Highly Entangled, Mechanically Robust Hydrogel Thin Films for Passive Cooling Materials via Open-Vessel Fabrication
Abstract
1. Introduction
2. Results and Discussion
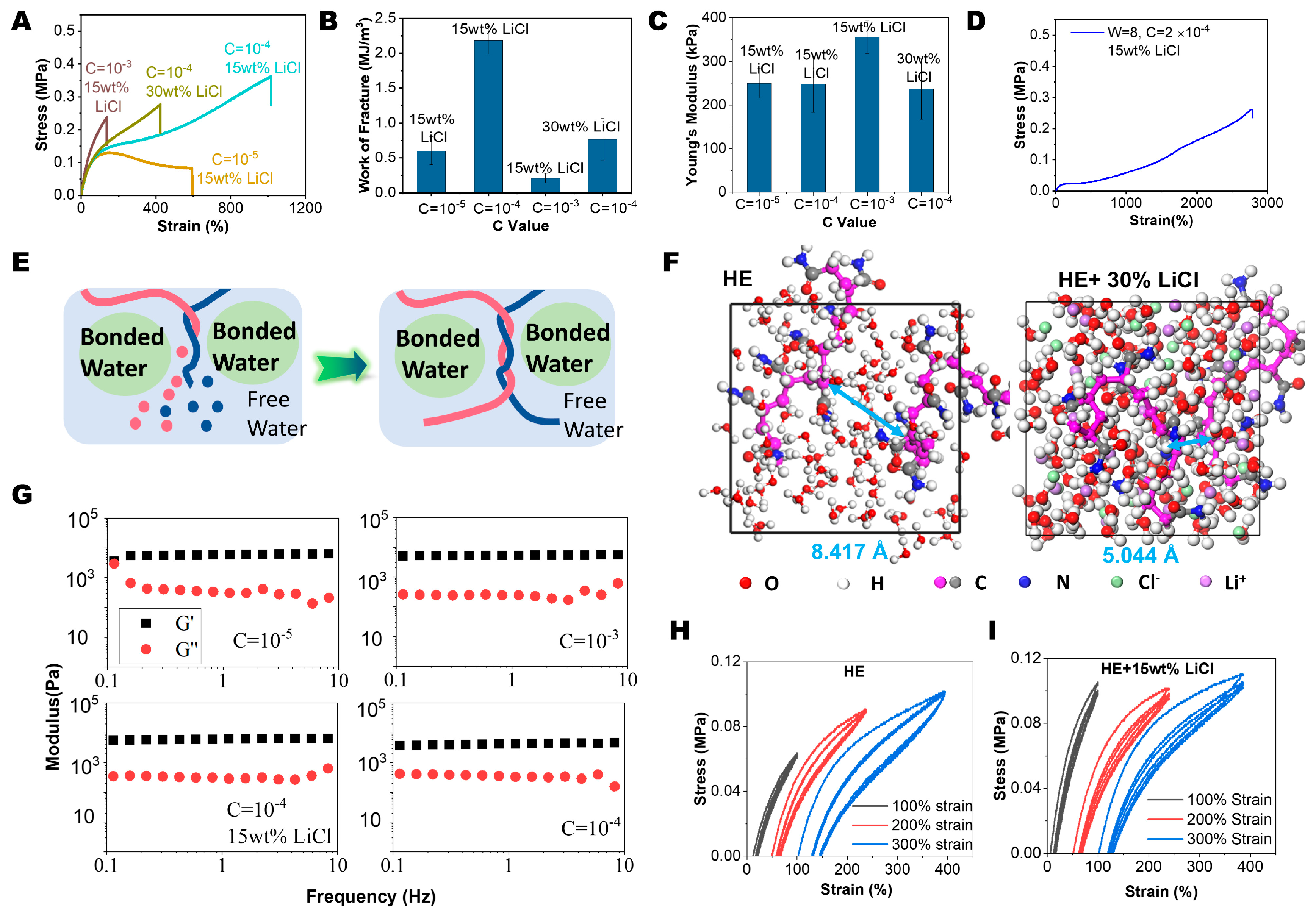
2.1. HE Hydrogel Preparation and Mechanical Properties
2.2. HE Hydrogel with Spatial Confinement
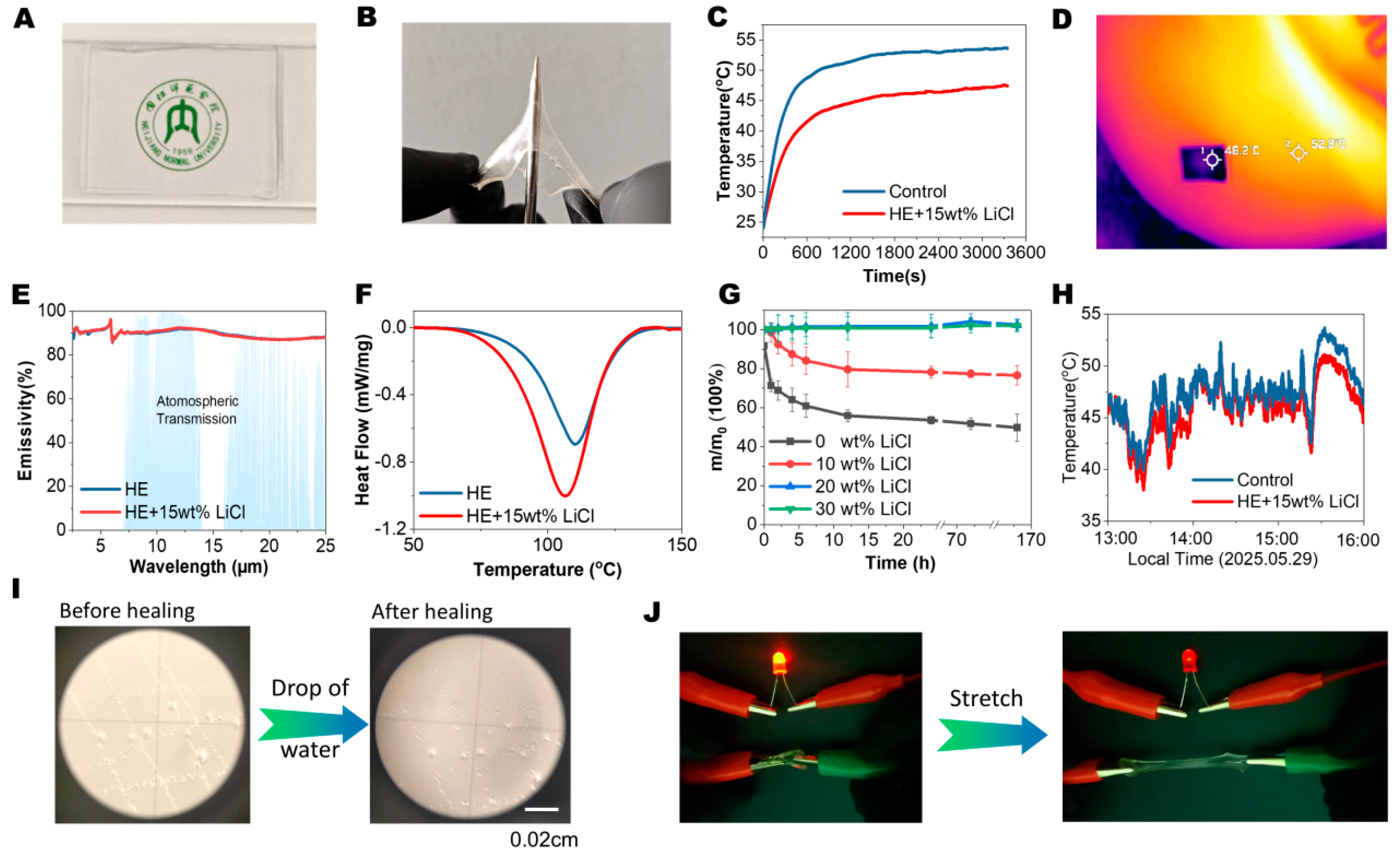
2.3. Passive Cooling Thin Film and Strain Sensor Applications
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Poly(acrylamide) (PAM) HE Hydrogels
4.3. Fabrication of HE Hydrogel Thin Film
4.4. Characterization
4.4.1. Mechanical Tests
4.4.2. Passive Cooling Tests
4.4.3. Self-Healing Observation and Strain Sensor Tests
4.4.4. Molecular Dynamics Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive polymer based hydrogels and their application in wearable sensors: A review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhao, F.; Lei, Y.; Wang, Y.-C. Hydrogel-based triboelectric devices for energy-harvesting and wearable sensing applications. Nano Energy 2022, 95, 106988. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- López-Díaz, A.; Vázquez, A.S.; Vázquez, E. Hydrogels in Soft Robotics: Past, Present, and Future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Abdullah, M.F.B.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental properties of smart hydrogels for tissue engineering applications: A review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, X.; Mi, Y.; Fan, F.; Xu, Q.; Zhao, H.; Wang, S.; Long, Y. Hydrogel smart windows. J. Mater. Chem. A 2020, 8, 10007–10025. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, Y.; Zhang, J.; Zhang, X.; An, Q. Hydrogel-Based Stimuli-Responsive Radiative and/or Evaporative Cooling Materials for Carbon Neutrality. ACS Energy Lett. 2024, 9, 594–626. [Google Scholar] [CrossRef]
- Cui, S.; Ahn, C.; Wingert, M.C.; Leung, D.; Cai, S.; Chen, R. Bio-inspired effective and regenerable building cooling using tough hydrogels. Appl. Energy 2016, 168, 332–339. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, D.; Zheng, Z.; Wang, X. Tough double network hydrogels with rapid self-reinforcement and low hysteresis based on highly entangled networks. Nat. Commun. 2024, 15, 1344. [Google Scholar] [CrossRef]
- Norioka, C.; Inamoto, Y.; Hajime, C.; Kawamura, A.; Miyata, T. A universal method to easily design tough and stretchable hydrogels. NPG Asia Mater. 2021, 13, 34. [Google Scholar] [CrossRef]
- Nian, G.; Kim, J.; Bao, X.; Suo, Z. Making Highly Elastic and Tough Hydrogels from Doughs. Adv. Mater. 2022, 34, 2206577. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Simič, R.; Mandal, J.; Zhang, K.; Spencer, N.D. Oxygen inhibition of free-radical polymerization is the dominant mechanism behind the “mold effect” on hydrogels. Soft Matter 2021, 17, 6394–6403. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Ma, Z.; Zuo, W.; Zhu, M. Unleashing the Power of PET-RAFT Polymerization: Journey from Porphyrin-Based Photocatalysts to Combinatorial Technologies and Advanced Bioapplications. Biomacromolecules 2024, 25, 1371–1390. [Google Scholar] [CrossRef]
- Allegrezza, M.L.; Konkolewicz, D. PET-RAFT Polymerization: Mechanistic Perspectives for Future Materials. ACS Macro Lett. 2021, 10, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Boyer, C.; Kwon, M.S. Photocontrolled RAFT polymerization: Past, present, and future. Chem. Soc. Rev. 2023, 52, 3035–3097. [Google Scholar] [CrossRef] [PubMed]
- Phommalysack-Lovan, J.; Chu, Y.; Boyer, C.; Xu, J. PET-RAFT polymerisation: Towards green and precision polymer manufacturing. Chem. Commun. 2018, 54, 6591–6606. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, D.; Chen, Y.; Yan, Q.; Liu, C.-Y.; Ling, K.; Liu, Y.; Lee, D.; Wu, X.; Senftle, T.P.; et al. Porphyrin-based donor–acceptor COFs as efficient and reusable photocatalysts for PET-RAFT polymerization under broad spectrum excitation. Chem. Sci. 2021, 12, 16092–16099. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jung, K.; Ma, Y.; Liu, W.; Boyer, C. Unravelling an oxygen-mediated reductive quenching pathway for photopolymerisation under long wavelengths. Nat. Commun. 2021, 12, 478. [Google Scholar] [CrossRef]
- Rigby, A.D.M.; Alipio, A.R.; Chiaradia, V.; Arno, M.C. Self-Healing Hydrogel Scaffolds through PET-RAFT Polymerization in Cellular Environment. Biomacromolecules 2023, 24, 3370–3379. [Google Scholar] [CrossRef]
- Jee, C.; Matsumoto, H.; Horiuchi, T.; Liu, Z.; Wang, Z.; Kakeru, O.; Kojio, K.; Nagao, M.; Miura, Y. Preparation of 4D hydrogels with PET-RAFT and orthogonal photo-reactions. RSC Appl. Polym. 2024, 3, 156–162. [Google Scholar] [CrossRef]
- Liu, J.; Miao, J.; Zhao, L.; Liu, Z.; Leng, K.; Xie, W.; Yu, Y. Versatile Bilayer Hydrogel for Wound Dressing through PET-RAFT Polymerization. Biomacromolecules 2022, 23, 1112–1123. [Google Scholar] [CrossRef]
- Tran, C.M.; Kim, J.; Yoon, J. Oxygen-Tolerant Fabrication of Large-Area Hydrogel Films via Photoinduced Electron/Energy Transfer-Reversible Addition–Fragmentation Chain Transfer Polymerization. ACS Appl. Polym. Mater. 2024, 6, 3326–3334. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wang, Z.; Xu, J.; Liu, J.; Xiao, R.; Qu, S.; Yang, W. A strategy for tough and fatigue-resistant hydrogels via loose cross-linking and dense dehydration-induced entanglements. Nat. Commun. 2024, 15, 5896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, J.P. Design principles for strong and tough hydrogels. Nat. Rev. Mater. 2024, 9, 380–398. [Google Scholar] [CrossRef]
- Irvine, G.; Myronidis, K.; Pinto, F.; Kopeć, M. Highly Entangled Hydrogels by Photoiniferter-Mediated Polymerization. Angew. Chem. Int. Ed. 2025, 64, e202421970. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Tamate, R.; Hiroi, T.; Samitsu, S.; Fujii, K.; Ueki, T. Highly stretchable and sself-healable polymer gels from physical entanglements of ultrahigh–molecular weight polymers. Sci. Adv. 2022, 8, eadd0226. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, S.; Li, Y.; Ma, W.; Zhang, G.; Yang, M.; Li, H.; Yang, Y.; Long, Y. Entanglement in Smart Hydrogels: Fast Response Time, Anti-Freezing and Anti-Drying. Adv. Funct. Mater. 2023, 33, 2211027. [Google Scholar] [CrossRef]
- Wang, J.; Tang, F.; Yao, C.; Li, L. Low Hysteresis Hydrogel Induced by Spatial Confinement. Adv. Funct. Mater. 2023, 33, 2214935. [Google Scholar] [CrossRef]
- Rong, L.-H.; Caldona, E.B.; Advincula, R.C. PET-RAFT polymerization under flow chemistry and surface-initiated reactions. Polym. Int. 2023, 72, 145–157. [Google Scholar] [CrossRef]


| Sample | W Value | C Value | LiCl (wt%) | AM (g) | MBAA (mg) | EY (mg) | TEOH (mg) | CDTPA (mg) | Water (mL) | LiCl (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| HE | 3.9 | 0 | 0 | 1.0 ± 0.1 | 0 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HC | 20 | 0 | 0 | 0.20 ± 0.02 | 0 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE | 3.9 | 10−5 | 0 | 1 ± 0.1 | 0.0025 ± 0.0002 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE | 3.9 | 10−4 | 0 | 1 ± 0.1 | 0.025 ± 0.002 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE | 3.9 | 10−3 | 0 | 1 ± 0.1 | 0.25 ± 0.02 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE | 3.9 | 10−2 | 0 | 1 ± 0.1 | 2.5 ± 0.2 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HC | 20 | 10−2 | 0 | 0.20 ± 0.02 | 2.5 ± 0.2 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE | 2 | 10−4 | 0 | 2 ± 0.1 | 0.025 ± 0.002 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0 |
| HE-LiCl | 3.9 | 10−5 | 15 | 1 ± 0.1 | 0.0025 ± 0.0002 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0.15 ± 0.02 |
| HE-LiCl | 3.9 | 10−4 | 15 | 1 ± 0.1 | 0.025 ± 0.002 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0.15 ± 0.02 |
| HE-LiCl | 3.9 | 10−3 | 15 | 1 ± 0.1 | 0.25 ± 0.02 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0.15 ± 0.02 |
| HE-LiCl | 3.9 | 10−3 | 30 | 1 ± 0.1 | 0.25 ± 0.02 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0.30 ± 0.04 |
| HE-LiCl | 8 | 2 × 10−4 | 15 | 0.50 ± 0.03 | 0.05 ± 0.04 | 0.025 ± 0.002 | 2.0 ± 0.1 | 0.25 ± 0.02 | 1.0 ± 0.1 | 0.15 ± 0.03 |
| Materials | Year | Tensile Strength (MPa) | Tensile Strain (%) |
|---|---|---|---|
| PAAm [31] | 2021 | 0.39 | ~420 |
| PAAm [17] | 2021 | ~0.3 | \ |
| PMPC [17] | 2021 | ~0.14 | ~330 |
| Poly(methyl methacrylate) (PMMA) [36] | 2022 | 0.12 | 570 |
| PEG [18] | 2022 | 0.46 | 590 |
| PAAm [32] | 2024 | 0.18 | \ |
| P(AAm-co-AMPS) [16] | 2024 | \ | ~320 |
| poly(N-iso-propylacrylamide)+hydroxypropyl cellulose(PNIPAM+HPC) [37] | 2023 | 0.05 | 500 |
| This work | 0.49~2.5 | 900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, L.; Xie, J.; Zhou, S.; Guan, T.; Fan, X.; Zhi, W.; Zhou, R.; Li, F.; Liu, Y.; Tang, T.; et al. Highly Entangled, Mechanically Robust Hydrogel Thin Films for Passive Cooling Materials via Open-Vessel Fabrication. Gels 2025, 11, 734. https://doi.org/10.3390/gels11090734
Rong L, Xie J, Zhou S, Guan T, Fan X, Zhi W, Zhou R, Li F, Liu Y, Tang T, et al. Highly Entangled, Mechanically Robust Hydrogel Thin Films for Passive Cooling Materials via Open-Vessel Fabrication. Gels. 2025; 11(9):734. https://doi.org/10.3390/gels11090734
Chicago/Turabian StyleRong, Lihan, Jiajiang Xie, Shigao Zhou, Tianqi Guan, Xinyi Fan, Wenjie Zhi, Rui Zhou, Feng Li, Yuyan Liu, Tingting Tang, and et al. 2025. "Highly Entangled, Mechanically Robust Hydrogel Thin Films for Passive Cooling Materials via Open-Vessel Fabrication" Gels 11, no. 9: 734. https://doi.org/10.3390/gels11090734
APA StyleRong, L., Xie, J., Zhou, S., Guan, T., Fan, X., Zhi, W., Zhou, R., Li, F., Liu, Y., Tang, T., Chen, X., & Zhang, L. (2025). Highly Entangled, Mechanically Robust Hydrogel Thin Films for Passive Cooling Materials via Open-Vessel Fabrication. Gels, 11(9), 734. https://doi.org/10.3390/gels11090734





