Development of an Innovative Nanosystem Based on Functionalized Albumin and Oxidized Gellan for the Synergistic Delivery of Curcumin and Temozolomide in the Treatment of Brain Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Obtaining Low-Molecular-Weight Protamine (LMWP) and Determining the Number of Moles of Free Amine Groups
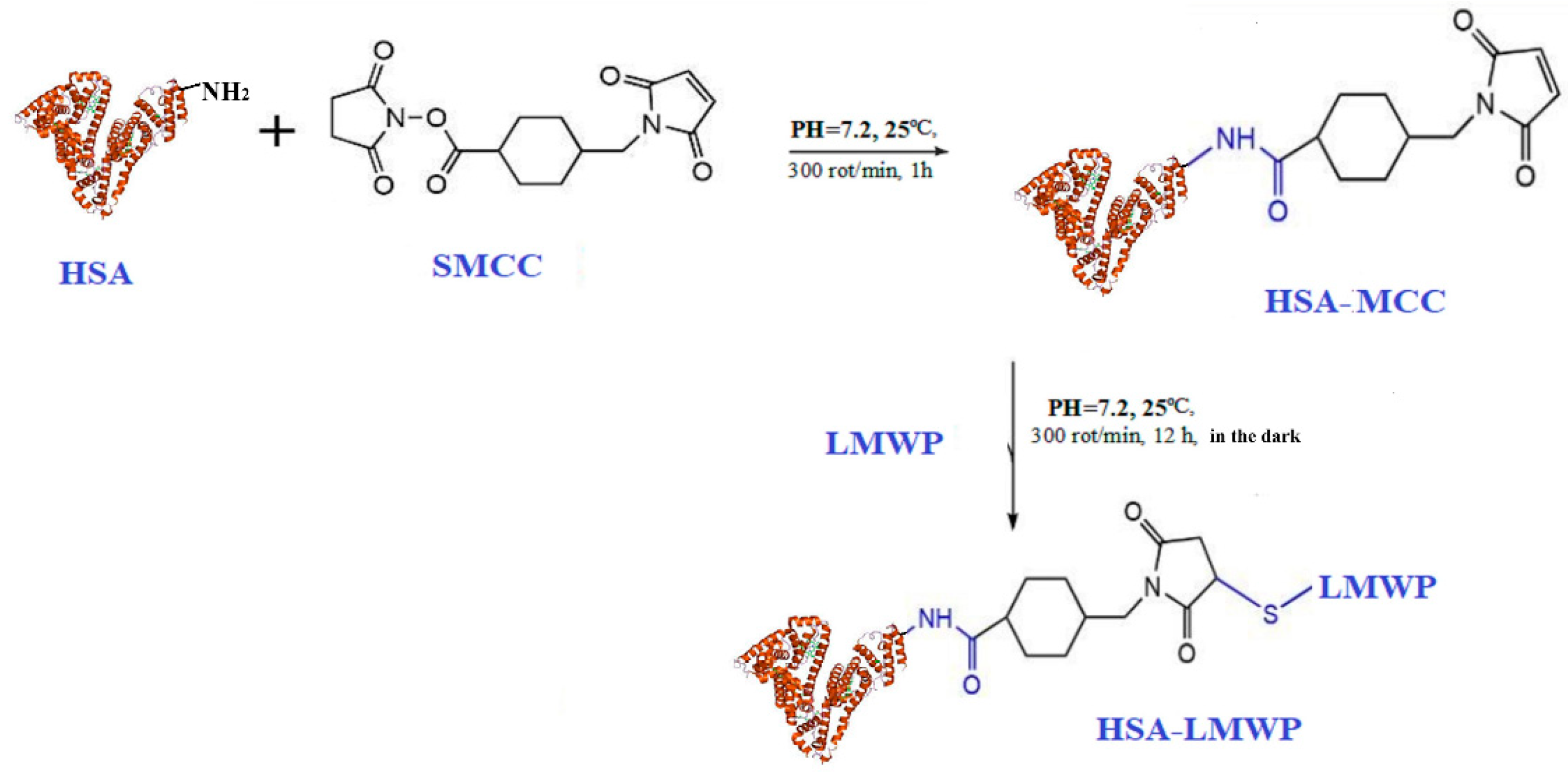
2.2. Modification of Human Serum Albumin with Low-Molecular-Weight Protamine
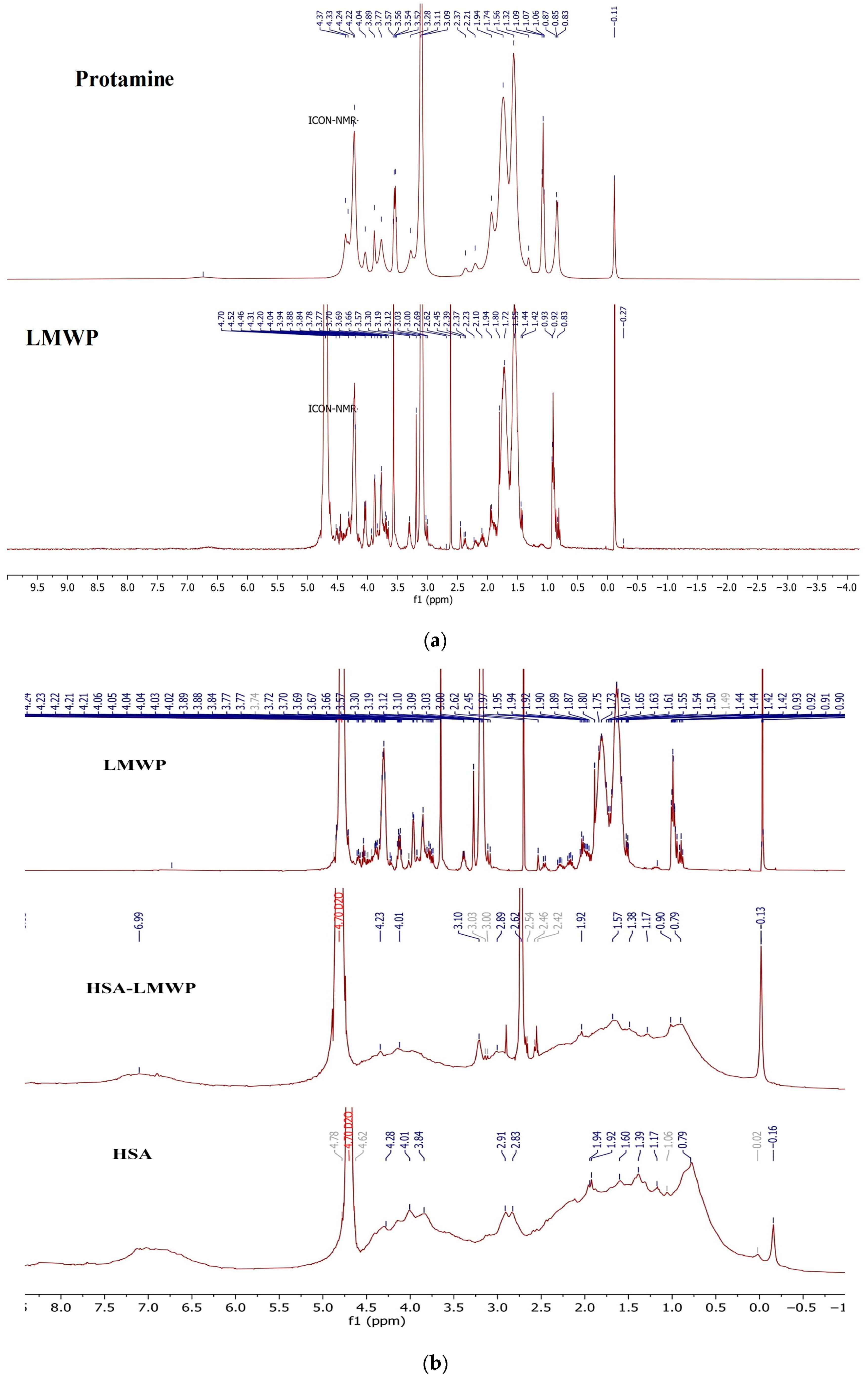
2.2.1. 1H-NMR Spectroscopy
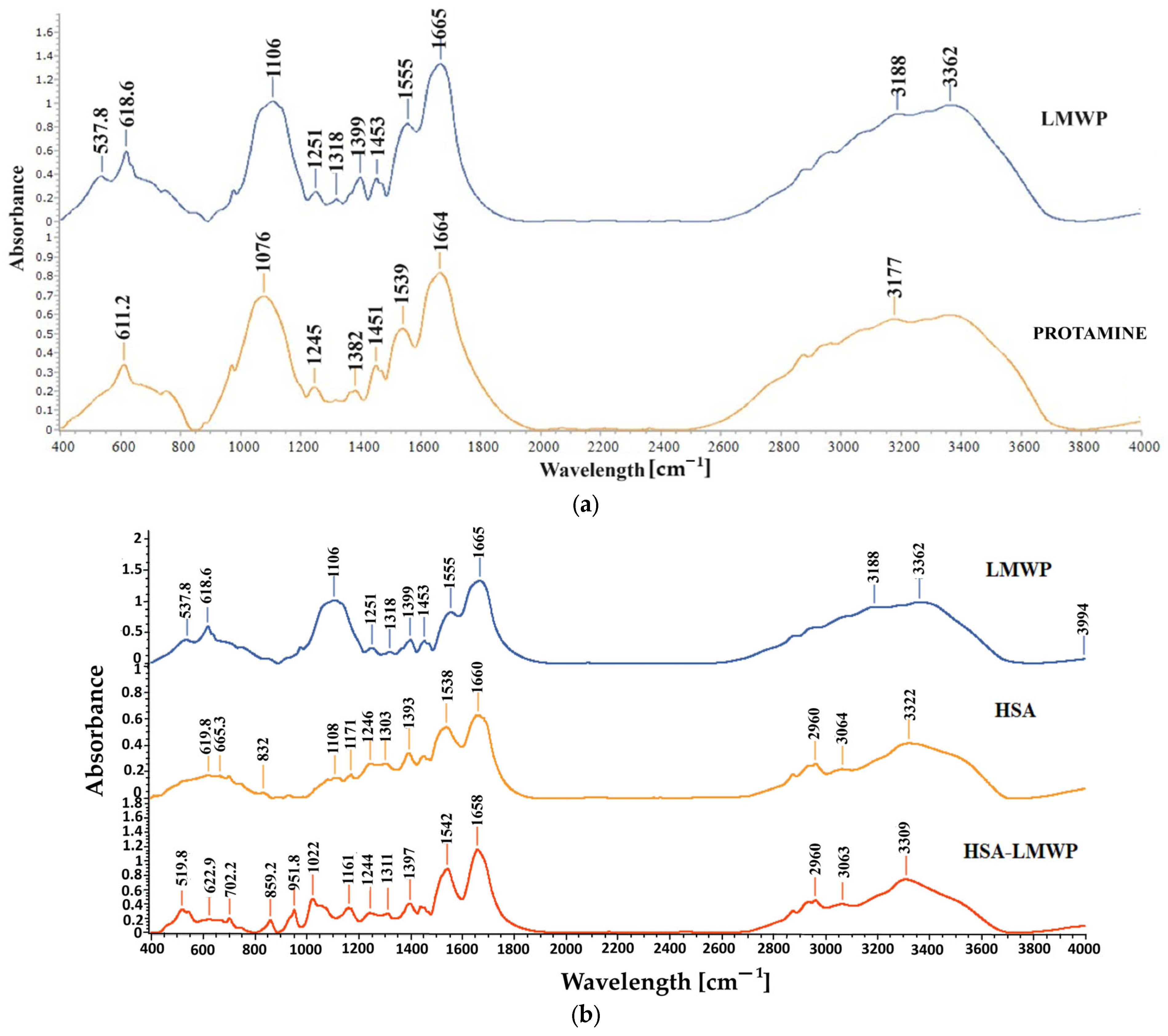
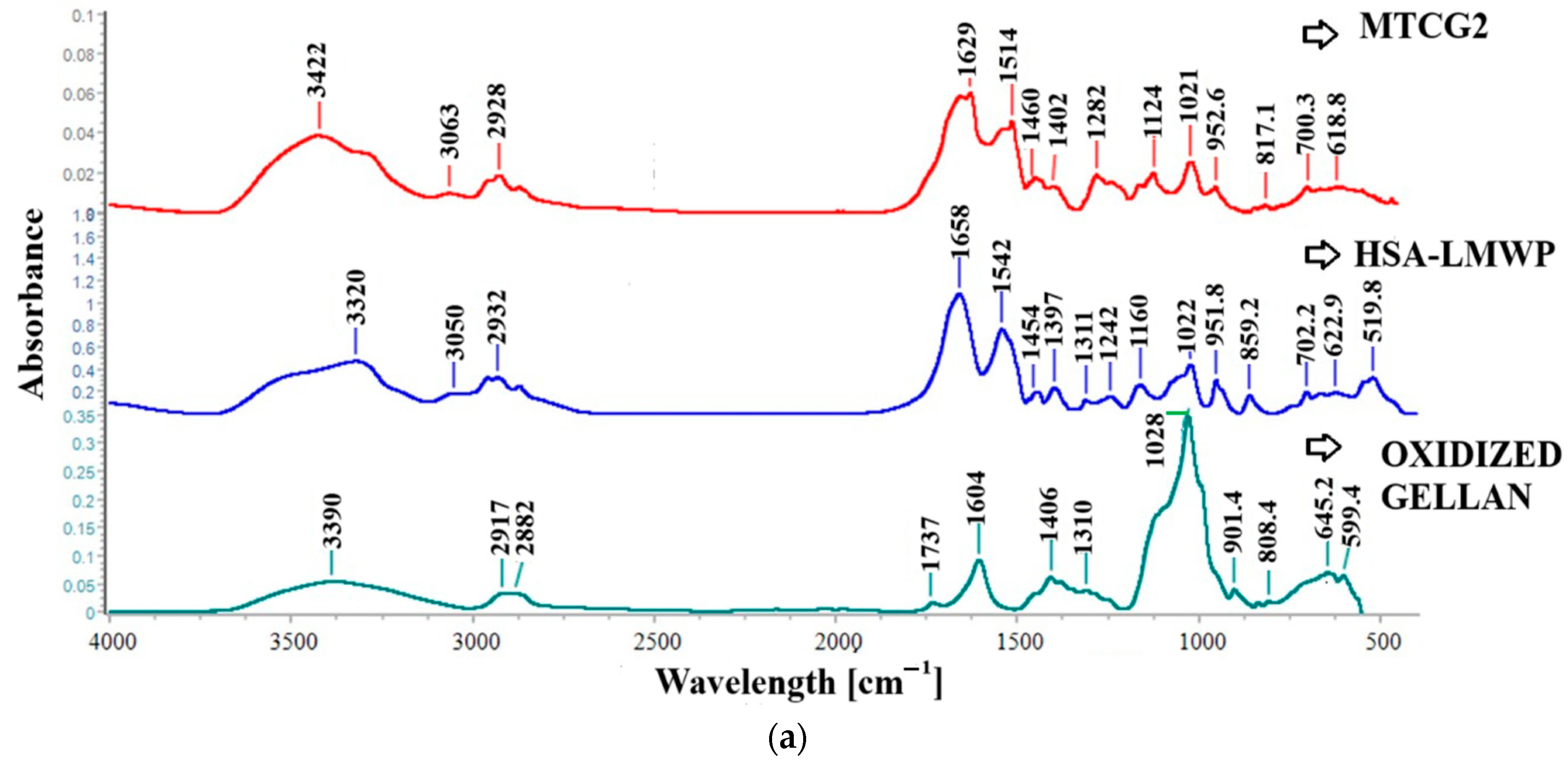
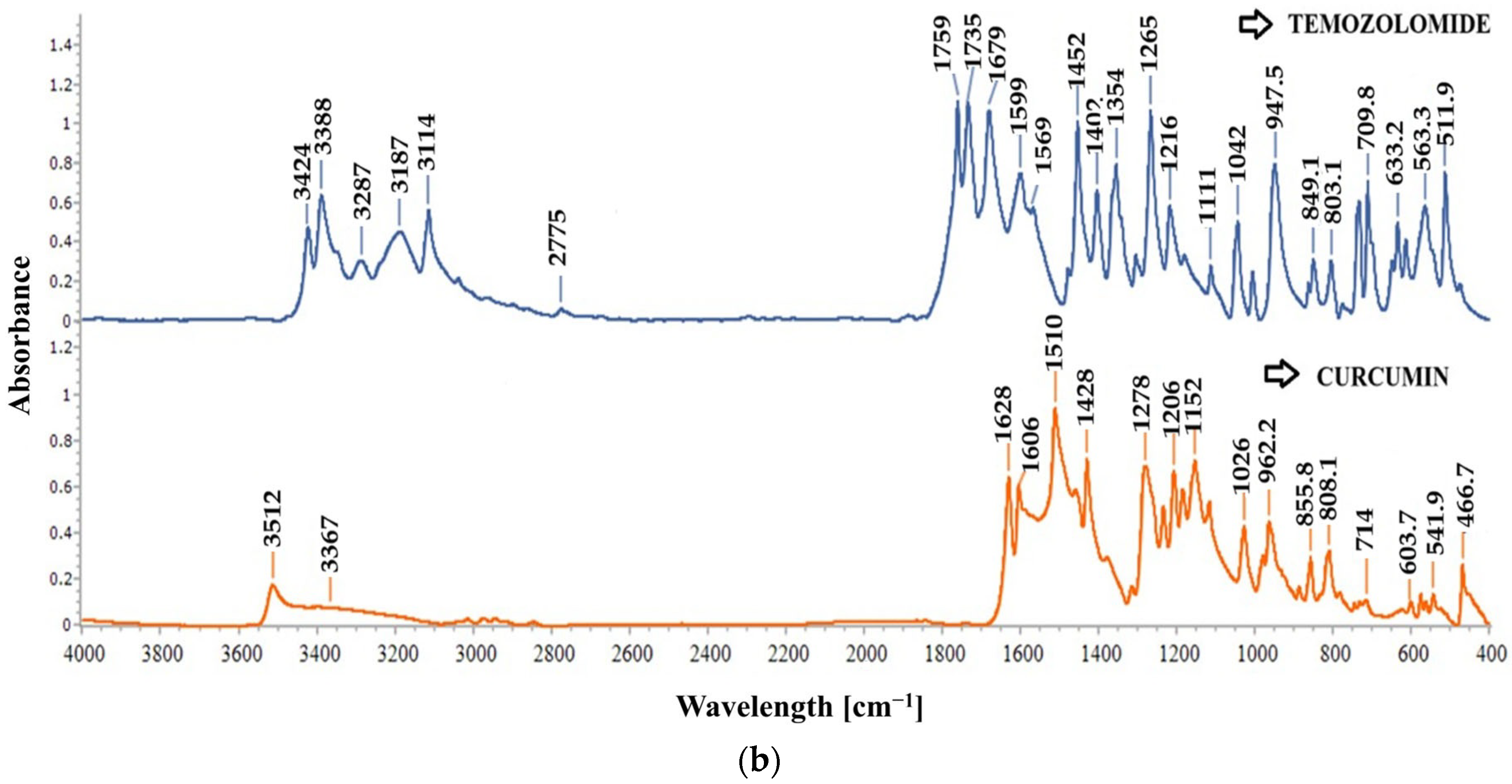
2.2.2. FT-IR Spectroscopy
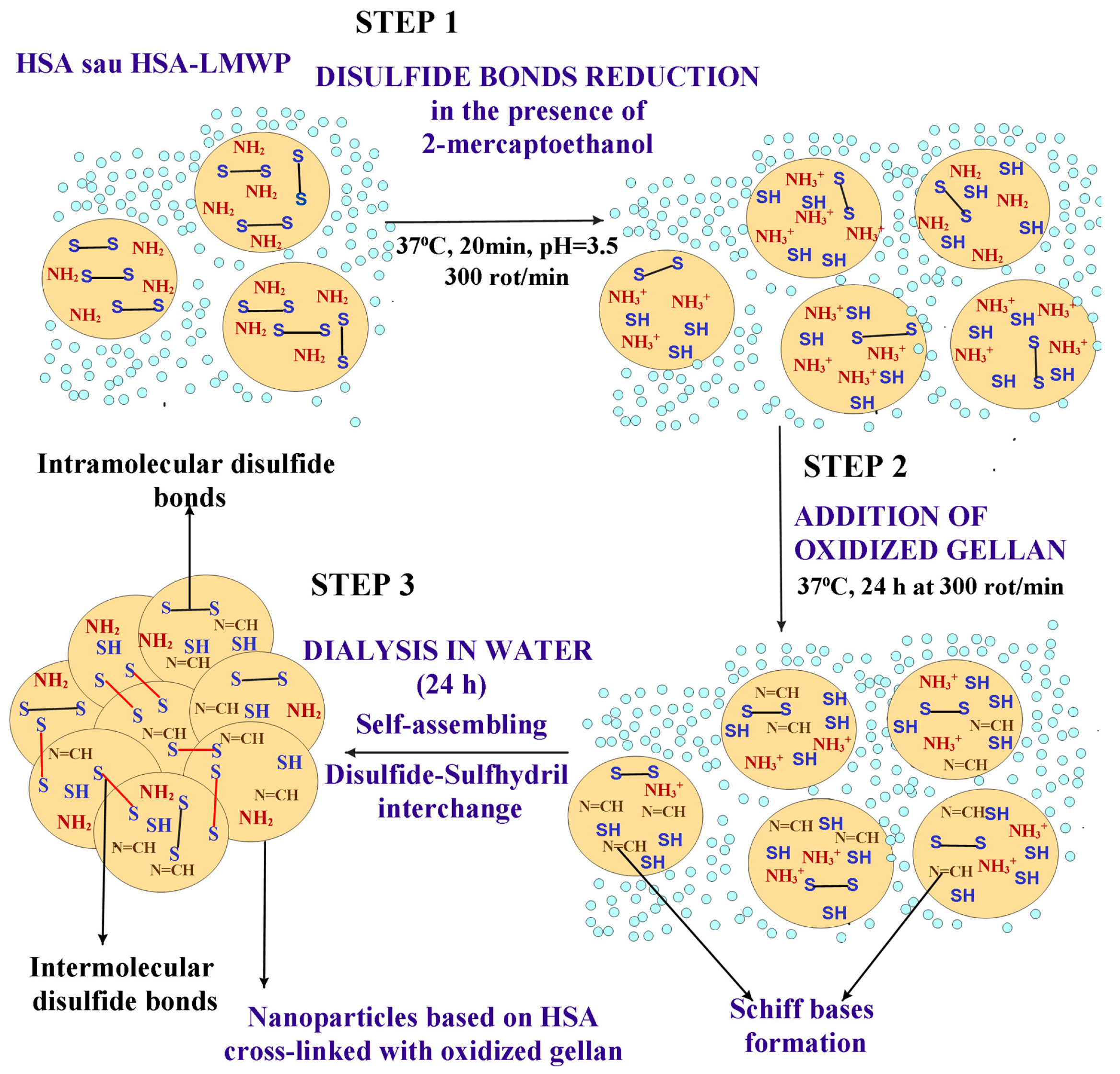
2.3. Obtaining and Characterization of the Nanoparticles
2.3.1. Obtaining Nanoparticles with and Without Encapsulated Drugs Through the Self-Assembly Method
2.3.2. Determination of the Mean Particle Size
2.3.3. Scanning Electron Microscopy
2.3.4. FT-IR Spectroscopy of Nanoparticles
2.3.5. Evaluation of the Drug Encapsulation Efficiency
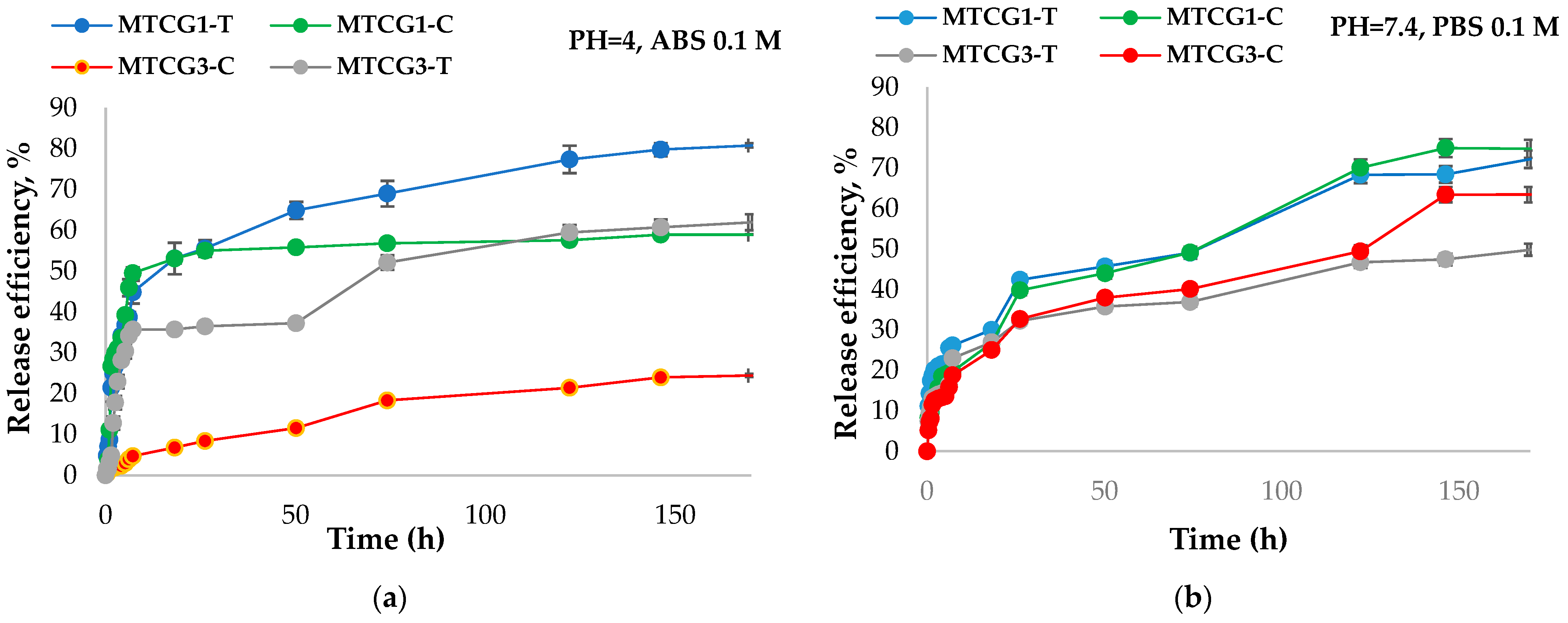
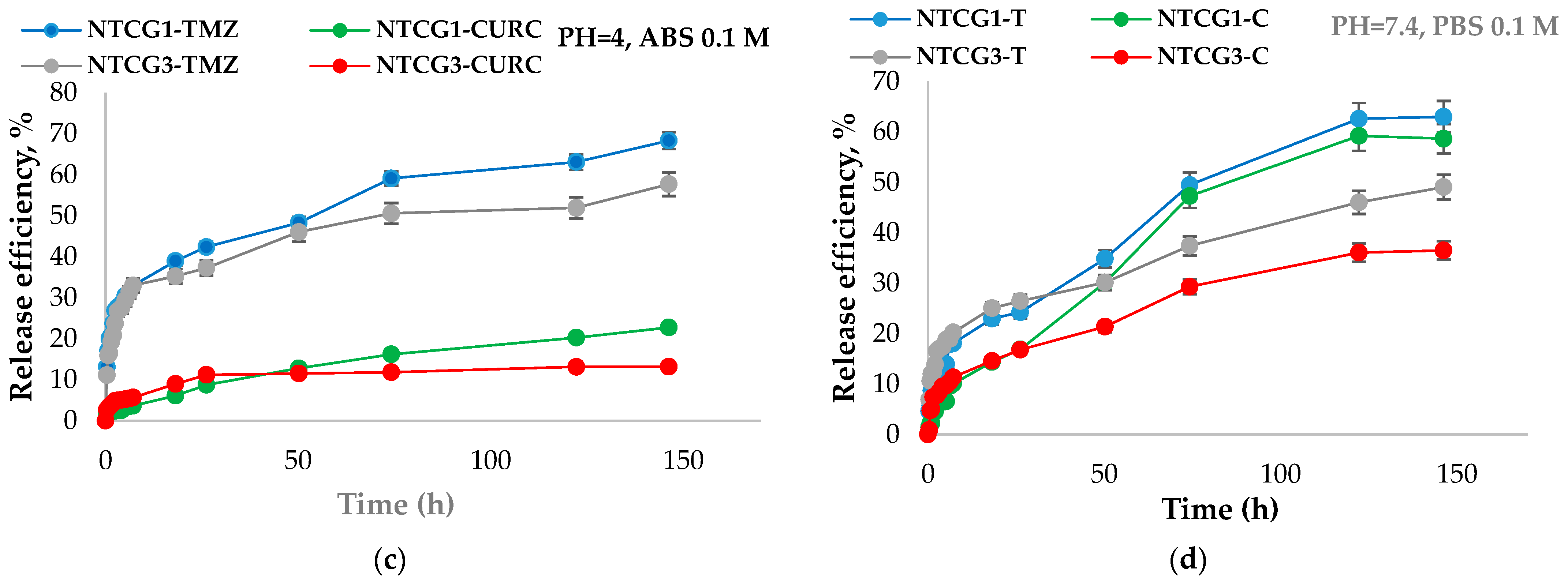
2.3.6. Study of the Release Kinetics of Curcumin and TMZ from Nanoparticles
2.4. Biological Properties and Functional Evaluation of the Functionalized Albumin Nanoparticles
2.4.1. Evaluating Cell Apoptosis Induced by Drug-Loaded Nanoparticles
2.4.2. Utilization of in Vitro Blood–Brain Barrier Models and Assessment of the Permeability of Functionalized Nanoparticles Containing Co-Encapsulated Drugs
2.4.3. Evaluation of Antioxidant Activity
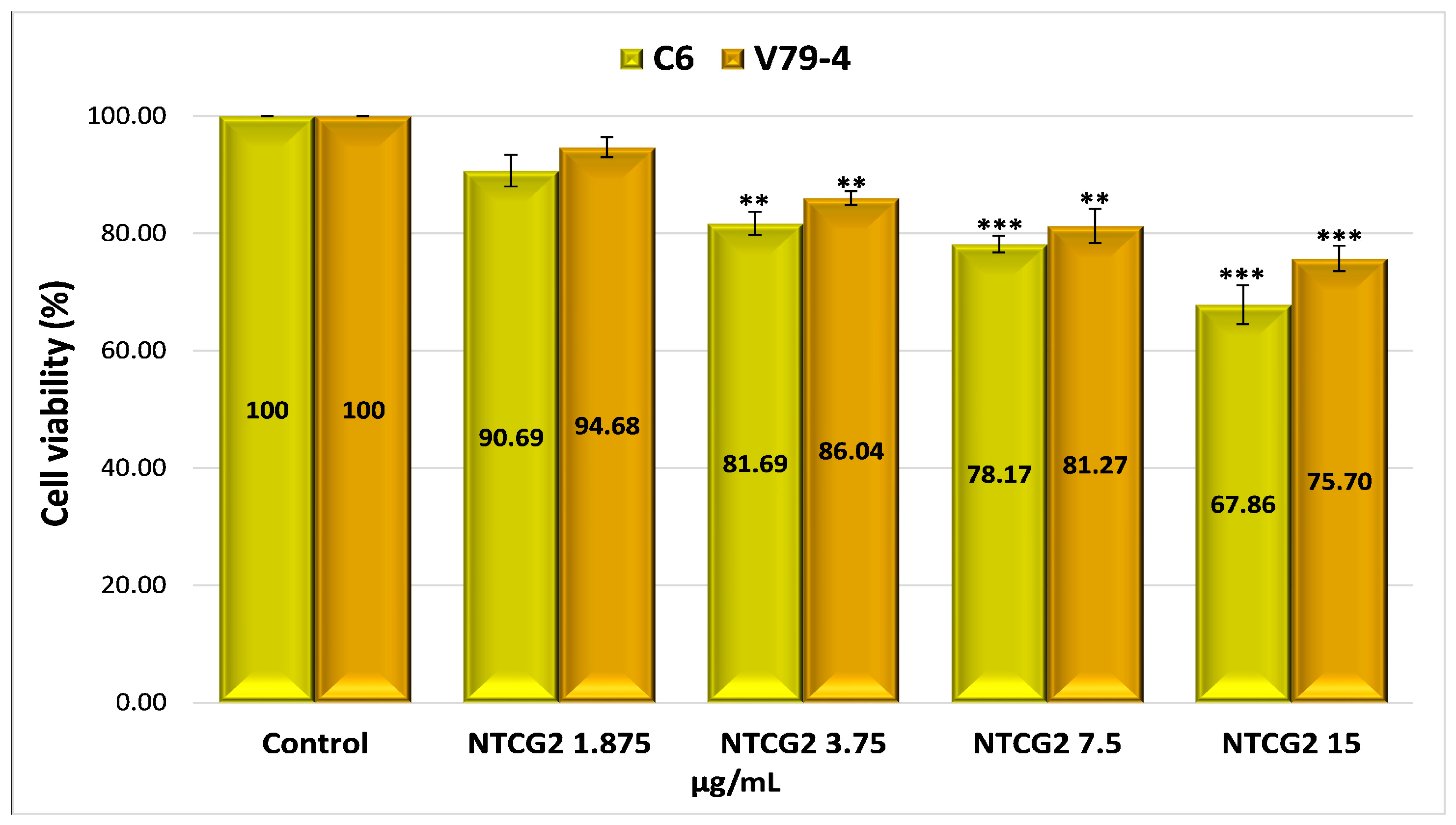
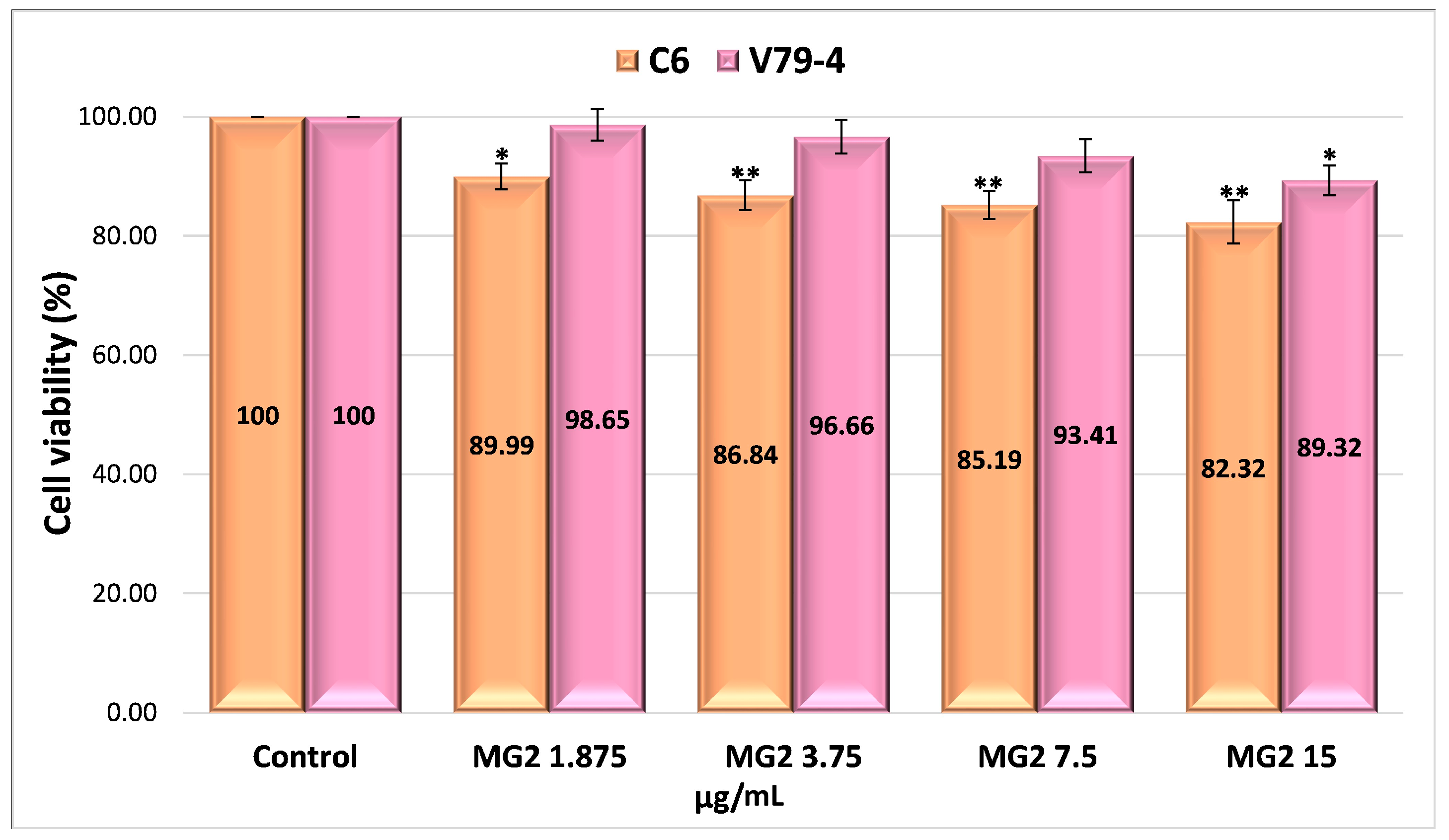
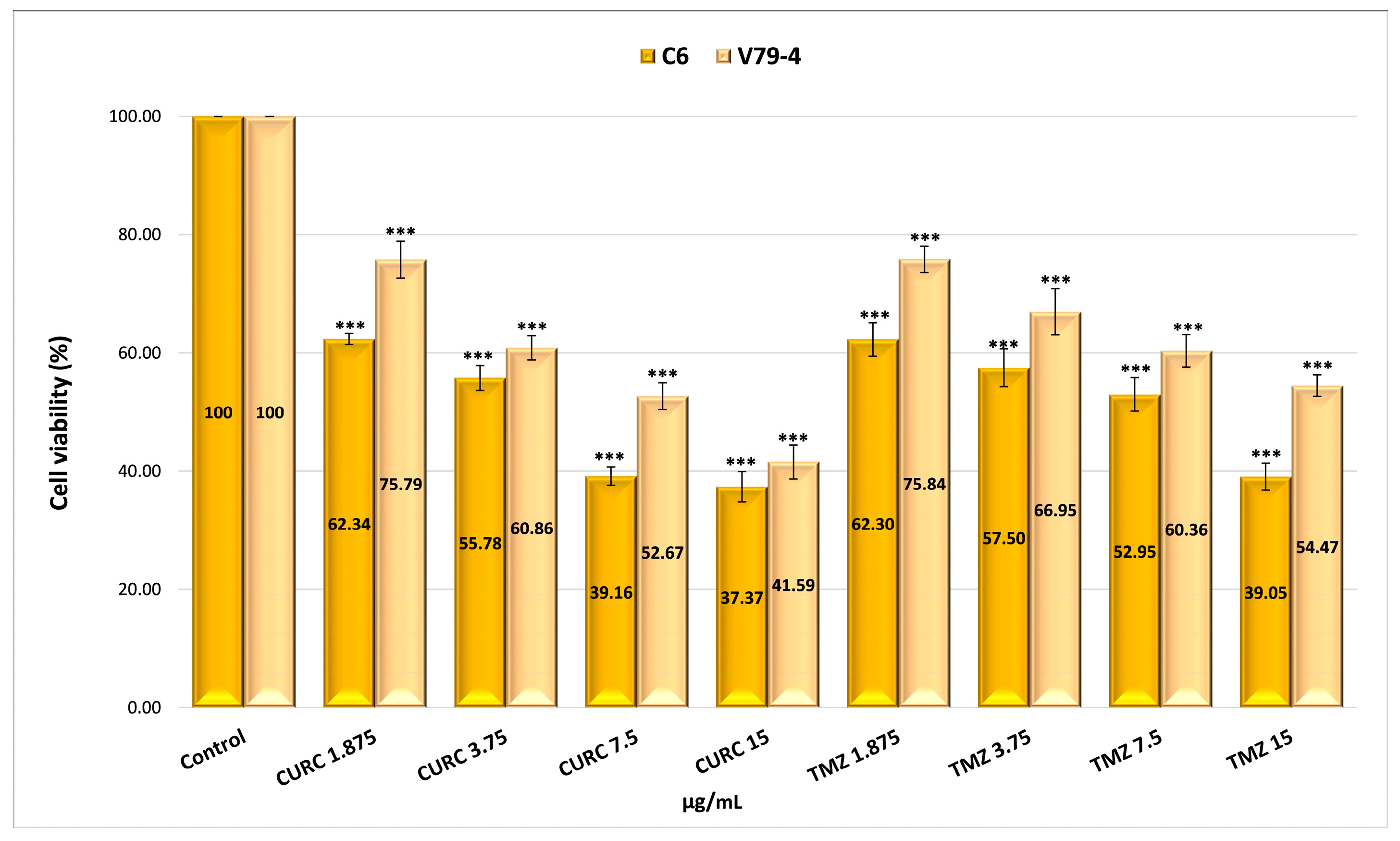
2.4.4. Evaluation of the Cytotoxicity of Drug-Encapsulated Particles via the MTT Assay
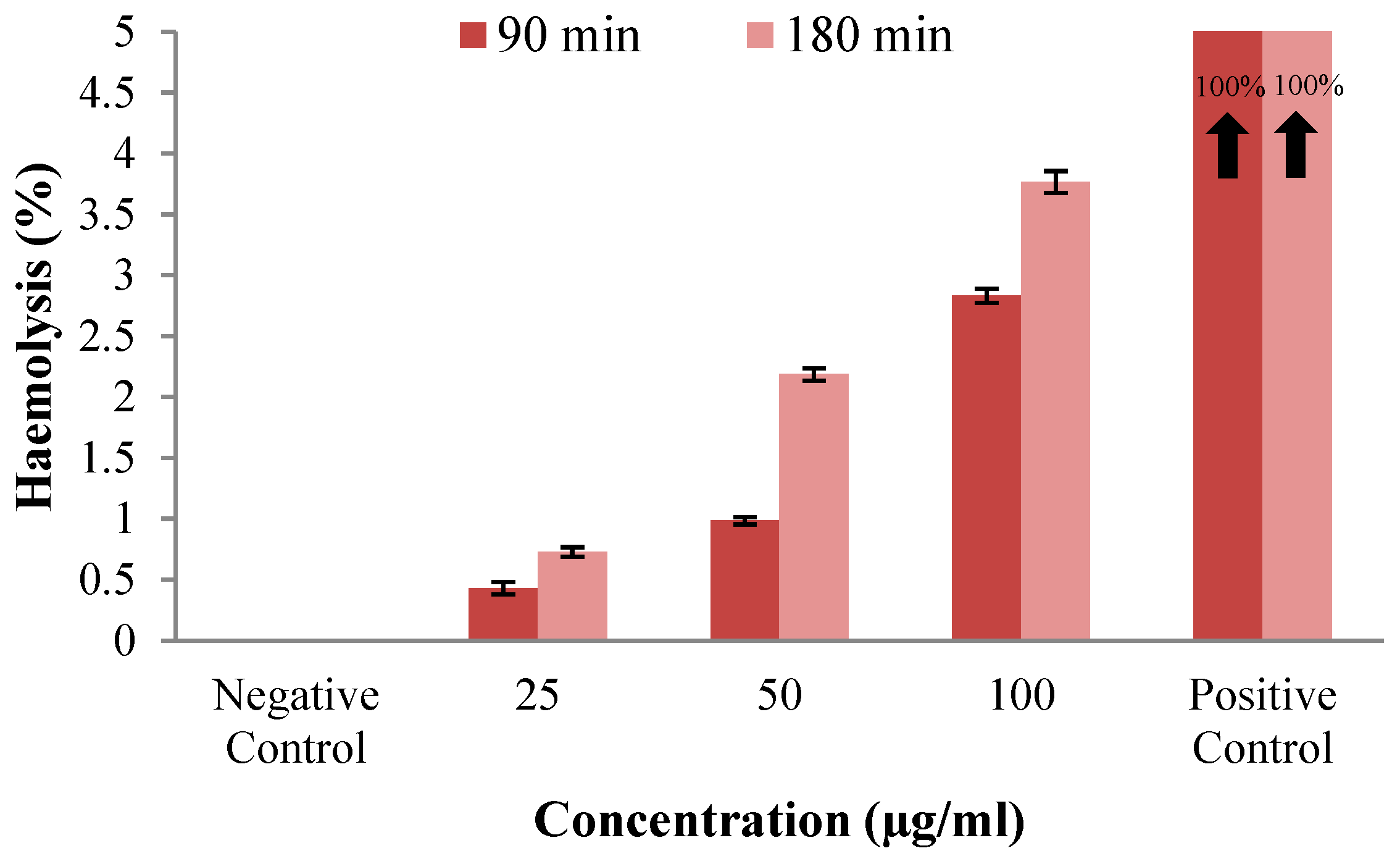
2.4.5. Analysis of the Hemocompatibility of Nanoparticles
2.5. Stability Evaluation
2.5.1. Study of the pH Stability of Drugs Encapsulated in Nanoparticles
2.5.2. Study of the Light Stability of Drugs Encapsulated in Nanoparticles
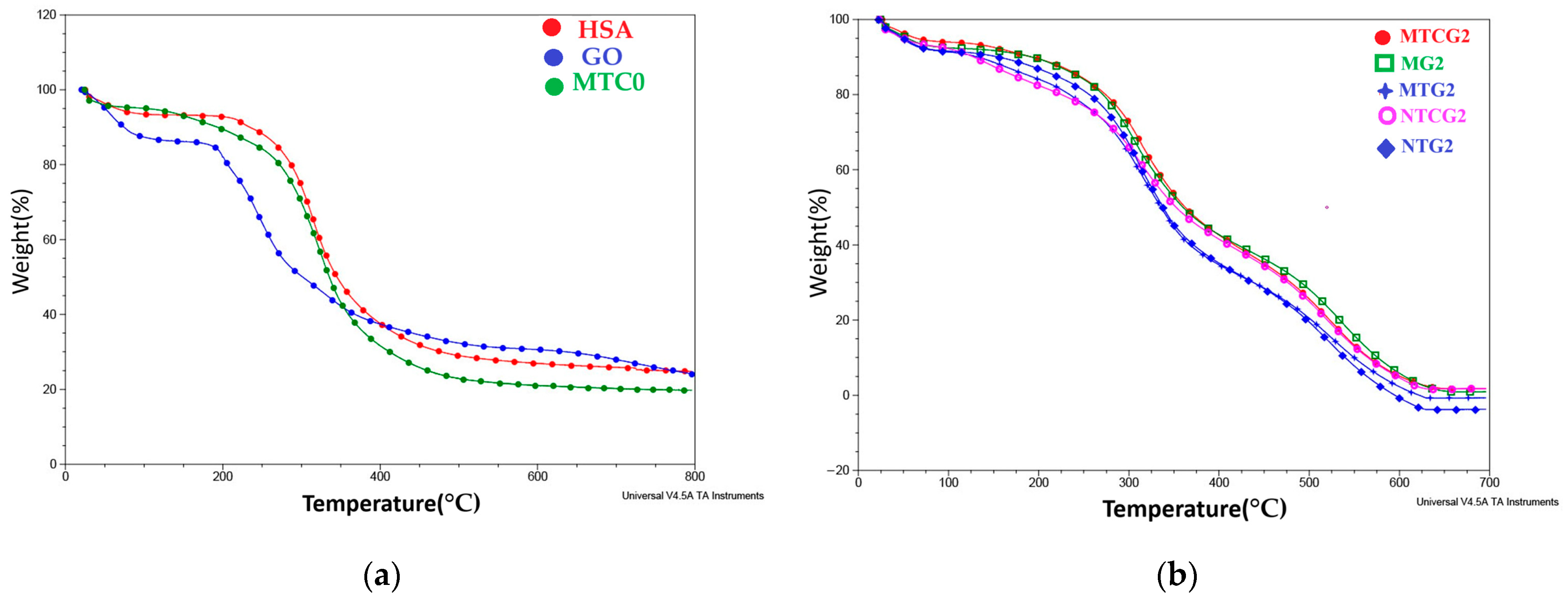
2.5.3. Thermogravimetric Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Obtaining Methods
4.2.1. Preparation of Low-Molecular-Weight Protamine (LMWP)
4.2.2. Obtaining Nanoparticles with and Without Encapsulated Drugs by the Self-Assembly Method
4.2.3. Determination of Free Amine Groups Using the Ninhydrin Solution Test
4.2.4. Modification of Human Serum Albumin Using Low-Molecular-Weight Protamine
4.3. Characterization Methods
4.3.1. 1H NMR Nuclear Magnetic Resonance Spectroscopy
4.3.2. FT-IR Spectroscopy
4.3.3. Determination of Average Particle Diameter
4.3.4. Scanning Electron Microscopy
4.3.5. Evaluation of the Encapsulation Efficiency (Ef%) for Temozolomide and Curcumin
4.3.6. The Study of Release Kinetics for Active Principles Co-Encapsulated in HSA-Based Nanoparticles in Two Different pH Environments Until Equilibrium
4.3.7. Evaluation of Cell Apoptosis Induced by Drug-Loaded Nanoparticles
4.3.8. Models of the Blood–Brain Barrier and Evaluation of the Permeability of Functionalized Nanoparticles with Co-Encapsulated Drugs
4.3.9. Assessment of the Antioxidant Properties of Curcumin Enclosed in Nanoparticles
4.3.10. Evaluation of Cytotoxicity of Drug-Encapsulated in Nanoparticles Using the MTT Assay
- Determination of cell viability—MTT method
4.3.11. Analysis of the Hemocompatibility of Nanoparticles
4.3.12. Analysis of pH Stability in Drugs Encapsulated Within Nanoparticles
4.3.13. Study of Light Stability in Drugs Encapsulated in Nanoparticles
4.3.14. Thermogravimetric Analyses
4.3.15. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, 2–8. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma Multiforme: State of the Art and Future Therapeutics. Surg. Neurol. Int. 2014, 5, 62–64. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fischer, J.L.; Langer, C.E.; Barnholtz-Sloan, J.S. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro-Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Mirimanoff, R.O. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.R.; Batista, C.; Afonso, B.d.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Moura Neto, V.; Mendes, F.d.A. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef]
- Shi, H.; Sun, S.; Xu, H.; Zhao, Z.; Han, Z.; Jia, J.; Wu, D.; Lu, J.; Liu, H.; Yu, R. Combined Delivery of Temozolomide and siPLK1 Using Targeted Nanoparticles to Enhance Temozolomide Sensitivity in Glioma. Int. J. Nanomed. 2020, 15, 3347–3362. [Google Scholar] [CrossRef]
- Petrenko, D.; Chubarev, V.; Syzrantsev, N.; Ismail, N.; Merkulov, V.; Sologova, S.; Grigorevskikh, E.; Smolyarchuk, E.; Alyautdin, R. Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems. Molecules 2022, 27, 3507. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.S.; Lee, S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Int. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Desjardins, A.; Weingart, J.; Brem, H.; Dolan, M.E.; Delaney, S.M.; Vredenburgh, J.; Rich, J.; Friedman, A.H.; Reardon, D.A.; et al. Phase I Trial of Temozolomide plus O6-Benzylguanine for Patients with Recurrent or Progressive Malignant Glioma. J. Clin. Oncol. 2009, 27, 7178–7187. [Google Scholar] [CrossRef] [PubMed]
- Krajcer, A.; Grzywna, E.; Lewandowska-Łańcucka, J. Strategies Increasing the Effectiveness of Temozolomide at Various Levels of Anti-GBL Therapy. Biomed. Pharmacother. 2023, 165, 115174. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, P.; Wiwattanawongsa, K.; Graidist, P.; Sangsen, Y.; Wiwattanapatapee, R. Enhanced Oral Bioavailability of Curcumin Using a Supersaturatable Self-Microemulsifying System Incorporating a Hydrophilic Polymer; In Vitro and In Vivo Investigations. AAPS PharmSciTech 2017, 19, 730–740. [Google Scholar] [CrossRef]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New Folic Acid-Chitosan Derivative Based Nanoparticles–Potential Applications in Cancer Therapy. Cellul. Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Y. Computer Simulation of the Role of Protein Corona in Cellular Delivery of Nanoparticles. Biomaterials 2014, 35, 8703–8710. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K.; Arihiro, K. Potential Functional Role of Plasmacytoid Dendritic Cells in Cancer Immunity. Immunology 2007, 121, 149–157. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Ukawala, M.; Manjappa, A.S.; Kumar, A.; Mundada, P.K.; Mishra, A.K.; Mathur, R.; Mönkkönen, J.; Murthy, R.S. Opsonization, Biodistribution, Cellular Uptake and Apoptosis Study of PEGylated PBCA Nanoparticle as Potential Drug Delivery Carrier. Pharm. Res. 2012, 29, 53–68. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and Metabolism of Phenolics from Digests of Polyphenol-Rich Potato Extracts Using the Caco-2/HepG2 Co-Culture System. Foods 2018, 7, 8. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming Blood–Brain Barrier with a Dual Purpose Temozolomide Loaded Lactoferrin Nanoparticles for Combating Glioma. Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Tincu, C.E.; Andrițoiu, C.V.; Popa, M.; Ochiuz, L. Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma. Polymers 2023, 15, 3969. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Dorai, T.; Cao, C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic Potential of Curcumin in Prostate Cancer—III: Curcumin Inhibits Proliferation, Induces Apoptosis, and Inhibits Angiogenesis of LNCaP Prostate Cancer Cells In Vivo. Prostate 2001, 47, 293–303. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood–Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2014, 141, 769. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Zheng, M.; Zou, Y.; Shi, B. Blood–Brain Barrier Penetrating Nanosystems Enable Synergistic Therapy of Glioblastoma. Nano Today 2024, 56, 102310. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J. Curcumin Targets Multiple Enzymes Involved in the ROS Metabolic Pathway to Suppress Tumor Cell Growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Damani, M.; Nilawar, N.; Momin, M.; Ningthoujham, R.S.; Khan, T. Nanoparticles Assisted Drug Delivery for Effective Management of Glioblastoma. Next Nanotechnol. 2024, 7, 100137. [Google Scholar] [CrossRef]
- Ramachandran, C.; Nair, S.M.; Escalon, E.; Melnick, S.J. Potentiation of Gemcitabine by Turmeric Force in Pancreatic Cancer Cell Lines. J. Complement. Integr. Med. 2012, 9, 20. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-Based Nanoparticles: A Promising Strategy to Overcome Cancer Drug Resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Brachmann, C.; Liu, X.; Pierce, D.W.; Dey, J.; Kerwin, W.S.; Li, Y.; Zhou, S.; Hou, S.; Carleton, M.; et al. Albumin-Bound Nanoparticle (nab) Paclitaxel Exhibits Enhanced Paclitaxel Tissue Distribution and Tumor Penetration. Cancer Chemother. Pharmacol. 2015, 76, 699. [Google Scholar] [CrossRef]
- Garg, S.M.; Vakili, M.R.; Lavasanifar, A. Polymeric Micelles Based on Poly(ethylene oxide) and α-Carbon Substituted Poly(ε-caprolactone): An in Vitro Study on the Effect of Core Forming Block on Polymeric Micellar Stability, Biocompatibility, and Immunogenicity. Colloids Surf. B Biointerfaces 2015, 132, 161–170. [Google Scholar] [CrossRef]
- Harries, M.; Ellis, P.; Harper, P. Nanoparticle Albumin-Bound Paclitaxel for Metastatic Breast Cancer. J. Clin. Oncol. 2005, 23, 7768–7771. [Google Scholar] [CrossRef]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly Nab-Paclitaxel in Combination with Carboplatin versus Solvent-Based Paclitaxel Plus Carboplatin as First-Line Therapy in Patients with Advanced Non-Small-Cell Lung Cancer: Final Results of a Phase III Trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W. US Food and Drug Administration Approves Paclitaxel Protein-Bound Particles (Abraxane®) in Combination with Gemcitabine as First-Line Treatment of Patients with Metastatic Pancreatic Cancer. JOP J. Pancreas 2013, 14, 686–688. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the Mysteries of Serum Albumin—More Than Just a Serum Protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Fanciullino, R.; Ciccolini, J.; Milano, G. Challenges, Expectations and Limits for Nanoparticles-Based Therapeutics in Cancer: A Focus on Nano-Albumin-Bound Drugs. Crit. Rev. Oncol. Hematol. 2013, 88, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Song, W.; Bergenfeldt, M.; Sass, P.; Malik, A.B. Gp60 Activation Mediates Albumin Transcytosis in Endothelial Cells by Tyrosine Kinase-Dependent Pathway. J. Biol. Chem. 1997, 272, 25968–25975. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E. Matricellular Proteins: Extracellular Modulators of Cell Function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a Versatile Platform for Drug Half-Life Extension. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 5526–5534. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73. [Google Scholar] [CrossRef]
- Chaudhury, C.; Brooks, C.L.; Carter, D.C.; Robinson, J.M.; Anderson, C.L. Albumin Binding to FcRn: Distinct from the FcRn-IgG Interaction. Biochemistry 2006, 45, 4983–4990. [Google Scholar] [CrossRef]
- Otterson, G.A.; Lavelle, J.; Villalona-Calero, M.A.; Shah, M.; Wei, X.; Chan, K.K.; Fischer, B.; Grever, M. A Phase I Clinical and Pharmacokinetic Study of Fenretinide Combined with Paclitaxel and Cisplatin for Refractory Solid Tumors. Investig. New Drugs 2005, 23, 555–562. [Google Scholar] [CrossRef]
- Tincu, C.E.; Daraba, O.M.; Jérôme, C.; Popa, M.; Ochiuz, L. Albumin-Based Hydrogel Films Covalently Cross-Linked with Oxidized Gellan with Encapsulated Curcumin for Biomedical Applications. Polymers 2024, 16, 1631. [Google Scholar] [CrossRef]
- Chang, L.C.; Liang, J.F.; Lee, H.F.; Lee, L.M.; Yang, V.C. Low Molecular Weight Protamine as a Non-Toxic Heparin/Low Molecular Weight Heparin Antidote (II): In Vitro Evaluation of Efficacy and Toxicity. AAPS PharmSciTech 2001, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Ripp, S.; Turunen, P.; Minot, E.D.; Rowan, A.E.; Blank, K.G. Deciphering Design Principles of Förster Resonance Energy Transfer-Based Protease Substrates: Thermolysin-Like Protease from Geobacillus stearothermophilus as a Test Case. ACS Omega 2018, 3, 4148–4156. [Google Scholar] [CrossRef]
- Kreij, A.; Venema, G.; van den Burg, B. Substrate Specificity in the Highly Heterogeneous M4 Peptidase Family Is Determined by a Small Subset of Amino Acids. J. Biol. Chem. 2000, 275, 31115–31120. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sheng, J.; David, A.E.; Kwon, Y.M.; Zhang, J.; Huang, Y.; Wang, J.; Yang, V.C. The Use of Low Molecular Weight Protamine Chemical Chimera to Enhance Monomeric Insulin Intestinal Absorption. Biomaterials 2013, 34, 7733–7743. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Wang, H.; Gong, J.; He, H.; Shin, M.C.; Yang, V.C.; Huang, Y. Low-Molecular-Weight Protamine-Modified PLGA Nanoparticles for Overcoming Drug-Resistant Breast Cancer. J. Control. Release 2014, 192, 47–56. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Wang, H.Y.; Zhao, Y.X.; Wang, H.; Zeng, F.; Hua, H.; Xu, Q.; Huang, Y. Cell-Penetrating Albumin Conjugates for Enhanced Doxorubicin Delivery. Polym. Chem. 2013, 4, 4584–4587. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Karthikeyan, S.; Kesherwani, M.; Velmurugan, D.; Hanagata, N. Underlying the Mechanism of 5-Fluorouracil and Human Serum Albumin Interaction: A Biophysical Study. J. Phys. Chem. Biophys. 2016, 6, 214. [Google Scholar] [CrossRef]
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules 2019, 25, 4854. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-Based Hydrogels for Regenerative Engineering and Cell Transplantation. Biotechnol. Bioeng. 2019, 116, 3457–3468. [Google Scholar] [CrossRef]
- Wolf, M.; Gulich, R.; Lunkenheimer, P.; Loidl, A. Relaxation Dynamics of a Protein Solution Investigated by Dielectric Spectroscopy. Biochim. Biophys. Acta BBA Proteins Proteom. 2012, 1824, 723–730. [Google Scholar] [CrossRef]
- Yilmaz, A.; Zengin, B.; Ulak, F.S. NMR Proton Spin-Lattice Relaxation Mechanism in D2O Solutions of Albumin Determined at 400 MHz. J. Appl. Spectrosc. 2014, 81, 365–370. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The Antioxidant Properties of Serum Albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Christodoulou, J.; Sadler, P.J.; Tucker, A. 1H NMR of Albumin in Human Blood Plasma: Drug Binding and Redox Reactions at Cys34. FEBS Lett. 1995, 376, 1–5. [Google Scholar] [CrossRef]
- Epasto, L.M.; Honegger, P.; Che, K.; Kozak, F.; Jörg, F.; Schröder, C.; Kurzbach, D. Nuclear Overhauser Spectroscopy in Hyperpolarized Water—Chemical vs. Magnetic Exchange. Chem. Commun. 2022, 58, 11661–11664. [Google Scholar] [CrossRef]
- Blaum, B.S.; Deakin, J.A.; Johansson, C.M.; Herbert, A.P.; Barlow, P.N.; Lyon, M.; Uhrín, D. Lysine and Arginine Side Chains in Glycosaminoglycan−Protein Complexes Investigated by NMR, Cross-Linking, and Mass Spectrometry: A Case Study of the Factor H−Heparin Interaction. J. Am. Chem. Soc. 2010, 132, 6374–6381. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Bigam, C.G.; Holm, A.; Hodges, R.S.; Sykes, B.D. 1H, 13C and 15N Random Coil NMR Chemical Shifts of the Common Amino Acids. I. Investigations of Nearest-Neighbor Effects. J. Biomol. NMR 1995, 5, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Oida, T. 1H-NMR Study on the Interactions of Human Serum Albumin with Free Fatty Acid. J. Biochem. 1986, 100, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent Trends in Peptide and Protein-Based Hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, A.A.; Carvalho, S.M.; Florentino, R.M.; Mansur, H.S. L-Cysteine and Poly-L-Arginine Grafted Carboxymethyl Cellulose/Ag-In-S Quantum Dot Fluorescent Nanohybrids for In Vitro Bioimaging of Brain Cancer Cells. Int. J. Biol. Macromol. 2019, 133, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997; p. 53. [Google Scholar]
- Thalhauser, S.; Breunig, M. Considerations for Efficient Surface Functionalization of Nanoparticles with a High Molecular Weight Protein as Targeting Ligand. Eur. J. Pharm. Sci. 2020, 155, 105520. [Google Scholar] [CrossRef]
- Asrorov, A.M.; Mukhamedov, N.; Kayumov, M.; Yashinov, A.S.; Wali, A.; Yili, A.; Mirzaakhmedov, S.Y.; Huang, Y. Albumin Is a Reliable Drug-Delivering Molecule: Highlighting Points in Cancer Therapy. Med. Drug Discov. 2024, 22, 100186. [Google Scholar] [CrossRef]
- Xu, R.; Fisher, M.; Juliano, R.L. Targeted Albumin-Based Nanoparticles for Delivery of Amphipathic Drugs. Bioconjugate Chem. 2011, 22, 870–879. [Google Scholar] [CrossRef]
- Liu, L.; Bi, Y.; Zhou, M.; Chen, X.; He, X.; Zhang, Y.; Sun, T.; Ruan, C.; Chen, Q.; Wang, H.; et al. Biomimetic Human Serum Albumin Nanoparticle for Efficiently Targeting Therapy to Metastatic Breast Cancers. ACS Appl. Mater. Interfaces 2017, 9, 7424–7435. [Google Scholar] [CrossRef]
- Asghar, S.; Salmani, J.M.M.; Hassan, W.; Xie, Y.; Meng, F.; Su, Z.; Sun, M.; Xiao, Y.; Ping, Q. A Facile Approach for Crosslinker-Free Nano Self-Assembly of Protein for Anti-Tumor Drug Delivery: Factors’ Optimization, Characterization and In Vitro Evaluation. Eur. J. Pharm. Sci. 2014, 63, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Matson, J.B. PH-Responsive Self-Assembling Peptide-Based Biomaterials: Designs and Applications. ACS Appl. Bio Mater. 2022, 5, 4635–4651. [Google Scholar] [CrossRef]
- Gong, G.; Xu, Y.; Zhou, Y.; Meng, Z.; Ren, G.; Zhao, Y.; Zhang, X.; Wu, J.; Hu, Y. Molecular Switch for the Assembly of Lipophilic Drug Incorporated Plasma Protein Nanoparticles and In Vivo Image. Biomacromolecules 2012, 13, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, M.; Cheng, A.; Lin, L.; Ho, Y.; Hsieh, C.; Lin, J. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Redox Responsive Curcumin-Loaded Human Serum Albumin Nanoparticles: Preparation, Characterization and In Vitro Evaluation. Int. J. Biol. Macromol. 2018, 114, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Asimakidou, E.; Tan, J.K.S.; Zeng, J.; Lo, C.H. Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals 2024, 17, 612. [Google Scholar] [CrossRef]
- Niknejad, H.; Mahmoudzadeh, R. Comparison of Different Cross-linking Methods for Preparation of Docetaxel-Loaded Albumin Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 385–394. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4403054/ (accessed on 2 March 2025). [PubMed]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2024, 19, 6945–6964. [Google Scholar] [CrossRef] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; Von Briesen, H.; Kreuter, J. Albumin Nanoparticles Targeted with Apo E Enter the CNS by Transcytosis and Are Delivered to Neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and Transferrin-Receptor-Antibody-Modified Nanoparticles Enable Drug Delivery across the Blood–Brain Barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Zhou, Y.; Shao, Y.; Zhang, H.; Zhu, J. An investigation into the effect of particle size distribution on the fluidization behavior of Group C and Group A particles. Powder Techn. 2021, 398, 117142. [Google Scholar] [CrossRef]
- Chua, A.; Tran, T.-T.; Pu, S.; Park, J.-W.; Hadinoto, K. Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability. Int. J. Mol. Sci. 2022, 23, 11731. [Google Scholar] [CrossRef]
- Helal, D.O.; Abdel-Mottaleb, M.M.A.; Kamel, A.O.; Rouatbi, N.; Han, S.; Geneidi, A.S.; Al-Jamal, K.T.; Awad, G.A.S. The Interplay of Solvent–Drug–Protein Interactions during Albumin Nanoparticles Formulations for Temozolomide Delivery to Brain Cancer Cells. J. Pharm. Pharmacol. 2023, 75, 921–930. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Hyaluronic Acid Conjugated Albumin Nanoparticles for Efficient Receptor Mediated Brain Targeted Delivery of Temozolomide. J. Drug Deliv. Sci. Technol. 2021, 61, 102129. [Google Scholar] [CrossRef]
- Jithan, A.; Madhavi, K.; Madhavi, M.; Prabhakar, K. Preparation and Characterization of Albumin Nanoparticles Encapsulating Curcumin Intended for the Treatment of Breast Cancer. Int. J. Pharm. Investig. 2011, 1, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.C.V.; Razzak, M.A.; Cho, J.H.; Kim, J.Y.; Choi, S.S. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-Like Symptoms in C. elegans. Nanomaterials 2022, 12, 758. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release, I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Baler, K.; Michael, R.; Szleifer, I.; Ameer, G.A. Albumin Hydrogels Formed by Electrostatically Triggered Self-Assembly and Their Drug Delivery Capability. Biomacromolecules 2014, 15, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Meussen, B.J.; van Zeeland, A.N.T.; Bruins, M.E.; Sanders, J.P.M. A Fast and Accurate UPLC Method for Analysis of Proteinogenic Amino Acids. Food Anal. Methods 2014, 7, 1047–1055. [Google Scholar] [CrossRef]
- Moawad, M.; Nasr, G.M.; Osman, A.S.; Shaker, E.S. Curcumin Nanocapsules Effect in Apoptotic Processes, Gene Expression, and Cell Cycle on Hep-G2 Cell Lines. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231176396. [Google Scholar] [CrossRef]
- Wong, S.C.; Kamarudin, M.N.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Havrdova, M.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Horakova, J.; et al. In Vitro Cytotoxicity Analysis of Doxorubicin-Loaded/Superparamagnetic Iron Oxide Colloidal Nanoassemblies on MCF7 and NIH3T3 Cell Lines. Int. J. Nanomed. 2015, 10, 949–961. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Ma, Y.; Gong, H.; Yang, S.; Fang, Q.; Hu, Z. Nanoparticle Abraxane Possesses Impaired Proliferation in A549 Cells Due to the Underexpression of Glucosamine 6-Phosphate N-Acetyltransferase 1 (GNPNAT1/GNA1). Int. J. Nanomed. 2017, 12, 1685–1695. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-Mediated Endocytosis Is Critical for Albumin Cellular Uptake and Response to Albumin-Bound Chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Sampron, N.; Matheu, A. Current Advances in Temozolomide Encapsulation for the Enhancement of Glioblastoma Treatment. Theranostics 2023, 13, 2734–2749. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-T.; Lee, M.-H.; Weng, C.-F.; Leong, M.K. In Silico Prediction of PAMPA Effective Permeability Using a Two-QSAR Approach. Int. J. Mol. Sci. 2019, 20, 3170. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Budai-Szűcs, M.; Balogh, G.T.; Veszelka, S.; Gróf, I.; Deli, M.A.; Volk, B.; Szabó-Révész, P.; Csóka, I. Development of In Situ Gelling Meloxicam–Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate. J. Agric. Food Chem. 2017, 65, 10812–10819. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved Antioxidant Activity and Physicochemical Properties of Curcumin by Adding Ovalbumin and Its Structural Characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.; Zhang, J.; Niu, B.; Li, W. Characterization, Release, and Antioxidant Activity of Curcumin-Loaded Sodium Alginate/ZnO Hydrogel Beads. Int. J. Biol. Macromol. 2018, 121, 1118–1125. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Ahad, A.; Shakeel, F.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Thermodynamic Solubility Profile of Temozolomide in Different Commonly Used Pharmaceutical Solvents. Molecules 2022, 27, 1437. [Google Scholar] [CrossRef]
- Colaço, M.; Roquito, T.; Costa, J.P.; Cruz, M.T.; Borges, O. The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute. Pharmaceutics 2023, 15, 623. [Google Scholar] [CrossRef]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-Brain Delivery of Temozolomide-Loaded PLGA Nanoparticles Functionalized with Anti-EPHA3 for Glioblastoma Targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef]
- Meteoglu, I.; Erdemir, A. Genistein and Temozolomide-Loaded Polymeric Nanoparticles: A Synergistic Approach for Improved Anti-Tumor Efficacy against Glioblastoma. Process Biochem. 2021, 110, 9–18. [Google Scholar] [CrossRef]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 2012, 34, 196–208. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Hoonjan, M.; Sachdeva, G.; Chandra, S.; Kharkar, P.S.; Sahu, N.; Bhatt, P. Investigation of HSA as a Biocompatible Coating Material for Arsenic Trioxide Nanoparticles. Nanoscale 2018, 10, 1436–1447. [Google Scholar] [CrossRef]
- Tacheva, B.; Paarvanova, B.; Ivanov, I.T.; Tenchov, B.; Georgieva, R.; Karabaliev, M. Drug Exchange between Albumin Nanoparticles and Erythrocyte Membranes. Nanomaterials 2019, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Elblbesy, M. Hemocompatibility of Albumin Nanoparticles as a Drug Delivery System—An In Vitro Study. J. Biomater. Nanobiotechnol. 2016, 7, 64–71. [Google Scholar] [CrossRef]
- Obuobi, S.; Wang, Y.; Khara, J.S.; Riegger, A.; Kuan, S.L.; Ee, P.L.R. Antimicrobial and Anti-Biofilm Activities of Surface Engineered Polycationic Albumin Nanoparticles with Reduced Hemolytic Activity. Macromol. Biosci. 2018, 18, 1800196. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, Z.; Yin, S.; Wang, Q.; Guo, F.; Zhang, Y.; Yu, R.; Liu, Y.; Su, Z. Extending Half-Life of H-Ferritin Nanoparticle by Fusing Albumin Binding Domain for Doxorubicin Encapsulation. Biomacromolecules 2018, 19, 773–781. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Zhu, X.; Liu, C.; Wu, M.; Li, C.; Li, X.; Gao, L.; Ding, Y. Tolerance, Variability and Pharmacokinetics of Albumin-Bound Paclitaxel in Chinese Breast Cancer Patients. Front. Pharmacol. 2018, 9, 419011. [Google Scholar] [CrossRef]
- Ding, D.; Tang, X.; Cao, X.; Wu, J.; Yuan, A.; Qiao, Q.; Pan, J.; Hu, Y. Novel Self-Assembly Endows Human Serum Albumin Nanoparticles with an Enhanced Antitumor Efficacy. AAPS PharmSciTech 2013, 15, 213. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A Novel Assay for Apoptosis: Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified Annexin V/Propidium Iodide Apoptosis Assay for Accurate Assessment of Cell Death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Esső, K.; Dargó, G.; Könczöl, Á.; Balogh, G.T. Tuning the Predictive Capacity of the PAMPA-BBB Model. Eur. J. Pharm. Sci. 2015, 79, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant Activity and Free Radical Scavenging Capacity between Korean Medicinal Plants and Flavonoids by Assay-Guided Comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Novakovic, V.G.; Freshney, R.I. Culture of Cells for Tissue Engineering, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2006; Available online: https://www.wiley.com/en-us/Culture+of+Cells+for+Tissue+Engineering-p-9780471741800 (accessed on 2 March 2025).
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J. Effects of Human Pharmaceuticals on Cytotoxicity, EROD Activity and ROS Production in Fish Hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture, 2nd ed.; Cree, I.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 731, pp. 237–245. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef] [PubMed]

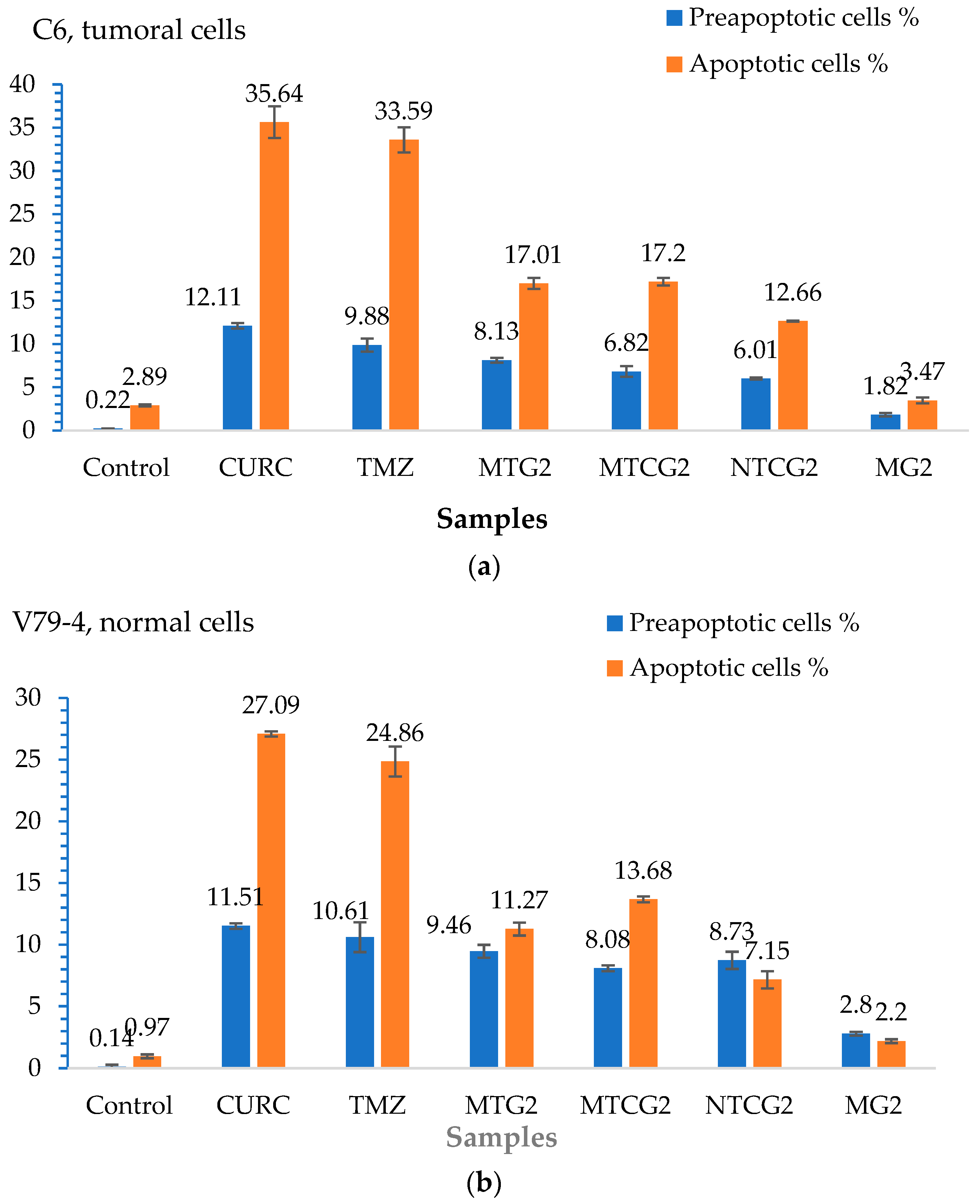
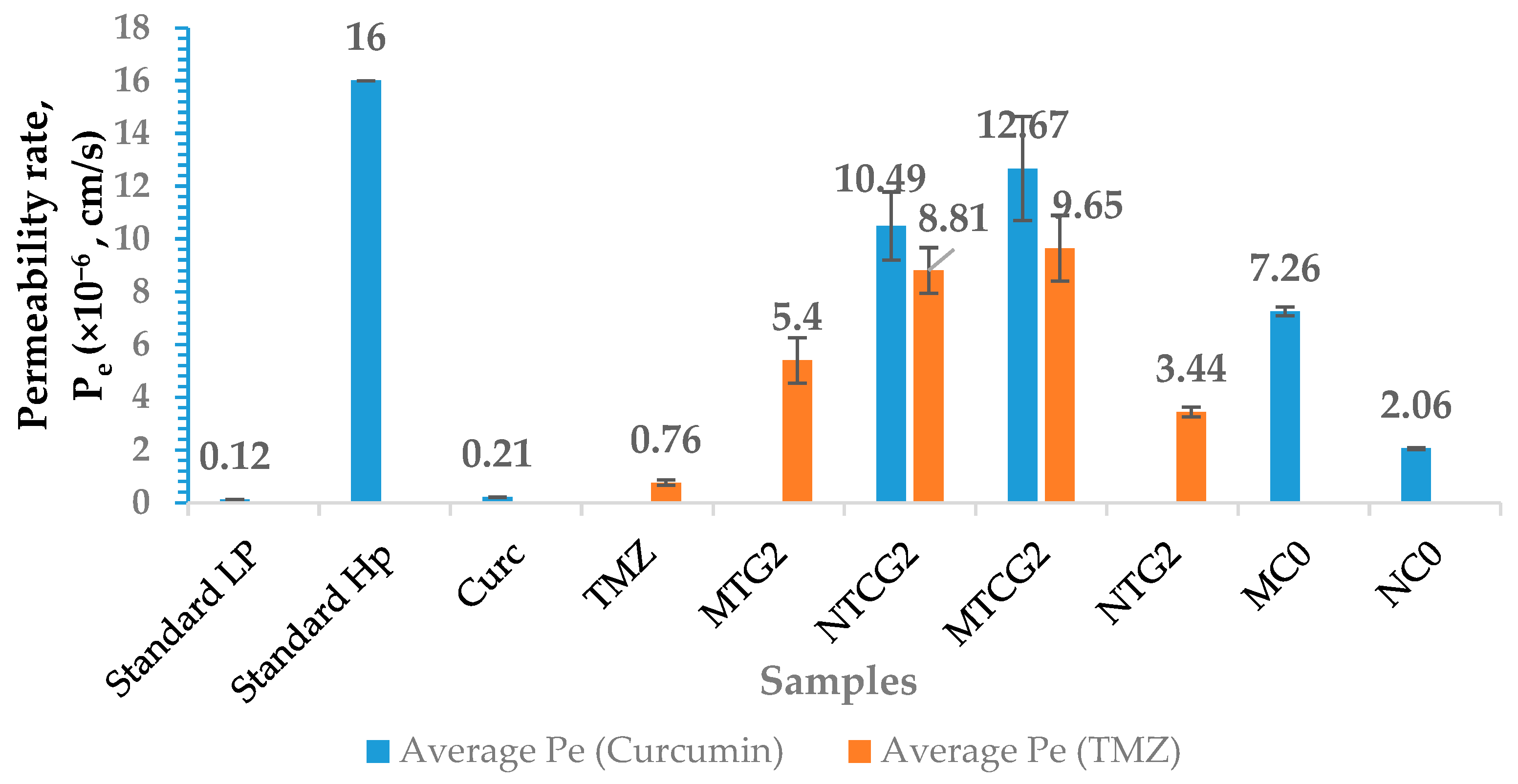

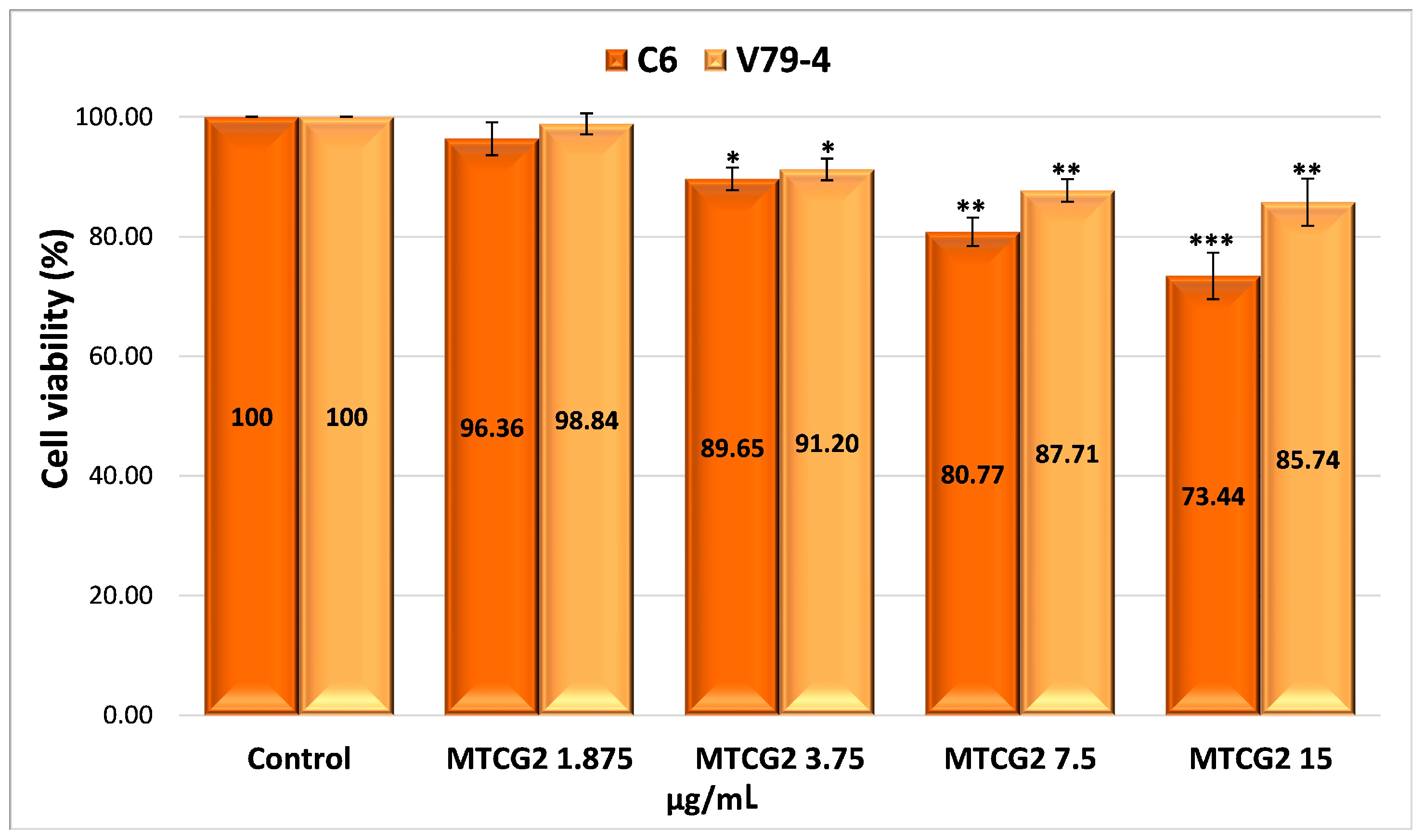
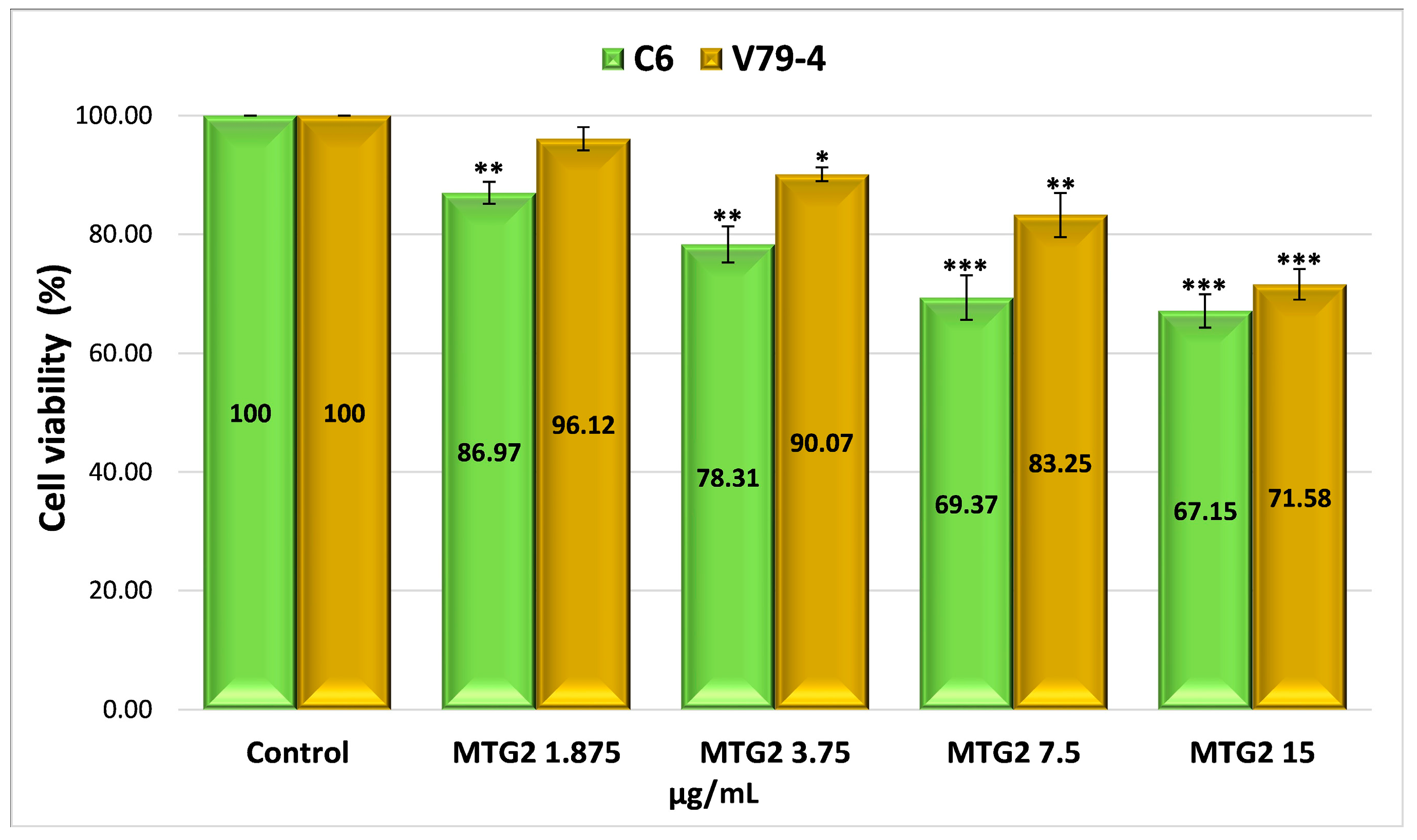
| Sample | Number of Moles of NH2 Groups × 10−4/g Protein |
|---|---|
| Protamine | 5.27 ± 1.22 |
| Fraction 4 | 8.31 ± 1.58 |
| Fraction 5 | 11.19 ± 3.47 |
| Fraction 6 | 13.91 ± 3.47 |
| Fraction 7 | 16.3 ± 2.46 |
| Fraction 8 | 18.75 ± 0.46 |
| Fraction 10 | 19.3 ± 0.56 |
| Fraction 11 | 23.57 ± 1.5 |
| Sample Code * | CHO/NH2 Ratio | HSA (mg) | OG (mg) | Drug(s) Encapsulated | Crosslinked with OG | Functionalized with LMWP |
|---|---|---|---|---|---|---|
| NT0 | 0 | 60 | 0 | TMZ | No | No |
| NCT0 | 0 | 0 | TMZ/curcumin | No | No | |
| NC0 | 0 | 0 | curcumin | No | No | |
| NG1 | 0.5/1 | 10 | No | Yes | No | |
| NG2 | 0.75/1 | 15 | No | Yes | No | |
| NG3 | 1/1 | 20 | No | Yes | No | |
| NTG1 | 0.5/1 | 10 | TMZ | Yes | No | |
| NTG2 | 0.75/1 | 15 | TMZ | Yes | No | |
| NTG3 | 01-Jan | 20 | TMZ | Yes | No | |
| NTCG1 | 0.5/1 | 10 | TMZ/curcumin | Yes | No | |
| NTCG2 | 0.75/1 | 15 | TMZ/curcumin | Yes | No | |
| NTCG3 | 1/1 | 20 | TMZ/curcumin | Yes | No | |
| NCG1 | 0.5/1 | 10 | curcumin | Yes | No | |
| NCG2 | 0.75/1 | 15 | curcumin | Yes | No | |
| NCG3 | 1/1 | 20 | curcumin | Yes | No | |
| MT0 | 0 | 0 | TMZ | No | Yes | |
| MCT0 | 0 | 0 | TMZ/curcumin | No | Yes | |
| MC0 | 0 | 0 | curcumin | No | Yes | |
| MG2 | 0.75/1 | 15 | No | Yes | Yes | |
| MTG2 | 0.75/1 | 15 | TMZ | Yes | Yes | |
| MTCG1 | 0.5/1 | 10 | TMZ/curcumin | Yes | Yes | |
| MTCG2 | 0.75/1 | 15 | TMZ/curcumin | Yes | Yes | |
| MTCG3 | 01-Jan | 20 | TMZ/curcumin | Yes | Yes | |
| MCG2 | 0.5/1 | 10 | curcumin | Yes | Yes |
| Sample | Average Diameter (nm) | SPAN |
|---|---|---|
| NT0 | 118 ± 1.5 | 0.44 ± 0.01 |
| NTC0 | 124.7 ± 2.9 | 0.35 ± 0.014 |
| NC0 | 83.7 ± 3.8 | 0.39 ± 0.02 |
| NTG1 | 119 ± 0.81 | 0.42 ± 0.009 |
| NTCG1 | 112 ± 1.0 | 0.41 ± 0.035 |
| NCG1 | 91.5 ± 0.5 | 0.45 ± 0.032 |
| NTG2 | 134.5 ± 11.3 | 0.36 ± 0.026 |
| NTCG2 | 143.3 ± 3.9 | 0.56 ± 0.07 |
| NCG2 | 122.7 ± 0.9 | 0.35 ± 0.003 |
| NTG3 | 145.3 ± 0.5 | 0.39 ± 0.008 |
| NTCG3 | 189.7 ± 8.3 | 0.38 ± 0.006 |
| NCG3 | 292.3 ± 0.5 | 0.26 ± 0.0004 |
| MT0 | 78 | 0.45 |
| MCT0 | 81 ± 1.2 | 0.31 ± 0.02 |
| MC0 | 96.7 ± 0.5 | 0.36 ± 0.003 |
| MTG2 | 67 | 0.42 ± 0.007 |
| MTCG2 | 79 ± 5.5 | 0.43 ± 0.03 |
| MCG2 | 104 | 0.375 |
| Sample | EF% TMZ | EF% CURC |
|---|---|---|
| NT0 | 18.6 ± 2.06 | 0 |
| NTG1 | 18.64 ± 1.1 | 0 |
| NTG2 | 17.44 ± 1.94 | 0 |
| NTG3 | 15.86 ± 0.93 | 0 |
| NTC0 | 28.07 ± 3.22 | 73.04 ± 1.24 |
| NTCG1 | 33.69 ± 0.78 | 94.68 ± 0.71 |
| NTCG2 | 24.29 ± 2.35 | 66.6 ± 5.48 |
| NTCG3 | 15.88 ± 0 | 48.71 ± 0.36 |
| NC0 | 0 | 92.35 ± 1.86 |
| NCG1 | 0 | 91.05 ± 0.45 |
| NCG3 | 0 | 34.11 ± 0.41 |
| MT0 | 14.74 ± 0.7 | 0 |
| MTG2 | 23.61 ± 0 | 0 |
| MC0 | 0 | 92.86 ± 0.4 |
| MCG2 | 0 | 88.92 ± 0 |
| MTC0 | 33.92 ± 3.01 | 69.73 ± 3.02 |
| MTCG2 | 31.29 ± 0.7 | 82.51 ± 7.8 |
| Sample | Release Efficiency, Ef% Curcumin | Release Efficiency, Ef% TMZ | Exponential Factor, n (Curcumin) | R2 (Curcumin) | Exponential Factor, n (TMZ) | R2 (TMZ) |
|---|---|---|---|---|---|---|
| NTCG1-4 | 22.76 ± 0.5 | 68.36 ± 1.9 | 0.25 | 0.8459 | 0.35 | 0.983 |
| NTCG2-4 | 20.24 ± 0.43 | 65.86 ± 2.6 | 0.26 | 0.8554 | 0.38 | 0.9505 |
| NTCG3-4 | 13.16 ± 0.5 | 57.71 ± 0.8 | 0.3 | 0.967 | 0.402 | 0.9781 |
| NTCG1-7.4 | 58.66 ± 3.46 | 63 ± 1.7 | 0.61 | 0.983 | 0.4135 | 0.973 |
| NTCG2-7.4 | 56.96 ± 0.5 | 61.76 ± 0.5 | 0.6 | 0.992 | 0.4746 | 0.9847 |
| NTCG3-7.4 | 36.5 ± 0.9 | 49.08 ± 1.4 | 0.76 | 0.9205 | 0.2837 | 0.9657 |
| MTCG1-4 | 59 ± 0.1 | 80.8 ± 0.6 | 1. | 0.851 | 0.8749 | 0.9324 |
| MTCG2-4 | 38.67 ± 0.5 | 66.7 ± 3.5 | 0.42 | 0.9647 | 0.7439 | 0.9282 |
| MTCG3-4 | 24.44 ± 0.5 | 61.99 ± 1.95 | 0.59 | 0.9774 | 0.6234 | 0.9876 |
| MTCG1-7.4 | 74.77 ± 0.4 | 72.23 ± 1.3 | 0.34 | 0.9784 | 0.253 | 0.9751 |
| MTCG2-7.4 | 60.38 ± 0.4 | 58.08 ± 0.3 | 0.31 | 0.9872 | 0.2597 | 0.9795 |
| MTCG3-7.4 | 63.45 ± 1.1 | 49.84 ± 0.9 | 0.34 | 0.9819 | 0.2819 | 0.9733 |
| Sample | IC50 μg/mL for TMZ on the C6 Cell Line | IC50 μg/mL for TMZ on the V79-4 Cell Line | IC50 for μg/mL for CURC on the C6 Cell Line | IC 50 μg/mL for CURC on the V79-4 Cell Line | IC50 μg/mL for TMZ/CURC on the C6 Cell Line | IC50 μg/mL for TMZ/CURC on the V79-4 Cell Line |
|---|---|---|---|---|---|---|
| MTCG2 | 1.13 | 2.24 | 1.8 | 3.58 | 2.93 | 5.81 |
| MTG2 | 0.51 | 0.53 | 0 | 0 | 0.51 | 0.53 |
| NTCG2 | 1.41 | 1.85 | 2.21 | 2.9 | 3.62 | 4.75 |
| TMZ | 8.75 | 16.84 | 0 | 0 | 8.75 | 16.84 |
| Curcumin | 0 | 0 | 6.31 | 10.36 | 6.31 | 10.36 |
| Sample | pH | Variation of Undegraded Curcumin (%) over Time | k | t1/2 (h) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 5 h | 8 h | ||||
| Free Curcumin | 3 | 100 | 76 | 55.7 | 47.6 | 0.091 | 7.7 |
| 6.8 | 100 | 76.4 | 56.8 | 43.7 | 0.107 | 6.5 | |
| 7.4 | 100 | 77 | 52.7 | 41.1 | 0.119 | 5.8 | |
| 9 | 100 | 61 | 21 | 8.6 | 0.301 | 2.3 | |
| MTCG2 | 3 | 100 | 95.8 | 65.2 | 53.8 | 0.0357 | 19.3 |
| 6.8 | 100 | 98.2 | 83.1 | 66.9 | 0.0213 | 32.5 | |
| 7.4 | 100 | 99.5 | 83 | 82.4 | 0.013 | 53.3 | |
| 9 | 100 | 93.87 | 64.8 | 58 | 0.0322 | 21.5 | |
| NTCG2 | 3 | 100 | 96.8 | 61.3 | 55.2 | 0.0363 | 19.1 |
| 6.8 | 100 | 91.8 | 77.4 | 66.6 | 0.0231 | 30 | |
| 7.4 | 100 | 97.5 | 81.2 | 75.9 | 0.0162 | 42.8 | |
| 9 | 100 | 99.5 | 72.8 | 59.7 | 0.0331 | 21.3 | |
| Sample | Percentage of Degradation for Free Versus Encapsulated Curcumin over Time, % | ||||
|---|---|---|---|---|---|
| Day 3 | Day 10 | Day 18 | Day 26 | Day 30 | |
| Curcumin | 6.8 | 13.5 | 16.9 | 26 | 32.3 |
| NC0 | 0.5 | 11.3 | 16.9 | 18.6 | 18.7 |
| MC0 | 3 | 7.3 | 8.7 | 10.4 | 10.7 |
| MTC0 | 6.2 | 9.9 | 12.5 | 19.7 | 19.8 |
| NTC0 | 5.4 | 3.2 | 9 | 12.4 | 17.5 |
| MTCG2 | 1 | 2.7 | 8.8 | 9.8 | 12.8 |
| NTCG2 | 0.8 | 7.2 | 12.3 | 15.3 | 16.3 |
| MTCG3 | 1.9 | 2.5 | 5.4 | 12.6 | 12.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iurciuc, C.E.; Vochița, G.; Gherghel, D.; Mihai, C.-T.; Vasiliu, S.; Racoviță, Ș.; Cadinoiu, A.N.; Logigan, C.L.; Hamcerencu, M.; Mitu, F.; et al. Development of an Innovative Nanosystem Based on Functionalized Albumin and Oxidized Gellan for the Synergistic Delivery of Curcumin and Temozolomide in the Treatment of Brain Cancer. Gels 2025, 11, 708. https://doi.org/10.3390/gels11090708
Iurciuc CE, Vochița G, Gherghel D, Mihai C-T, Vasiliu S, Racoviță Ș, Cadinoiu AN, Logigan CL, Hamcerencu M, Mitu F, et al. Development of an Innovative Nanosystem Based on Functionalized Albumin and Oxidized Gellan for the Synergistic Delivery of Curcumin and Temozolomide in the Treatment of Brain Cancer. Gels. 2025; 11(9):708. https://doi.org/10.3390/gels11090708
Chicago/Turabian StyleIurciuc (Tincu), Camelia Elena, Gabriela Vochița, Daniela Gherghel, Cosmin-Teodor Mihai, Silvia Vasiliu, Ștefania Racoviță, Anca Niculina Cadinoiu, Corina Lenuța Logigan, Mihaela Hamcerencu, Florin Mitu, and et al. 2025. "Development of an Innovative Nanosystem Based on Functionalized Albumin and Oxidized Gellan for the Synergistic Delivery of Curcumin and Temozolomide in the Treatment of Brain Cancer" Gels 11, no. 9: 708. https://doi.org/10.3390/gels11090708
APA StyleIurciuc, C. E., Vochița, G., Gherghel, D., Mihai, C.-T., Vasiliu, S., Racoviță, Ș., Cadinoiu, A. N., Logigan, C. L., Hamcerencu, M., Mitu, F., Popa, M., & Ochiuz, L. (2025). Development of an Innovative Nanosystem Based on Functionalized Albumin and Oxidized Gellan for the Synergistic Delivery of Curcumin and Temozolomide in the Treatment of Brain Cancer. Gels, 11(9), 708. https://doi.org/10.3390/gels11090708



.png)






