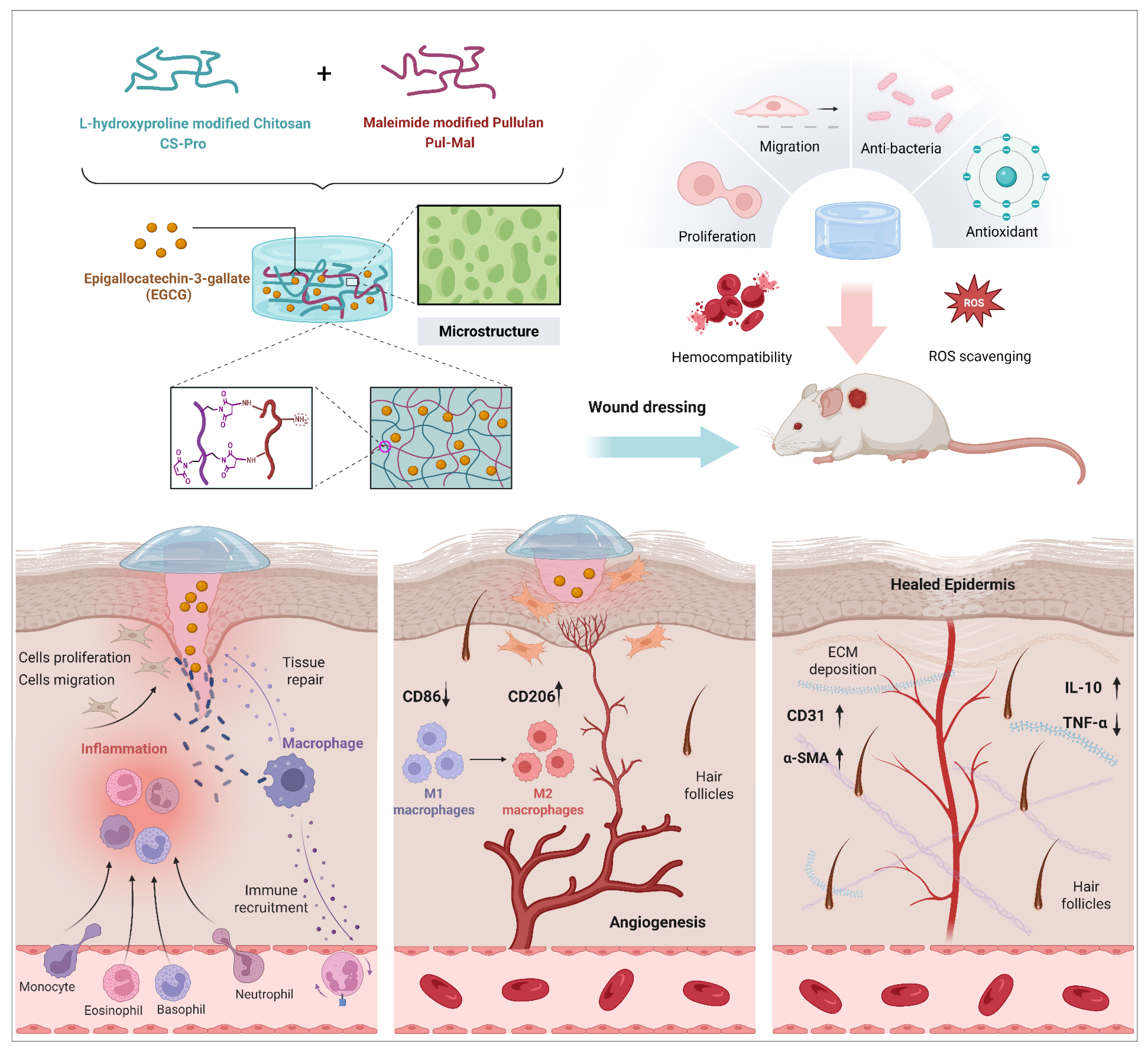
Hydroxyproline-Modified Chitosan-Based Hydrogel Dressing Incorporated with Epigallocatechin-3-Gallate Promotes Wound Healing Through Immunomodulation
Abstract
1. Introduction
2. Results and Discussion
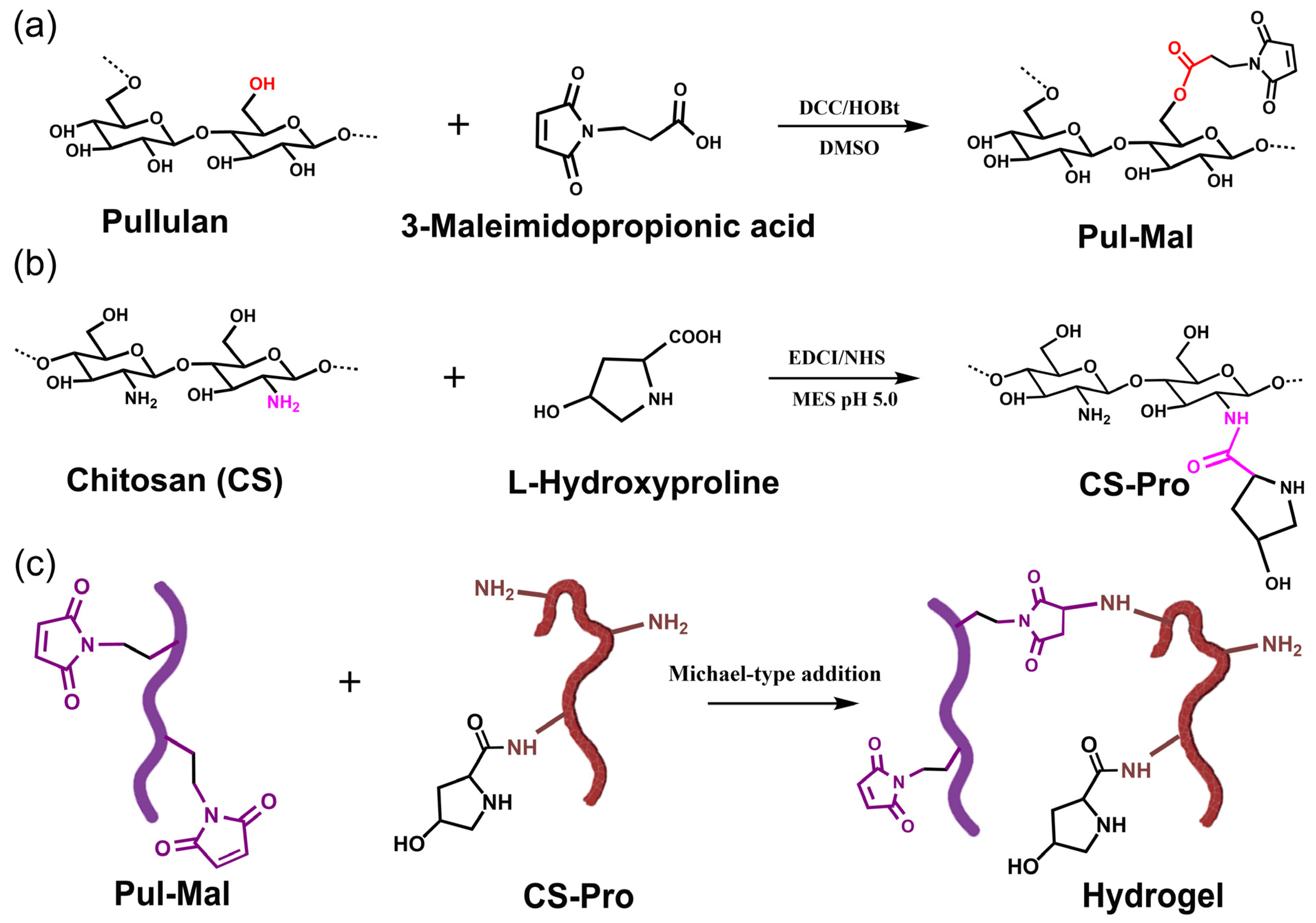
2.1. Synthesis of Pul-Mal and CS-Pro
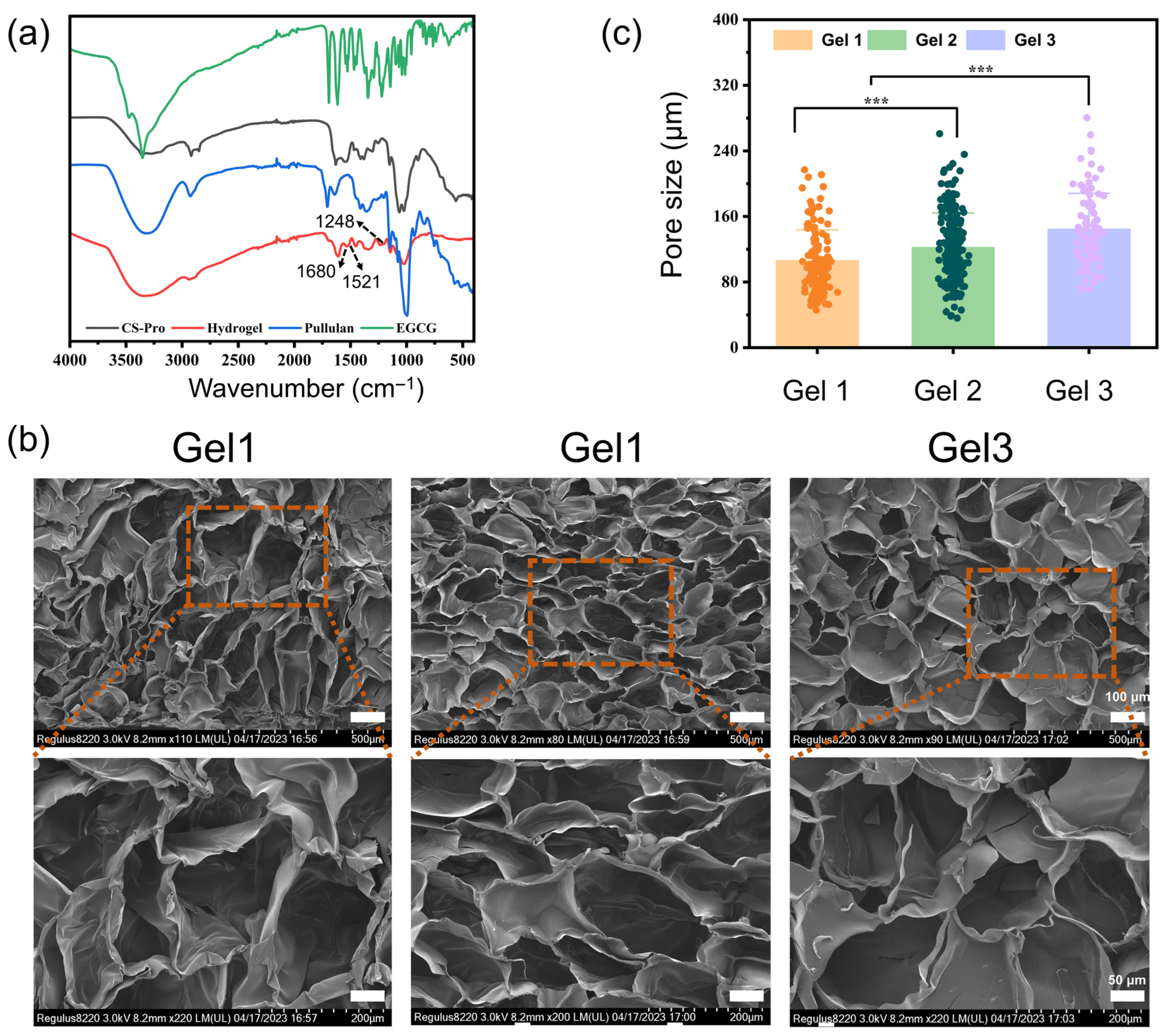
2.2. Physicochemical Properties of the Composite Hydrogels
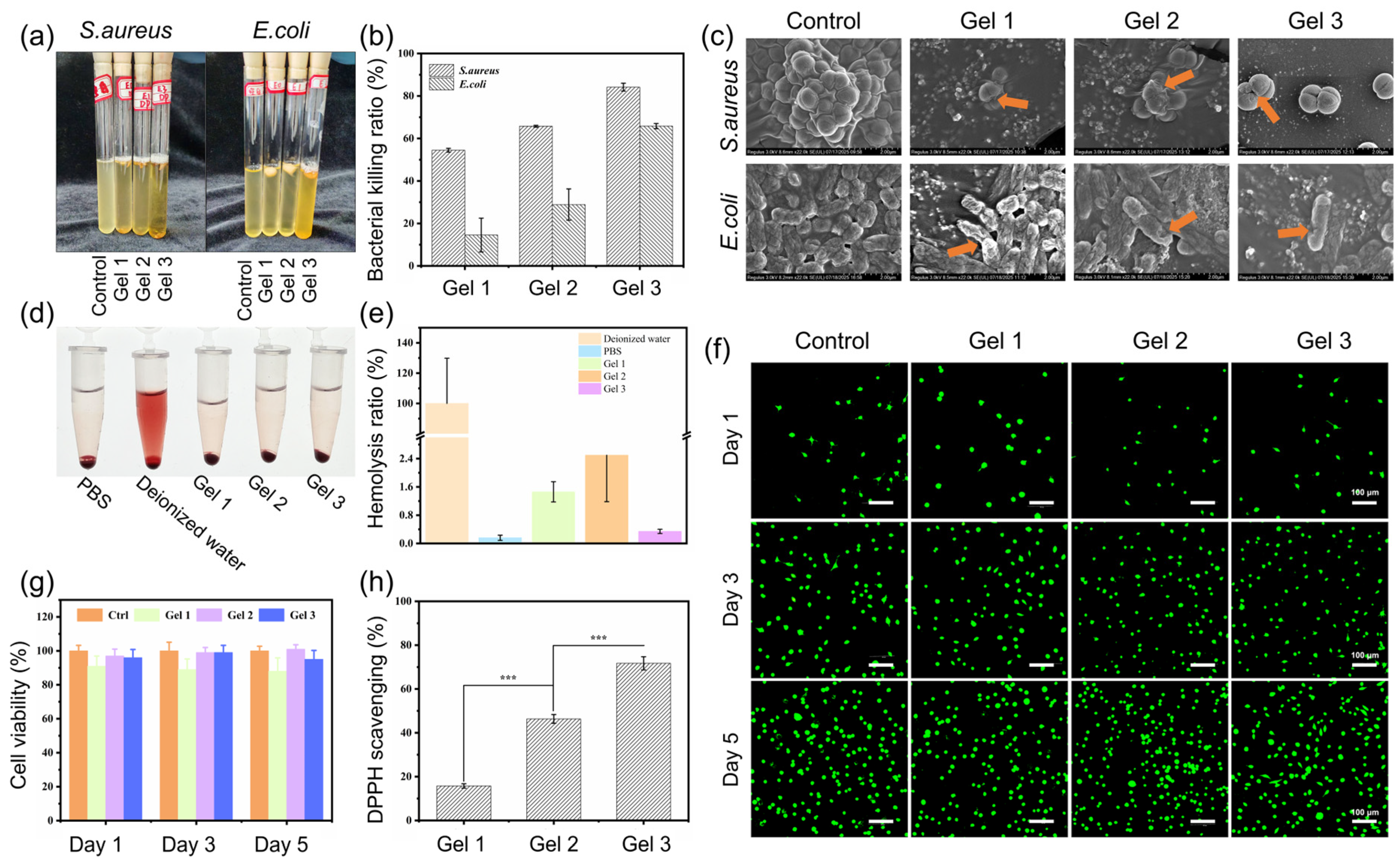
2.3. Antibacterial Properties of Hydrogels
2.4. Biocompatibility of Hydrogels
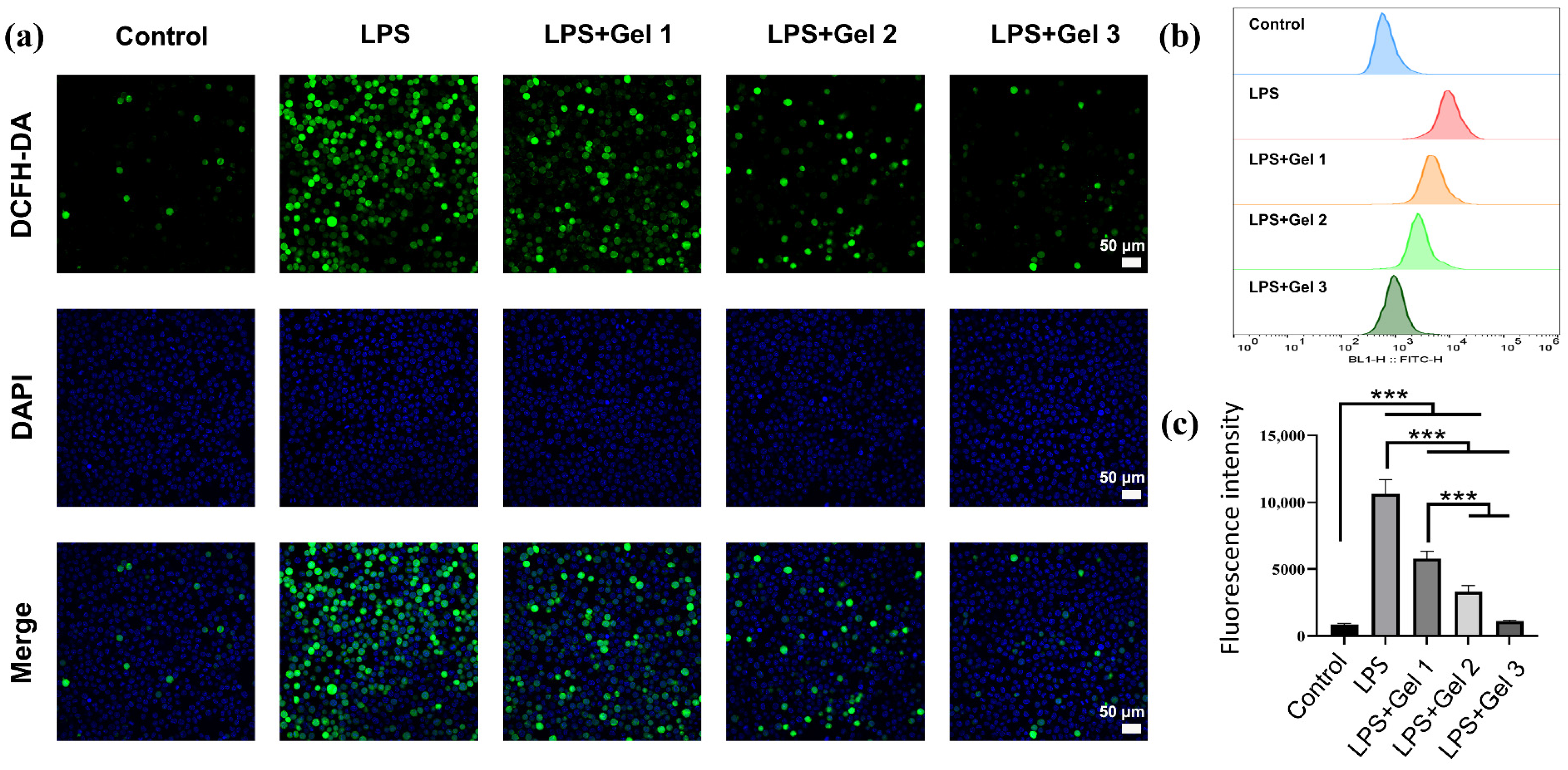
2.5. Antioxidant and Anti-Inflammatory Activities
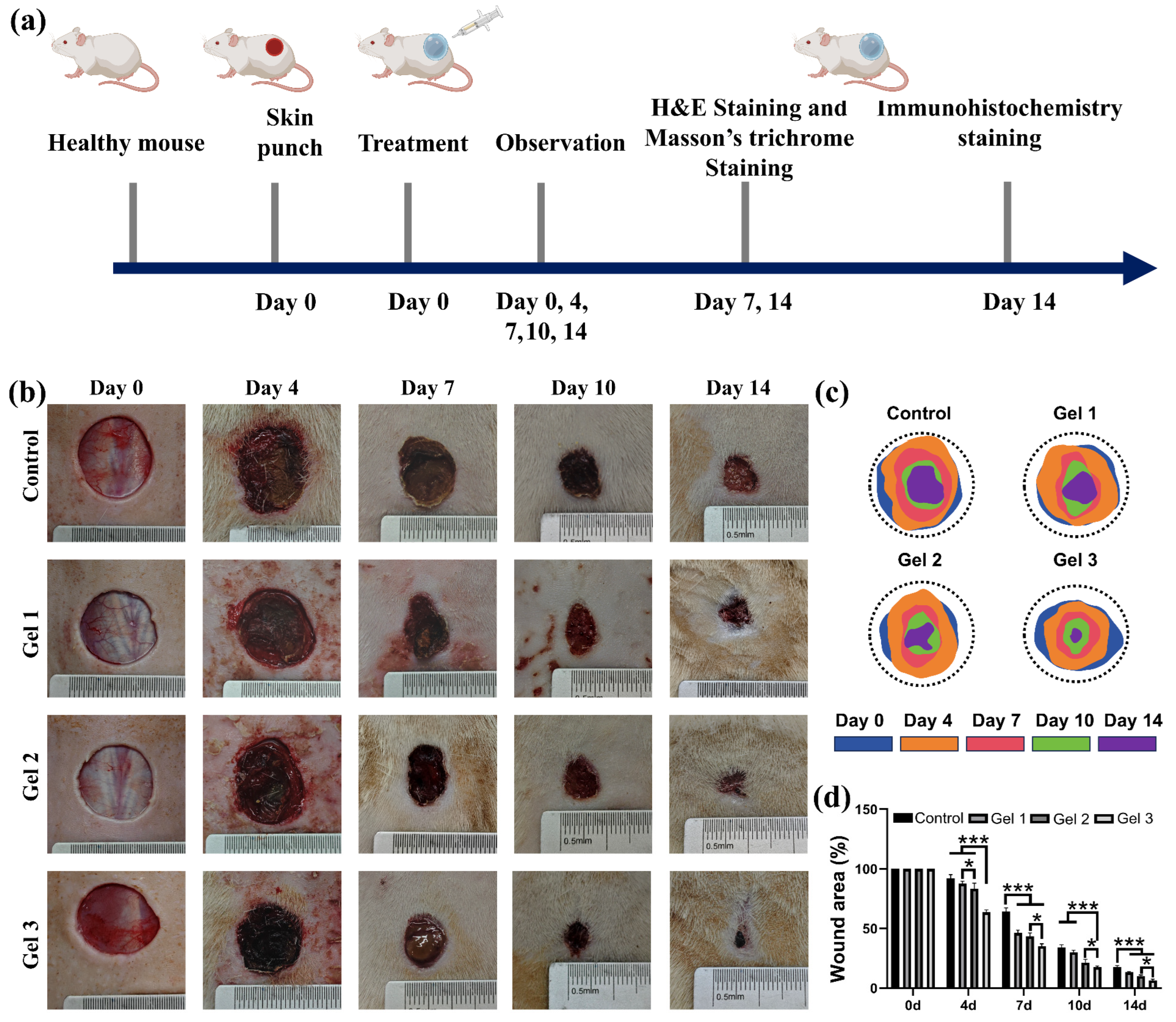
2.6. In Vivo Wound Closure and Healing
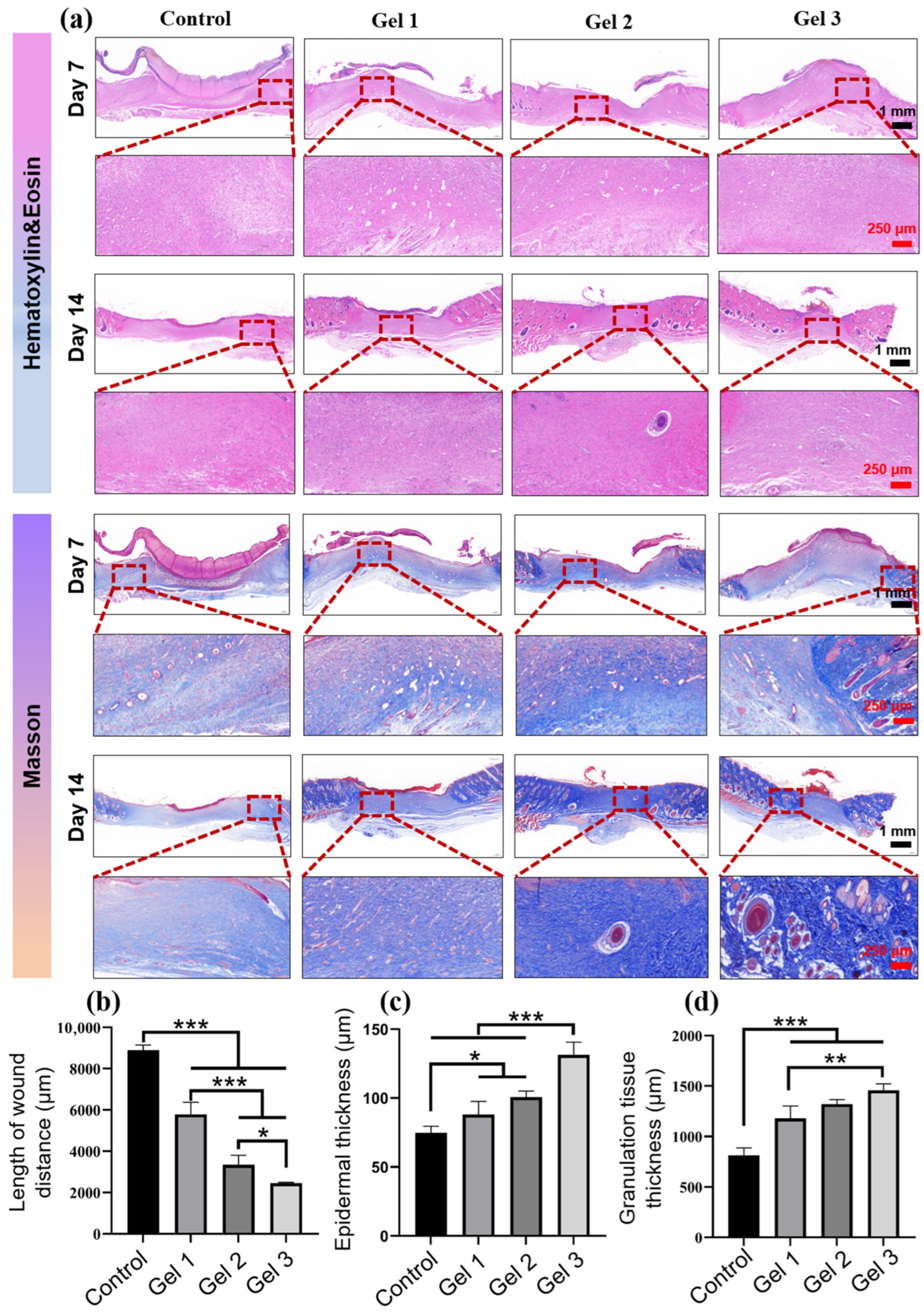
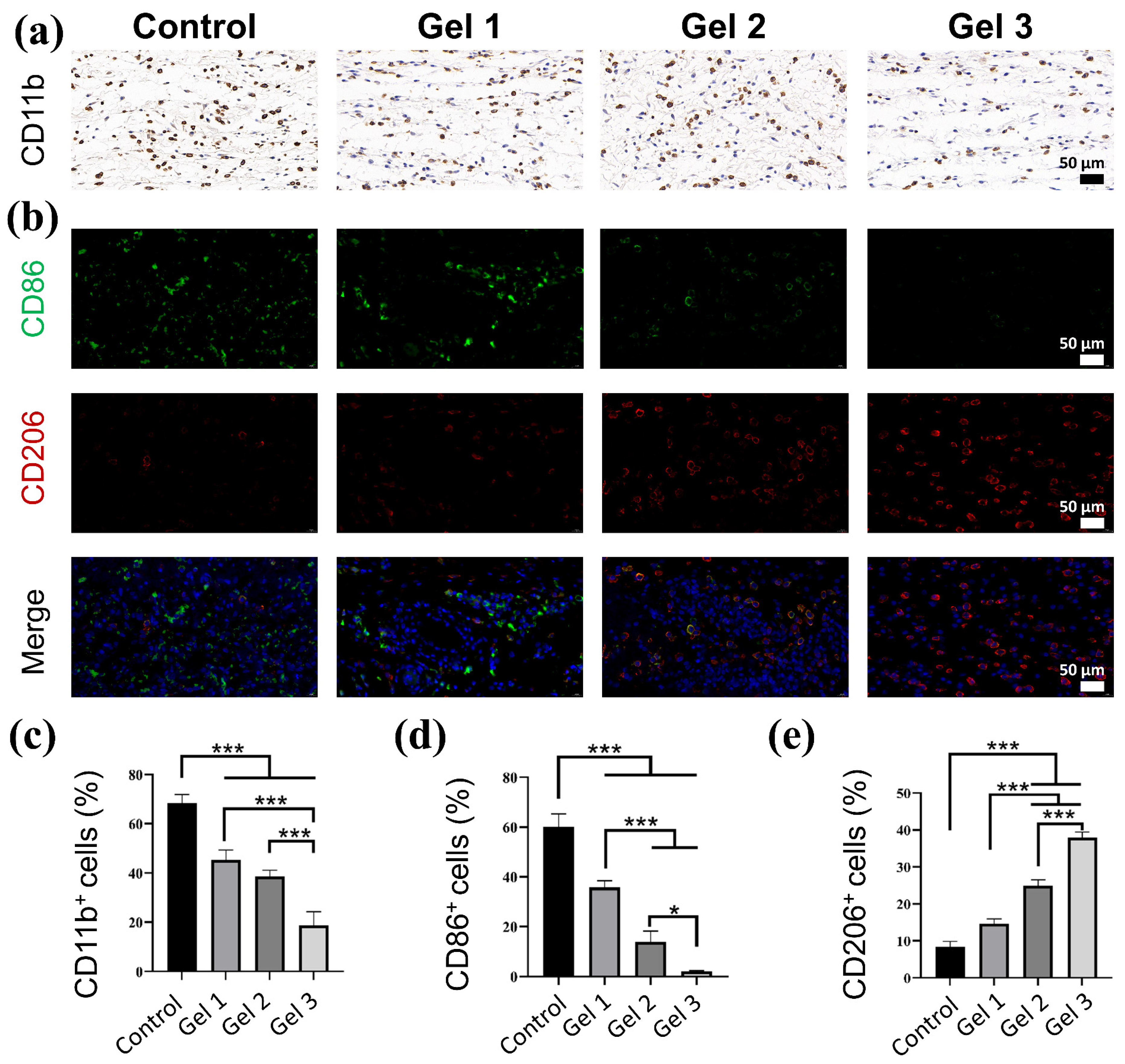
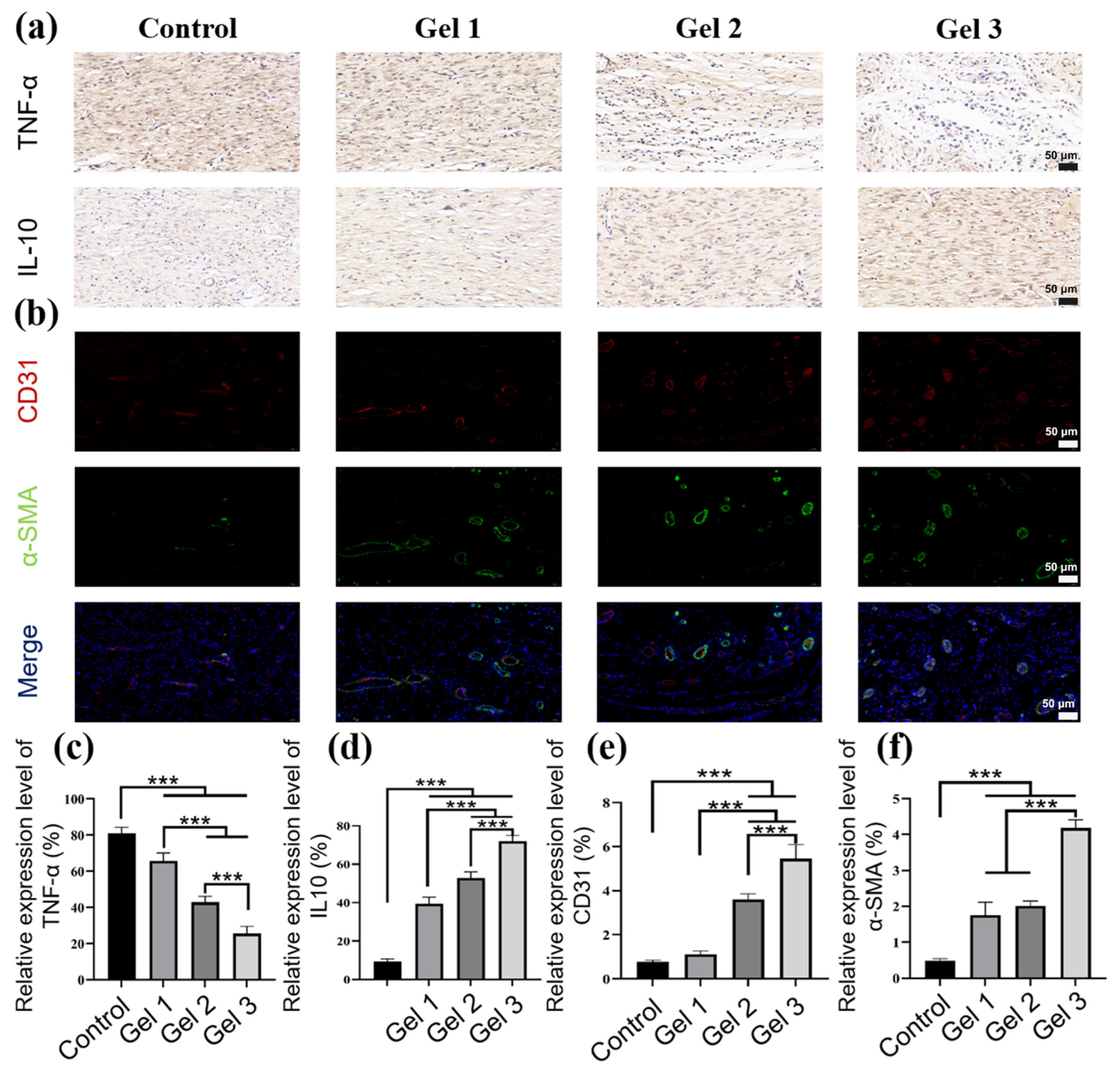
2.7. Histological and Immunohistochemical Evaluation of Wound Regeneration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Pullulan Modified with Maleimide (Pul-Mal)
4.3. Chitosan Functionalized with L-Hydroxyproline (CS-Pro)
4.4. Chemical Characterization of Synthesized Polymers
4.5. Preparation of the Composite Hydrogels
4.6. Characterization of Hydrogels
4.6.1. Hydrogel Morphology
4.6.2. Swelling Rate Test
4.6.3. Release Profile of EGCG in Hydrogels
4.7. Rheological Analysis
4.8. Antibacterial Activity
4.9. Antioxidant Performance
4.10. Intracellular ROS Scavenging Activity
4.11. Hemolysis Test
4.12. Cytocompatibility Test
4.13. Full-Thickness Skin Wound Model
4.14. Histology and Immunohistochemical Staining
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratsinis, H.; Mavrogonatou, E.; Kletsas, D.J. Scarless wound healing: From development to senescence. Adv. Drug Deliv. Rev. 2019, 146, 325–343. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B.J.A.F.M. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.J. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, Y.; Lim, C.J. Tissue scaffolds for skin wound healing and dermal reconstruction. WIREs Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol. Res. 2008, 58, 112–116. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Lauer, G.; Sollberg, S.; Cole, M.; Krieg, T.; Eming, S.A.; Flamme, I.; Stürzebecher, J.; Mann, K. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J. Investig. Dermatol. 2000, 115, 12–18. [Google Scholar] [CrossRef]
- Wallace, H.J.; Stacey, M.C. Levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in chronic venous leg ulcers–correlations to healing status. J. Investig. Dermatol. 1998, 110, 292–296. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Shen, Y.-I.; Cho, H.; Papa, A.E.; Burke, J.A.; Chan, X.Y.; Duh, E.J.; Gerecht, S. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef]
- Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Ahmed, A.A.Q.; Lamboni, L.; Shi, Z.; Mukole, B.M.; Zheng, R.; Mbang, M.P.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef]
- Rungrod, A.; Kapanya, A.; Punyodom, W.; Molloy, R.; Meerak, J.; Somsunan, R.J.B. Synthesis of poly (ε-caprolactone) diacrylate for micelle-cross-linked sodium AMPS hydrogel for use as controlled drug delivery wound dressing. Biomacromolecules 2021, 22, 3839–3859. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, K.; Li, J.; Su, J.; Guan, F.; Li, J.J.M. Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater. Des. 2022, 222, 111109. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Farjadian, F.; Mirkiani, S.; Ghasemiyeh, P.; Kafshboran, H.R.; Mehdi-Alamdarlou, S.; Raeisi, A.; Esfandiarinejad, R.; Soleymani, S.; Goshtasbi, G.; Firouzabadi, N. Smart nanogels as promising platform for delivery of drug, gene, and vaccine; therapeutic applications and active targeting mechanism. Eur. Polym. J. 2024, 219, 113400. [Google Scholar] [CrossRef]
- Luo, P.; Huang, R.; Wu, Y.; Liu, X.; Shan, Z.; Gong, L.; Deng, S.; Liu, H.; Fang, J.; Wu, S. Tailoring the multiscale mechanics of tunable decellularized extracellular matrix (dECM) for wound healing through immunomodulation. Bioact. Mater. 2023, 28, 95–111. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Mao, J.; Chen, L.; Cai, Z.; Qian, S.; Liu, Z.; Zhao, B.; Zhang, Y.; Sun, X.; Cui, W. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv. Funct. Mater. 2022, 32, 2111003. [Google Scholar] [CrossRef]
- Kim, S.-W.; Im, G.-B.; Jeong, G.-J.; Baik, S.; Hyun, J.; Kim, Y.-J.; Pang, C.; Jang, Y.C.; Bhang, S.H. Delivery of a spheroids-incorporated human dermal fibroblast sheet increases angiogenesis and M2 polarization for wound healing. Biomaterials 2021, 275, 120954. [Google Scholar] [CrossRef]
- Saoudi, M.; Badraoui, R.; Chira, A.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Kallel, C.; El Feki, A. The Role of Allium subhirsutum L. in the attenuation of dermal wounds by modulating oxidative stress and inflammation in Wistar albino rats. Molecules 2021, 26, 4875. [Google Scholar] [CrossRef]
- Liu, R.; Chen, S.; Huang, P.; Liu, G.; Luo, P.; Li, Z.; Xiao, Y.; Chen, Z.; Chen, Z. Immunomodulation-based strategy for improving soft tissue and metal implant integration and its implications in the development of metal soft tissue materials. Adv. Funct. Mater. 2020, 30, 1910672. [Google Scholar] [CrossRef]
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic actions of microRNAs in diabetic wound healing. Cells 2020, 9, 2228. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Shah, A.H.; Ruzga, M.N.; Olivares-Navarrete, R. Surface characteristics on commercial dental implants differentially activate macrophages in vitro and in vivo. Clin. Oral Implant. Res. 2021, 32, 487–497. [Google Scholar] [CrossRef]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef]
- Feng, Z.; Su, Q.; Zhang, C.; Huang, P.; Song, H.; Dong, A.; Kong, D.; Wang, W. Bioinspired nanofibrous glycopeptide hydrogel dressing for accelerating wound healing: A cytokine-free, M2-type macrophage polarization approach. Adv. Funct. Mater. 2020, 30, 2006454. [Google Scholar] [CrossRef]
- Das, S.; Majid, M.; Baker, A.B. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomater. 2016, 42, 56–65. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Strudwick, X.L.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021, 128, 420–434. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S. Biomaterials for soft tissue repair and regeneration: A focus on Italian research in the field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, X.; Zhang, H.; Liu, Z.; Han, Y.; Wei, Q.; Okoro, O.V.; Shavandi, A.; Nie, L. Recent advances of chitosan-based hydrogels for skin-wound dressings. Gels 2024, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, H.; Zhao, Y.; Zhen, Q.; Shi, D.; Chen, J.; Chen, X. Pullulan dialdehyde cross-linked dual-action adhesive with high adhesion to lung tissue and the capability of pH-responsive drug release. Carbohydr. Polym. 2025, 348, 122906. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiong, Y.; Zhu, Y.; Zhou, F.; Liu, X.; Chen, S.; Li, Z.; Qi, S.; Chen, L. Copper-epigallocatechin gallate enhances therapeutic effects of 3D-printed dermal scaffolds in mitigating diabetic wound scarring. ACS Appl. Mater. Interfaces 2023, 15, 38230–38246. [Google Scholar] [CrossRef]
- Ud-Din, S.; Foden, P.; Mazhari, M.; Al-Habba, S.; Baguneid, M.; Bulfone-Paus, S.; McGeorge, D.; Bayat, A. A double-blind, randomized trial shows the role of zonal priming and direct topical application of epigallocatechin-3-gallate in the modulation of cutaneous scarring in human skin. J. Investig. Dermatol. 2019, 139, 1680–1690.e16. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- He, Z.; Luo, H.; Wang, Z.; Chen, D.; Feng, Q.; Cao, X. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohydr. Polym. 2023, 299, 120180. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Lv, X.; Han, B.; Jiang, Z. Multifunctional carboxymethyl chitosan-based sponges loaded with epigallocatechin-3-gallate for accelerating wound healing in diabetic rats with full-thickness burns. Carbohydr. Polym. 2025, 350, 123025. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Zhu, Q.-Q.; Yang, X.-Y.; Xu, H.-H.; Sun, B.; Wang, X.-J.; Sheng, J. Wound healing can be improved by (—)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Ning, Y.; Yuan, Z.; Wang, Q.; He, J.; Zhu, W.; Ren, D.n.; Wo, D. Epigallocatechin-3-gallate promotes wound healing response in diabetic mice by activating keratinocytes and promoting re-epithelialization. Phytother. Res. 2024, 38, 1013–1027. [Google Scholar] [CrossRef]
- Kumar Srivastava, A.; Khare, P.; Kumar Nagar, H.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- Torkaman, S.; Najafi, S.H.M.; Ashori, A.; Mohseni, F.A. Chemoselective modification of chitosan with arginine and hydroxyproline: Development of antibacterial composite films for wound healing applications. Int. J. Biol. Macromol. 2024, 282, 137081. [Google Scholar] [CrossRef]
- Chen, L.; Cao, P.; Zhao, P.; Xu, Y.; Lv, G.; Yu, D. Photodynamic-Controllable microneedle composite with Antibacterial, Antioxidant, and angiogenic effects to expedite infected diabetic wound healing. Mater. Des. 2024, 241, 112971. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Li, M.; Nie, L.; Wei, Q.; Okoro, O.V.; Jafari, H.; Wang, S.; Deng, J.; Chen, J. Bioactive wound dressing based on decellularized tendon and GelMA with incorporation of PDA-loaded asiaticoside nanoparticles for scarless wound healing. Chem. Eng. J. 2023, 466, 143016. [Google Scholar] [CrossRef]
- Yan, L.; Liu, S.; Wang, J.; Ding, X.; Zhao, Y.; Gao, N.; Xia, Z.; Li, M.; Wei, Q.; Okoro, O.V. Constructing nerve guidance conduit using dECM-doped conductive hydrogel to promote peripheral nerve regeneration. Adv. Funct. Mater. 2024, 34, 2402698. [Google Scholar] [CrossRef]
- Ishii, S.; Kokubo, H.; Hashimoto, K.; Imaizumi, S.; Watanabe, M. Tetra-PEG network containing ionic liquid synthesized via michael addition reaction and its application to polymer actuator. Macromolecules 2017, 50, 2906–2915. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Yang, C.; Liu, X.; Huangfu, Y.; Zhang, C.; Huang, P.; Dong, A.; Liu, J.; Liu, J. Bioinspired and inflammation-modulatory glycopeptide hydrogels for radiation-induced chronic skin injury repair. Adv. Healthc. Mater. 2023, 12, 2201671. [Google Scholar] [CrossRef]
- Rahayu, D.P.; De Mori, A.; Yusuf, R.; Draheim, R.; Lalatsa, A.; Roldo, M. Enhancing the antibacterial effect of chitosan to combat orthopaedic implant-associated infections. Carbohydr. Polym. 2022, 289, 119385. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Z.; Du, Y.; Kennedy, J.F. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Tu, C.-W.; Tsai, F.-C.; Chen, J.-K.; Wang, H.-P.; Lee, R.-H.; Zhang, J.; Chen, T.; Wang, C.-C.; Huang, C.-F. Preparations of tough and conductive PAMPS/PAA double network hydrogels containing cellulose nanofibers and polypyrroles. Polymers 2020, 12, 2835. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Li, J.; Lu, G.; Wei, X.; Deng, Y.; Liu, S.; Zhong, S.; Shi, Q.; Hou, R.; Sun, Y. Temperature responsive hydrogel for cells encapsulation based on graphene oxide reinforced poly (N-isopropylacrylamide)/hydroxyethyl-chitosan. Mater. Today Commun. 2022, 31, 103697. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery–an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef]
- Deng, Z.; Shi, F.; Zhou, Z.; Sun, F.; Sun, M.-H.; Sun, Q.; Chen, L.; Li, D.; Jiang, C.-Y.; Zhao, R.-Z.J.T.; et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 366, 83–95. [Google Scholar] [CrossRef]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guérin, S.L. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, C.; Xie, B.; Xu, W.; Rao, Z.; Zhou, P.; Ma, X.; Chen, J.; Cai, R.; Tao, G.; et al. Multifunctional microneedle patch based on metal-phenolic network with photothermal antimicrobial, ROS scavenging, immunomodulatory, and angiogenesis for programmed treatment of diabetic wound healing. ACS Appl. Mater. Interfaces 2024, 16, 33205–33222. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, L.; Guo, H.; Xu, R.; Liang, X.; Chen, Y.; Li, H.; Fu, X.; Wang, X.; Chen, H. Redox modulatory Cu (II)-baicalein microflowers prepared in one step effectively promote therapeutic angiogenesis in diabetic mice. Adv. Healthc. Mater. 2023, 12, 2202010. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, M.; Wang, M.; Shao, Z.; Jiang, X.; Wang, K.; Yao, Z.; Yang, S.; Zhang, X.; Gao, W. Engineering bioactive M2 macrophage-polarized anti-inflammatory, antioxidant, and antibacterial scaffolds for rapid angiogenesis and diabetic wound repair. Adv. Funct. Mater. 2021, 31, 2100924. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Yang, C.; Zhang, H.; Fareed, M.S.; He, Y.; Su, J.; Wang, P.; Shen, Z.; Yan, W.; et al. A carrier-free, dual-functional hydrogel constructed of antimicrobial peptide Jelleine-1 and 8Br-cAMP for MRSA infected diabetic wound healing. Acta Biomater. 2022, 151, 223–234. [Google Scholar] [CrossRef]
- Ding, P.; Ding, X.; Liu, X.; Lu, Y.; Zhao, Y.; Chu, Y.; Fan, L.; Nie, L. Injectable, self-healing, antibacterial hydrogel dressing based on oxidized dextran and sialic acid substituted chitosan with incorporation of tannic acid. Eur. Polym. J. 2024, 216, 113297. [Google Scholar] [CrossRef]
- Nie, L.; Wei, Q.; Sun, M.; Ding, P.; Wang, L.; Sun, Y.; Ding, X.; Okoro, O.V.; Jiang, G.; Shavandi, A. Injectable, self-healing, transparent, and antibacterial hydrogels based on chitosan and dextran for wound dressings. Int. J. Biol. Macromol. 2023, 233, 123494. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, J.; Feng, W.; Xu, B.; Zhang, K.; Ma, G.; Li, Y.; Yang, M.; Xu, F.-J. Glycosaminoglycan-based hydrogel delivery system regulates the wound microenvironment to rescue chronic wound healing. ACS Appl. Mater. Interfaces 2022, 14, 31737–31750. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, P.; Sun, Y.; Jiang, G.; Nie, L. Hydroxyproline-Modified Chitosan-Based Hydrogel Dressing Incorporated with Epigallocatechin-3-Gallate Promotes Wound Healing Through Immunomodulation. Gels 2025, 11, 732. https://doi.org/10.3390/gels11090732
Ding P, Sun Y, Jiang G, Nie L. Hydroxyproline-Modified Chitosan-Based Hydrogel Dressing Incorporated with Epigallocatechin-3-Gallate Promotes Wound Healing Through Immunomodulation. Gels. 2025; 11(9):732. https://doi.org/10.3390/gels11090732
Chicago/Turabian StyleDing, Peng, Yanfang Sun, Guohua Jiang, and Lei Nie. 2025. "Hydroxyproline-Modified Chitosan-Based Hydrogel Dressing Incorporated with Epigallocatechin-3-Gallate Promotes Wound Healing Through Immunomodulation" Gels 11, no. 9: 732. https://doi.org/10.3390/gels11090732
APA StyleDing, P., Sun, Y., Jiang, G., & Nie, L. (2025). Hydroxyproline-Modified Chitosan-Based Hydrogel Dressing Incorporated with Epigallocatechin-3-Gallate Promotes Wound Healing Through Immunomodulation. Gels, 11(9), 732. https://doi.org/10.3390/gels11090732








