Inflammation-Responsive Hydrogels in Perioperative Pain and Wound Management: Design Strategies and Emerging Potential
Abstract
1. Introduction
2. Pathophysiology and Design Implications of Perioperative Inflammation
2.1. Inflammatory Cascade Triggered by Surgical Trauma
2.2. Spatiotemporal Dynamics of the Inflammatory Response
2.3. Interplay Between Inflammation, Nociception, and Tissue Regeneration
2.4. Biomarkers as Triggers for Responsive Drug Release
- pH shifts: Local acidosis, typically driven by hypoxia and lactic acid accumulation, can lower the extracellular pH to approximately 6.5 during the acute inflammatory phase [45]. Hydrogels incorporating acid-labile linkers, such as orthoesters, imines, and acetals, can be engineered to swell, degrade, or release therapeutic agents selectively under mildly acidic conditions, thereby enabling rapid and localized drug delivery [46].
- ROS: Neutrophil-derived ROS, including hydrogen peroxide and superoxide, are elevated in inflamed and ischemic tissues following surgical injury [47]. Hydrogels containing ROS-sensitive moieties such as thioketal or boronic ester groups can undergo cleavage or structural transitions in response to oxidative stress, allowing either burst or sustained drug release [19].
- Proteolytic enzymes: Proteases such as MMP-2, MMP-9, and NE are commonly upregulated in inflamed surgical wounds [32]. Peptide-based crosslinkers designed to be cleaved by these enzymes enable site-specific degradation of hydrogels, facilitating targeted therapeutic release at protease-rich sites [48].
- Cytokine gradients: Although more challenging to directly exploit, inflammatory cytokines such as IL-1β and TNF-α can inform the development of feedback-controlled delivery systems. Aptamers are short, single-stranded nucleic acids that bind specific molecular targets with high affinity and selectivity. When incorporated into a hydrogel matrix, these aptamer-functionalized systems can undergo conformational or charge-based changes upon binding their target cytokine, triggering structural transitions or payload release. Alternatively, nanoparticle-integrated hydrogel matrices can be engineered to sense cytokine concentrations through particle surface chemistry or embedded biosensing elements, enabling dynamic modulation of drug release in response to inflammatory status [49,50].
3. Design Principles of Inflammation-Responsive Hydrogels
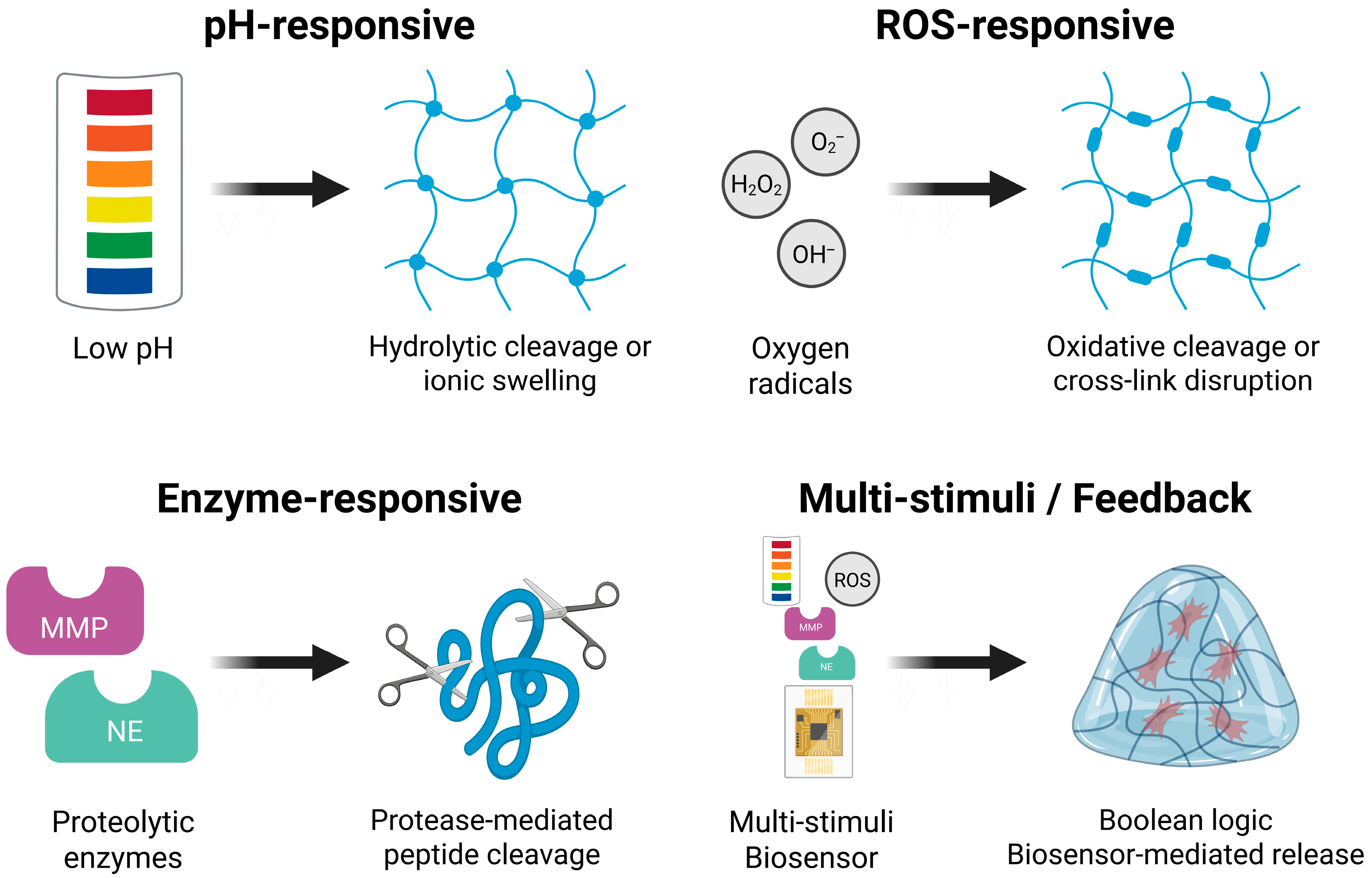
3.1. pH-Responsive Hydrogels
- Schiff base (imine) bonds: Formed through aldehyde–amine condensation, these bonds are relatively stable at physiological pH, but hydrolyze rapidly under acidic conditions [57].
- Poly(acrylic acid) (PAA) derivatives: These polymers undergo pH-dependent ionization of the carboxyl groups, enabling reversible swelling through protonation and deprotonation [58].
- A mesoporous glass–hydroxyapatite scaffold incorporating orthoester linkers achieved pH-triggered levofloxacin release under acidic conditions (pH 5.5–6.7), enhancing bacterial clearance while preserving osteoblast viability [60]. Although developed for bone infections, this platform can be adapted for localized anti-inflammatory delivery in acidified surgical wounds.
- A self-healing polyethylene glycol (PEG)-based hydrogel, crosslinked via imine bonds between aldehyde- and amine-functionalized poly(ether urethane)s, remained stable at pH 7.4, but was hydrolyzed in acidic environments. It supported sustained drug release for up to 17 days and was injectable through a 21 G needle, suggesting promise for minimally invasive perioperative applications in spatially and temporally confined inflammation [61].
- A semi-interpenetrating κ-carrageenan–PAA hydrogel, originally developed for gastrointestinal diclofenac delivery, exhibited pH-dependent diffusion (~80% at pH 7.4 vs. ~40% at pH 1.2) [62]. This behavior may be repurposed to match drug availability with wound pH normalization during postoperative recovery.
3.2. ROS-Responsive Hydrogels
- Thioketal linkages (–S–C–S–): These linkages are selectively cleaved in the presence of ROS, resulting in hydrogel disassembly and controlled drug release [65].
- Boronic esters or acids: These groups react specifically with hydrogen peroxide to form phenols and boric acid, triggering gel degradation or drug release [66].
- Selenium-containing moieties (e.g., diselenide bonds): Upon oxidation, these are converted to selenoxide derivatives, modulating the crosslink density and often imparting intrinsic antioxidant activity [67].
- A PEG hydrogel crosslinked with thioketal linkages was developed for the ROS-responsive delivery of epidermal growth factor. Upon ROS exposure, thioketal cleavage accelerated growth factor release, leading to enhanced angiogenesis, reduced oxidative stress, and improved re-epithelialization in a full-thickness wound model [68]. Compared with non-responsive controls, this system significantly improved wound closure, demonstrating direct applicability to surgical incisions with oxidative injury.
- A dual-responsive gelatin–poly(vinyl alcohol) (PVA) hydrogel incorporating boronic ester crosslinks and pH-sensitive micelles enabled the sequential release of vancomycin–silver nanoclusters and nimesulide. ROS triggered rapid antimicrobial delivery, while acidic pH sustained anti-inflammatory release. In a diabetic infected wound model, this system outperformed commercial dressings and single-drug controls in bacterial clearance, cytokine suppression, and hemostasis [69]. Although developed for chronic wounds, its dual-triggered logic and infection-modulating capacity are relevant in perioperative settings, particularly at contaminated or high-risk surgical sites.
- A selenium-based polyurethane hydrogel incorporating diselenide crosslinkers responded to oxidative stress by forming selenoxide, softening the matrix, and delivering antioxidant effects. In a rat myocardial infarction model, this system reduced fibrosis, suppressed inflammatory cytokines, and improved cardiac function relative to non-selenium controls [70]. Its redox-adaptive behavior is especially pertinent to cardiovascular and thoracic surgeries that involve localized oxidative injury.
3.3. Enzyme-Responsive Hydrogels
- MMP-cleavable linkers (e.g., GPLGIAGQ, CVPLSLYSG) integrated into PEG-based matrices to enable enzyme-responsive degradation and drug release [72].
- NE-sensitive motifs (e.g., AAPV) for targeted activation in neutrophil-rich tissues, especially during early postoperative inflammation [73].
- Microneedle and nanoparticle–hydrogel hybrids that combine physical precision with enzyme-triggered delivery, supporting minimally invasive surgical applications [74].
- A tetra-PEG hydrogel crosslinked with GPLGIAGQ peptides was used for the MMP-2-responsive delivery of phosphatidylserine. This system promoted M2 macrophage polarization, suppressed IL-1β and TNF-α expression, and enhanced bone regeneration in a rat calvarial defect model. Compared with drug-free and non-responsive controls, the hydrogel showed superior immunomodulatory effects, supporting its relevance for surgical tissue repair [75].
- A PEG hydrogel incorporating CVPLSLYSG linkers was engineered for MMP-2/9-responsive delivery of docetaxel-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles. Upon enzyme exposure, the matrix degraded, releasing nanoparticles that achieved over five-fold higher cytotoxicity against glioma cells compared to free docetaxel, and over 20-fold compared to non-degradable controls [76]. Although developed for post-resection chemotherapy, this design underscores the spatial precision achievable in surgical oncology.
- An NE-responsive RADA16-I hydrogel functionalized with AAPV-cleavable sequences and regenerative peptides (GHK, KGHK, and RDKVYR) released bioactive cues upon NE exposure. In a murine full-thickness wound model, the system accelerated re-epithelialization, enhanced fibroblast activity, and promoted collagen deposition, demonstrating its efficacy in protease-rich wound environments [77].
- A microneedle patch composed of gelatin methacryloyl (GelMA) and ε-poly-L-lysine loaded with curcumin nanoparticles was developed for MMP-triggered degradation. In infected wound models, the patch enhanced closure, suppressed TNF-α, and upregulated IL-10 and vascular endothelial growth factor (VEGF) expression [78].
3.4. Multi-Stimuli and Feedback-Controlled Systems
4. Anti-Inflammatory and Analgesic Payloads for Hydrogel Delivery
4.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
4.2. Corticosteroids
4.3. α2-Adrenergic Agonists
4.4. Biologics and Regenerative Factors
4.5. Dual and Synergistic Payload Systems
5. Preclinical Applications in Perioperative Models
5.1. Soft Tissue Surgery
5.2. Orthopedic Surgery
5.3. Thoracic and Abdominal Surgery
5.4. Comparative Delivery Strategies in Preclinical Perioperative Models
6. Translational Outlook and Future Directions: From Proof-of-Concept to Surgical Integration
6.1. Translational Gaps and Preclinical Readiness
- Trigger fidelity—specificity and sensitivity to pathological pH, ROS, or protease thresholds.
- Release kinetics—under both simulated and in vivo perioperative inflammatory conditions.
- Mechanical stability and degradation—including timeline and identification of degradation byproducts.
- Inflammatory biomarker modulation—e.g., TNF-α, IL-6 at defined postoperative timepoints.
- Therapeutic window—duration of analgesic or anti-inflammatory effects compared with current clinical standards.
- Functional endpoints—standardized nociceptive assays, mobility scores, or tissue regeneration quality.
- Safety—assessment of local tissue compatibility and systemic toxicity.
6.2. Regulatory Compatibility and Surgical Integration
6.3. Key Barriers: Biocompatibility and Responsiveness
6.4. Next-Generation Strategies for Precision Translation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSAID | Non-steroidal anti-inflammatory drug |
| ROS | Reactive oxygen species |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| NE | Neutrophil elastase |
| MMP | Matrix metalloproteinase |
| ECM | Extracellular matrix |
| COX | Cyclooxygenase |
| PAA | Poly(acrylic acid) |
| PEG | Polyethylene glycol |
| PVA | Poly(vinyl alcohol) |
| PLGA | Poly(lactic-co-glycolic acid) |
| GelMA | Gelatin methacryloyl |
| VEGF | Vascular endothelial growth factor |
| OCP | Octacalcium phosphate |
| CPBA | Carboxyphenylboronic acid |
| CS | Chondroitin sulfate |
| CMCS | Carboxymethyl chitosan |
| HA | Hyaluronic Acid |
| BMP | Bone morphogenetic protein |
| AI | Artificial intelligence |
| FDA | Food and Drug Administration |
| ISO | International Organization for Standardization |
| GMP | Good Manufacturing Practice |
References
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Bain, C.R.; Myles, P.S.; Martin, C.; Wallace, S.; Shulman, M.A.; Corcoran, T.; Bellomo, R.; Peyton, P.; Story, D.A.; Leslie, K.; et al. Postoperative systemic inflammation after major abdominal surgery: Patient-centred outcomes. Anaesthesia 2023, 78, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Maisat, W.; Yuki, K. Narrative review of systemic inflammatory response mechanisms in cardiac surgery and immunomodulatory role of anesthetic agents. Ann. Card. Anaesth. 2023, 26, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Tedesco, G.; Sartori, M.; Contartese, D.; Asunis, E.; Cini, C.; Veronesi, F.; Martikos, K.; Fini, M.; Giavaresi, G.; et al. Clinical Outcomes and Inflammatory Response to the Enhanced Recovery After Surgery (ERAS) Protocol in Adolescent Idiopathic Scoliosis Surgery: An Observational Study. Int. J. Mol. Sci. 2025, 26, 3723. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Komatsu, R.; Singleton, M.D.; Dinges, E.M.; Wu, J.; Bollag, L.A. Association between perioperative non-steroidal anti-inflammatory drug use and cardiovascular complications after non-cardiac surgery in older adult patients. JA Clin. Rep. 2024, 10, 29. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- Al-Saeed, A. Gastrointestinal and Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs. Oman Med. J. 2011, 26, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Shanthanna, H.; Ladha, K.S.; Kehlet, H.; Joshi, G.P. Perioperative Opioid Administration. Anesthesiology 2021, 134, 645–659. [Google Scholar] [CrossRef]
- Rose, L.F.; Chan, R.K. The Burn Wound Microenvironment. Adv. Wound Care 2016, 5, 106–118. [Google Scholar] [CrossRef]
- Capobianco, A.; Cottone, L.; Monno, A.; Manfredi, A.A.; Rovere-Querini, P. The peritoneum: Healing, immunity, and diseases. J. Pathol. 2017, 243, 137–147. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Sig. Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Patroklou, G.; Triantafyllopoulou, E.; Goula, P.-E.; Karali, V.; Chountoulesi, M.; Valsami, G.; Pispas, S.; Pippa, N. pH-Responsive Hydrogels: Recent Advances in Pharmaceutical Applications. Polymers 2025, 17, 1451. [Google Scholar] [CrossRef]
- Liu, J.; Jia, B.; Li, Z.; Li, W. Reactive oxygen species-responsive polymer drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1115603. [Google Scholar] [CrossRef] [PubMed]
- Noddeland, H.K.; Lind, M.; Jensen, L.B.; Petersson, K.; Skak-Nielsen, T.; Larsen, F.H.; Malmsten, M.; Heinz, A. Design and characterization of matrix metalloproteinase-responsive hydrogels for the treatment of inflammatory skin diseases. Acta Biomater. 2023, 157, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Pandey, V.; Pandey, T. A Mechanistic Understanding of Reactive Oxygen Species (ROS)-Responsive Bio-Polymeric Nanoparticles: Current State, Challenges and Future Toward Precision Therapeutics. Biopolymers 2025, 116, e70027. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, C.; Wang, Y.; Gu, Q.; Shao, Y.; Jin, A.; Zhang, X.; Lei, L.; Li, Y. Stimulus-responsive cellulose hydrogels in biomedical applications and challenges. Mater. Today Bio 2025, 32, 101814. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Jurczak, K.M.; Van Der Boon, T.A.B.; Devia-Rodriguez, R.; Schuurmann, R.C.L.; Sjollema, J.; Van Huizen, L.; De Vries, J.P.P.M.; Van Rijn, P. Recent regulatory developments in EU Medical Device Regulation and their impact on biomaterials translation. Bioeng. Transl. Med. 2025, 10, e10721. [Google Scholar] [CrossRef]
- Reis, M.E.; Bettencourt, A.; Ribeiro, H.M. The regulatory challenges of innovative customized combination products. Front. Med. 2022, 9, 821094. [Google Scholar] [CrossRef]
- Tettey, F.; Parupelli, S.K.; Desai, S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. Biomed. Mater. Devices 2024, 2, 316–341. [Google Scholar] [CrossRef]
- Muire, P.J.; Mangum, L.H.; Wenke, J.C. Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds. Front. Immunol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Caballero-Herrero, M.J.; Jumilla, E.; Buitrago-Ruiz, M.; Valero-Navarro, G.; Cuevas, S. Role of Damage-Associated Molecular Patterns (DAMPS) in the Postoperative Period after Colorectal Surgery. Int. J. Mol. Sci. 2023, 24, 3862. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, J.; Cai, X.; Liou, Y.-C.; Shen, H.-M.; Hao, J.; Huang, C.; Luo, G.; He, W. Macrophage plasticity: Signaling pathways, tissue repair, and regeneration. MedComm 2024, 5, e658. [Google Scholar] [CrossRef]
- Paruk, F.; Chausse, J.M. Monitoring the post surgery inflammatory host response. J. Emerg. Crit. Care Med. 2019, 3, 47. [Google Scholar] [CrossRef]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Nauta, T.D.; Van Hinsbergh, V.W.M.; Koolwijk, P. Hypoxic Signaling During Tissue Repair and Regenerative Medicine. Int. J. Mol. Sci. 2014, 15, 19791–19815. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Chung, G.; Kim, Y.H.; Park, C.-K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.-M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q.; et al. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024, 15, 6820. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-scavenging materials for skin wound healing: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef]
- Liang, R.; Pan, R.; He, L.; Dai, Y.; Jiang, Y.; He, S.; Li, B.; Li, Y. Decellularized Extracellular Matrices for Skin Wound Treatment. Materials 2025, 18, 2752. [Google Scholar] [CrossRef] [PubMed]
- Tronci, G.; Yin, J.; Holmes, R.A.; Liang, H.; Russell, S.J.; Wood, D.J. Protease-sensitive atelocollagen hydrogels promote healing in a diabetic wound model. J. Mater. Chem. B 2016, 4, 7249–7258. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Kirschbaum, M.; Kong, S.C.; Gehmert, S.; Felthaus, O.; Küchler, S.; Sharpe, J.R.; Wöltje, K.; Weiß, K.T.; et al. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics 2014, 4, 721–735. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. pH-Responsive Drug-Delivery Systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Minehan, R.L.; Del Borgo, M.P. Controlled Release of Therapeutics From Enzyme-Responsive Biomaterials. Front. Biomater. Sci. 2022, 1, 916985. [Google Scholar] [CrossRef]
- Abune, L.; Davis, B.; Wang, Y. Aptamer-functionalized hydrogels: An emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1731. [Google Scholar] [CrossRef]
- Ryan, A.K.; Rahman, S.; Williams, R.M. Optical Aptamer-Based Cytokine Nanosensor Detects Macrophage Activation by Bacterial Toxins. ACS Sens. 2024, 9, 3697–3706. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Zeng, Y.; Lin, L.; Yu, H.; Zhang, S.; Yang, W. Hydrogel systems for spatiotemporal controlled delivery of immunomodulators: Engineering the tumor immune microenvironment for enhanced cancer immunotherapy. Front. Cell Dev. Biol. 2024, 12, 1514595. [Google Scholar] [CrossRef]
- Manshor, M.R.; Alli, Y.A.; Anuar, H.; Ejeromedoghene, O.; Omotola, E.O.; Suhr, J. 4D printing: Historical evolution, computational insights and emerging applications. Mater. Sci. Eng. B 2023, 295, 116567. [Google Scholar] [CrossRef]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef]
- Haller, H.L.; Sander, F.; Popp, D.; Rapp, M.; Hartmann, B.; Demircan, M.; Nischwitz, S.P.; Kamolz, L.P. Oxygen, pH, Lactate, and Metabolism-How Old Knowledge and New Insights Might Be Combined for New Wound Treatment. Medicina 2021, 57, 1190. [Google Scholar] [CrossRef]
- Gannimani, R.; Walvekar, P.; Naidu, V.R.; Aminabhavi, T.M.; Govender, T. Acetal containing polymers as pH-responsive nano-drug delivery systems. J. Control. Release 2020, 328, 736–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Yan, G.; Fu, S.; Tang, R. pH-sensitive nanogels with ortho ester linkages prepared via thiol-ene click chemistry for efficient intracellular drug release. J. Colloid. Interface Sci. 2017, 508, 282–290. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.-h. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, G.; Wu, T.; Li, P.; Zhan, P.; Liu, N.; Wu, Z. Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance. Gels 2025, 11, 241. [Google Scholar] [CrossRef]
- Zarur, M.; Seijo-Rabina, A.; Goyanes, A.; Concheiro, A.; Alvarez-Lorenzo, C. pH-responsive scaffolds for tissue regeneration: In vivo performance. Acta Biomater. 2023, 168, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, R.; Boffito, M.; Cassino, C.; Caccamo, V.; Chiono, V.; Ciardelli, G. Schiff-Base Cross-Linked Hydrogels Based on Properly Synthesized Poly(ether urethane)s as Potential Drug Delivery Vehicles in the Biomedical Field: Design and Characterization. ACS Omega 2024, 9, 45774–45788. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H. Controlled release of diclofenac sodium from pH-responsive carrageenan-g-poly (acrylic acid) superabsorbent hydrogel. J. Chem. Sci. 2010, 122, 651–659. [Google Scholar] [CrossRef]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound. J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Rinaldi, A.; Caraffi, R.; Grazioli, M.V.; Oddone, N.; Giardino, L.; Tosi, G.; Vandelli, M.A.; Calzà, L.; Ruozi, B.; Duskey, J.T. Applications of the ROS-Responsive Thioketal Linker for the Production of Smart Nanomedicines. Polymers 2022, 14, 687. [Google Scholar] [CrossRef]
- Terriac, L.; Helesbeux, J.-J.; Maugars, Y.; Guicheux, J.; Tibbitt, M.W.; Delplace, V. Boronate Ester Hydrogels for Biomedical Applications: Challenges and Opportunities. Chem. Mater. 2024, 36, 6674–6695. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef]
- An, Z.; Zhang, L.; Liu, Y.; Zhao, H.; Zhang, Y.; Cao, Y.; Zhang, Y.; Pei, R. Injectable thioketal-containing hydrogel dressing accelerates skin wound healing with the incorporation of reactive oxygen species scavenging and growth factor release. Biomater. Sci. 2021, 10, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, C.; Li, Y.; Li, Z.; Zhang, Z.; Xu, J.; Chen, M.; Li, R.; Liu, S.; Wu, Y.; et al. Injectable selenium-containing polymeric hydrogel formulation for effective treatment of myocardial infarction. Front. Bioeng. Biotechnol. 2022, 10, 912562. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, L.; Zhang, L.; Tai, Y.; Lin, X.; Cai, Z. Roles of MMP-2 and MMP-9 and their associated molecules in the pathogenesis of keloids: A comprehensive review. Front. Pharmacol. 2024, 15, 1444653. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Chen, D.; Li, Y.; Zhang, Q.; Su, J. Bone Regeneration Using MMP-Cleavable Peptides-Based Hydrogels. Gels 2021, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Song, Y.; Wang, R.; He, R.; Wang, T. Neutrophil elastase: From mechanisms to therapeutic potential. J. Pharm. Anal. 2023, 13, 355–366. [Google Scholar] [CrossRef]
- Qi, Z.; Yan, Z.; Tan, G.; Kundu, S.C.; Lu, S. Smart Responsive Microneedles for Controlled Drug Delivery. Molecules 2023, 28, 7411. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, T.; Li, J.; Yan, H.; Lyu, L.; Yu, Y.; Yang, G.; Zhang, T.; Zhou, Y.; Wang, X.; et al. Matrix Metalloproteinase-Responsive Hydrogel with On-Demand Release of Phosphatidylserine Promotes Bone Regeneration Through Immunomodulation. Adv. Sci. 2024, 11, 2306924. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.B.; Palange, A.; Schlich, M.; Bellotti, E.; Brahmachari, S.; Di Francesco, M.; Decuzzi, P. Matrix metalloproteinase responsive hydrogel microplates for programmed killing of invasive tumour cells. RSC Appl. Polym. 2023, 1, 19–29. [Google Scholar] [CrossRef]
- Dzierżyńska, M.; Sawicka, J.; Deptuła, M.; Sosnowski, P.; Sass, P.; Peplińska, B.; Pietralik-Molińska, Z.; Fularczyk, M.; Kasprzykowski, F.; Zieliński, J.; et al. Release systems based on self-assembling RADA16-I hydrogels with a signal sequence which improves wound healing processes. Sci. Rep. 2023, 13, 6273. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Ding, Y.; Huang, S.; Chen, S.; Huang, H.; Yang, S.; Xiao, F. Metalloproteinase-responsive gelatin/polylysine hydrogel microneedles for on-demand curcumin delivery in bacteria-infected wound healing. Colloids Surf. B Biointerfaces 2025, 254, 114797. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Maruf, D.S.A.A.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emerg. Mater. 2024, 8, 1339–1356. [Google Scholar] [CrossRef]
- Ruskowitz, E.R.; Comerford, M.P.; Badeau, B.A.; DeForest, C.A. Logical stimuli-triggered delivery of small molecules from hydrogel biomaterials. Biomater. Sci. 2019, 7, 542–546. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Kovrlija, I.; Locs, J.; Loca, D.; Gasik, M. Octacalcium Phosphate-Laden Hydrogels on 3D-Printed Titanium Biomaterials Improve Corrosion Resistance in Simulated Biological Media. Int. J. Mol. Sci. 2023, 24, 13135. [Google Scholar] [CrossRef]
- Bressler, E.M.; Adams, S.; Liu, R.; Colson, Y.L.; Wong, W.W.; Grinstaff, M.W. Boolean logic in synthetic biology and biomaterials: Towards living materials in mammalian cell therapeutics. Clin. Transl. Med. 2023, 13, e1244. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Fan, H.; Cui, J.; Wang, Y.; Wang, Z. ROS/pH Dual-Responsive Hydrogel Dressings Loaded with Amphiphilic Structured Nano Micelles for the Repair of Infected Wounds. Int. J. Nanomed. 2025, 20, 8119–8142. [Google Scholar] [CrossRef]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Roost, K.; Stuber, A.; Wei, K.; De Lapeyrière, M.; Yang, K.; Gantenbein, V.; Pané, S.; Corbeski, I.; Maniura-Weber, K.; Nakatsuka, N. Spatially Controlled 3-D Multiplexed Aptamer Patterning in Hydrogels. Adv. Mater. Interfaces 2025, 2400986. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Jiao, C.; Obst, F.; Geisler, M.; Che, Y.; Richter, A.; Appelhans, D.; Gaitzsch, J.; Voit, B. Reversible Protein Capture and Release by Redox-Responsive Hydrogel in Microfluidics. Polymers 2022, 14, 267. [Google Scholar] [CrossRef]
- Khadka, B.; Lee, B.; Kim, K.-T. Drug Delivery Systems for Personal Healthcare by Smart Wearable Patch System. Biomolecules 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.B.; Decuzzi, P. Harnessing Endogenous Stimuli for Responsive Materials in Theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; He, L.; Chen, F.; Fu, T.; Zhang, P.; Xiao, Z.; Liu, Y.; Tan, W. Stimulus-responsive nanomaterials containing logic gates for biomedical applications. Cell Rep. Phys. Sci. 2021, 2, 100350. [Google Scholar] [CrossRef]
- Cedillo-Servin, G.; Al-Jehani, E.A.A.; Rossy, T.; Teixeira, S.P.B.; Sage, F.; Domingues, R.M.A.; Raman, R.; Castilho, M. Meta-adaptive biomaterials: Multiscale, spatiotemporal organization and actuation in engineered tissues. Trends Biotechnol. 2025. [Google Scholar] [CrossRef]
- Kianian, S.; Bansal, J.; Lee, C.; Zhang, K.; Bergese, S.D. Perioperative multimodal analgesia: A review of efficacy and safety of the treatment options. Anesthesiol. Perioper. Sci. 2024, 2, 9. [Google Scholar] [CrossRef]
- Gade, L.; Boyd, B.J.; Malmsten, M.; Heinz, A. Stimuli-responsive drug delivery systems for inflammatory skin conditions. Acta Biomater. 2024, 187, 1–19. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, E.; Qian, X. A Narrative Review on Perioperative Pain Management Strategies in Enhanced Recovery Pathways-The Past, Present and Future. J. Clin. Med. 2021, 10, 2568. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Z.; Yang, S.; Li, T.; Wang, Y.; Shi, L.; Yuan, F.-l.; Dong, W. pH-responsive diclofenac sodium releasing adhesive hydrogel with wound hypoxia-relieving capacity for promoting wound repairing. Colloids Surf. A 2025, 716, 136679. [Google Scholar] [CrossRef]
- Otto, F.; Froelich, A. Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies. Gels 2024, 10, 435. [Google Scholar] [CrossRef]
- Mammella, A.; Bhavana, V.; Chary, P.S.; Anuradha, U.; Mehra, N.K. Modulation of chondroprotective hyaluronic acid and poloxamer gel with Ketoprofen loaded transethosomes: Quality by design-based optimization, characterization, and preclinical investigations in osteoarthritis. Int. J. Biol. Macromol. 2024, 280, 135919. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, C.; Xiong, Y.; Wang, Y.; Pu, C.; He, J.; Chen, L.; Jiang, K.; Zhao, W.; Yang, H.; et al. MMP13-responsive hydrogel microspheres for osteoarthritis treatment by precise delivery of celecoxib. Mater. Des. 2024, 241, 112966. [Google Scholar] [CrossRef]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef]
- Oshima, A.; Hatayama, K.; Terauchi, M.; Kakiage, H.; Hashimoto, S.; Chikuda, H. The comparison of dexamethasone and triamcinolone periarticular administration in total knee arthroplasty: Retrospective cohort study. BMC Musculoskelet. Disord. 2022, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiong, H.; Wang, S.Q.; Zhang, H.L.; Zheng, W.W.; Gou, Z.R.; Fan, C.Y.; Gao, C.Y. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis. Mater. Today Nano 2022, 17, 100164. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhao, A.; Chen, Z.; Liang, G.; Liu, H.; Wu, C. Weak acid-initiated slow release of Dexamethasone from hydrogel to treat orbital inflammation. Theranostics 2023, 13, 4030–4041. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Soeranaya, B.H.T.; Truong, T.H.A.; Dang, T.T. Modular design of a hybrid hydrogel for protease-triggered enhancement of drug delivery to regulate TNF-α production by pro-inflammatory macrophages. Acta Biomater. 2020, 117, 167–179. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, N.; He, T.; Shang, J.; Li, L.; Song, L.; Yang, X.; Li, X.; Luo, N.; Zhang, W.; et al. Thermosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci. Rep. 2015, 5, 13553. [Google Scholar] [CrossRef] [PubMed]
- Linderman, S.W.; Barber, G.F.; DeVeaux, S.A.; Botchwey, E.A.; Refai, D.; Klein, A.M.; García, A.J. Dexamethasone Delivery via Amphiphilic, Low-swelling Hydrogels Treats Postoperative Inflammation in Cervical Spine Applications. Adv. Healthc. Mater. 2025, 14, e2404292. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Kuttler, A.; Beckmann, N.; Nam, S.; Kent, D.; Schuleit, M.; Ramazani, F.; Accart, N.; Rock, A.; Li, J.; et al. Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity. Nat. Biomed. Eng. 2022, 6, 1167–1179. [Google Scholar] [CrossRef]
- Nakajima, N.; Hashimoto, S.; Sato, H.; Takahashi, K.; Nagoya, T.; Kamimura, K.; Tsuchiya, A.; Yokoyama, J.; Sato, Y.; Wakatsuki, H.; et al. Efficacy of gelatin hydrogels incorporating triamcinolone acetonide for prevention of fibrosis in a mouse model. Regen. Ther. 2019, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Sunkureddi, P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J. Clin. Rheumatol. 2013, 19, 19–29. [Google Scholar] [CrossRef]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Y.; Lv, J.; Dou, X.; Dai, M.; Sun, S.; Lin, Y. Effects of Dexmedetomidine on Immune Cells: A Narrative Review. Front. Pharmacol. 2022, 13, 829951. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Seon, K.; Kim, M.-Y.; Kim, S.W.; Yoo, Y.C. Effects of Perioperative Dexmedetomidine on Immunomodulation in Uterine Cancer Surgery: A Randomized, Controlled Trial. Front. Oncol. 2021, 11, 749003. [Google Scholar] [CrossRef]
- Flanders, C.A.; Rocke, A.S.; Edwardson, S.A.; Baillie, J.K.; Walsh, T.S. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: A systematic review of animal and human studies. Crit. Care. 2019, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Sun, S.; Li, Y.; Dou, X.; Dai, M.; Wu, Y.; Lin, Y. Efficacy and safety evaluation of dexmedetomidine for postoperative patient controlled intravenous analgesia: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1028704. [Google Scholar] [CrossRef]
- Wang, K.; Wu, M.; Xu, J.; Wu, C.; Zhang, B.; Wang, G.; Ma, D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br. J. Anaesth. 2019, 123, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Che, J.-X.; Chen, L.; Song, T.-T.; Qu, J.-T. Effect of Dexmedetomidine on Intraoperative Hemodynamics and Blood Loss in Patients Undergoing Spine Surgery: A Systematic Review and Meta-Analysis. Chin. Med. Sci. J. 2024, 39, 54–68. [Google Scholar] [CrossRef]
- Grest, A.; Kurmann, J.; Müller, M.; Jeger, V.; Krüger, B.; Spahn, D.R.; Bettex, D.; Rudiger, A. Cardiovascular Safety of Clonidine and Dexmedetomidine in Critically Ill Patients after Cardiac Surgery. Crit. Care Res. Pract. 2020, 2020, 4750615. [Google Scholar] [CrossRef]
- Allam, A.A.; Eleraky, N.E.; Diab, N.H.; Elsabahy, M.; Mohamed, S.A.; Abdel-Ghaffar, H.S.; Hassan, N.A.; Shouman, S.A.; Omran, M.M.; Hassan, S.B.; et al. Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations. Pharmaceutics 2022, 14, 220. [Google Scholar] [CrossRef]
- Chen, S.; Yao, W.; Wang, H.; Wang, T.; Xiao, X.; Sun, G.; Yang, J.; Guan, Y.; Zhang, Z.; Xia, Z.; et al. Injectable electrospun fiber-hydrogel composite sequentially releasing clonidine and ropivacaine for prolonged and walking regional analgesia. Theranostics 2022, 12, 4904–4921. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Fan, X.; Zhang, T.; Qiu, R.; Wu, Y.; Wang, M.; Zhang, Y.; Li, M.; Cai, N.; et al. Advances in rationally engineered cytokines for precision therapy in diabetic wound healing. Explor. Immunol. 2024, 4, 533–553. [Google Scholar] [CrossRef]
- Gutowski, S.M.; Shoemaker, J.T.; Templeman, K.L.; Wei, Y.; Latour, R.A., Jr.; Bellamkonda, R.V.; LaPlaca, M.C.; García, A.J. Protease-degradable PEG-maleimide coating with on-demand release of IL-1Ra to improve tissue response to neural electrodes. Biomaterials 2015, 44, 55–70. [Google Scholar] [CrossRef]
- Robert, M.-C.; Frenette, M.; Zhou, C.; Yan, Y.; Chodosh, J.; Jakobiec, F.A.; Stagner, A.M.; Vavvas, D.; Dohlman, C.H.; Paschalis, E.I. A Drug Delivery System for Administration of Anti–TNF-α Antibody. Transl. Vis. Sci. Technol. 2016, 5, 11. [Google Scholar] [CrossRef]
- Xi, L.; Wang, L.; Zhang, M.; He, C.; Yang, X.; Pang, Y.; Chen, H.; Cheng, F. TNF-R1 Cellular Nanovesicles Loaded on the Thermosensitive F-127 Hydrogel Enhance the Repair of Scalded Skin. ACS Biomater. Sci. Eng. 2023, 9, 5843–5854. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, H.; Chen, J.; Chen, G.; Yu, X.; Ye, Z. BMP-2 releasing mineral-coated microparticle-integrated hydrogel system for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1217335. [Google Scholar] [CrossRef] [PubMed]
- García, J.R.; Clark, A.Y.; García, A.J. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J. Biomed. Mater. Res. A 2016, 104, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S.; Lee, J.M.; Jo, M.J.; Kang, S.J.; Yoo, M.K.; Park, S.Y.; Bong, S.; Park, C.-S.; Park, C.-W.; Kim, J.-S.; et al. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels 2025, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Xue, Y.; Jin, J.; Xu, Y.; Zeng, W.; Liu, J.; Xie, J. Injectable Hydrogel Delivery System with High Drug Loading for Prolonging Local Anesthesia. Adv. Sci. 2024, 11, 2309482. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Boyd, B.J.; Hill, A.; Sharma, M. Injectable thermoresponsive gels offer sustained dual release of bupivacaine hydrochloride and ketorolac tromethamine for up to two weeks. Int. J. Pharm. 2021, 604, 120748. [Google Scholar] [CrossRef]
- Shamszadeh, S.; Asgary, S.; Akrami, M.; Mashhadiabbas, F.; Akbarzadeh Baghban, A.; Shams, F. Development of a Thermoresponsive Core–Shell Hydrogel for Sequential Delivery of Antibiotics and Growth Factors in Regenerative Endodontics. Front. Biosci. 2024, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain-from mechanisms to treatment. Pain. Rep. 2017, 2, e588. [Google Scholar] [CrossRef]
- Ji, Q.; Chen, K.; Yi, H.; He, B.; Jiang, T. A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing. Gels 2024, 10, 621. [Google Scholar] [CrossRef]
- Garcia, P.; Histing, T.; Holstein, J.H.; Klein, M.; Laschke, M.W.; Matthys, R.; Ignatius, A.; Wildemann, B.; Lienau, J.; Peters, A.; et al. Rodent animal models of delayed bone healing and non-union formation: A comprehensive review. Eur. Cell. Mater. 2013, 26, 1–12, discussion 12–14. [Google Scholar] [CrossRef]
- Huang, Q.; Qu, Y.; Tang, M.; Lan, K.; Zhang, Y.; Chen, S.; Li, W.; Gu, L. ROS-responsive hydrogel for bone regeneration: Controlled dimethyl fumarate release to reduce inflammation and enhance osteogenesis. Acta Biomater. 2025, 195, 183–200. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, K.; Wan, G.; Liao, W.; Jin, J.; Wang, P.; Sun, X.; Wang, W.; Jiang, Q. A ROS-responsive hydrogel encapsulated with matrix metalloproteinase-13 siRNA nanocarriers to attenuate osteoarthritis progression. J. Nanobiotechnol. 2025, 23, 18. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, Q.; Zhang, W.; Wang, H.; Zhang, W.; Li, Z.; Ji, M.; You, Y.; Lu, J. Injectable Piezoelectric Hydrogel Promotes Tendon–Bone Healing via Reshaping the Electrophysiological Microenvironment and M2 Macrophage Polarization. ACS Appl. Mater. Interfaces 2025, 17, 22210–22231. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Lu, B.; Mei, J.; Xu, L.; Zhang, X.; Su, Z.; Xu, W.; Fang, S.; Zhu, C.; et al. Inflammation-Responsive Hydrogel Spray for Synergistic Prevention of Traumatic Heterotopic Ossification via Dual-Homeostatic Modulation Strategy. Adv. Sci. 2023, 10, 2302905. [Google Scholar] [CrossRef]
- Wang, J.C.; Strichartz, G.R. Prevention of Chronic Post-Thoracotomy Pain in Rats By Intrathecal Resolvin D1 and D2: Effectiveness of Perioperative and Delayed Drug Delivery. J. Pain 2017, 18, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Vediappan, R.S.; Bennett, C.; Bassiouni, A.; Smith, M.; Finnie, J.; Trochsler, M.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. A Novel Rat Model to Test Intra-Abdominal Anti-adhesive Therapy. Front. Surg. 2020, 7, 12. [Google Scholar] [CrossRef]
- Åkerberg, D.; Posaric-Bauden, M.; Isaksson, K.; Andersson, R.; Tingstedt, B. Prevention of Pleural Adhesions by Bioactive Polypeptides—A Pilot Study. Int. J. Med. Sci. 2013, 10, 1720–1726. [Google Scholar] [CrossRef][Green Version]
- Luo, Q.; Cheng, N.; Yang, Y.; Shao, N.; Nie, T.; Chen, J.; Huang, C.; Zhang, S.; Huang, Y.; Ieong, C.M.; et al. Multi-stage cooperative ROS-responsive hydrogel platform for drug delivery in myocardial ischemia-reperfusion injury repair. Mater. Today Bio 2025, 32, 101854. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Liu, W.; Li, W.; Huang, Y.; Jin, Q.; Zhang, L.; Jiang, Y.; Luo, Z. Highly-stable, injectable, conductive hydrogel for chronic neuromodulation. Nat. Commun. 2024, 15, 7993. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, X.; Gong, Y.; Ren, L.; Luo, Y.; Sun, Y.; Chen, M.; Jiang, J.; Guan, Z.; Zhao, C. ROS-responsive sprayable hydrogel as ROS scavenger and GATA6+ macrophages trap for the prevention of postoperative abdominal adhesions. J. Control. Release. 2024, 369, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, Y.; Gong, Y.; Shi, X.; Xiao, D.; Ren, L.; Dai, X.; Zeng, Z.; Zhao, C. A ROS-responsive and scavenging hydrogel for postoperative abdominal adhesion prevention. Acta Biomater. 2024, 184, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, T.; Wang, K.; Chang, B.; Fu, D.; Chen, X. Postoperative Multimodal Analgesia Strategy for Enhanced Recovery After Surgery in Elderly Colorectal Cancer Patients. Pain Ther. 2024, 13, 745–766. [Google Scholar] [CrossRef]
- Brigham, N.C.; Ji, R.-R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 1367. [Google Scholar] [CrossRef]
- Chen, P.C.; Kohane, D.S.; Park, Y.J.; Bartlett, R.H.; Langer, R.; Yang, V.C. Injectable microparticle-gel system for prolonged and localized lidocaine release. II. In vivo anesthetic effects. J. Biomed. Mater. Res. A 2004, 70A, 459–466. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Ning, C.; Li, M.; Zhao, G.; Jiang, W.; Ding, J.; Chen, X. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials 2018, 181, 378–391. [Google Scholar] [CrossRef]
- Alka; Singh, P.; Pal, R.R.; Mishra, N.; Singh, N.; Verma, A.; Saraf, S.A. Development of pH-Sensitive hydrogel for advanced wound Healing: Graft copolymerization of locust bean gum with acrylamide and acrylic acid. Int. J. Pharm. 2024, 661, 124450. [Google Scholar] [CrossRef]
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.; Riegel, L.; He, X.; et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 2018, 9, 1275. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Ning, F.; Du, C.; Chen, M.; Feng, C.; Dong, C.-M. Dynamic Hyaluronic Acid Hydrogels for Comprehensively Regulating Inflammation, Angiogenesis, and Metabolism to Effectively Proheal Diabetic Wounds. ACS Appl. Mater. Interfaces 2024, 16, 70256–70273. [Google Scholar] [CrossRef] [PubMed]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Q.; Zhou, J.; Li, Y.; Zheng, Y.; Chen, L. A reactive oxygen species-responsive hydrogel loaded with Apelin-13 promotes the repair of spinal cord injury by regulating macrophage M1/M2 polarization and neuroinflammation. J. Nanobiotechnol. 2025, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Wang, T. Reactive oxygen species (ROS)-responsive biomaterials for treating myocardial ischemia-reperfusion injury. Front. Bioeng. Biotechnol. 2024, 12, 1469393. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; He, C.; Zhang, Z.; Yu, J. Stimulus-responsive hydrogels for diabetic wound management via microenvironment modulation. Biomater. Sci. 2025, 13, 3192–3212. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.T.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and clinical application of injectable hydrogels for musculoskeletal therapy. Bioeng. Transl. Med. 2022, 7, e10295. [Google Scholar] [CrossRef]
- Raeisi, A.; Farjadian, F. Commercial hydrogel product for drug delivery based on route of administration. Front. Chem. 2024, 12, 1336717. [Google Scholar] [CrossRef]
- Xie, B.; Xie, H. Application of stimuli-responsive hydrogel in brain disease treatment. Front. Bioeng. Biotechnol. 2024, 12, 1450267. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Dan, Y.; Shao, Z.; Yang, S.; Yang, C.; Liu, G.; Duan, D. MMP-sensitive PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC-mediated angiogenesis. Exp. Ther. Med. 2019, 18, 2933–2941. [Google Scholar] [CrossRef]
- Liang, H.; Russell, S.J.; Wood, D.J.; Tronci, G. A hydroxamic acid–methacrylated collagen conjugate for the modulation of inflammation-related MMP upregulation. J. Mater. Chem. B 2018, 6, 3703–3715. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Y.; Ju, Y.; Yang, P.; Shen, N.; Yang, A.; Wu, R.; Fang, B.; Liu, L. Immunomodulatory hydrogels for tissue repair and regeneration. APL Mater. 2024, 12, 080603. [Google Scholar] [CrossRef]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Pellegrini, V.; La Grotta, R.; Carreras, F.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; Berra, C.C.; Ceriello, A.; Prattichizzo, F. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells 2024, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Xiong, D.; Geng, H.; Lv, X.; Wang, S.; Jia, L. Inflammatory Response and Anti-Inflammatory Treatment in Persistent Inflammation-Immunosuppression-Catabolism Syndrome (PICS). J. Inflamm. Res. 2025, 18, 2267–2281. [Google Scholar] [CrossRef]
- Elesber, A.A.; Best, P.J.; Lennon, R.J.; Mathew, V.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Plasma 8-iso-prostaglandin F2alpha, a marker of oxidative stress, is increased in patients with acute myocardial infarction. Free Radic. Res. 2006, 40, 385–391. [Google Scholar] [CrossRef]
- van der Plas, A.; Pouly, S.; de La Bourdonnaye, G.; Baker, G.; Lüdicke, F. Influence of smoking on levels of urinary 8-iso Prostaglandin F2α. Toxicol. Rep. 2019, 6, 18–25. [Google Scholar] [CrossRef]
- Tayler, I.M.; Stowers, R.S. Engineering hydrogels for personalized disease modeling and regenerative medicine. Acta Biomater. 2021, 132, 4–22. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Mohseni-Motlagh, S.F.; Dolatabadi, R.; Baniassadi, M.; Baghani, M. Application of the Quality by Design Concept (QbD) in the Development of Hydrogel-Based Drug Delivery Systems. Polymers 2023, 15, 4407. [Google Scholar] [CrossRef] [PubMed]
- Pielenhofer, J.; Meiser, S.L.; Gogoll, K.; Ciciliani, A.-M.; Denny, M.; Klak, M.; Lang, B.M.; Staubach, P.; Grabbe, S.; Schild, H.; et al. Quality by Design (QbD) Approach for a Nanoparticulate Imiquimod Formulation as an Investigational Medicinal Product. Pharmaceutics 2023, 15, 514. [Google Scholar] [CrossRef]
- Parshad, B.; Arora, S.; Singh, B.; Pan, Y.; Tang, J.; Hu, Z.; Patra, H.K. Towards precision medicine using biochemically triggered cleavable conjugation. Commun. Chem. 2025, 8, 100. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Zhang, X.; Wang, X.; Xiong, M.; Luo, D. Stimuli-responsive linkers and their application in molecular imaging. Exploration 2024, 4, 20230027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, B.M.; Schwendeman, S.P. Characterization of the initial burst release of a model peptide from poly (d,l-lactide-co-glycolide) microspheres. J. Control. Release 2002, 82, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Griffin, J.I.; Inturi, S.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Moghimi, S.M.; Simberg, D. In Vitro and In Vivo Differences in Murine Third Complement Component (C3) Opsonization and Macrophage/Leukocyte Responses to Antibody-Functionalized Iron Oxide Nanoworms. Front. Immunol. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Kanďárová, H.; Pôbiš, P. The “Big Three” in biocompatibility testing of medical devices: Implementation of alternatives to animal experimentation-are we there yet? Front. Toxicol. 2023, 5, 1337468. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhao, Z.; Yang, L.; Lv, D.; Sun, R.; Zhang, T.; Li, Y.; Bao, Q.; Zhang, M.; Wang, L.; et al. Towards Intelligent Wound Care: Hydrogel-Based Wearable Monitoring and Therapeutic Platforms. Polymers 2025, 17, 1881. [Google Scholar] [CrossRef]
- Rothe, R.; Xu, Y.; Thomas, A.K.; Meister, S.; Zhang, Y.; Pietzsch, J.; Hauser, S. A modular, injectable, non-covalently assembled hydrogel system features widescale tunable degradability for controlled release and tissue integration. Biomaterials 2021, 269, 120637. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Fang, Y.; Xiong, Z.; Zhang, T. AI-driven 3D bioprinting for regenerative medicine: From bench to bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, R.; Peng, I.; Xu, Z.; Jiang, Y. Wearable and implantable biosensors: Mechanisms and applications in closed-loop therapeutic systems. J. Mater. Chem. B 2024, 12, 8577–8604. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI energized hydrogel design, optimization and application in biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef]
- Liao, H.; Hu, S.; Yang, H.; Wang, L.; Tanaka, S.; Takigawa, I.; Li, W.; Fan, H.; Gong, J.P. Data-driven de novo design of super-adhesive hydrogels. Nature 2025, 644, 89–95. [Google Scholar] [CrossRef]
- Arsuffi, B.; Siqueira, G.; Nyström, G.; Titotto, S.; Magrini, T.; Daraio, C. Programmable Multi-Responsive Nanocellulose-Based Hydrogels With Embodied Logic. Adv. Funct. Mater. 2024, 34, 2409864. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, H.; Zhang, W.; Hu, J.; Zeng, Y.; Hu, H.; Shi, L.; Xia, J.; Xu, F. Inflammatory Microenvironment-Responsive Hydrogels Enclosed with Quorum Sensing Inhibitor for Treating Post-Traumatic Osteomyelitis. Adv. Sci. 2024, 11, e2307969. [Google Scholar] [CrossRef] [PubMed]
- Cristea, A.-G.; Lisă, E.-L.; Iacob, S.; Dragostin, I.; Ștefan, C.S.; Fulga, I.; Anghel, A.M.; Dragan, M.; Morariu, I.D.; Dragostin, O.-M. Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals 2025, 18, 825. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Lamture, Y.; Krishna, M. Enhanced Recovery After Surgery: Exploring the Advances and Strategies. Cureus 2023, 15, e47237. [Google Scholar] [CrossRef] [PubMed]

| Inflammatory Phase | Key Triggers | Design Implications |
|---|---|---|
| Acute (0–72 h) | ROS, low pH, NE, MMPs, IL-1β | Burst or early-phase release via ROS-, pH-, or enzyme-responsive linkers |
| Resolution (>72 h) | MMP-2/9, IL-10 | Sustained release of regenerative agents; ECM-mimetic or degradable scaffolds |
| Spatial variation | Local hypoxia, edema, cytokine gradients | Injectable, conformal, or multi-stimuli systems for site-specific delivery |
| Hydrogel Class | Primary Trigger | Mechanism of Response | Representative Design Strategy |
|---|---|---|---|
| pH-responsive | Local acidosis (pH 6.0–6.8) | Hydrolytic cleavage or ionic swelling | Orthoester, acetal, imine linkers; ionizable PAA |
| ROS-responsive | H2O2, O2−, OH− radical | Oxidative cleavage or crosslink disruption | Thioketal, boronic ester, diselenide bonds |
| Enzyme-responsive | MMP-2/9, NE | Protease-mediated peptide cleavage | GPLGIAGQ or AAPV peptide crosslinkers |
| Multi-stimuli/feedback | ROS + pH, MMP + pH, cytokines | Boolean logic (AND/OR), biosensor-mediated release | Dual-labile networks, aptamer-functionalized matrices, redox-switchable gels |
| Payload Class | Key Examples | Delivery Features |
|---|---|---|
| NSAIDs | Diclofenac, Ketoprofen, Celecoxib | pH-/ROS-responsive; improved local efficacy |
| Corticosteroids | Dexamethasone, Triamcinolone | Sustained release; enzyme-triggered systems |
| α2-Adrenergic Agonists | Dexmedetomidine, Clonidine | Mucoadhesive or thermosensitive hydrogels |
| Biologics/Regenerative Factors | IL-1Ra, TNF-α blockers, BMP-2, VEGF | Cytokine/ECM-targeted; protease-responsive |
| Dual-Payload Systems | Ropivacaine + Dexmedetomidine or Ketorolac | Synergistic release; compartmental structures |
| Hydrogel Example | Trigger Type | Payload | Model | Comparator | Key Quantitative Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Dual-responsive gelatin–PVA | ROS + pH | Vancomycin–Ag NCs + nimesulide | Rat diabetic infected wound | Commercial dressing | Wound closure ↑ (92% vs. 74%, p < 0.01), TNF-α ↓ (~45%, p < 0.01), Bacterial clearance ↑ (96% vs. 62%, p < 0.001) | [69] |
| MMP-cleavable tetra-PEG | Enzyme (MMP-2/9) | Phosphatidylserine | Rat calvarial defect | Non-responsive hydrogel | Bone regeneration ↑ (78% vs. 54%, p < 0.05), IL-1β ↓ (~40%, p < 0.05), M2/M1 ratio ↑ (~3.2-fold, p < 0.01) | [75] |
| MMP-13-responsive HAMA microsphere | Enzyme (MMP-13) | Celecoxib | Rat OA model (ACLT + MMx) | Non-responsive hydrogel | OARSI score ↓ (~75%, p < 0.05), Joint space width maintained, Col2 preserved | [100] |
| MMP-cleavable PEG | Enzyme (MMP-1/2) | IL-1Ra | Rat cortical LPS-induced neuroinflammation model | Uncoated implant | IgG leakage ↓ to sham level (p < 0.05), Neuronal survival ↑ (p < 0.05) | [123] |
| ROS-degradable HA–EGCG | ROS | EGCG | Rat cecum–sidewall abrasion adhesion model | Commercial HA hydrogel | Adhesion score ↓ (1.2 vs. 3.8, p < 0.05), Fibrosis markers ↓, M2 macrophages ↑ | [145] |
| ROS-responsive HA–PBA/ADH + PVA–SB–CHO | ROS | Chlorogenic acid | Rat cecum–sidewall abrasion adhesion model | Commercial HA hydrogel | Adhesion score ↓ (1.0–1.125 vs. 3.5+, p < 0.0001), 20% adhesion-free | [146] |
| Surgical Model | Responsive Cue | Main Outcome |
|---|---|---|
| Incisional wound | ROS, pH | Reduced inflammation, faster healing |
| Burn injury | ROS | Lower cytokines, improved repair |
| Bone fracture | MMP, pH | Enhanced bone regeneration |
| Joint surgery | Enzyme | Cartilage protection |
| VATS/laparotomy | pH, ROS | Nerve protection, reduced pain |
| Challenge Area | Current Limitation | Emerging Strategy |
|---|---|---|
| Preclinical Models | Non-surgical, short-term, poor benchmarking | Standardized surgical inflammation models incorporating clinically relevant injury mechanisms (e.g., electrocautery, ischemia–reperfusion, multi-tissue handling) and harmonized benchmark endpoints (trigger fidelity, release kinetics, mechanical stability, biomarker modulation, therapeutic window, functional recovery, safety) |
| Responsiveness Fidelity | Off-target release, low specificity | Logic-gated or biosensor-integrated systems |
| Biocompatibility | Immunogenicity, unstable degradation byproducts | Modular, clinically validated chemistries |
| Workflow Integration | Complex delivery, poor fit with surgical pace | Injectable, sprayable, or thermogelling formats |
| Regulatory Translation | Combination product hurdles, GMP gaps | Use of ISO-approved polymers, early alignment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.E.; Jeong, J.-O.; Choi, H. Inflammation-Responsive Hydrogels in Perioperative Pain and Wound Management: Design Strategies and Emerging Potential. Gels 2025, 11, 691. https://doi.org/10.3390/gels11090691
Moon YE, Jeong J-O, Choi H. Inflammation-Responsive Hydrogels in Perioperative Pain and Wound Management: Design Strategies and Emerging Potential. Gels. 2025; 11(9):691. https://doi.org/10.3390/gels11090691
Chicago/Turabian StyleMoon, Young Eun, Jin-Oh Jeong, and Hoon Choi. 2025. "Inflammation-Responsive Hydrogels in Perioperative Pain and Wound Management: Design Strategies and Emerging Potential" Gels 11, no. 9: 691. https://doi.org/10.3390/gels11090691
APA StyleMoon, Y. E., Jeong, J.-O., & Choi, H. (2025). Inflammation-Responsive Hydrogels in Perioperative Pain and Wound Management: Design Strategies and Emerging Potential. Gels, 11(9), 691. https://doi.org/10.3390/gels11090691







