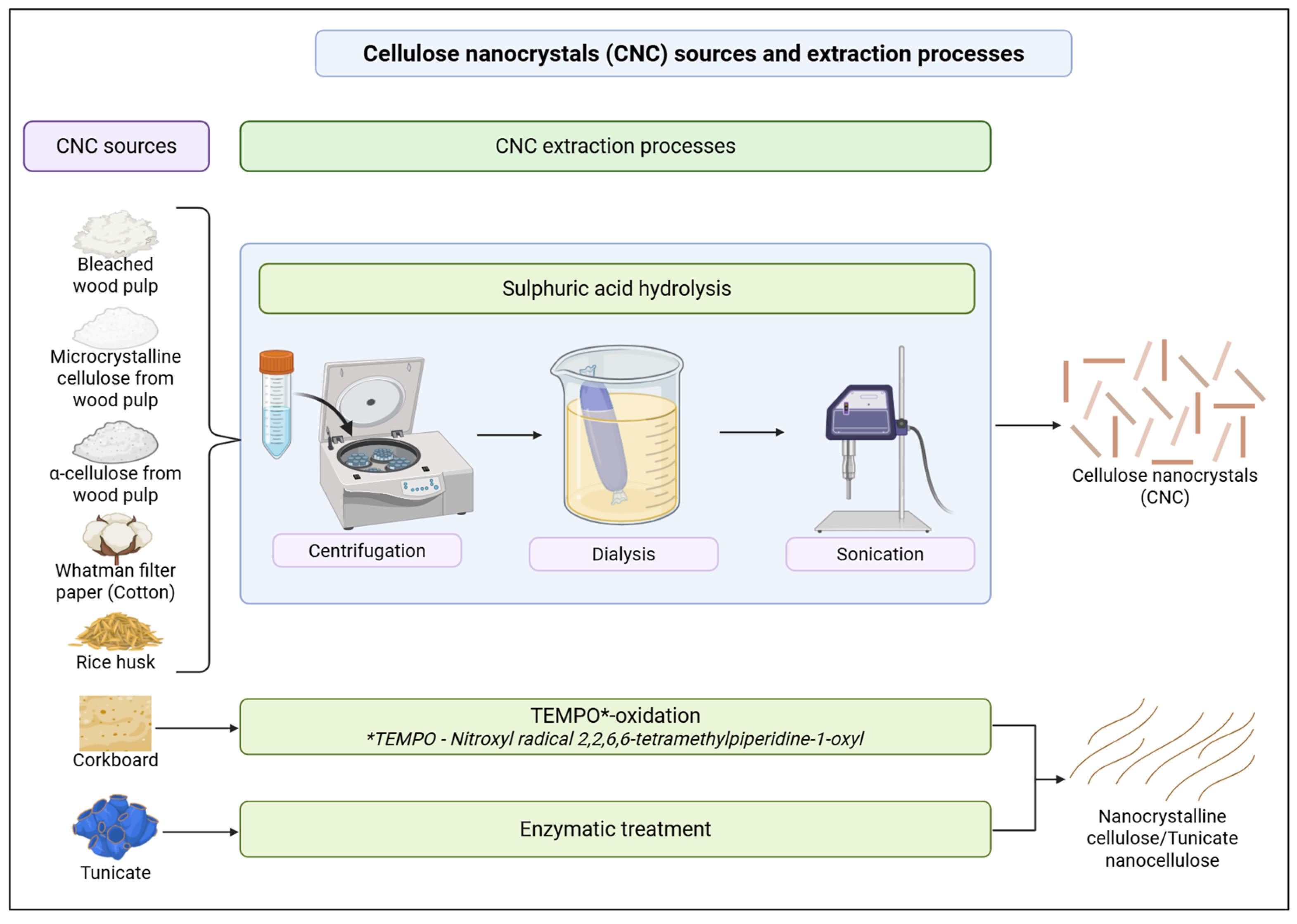


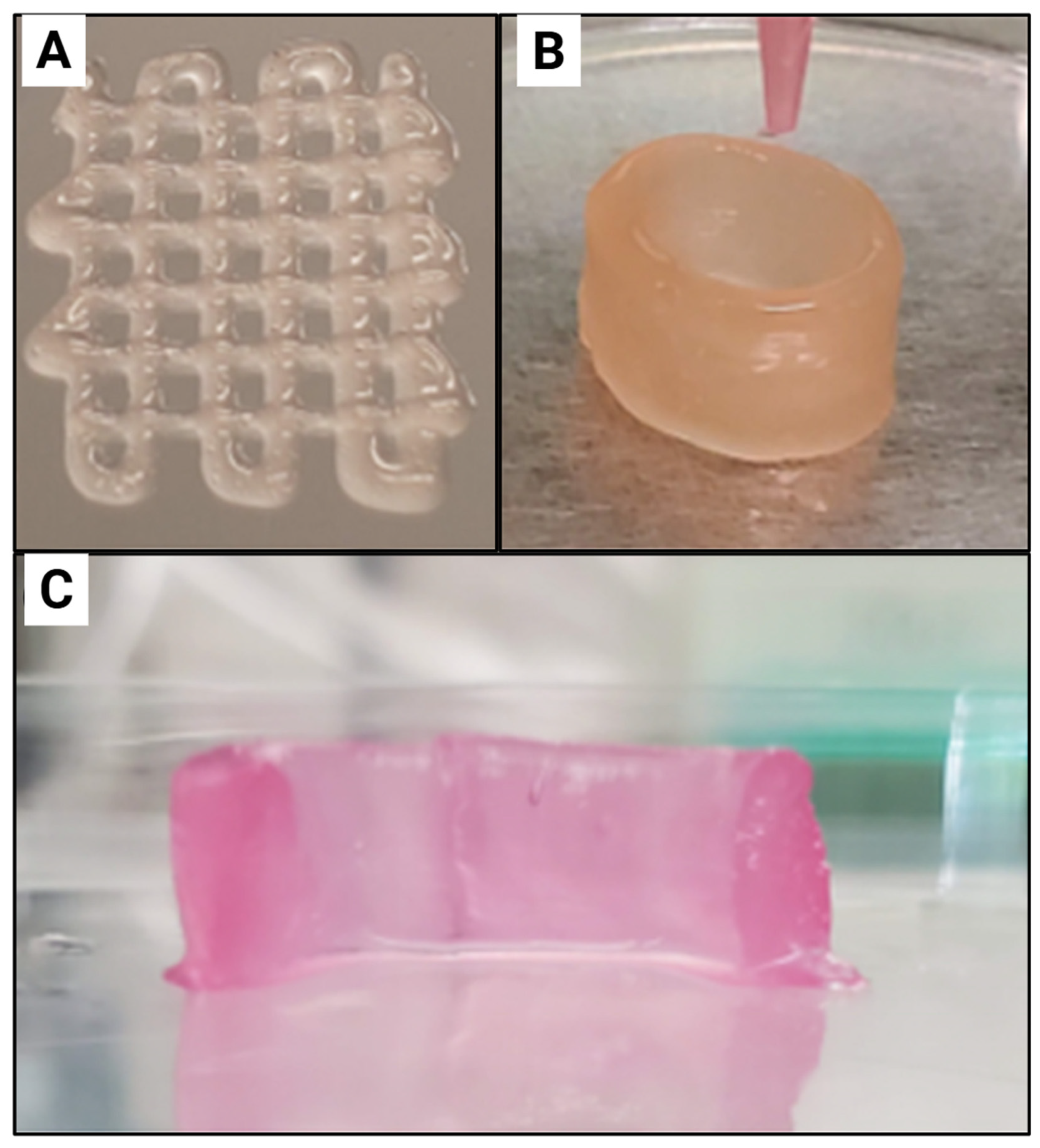
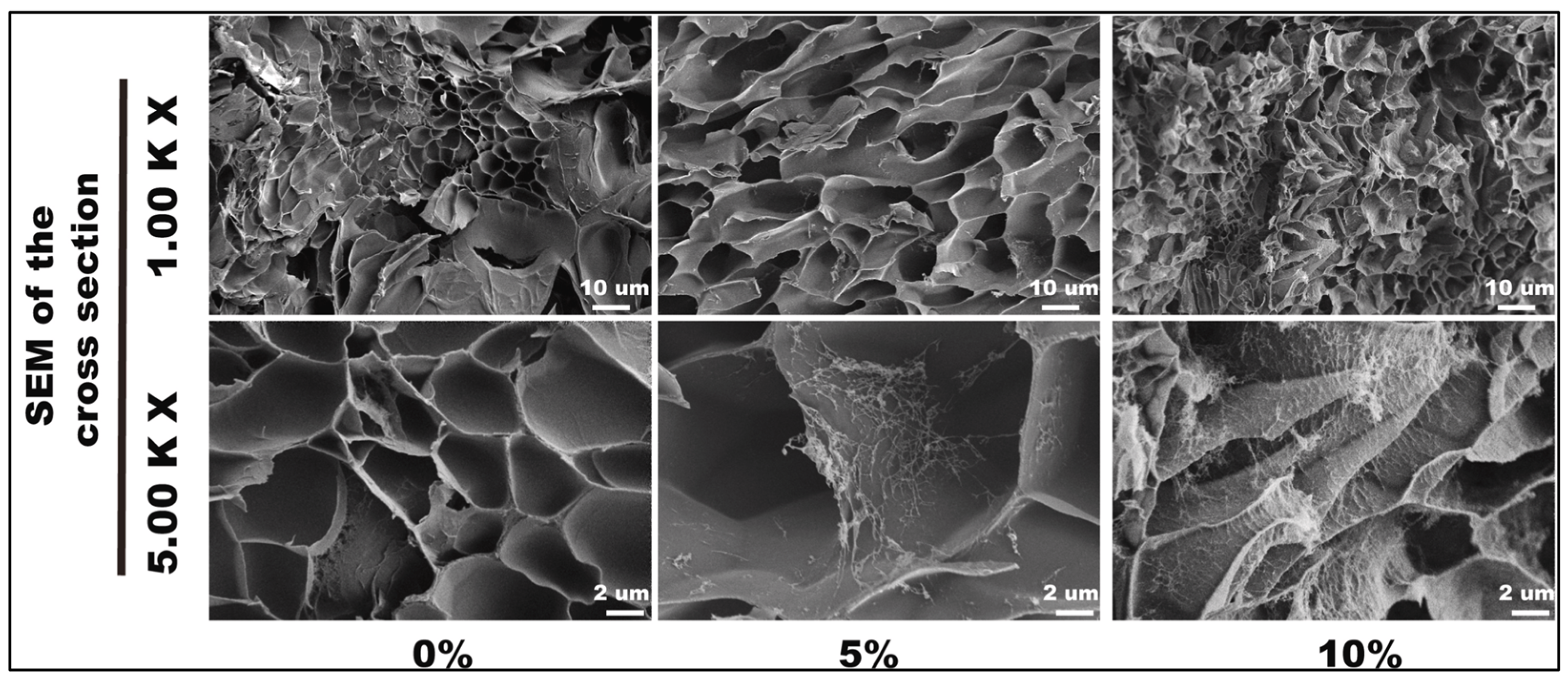
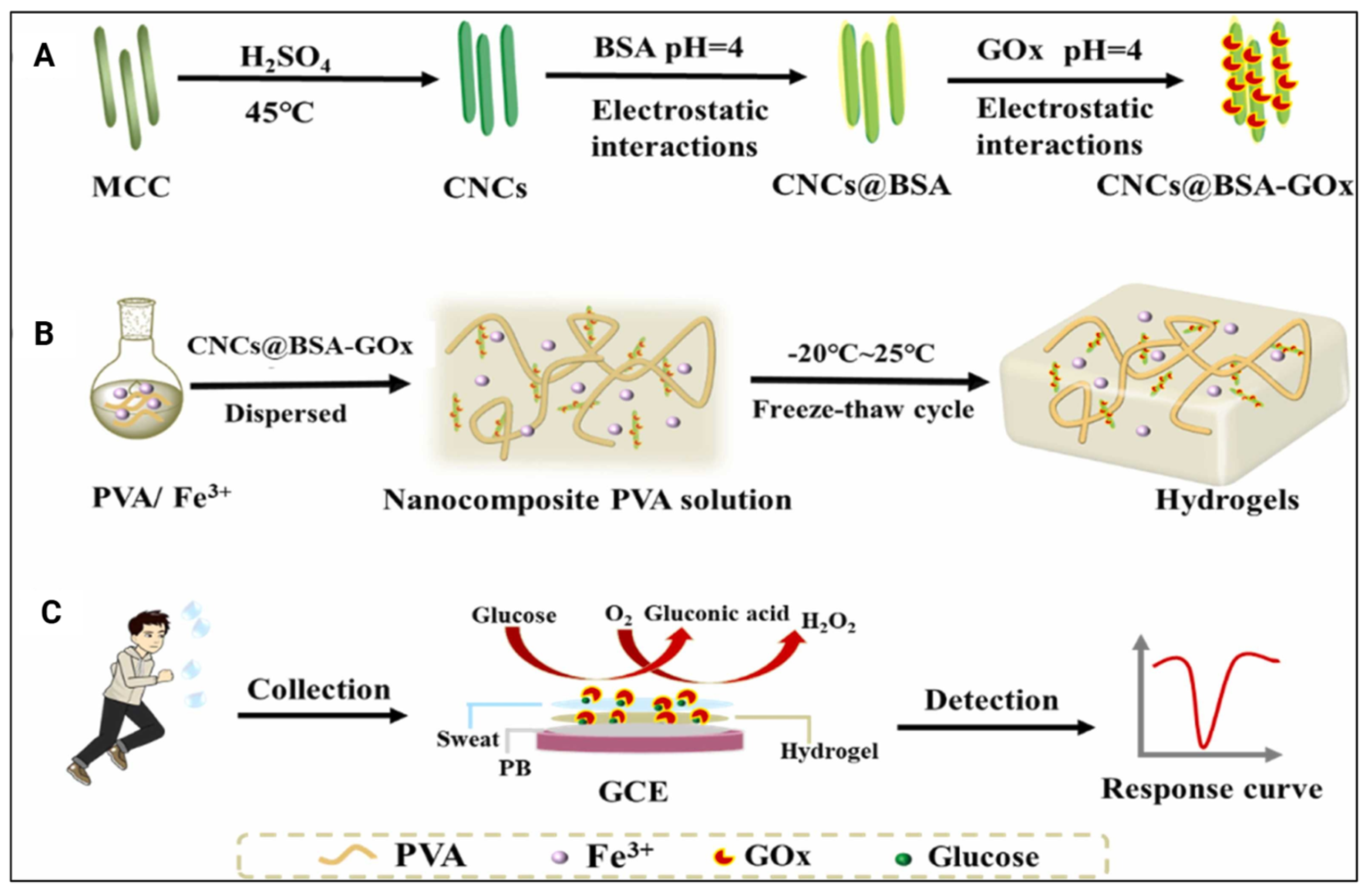
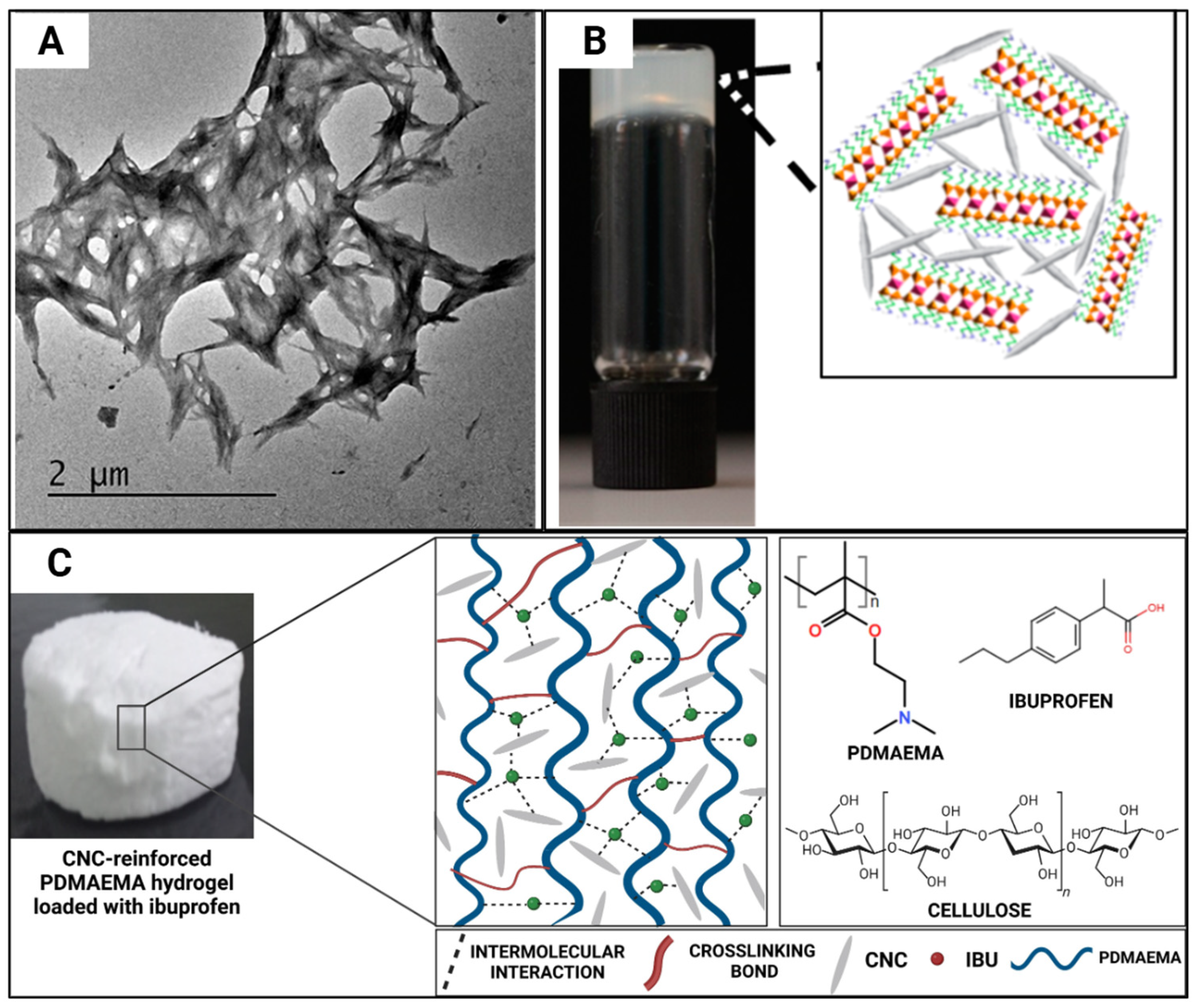
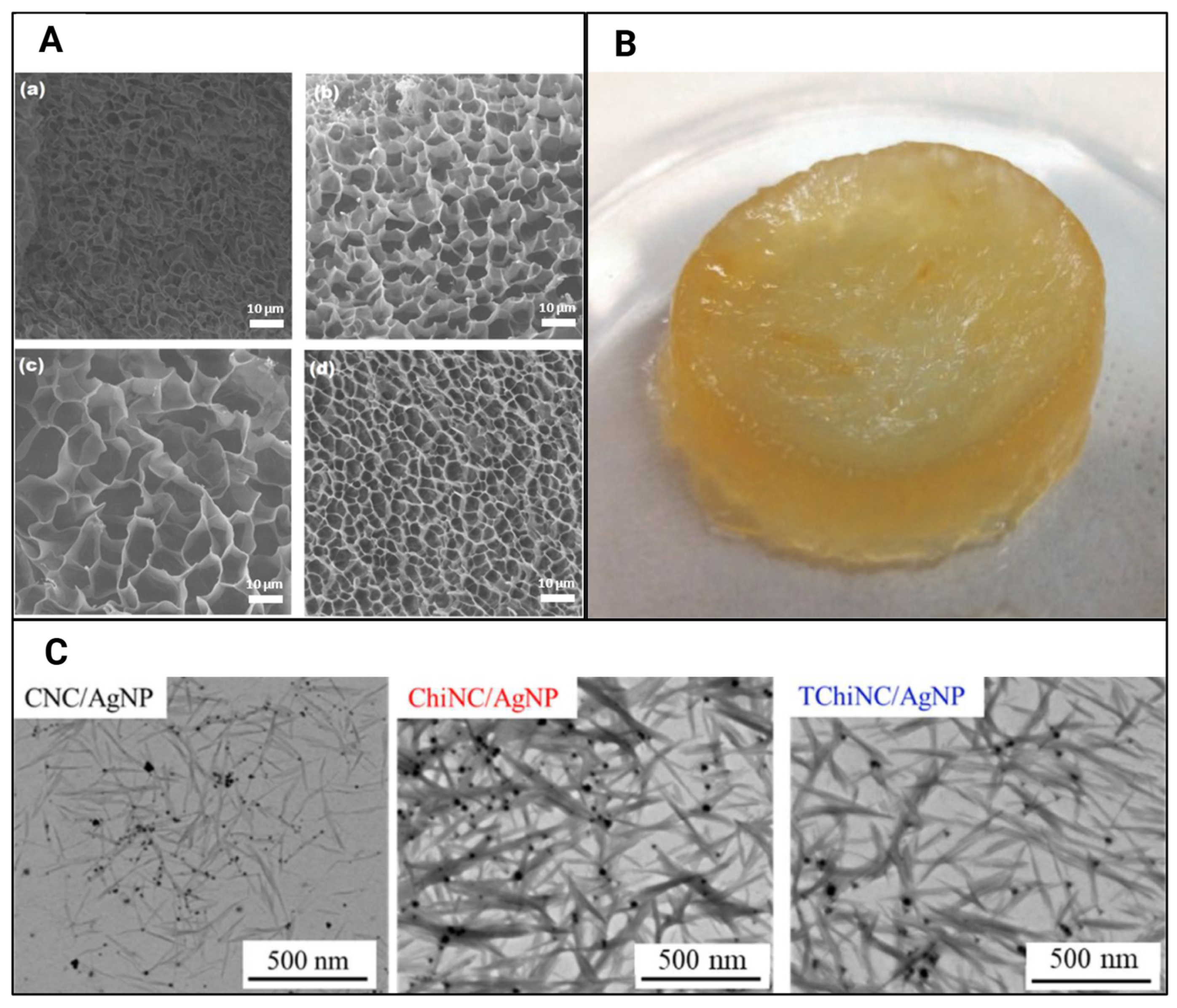
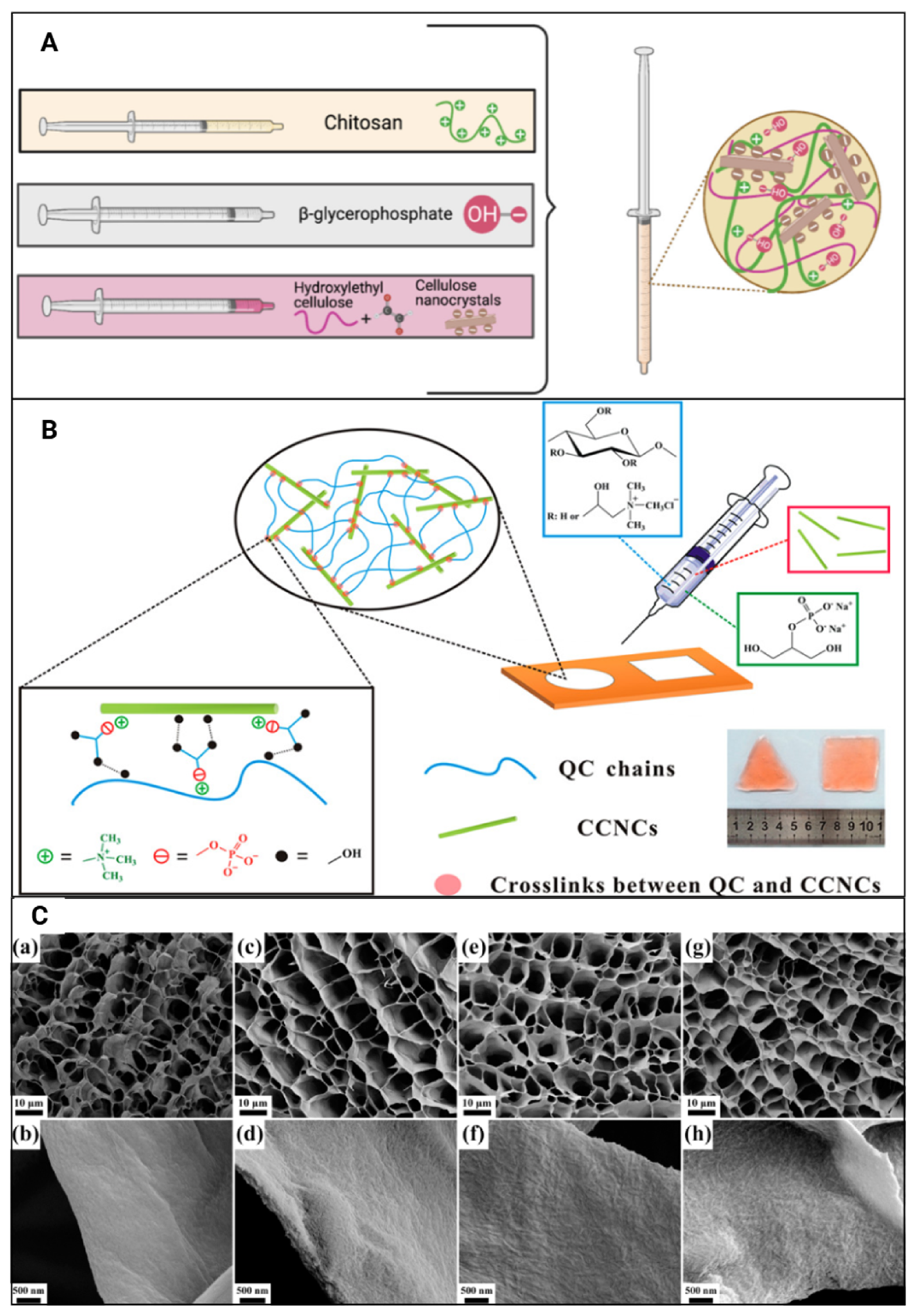
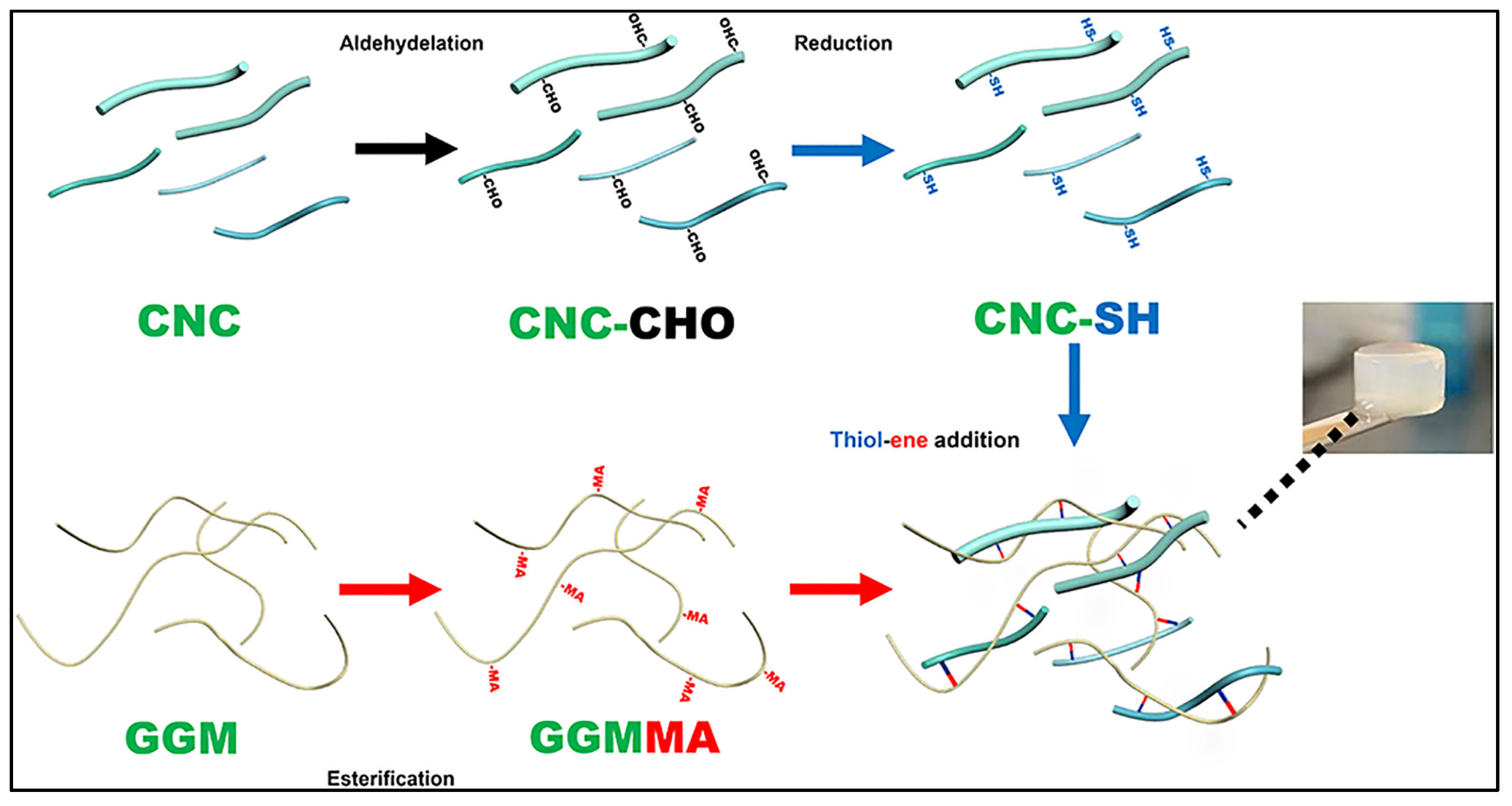
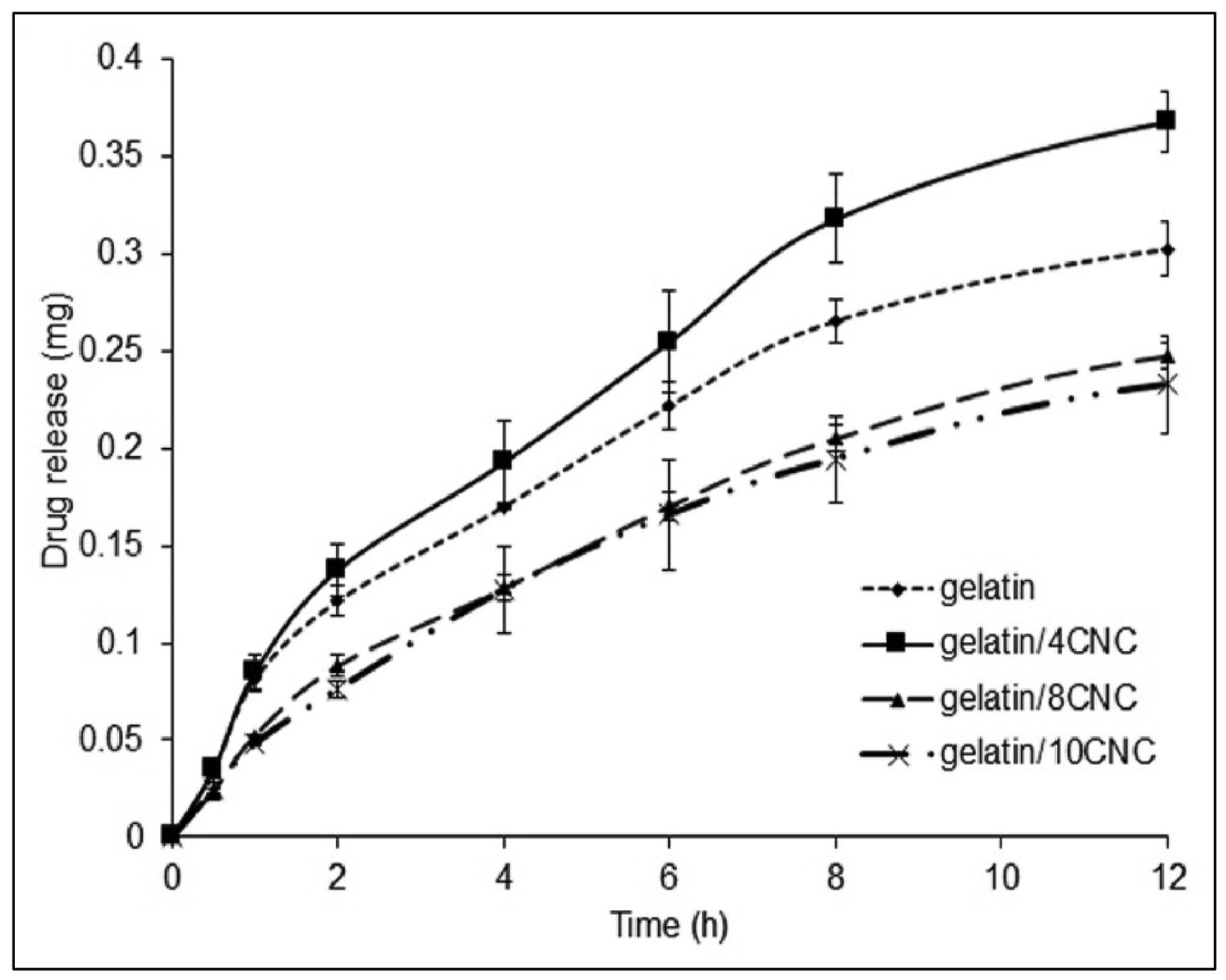

| Reference | CNC Source | CNC Extraction Method | CNC Modifications |
|---|---|---|---|
| [23] | Microcrystalline cellulose (MCC; Avicel PH-101; Fluka) | Sulphuric acid hydrolysis | Thiol-grafted CNC (CNC-SH); L-cysteine-grafted oxidised CNC |
| [24] | MCC (Tianjin Komeo Chemical Reagent) | Glucose oxidase (Gox)-loaded CNC using CNCs@BSA-Gox (CBG) | |
| [25] | MCC with a degree of polymerisation of 100–300 (Asahi Kasei Corporation, Japan) | Cationic CNC (CCNC) | |
| [26] | Cotton (from a local retailer, Shanghai, China) | Dialdehyde CNC | |
| [27] | Cotton cellulose from Whatman ashless filter-aid paper (Sigma-Aldrich) | - | |
| [28] | Rice husk (Bernas Sdn. Bhd., Shah Alam, Selangor, Malaysia) | ||
| [29] | MCC (manufacturer details not provided) | ||
| [30] | MCC (Sigma-Aldrich) | ||
| [31] | |||
| [32] | Microcrystalline fibrous cellulose powder from Whatman-CF11 filter paper (Sigma-Aldrich Co.) | ||
| [33] | MCC (R&M Chemicals, Essex, UK) | ||
| [34] | MCC (Avicel PH-101, particle size of 50 µm; Sigma-Aldrich, Toluca, Mexico) | ||
| [35] | α-cellulose (Sigma-Aldrich) | ||
| [36] | Commercially purchased (solid content of 6%, Cellulose Lab) | - | TEMPO-oxidised CNC |
| [37] | Commercially purchased (CelluForce Inc., Canada) | Magnetic CNC; shear-induced preferential CNC alignment | |
| [38] | Commercially purchased (product number 2015–009, CelluForce, Canada) | Silver nanoparticle-CNC hybrid suspensions via dissolution of AgNO3 in CNC | |
| [39] | Commercially purchased (CELLUFORCE NCV100–NASD90; CelluForce, Montreal, QC, Canada) | - | |
| [40] | Commercially purchased (particle size < 150 nm, CELLUFORCE NCV100—NASD90, Windsor, ON, Canada) | ||
| [41] | Corkboard | TEMPO-oxidation of corkboard and mild disintegration in water | - |
| [42] | Not provided | - | - |
| [43] | Tunicate CNC (TCNC) | ||
| [44] | Commercially purchased (enzymatically pretreated tunicate nanocellulose; Ocean TuniCell AS, Blomsterdalen, Norway) | - |
| Reference | Components of the Hydrogel | Influence of CNC | Biomedical Applications |
|---|---|---|---|
| [27] | Chitosan, CNCs | Increased viscosity of hydrogel bioinks; promoted osteogenesis onset, collagen formation in extracellular matrix (ECM) and calcium deposition; reinforcing agent of the hydrogel; no impact on cell viability or proliferation of clonal non-transformed newborn mouse calvaria MC3T3-E1 pre-osteoblast cartilage-like cells | Bone tissue engineering and bone defect repairing |
| [43] | Chitosan, CNCs | Increased proliferation and survival of clonal non-transformed newborn mouse calvaria MC3T3-E1 pre-osteoblast cartilage-like cells; increased osteogenic differentiation of MC3T3-E1 cells, collagen formation in ECM and calcium deposition | Bone tissue engineering |
| [29] | Sodium alginate, sericin, CNCs | Supported clonal non-transformed newborn mouse calvaria MC3T3-E1 pre-osteoblast cartilage-like cell survival and proliferation; diminished biodegradation via strong crosslinking; promoted osteogenic differentiation and biomineralisation capacity | |
| [35] | MeGel, hyaluronic acid methacrylate (HAMA), CNCs, lithium phenyl 2,4,6-trimethyl-benzoylphosphinate (LAP) | Increased chondrogenic ATDC5 cell proliferation | Cartilage tissue repair and regeneration |
| [39] | Alginate, CNCs | Improved human-derived TC28a2 immortalised chondrocyte viability, especially in bioprinted hydrogels and maintenance of cell morphology | Bioink formulation for the fabrication of cartilage soft tissue |
| Reference | Components of the Hydrogel | Influence of CNCs (Compared to No-CNC Hydrogel Control) | Biomedical Applications |
|---|---|---|---|
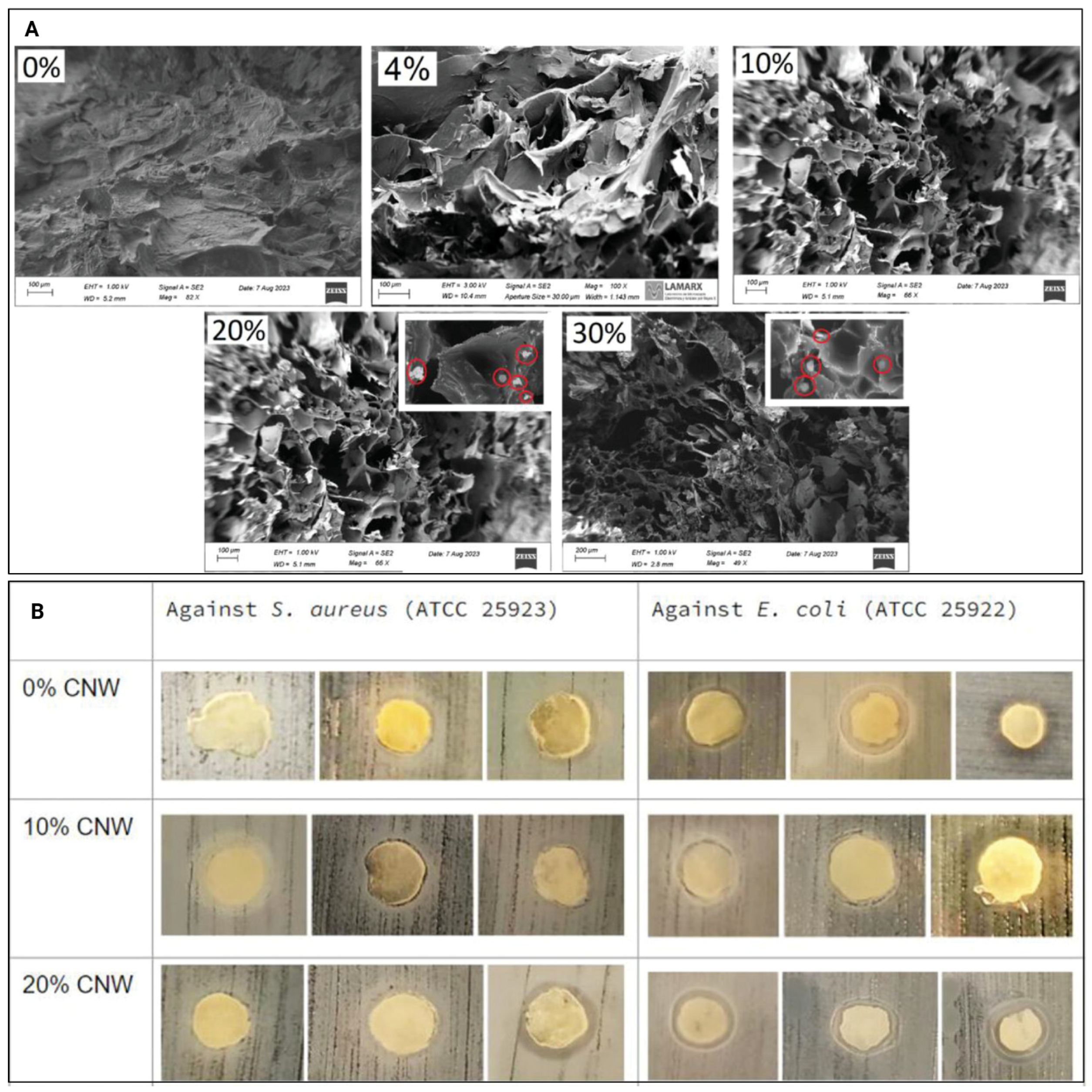
| [30] | CNCs, pectin, chitosan | Increased adult human dermal fibroblast cell numbers over seven days for 10% CNC; increased antibacterial activity against Staphylococcus aureus and Escherichia coli at 10% and 20% CNCs (Figure 6B) | Wound healing and antimicrobial activity |
| Reference | Components of the Hydrogel | Influence of CNC | Biomedical Applications |
|---|---|---|---|
| [36] | Methacrylated-gelatine (MeGel), TEMPO-oxidised CNC | Improved stiffness of the hydrogel via intra- and intermolecular hydrogen bonds; promoted human adipose-derived mesenchymal stem cell migration and metabolic activity; increased GAG deposition; decreased hydroxyproline content and calcification; inhibited osteogenic differentiation of human adipose-derived mesenchymal stem cells; reduced SMA and MMP2 expressions and increased vimentin expression to promote a fibroblastic phenotype of human adipose-derived mesenchymal stem cells | Cardiac valve engineering |
| [41] | CNCs, gelatine, genipin, astragaloside IV (AS-IV) | Good human umbilical vein endothelial cell proliferation and local tissue compatibility with no significant impact on important organs and inflammatory responses; controlled biodegradation and drug release | Extravascular stents inhibiting restenosis via preventing endothelial-to-mesenchymal transition (EndMT) after coronary artery bypass grafting and haemodialysis access; Drug delivery of astragaloside IV (AS-IV) |
| [44] | Lipoaspirate adipose (LAT) fraction, enzymatically pretreated tunicate nanocellulose (ETC), sodium alginate, CaCO3 microparticles (CMP), Glucono-δ-lactone (gluconolactone; GDL) | Assistance in shape and volume retention in grafts, resulting in retention of more adipocytes | Volume and shape retention and distribution of adipose cells in autologous fat-hydrogel grafts |
| [24] | PVA, glucose oxidase (Gox)-loaded CNC using BSA, iron (III) ions | Carrier of BSA and Gox via adsorption and surface grafting, respectively; no significant effect on adherence properties of the hydrogel or the current response to various glucose concentrations | Glucose level sensor in human sweat |
| Reference | Components of the Hydrogel | Influence of CNC | Biomedical Applications |
|---|---|---|---|
| [32] | Aminopropyl-functionalised magnesium phyllosilicate (Organoclay), CNC | Physical immobilisation and controlled release of the drug | Anti-inflammatory drug, ibuprofen, loading and releasing |
| [40] | Poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA), CNC | No effect on drug absorption or altering the thermal degradation of hydrogels; Improved encapsulation efficiency of drugs and controlled drug release pattern | |
| [31] | Gelatinised starch (S) with furfuryl isocyanate (FI) (S-FI), CNCs, tetra maleimide (TTMI), chloramphenicol | Improved mouse L929 fibroblast viability and drug loading; controlled drug release | Antimicrobial drug, chloramphenicol, loading and releasing |
| [34] | Aqueous solutions of acrylamide (net-AAm), CNC | Increased drug loading and retention capabilities as the CNC content increases | Antibiotic drug, ciprofloxacin, loading and releasing with potential for wound healing and treating infections |
| [38] | Calcium alginate, silver nanoparticles, CNCs, or TEMPO-oxidised chitin nanocrystals | Improved silver nanoparticle encapsulation and retention; a biopolymeric template for grafting silver nanoparticles and stabilising them | Antifungal drug, silver, encapsulation and release |
| [42] | Chitosan, CNC | Reinforcing agent; improved gelation kinetics; prolonged hydrogel degradation; assisted in sustained release of therapeutic C17 mouse neural stem cells from hydrogels with no significant difference in cell viability; controlled release of tumour necrosis factor-α (TNF-α)-related apoptosis-inducing ligand (TRAIL) protein | Neural stem cell-laden TRAIL proapoptotic agent-incorporated hydrogel for post-surgical glioblastoma multiforme treatment |
| [25] | Quaternised cellulose (QC), CNCs, β-glycerophosphate (β-GP) | Initially triggered inflammatory responses gradually diminished; no evidence of necrosis, haemorrhaging, oedema, or muscle damage; improved dimensional stability; slow degradation rate; controlled drug release | Localised and sustained antitumour drug, doxorubicin, delivery |
| [26] | CCHO, nano carbon dots | High biosafety and negligible cytotoxicity as evaluated on mouse B16F10 melanoma cells and HeLa cervical cancer cells; irradiated hydrogels have tumour-killing ability towards B16F10 tumour-bearing nude mice through simultaneous photothermal therapy and photodynamic therapy | Injectable tumour therapy platform |
| [37] | CNCs or magnetic CNCs with and without preferential alignment, 3-dimethyl (methacryloyloxyethyl) ammonium propane sulfonate (DMAPS, 95%), methacrylic acid (MAA, 99%) | Magnetic CNC: Magnetic navigation of the soft robot using superparamagnetic behaviour; increased mouse NIH-3T3 cell viability and proliferation; controlled biodegradation | Soft robot for grabbing, moving, and releasing soft and light biological cargo |
| Shear-induced preferential CNC alignment: Inducing structural anisotropy, application of shear leads to shear thinning, reorientation, and alignment of the hydrogel | |||
| [23] | Methacrylated-galactoglucomannan (GGMMA); CNC-SH or L-cysteine-grafted oxidised CNC; bioactive glass nanoparticle (BaGNP); Copper-BaGNP | Tailor degradation of hydrogels; localised and controlled drug delivery | Extended therapeutic release of silicon and calcium ions for wound healing |
| [28] | CNCs, gelatine, riboflavin | Good biocompatibility with slight toxicity towards mouse L929 fibroblast cells; inverse proportionality between CNC content and drug loading and releasing | Drug loading and release in the gut |
| [33] | CNCs, chitosan, curcumin | Enhancing drug loading capacity; controlled biodegradability and drug release capability at the appropriate site to enhance bioavailability of stomach and upper intestinal tract-related drugs | Stomach and upper intestinal tract-related drug, curcumin, loading and releasing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seneviratne, D.M.; Whiteside, E.J.; Windus, L.C.E.; Burey, P.; Ward, R.; Annamalai, P.K. Emerging Biomedical Applications of Sustainable Cellulose Nanocrystal-Incorporated Hydrogels: A Scoping Review. Gels 2025, 11, 740. https://doi.org/10.3390/gels11090740
Seneviratne DM, Whiteside EJ, Windus LCE, Burey P, Ward R, Annamalai PK. Emerging Biomedical Applications of Sustainable Cellulose Nanocrystal-Incorporated Hydrogels: A Scoping Review. Gels. 2025; 11(9):740. https://doi.org/10.3390/gels11090740
Chicago/Turabian StyleSeneviratne, Dinuki M., Eliza J. Whiteside, Louisa C. E. Windus, Paulomi (Polly) Burey, Raelene Ward, and Pratheep K. Annamalai. 2025. "Emerging Biomedical Applications of Sustainable Cellulose Nanocrystal-Incorporated Hydrogels: A Scoping Review" Gels 11, no. 9: 740. https://doi.org/10.3390/gels11090740
APA StyleSeneviratne, D. M., Whiteside, E. J., Windus, L. C. E., Burey, P., Ward, R., & Annamalai, P. K. (2025). Emerging Biomedical Applications of Sustainable Cellulose Nanocrystal-Incorporated Hydrogels: A Scoping Review. Gels, 11(9), 740. https://doi.org/10.3390/gels11090740









