Phenylethyl Alcohol-Based Polymeric Nanogels Obtained Through Polymerization-Induced Self-Assembly Toward Achieving Broad-Spectrum Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
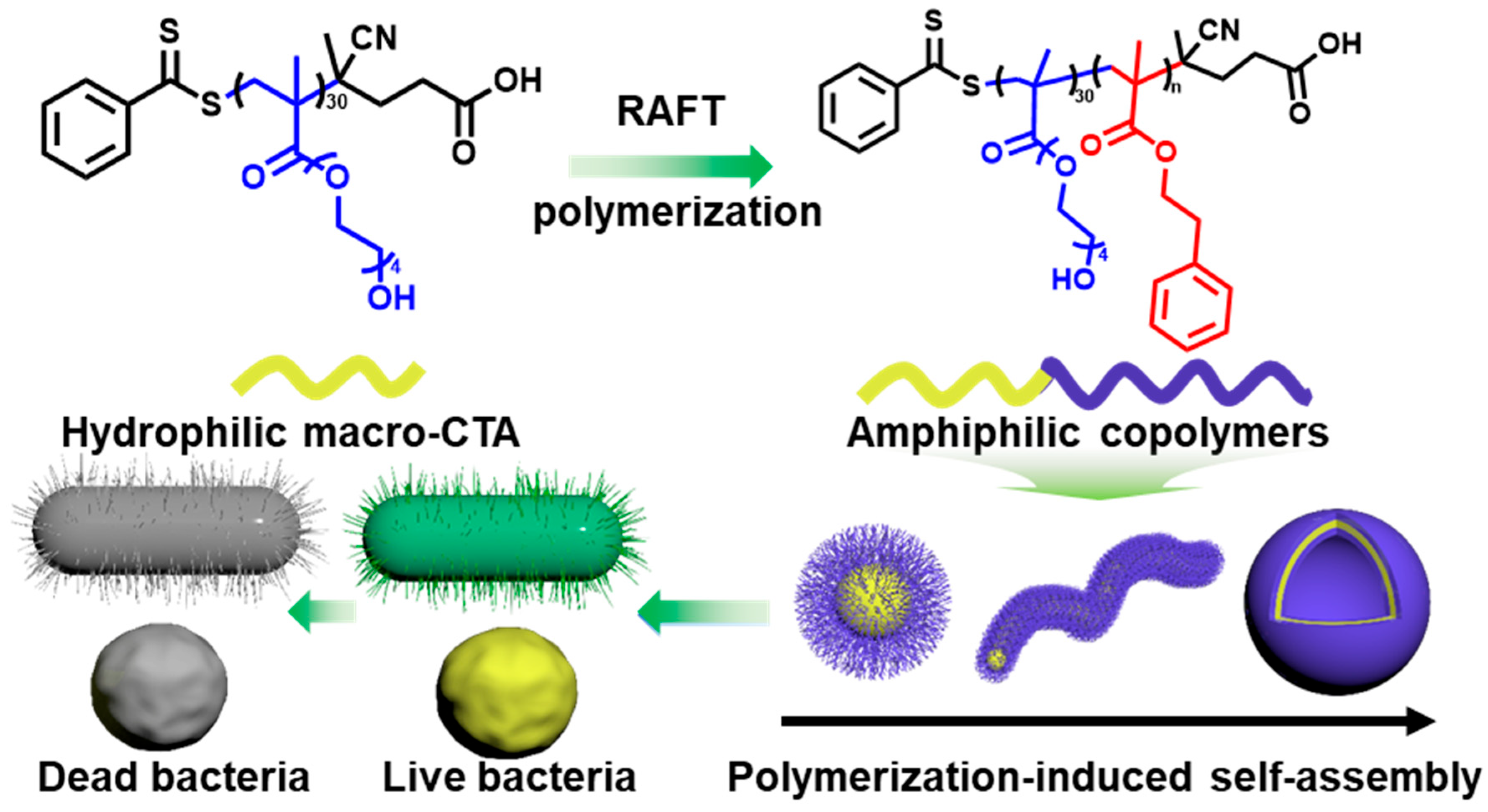
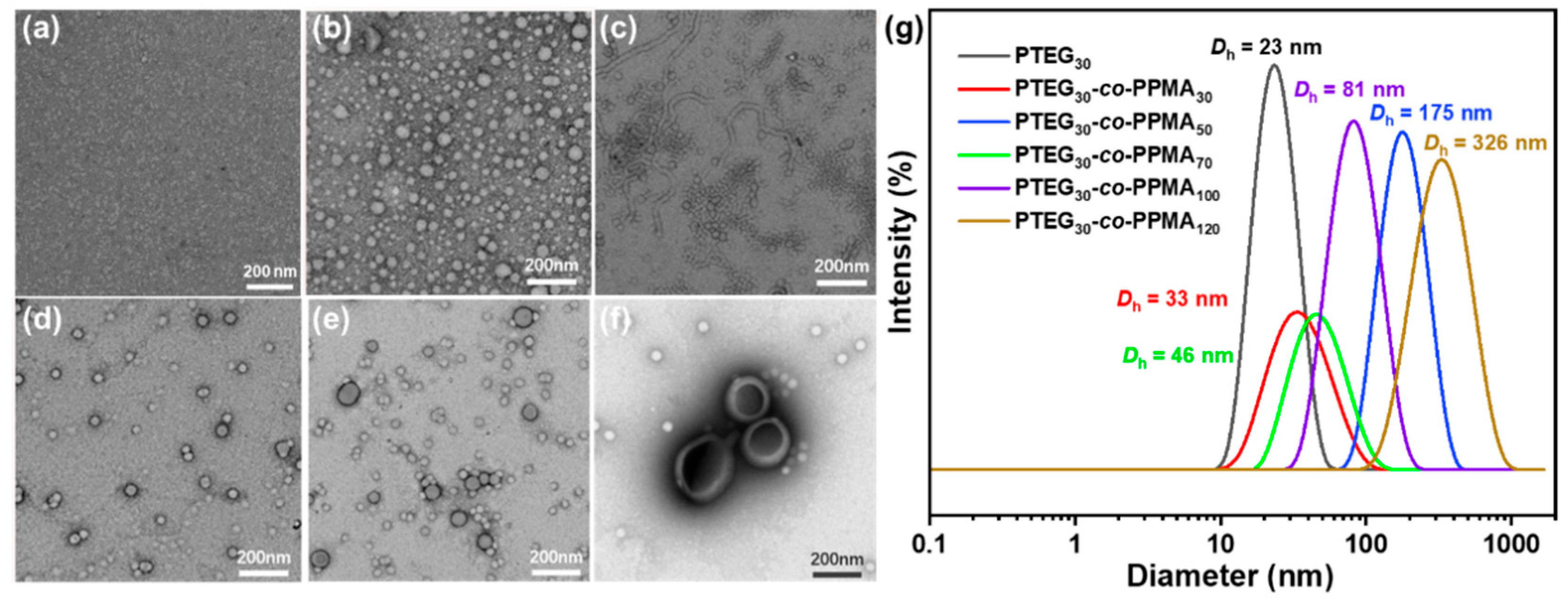
2.1. Synthesis and Characterization of Phenylethyl Alcohol-Based Polymers
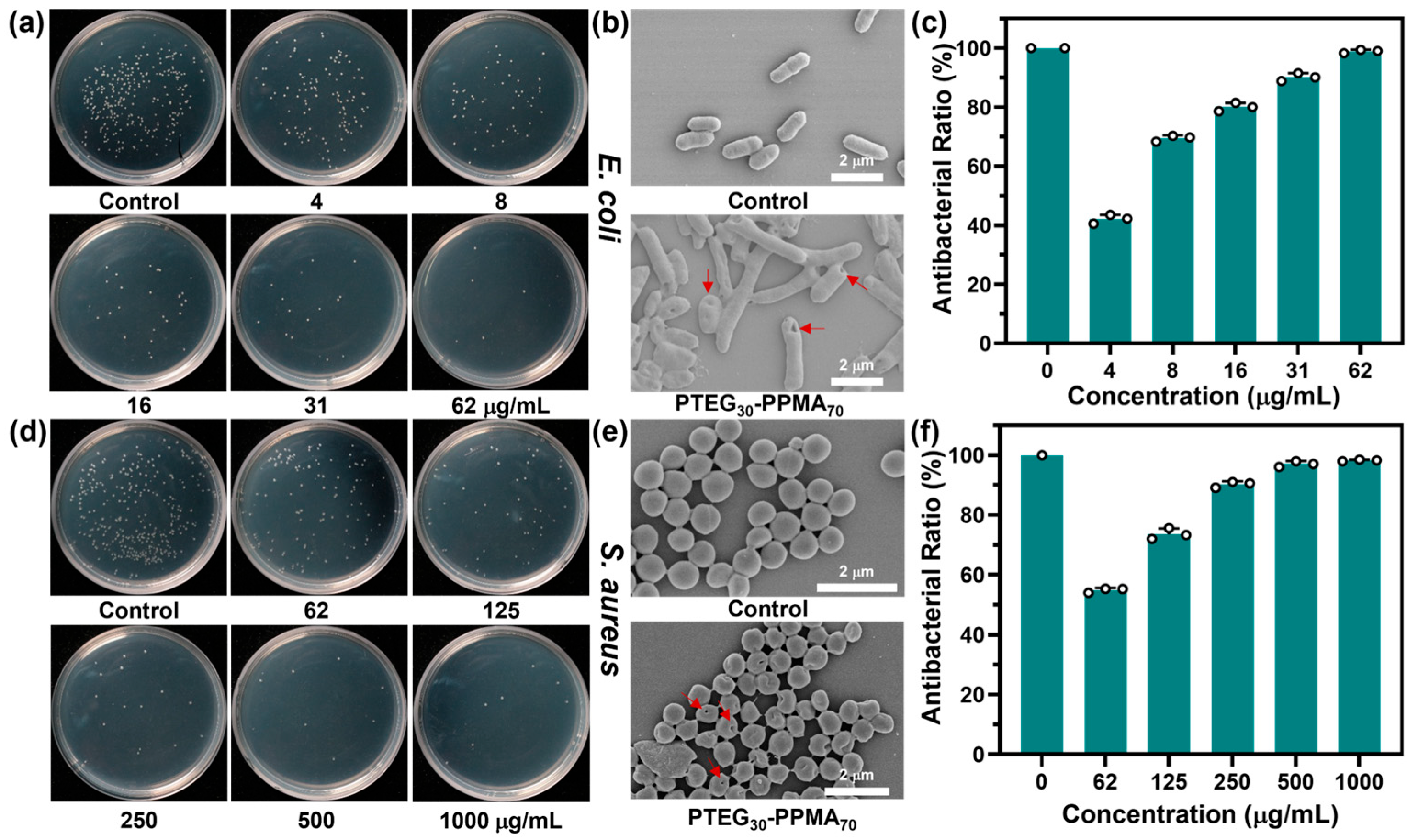
2.2. Antibacterial Activity of PTEG30-co-PPMAn
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Synthesis of TEG
4.4. Synthesis of PMA
4.5. Synthesis of Macro-CTA PTEG30
4.6. Representative Synthesis of PTEG30-co-PPMA100
4.7. MIC Measurements
4.8. In Vitro Antibacterial Test
4.9. Bacterial Morphological Observation
4.10. Zeta Potential Measurement
4.11. Hemolysis Assay
control − OD560 negative control)] × 100%
4.12. MTT Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mather, A.E.; Gilmour, M.W.; Reid, S.W.J.; French, N.P. Foodborne bacterial pathogens: Genome-based approaches for enduring and emerging threats in a complex and changing world. Nat. Rev. Microbiol. 2024, 22, 543–555. [Google Scholar] [CrossRef]
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural products in antibiotic drug discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.; Jiang, H. Metal-Organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Wijesundara, Y.H.; Howlett, T.S.; Kumari, S.; Gassensmith, J.J. The promise and potential of metal-organic frameworks and covalent organic frameworks in vaccine nanotechnology. Chem. Rev. 2024, 124, 3013–3036. [Google Scholar] [CrossRef] [PubMed]
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef]
- Katz, M.S. Therapy Insight: Potential of statins for cancer chemoprevention and therapy. Nat. Clin. Pract. Oncol. 2005, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, Y.; He, W.; Chen, T.; Liu, N.; Ma, L.; Qiu, Z.; Shang, Z.; Wang, Z. A polyene macrolide targeting phospholipids in the fungal cell membrane. Nature 2025, 640, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Brown, T.E.; Anseth, K.S. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. [Google Scholar] [CrossRef]
- Gan, D.; Shuai, T.; Wang, X.; Huang, Z.; Ren, F.; Fang, L.; Wang, K.; Xie, C.; Lu, X. Mussel-inspired redox-active and hydrophilic conductive polymer nanoparticles for adhesive hydrogel bioelectronics. Nanomicro. Lett. 2020, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, W.; Hu, K.; Luo, Y.; Zeng, W.; He, X.; Li, T.; Ouyang, J.; Li, Y.; Xie, L.; et al. Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment. Nat. Nanotechnol. 2024, 19, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Samanta, S.K. Soft-nanocomposites of nanoparticles and nanocarbons with supramolecular and polymer gels and their applications. Chem. Rev. 2016, 116, 11967–12028. [Google Scholar] [CrossRef]
- Spring, B.Q.; Sears, R.B.; Zheng, L.Z.; Mai, Z.M.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E.; et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotechnol. 2016, 11, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chountoulesi, M.; Antoniadi, L.; Angelis, A.; Lei, J.; Halabalaki, M.; Demetzos, C.; Mitakou, S.; Skaltsounis, L.A.; Wang, C. Development and physicochemical characterization of nanoliposomes with incorporated oleocanthal, oleacein, oleuropein and hydroxytyrosol. Food Chem. 2022, 384, 132470. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Kitayama, Y.; Yoshimatsu, E.; Harada, A. Polymer nanogels with albumin reporting interface created using RAFT precipitation polymerization for disease diagnosis and food testing. Adv. Mater. 2025, 12, 2500147. [Google Scholar] [CrossRef]
- Tao, X.; Wang, Z.; Zhang, Q.; Liu, N.; Sun, Y.; Niu, R.; Sun, R.; Wang, X.; Tan, B.; Zhang, C. Covalent Organic Framework Nanohydrogels. J. Am. Chem. Soc. 2023, 145, 25471–25477. [Google Scholar] [CrossRef]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.K.; Leal, C. Polymer-lipid hybrid materials. Chem. Rev. 2021, 121, 13996–14030. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Kim, Y.; Choi, M.; Lee, I.; Kim, E.; Yoon, C.G.; Pu, K.; Kang, H.M.; Kim, J.S. In situ self-assembly for cancer therapy and imaging. Nat. Rev. Mater. 2023, 8, 710–725. [Google Scholar] [CrossRef]
- Yan, X.; Chai, L.; Fleury, E.; Ganachaud, F.; Bernard, J. ‘Sweet as a Nut’: Production and use of nanocapsules made of glycopolymer or polysaccharide shell. Prog. Polym. Sci. 2021, 120, 44. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Clothier, G.K.K.; Guimaraes, T.R.; Thmpson, S.W.; Rho, J.Y.; Perrier, S.; Moad, G.; Zetterlund, P.B. Multiblock copolymer synthesis via RAFT emulsion polymerization. Chem. Soc. Rev. 2023, 52, 3438–3469. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Farsi, M.; Mobini, A.; Sang, G. Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Mater. Sci. Eng. C 2017, 72, 558–565. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Zhang, C.; Chen, Y.; Li, B. Self-assembled lysozyme/carboxymethylcellulose nanogels for delivery of methotrexate. Int. J. Biol. Macromol. 2015, 75, 166–172. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Chang, J.; Zhou, H.; Wang, D.; Sun, J.; Liu, T.; Peng, H.; Wang, Q.; Li, Y.; et al. Advances and prospects of RAFT polymerization-derived nanomaterials in MRI-assisted biomedical applications. Prog. Polym. Sci. 2023, 146, 30. [Google Scholar] [CrossRef]
- Doolan, J.A.; Williams, G.T.; Hilton, K.L.F.; Chaudhari, R.; Fossey, J.S.; Goult, B.T.; Hiscock, J.R. Advancements in antimicrobial nanoscale materials and self-assembling systems. Chem. Soc. Rev. 2022, 51, 8696–8755. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, N.; Wan, Z.; Ye, X.; Zhan, J.; Wa, X. High-performance nano-splitters containing aggregation-induced emission luminogens for stereoselective crystallization obtained via polymerization-induced self-assembly. Aggregate 2021, 2, e129. [Google Scholar] [CrossRef]
- Agost, F.D.; Rieger, J.; Lansalot, M. RAFT-mediated polymerization-induced self-assembly. Angew. Chem. Int. Ed. 2020, 59, 8368–8392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lei, S.; Zeng, M.; Huo, M. Recent progress in polymerization-induced self-assembly: From the perspective of driving forces. Aggregate 2023, 5, e418. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; An, Z. Degradable block copolymer nanoparticles synthesized by polymerization-induced self-assembly. Angew. Chem. Int. Ed. 2024, 63, e202315849. [Google Scholar] [CrossRef]
- György, C.; Armes, S.P. Recent advances in polymerization-induced self-Assembly (PISA) syntheses in non-polar media. Angew. Chem. Int. Ed. 2023, 62, e202308372. [Google Scholar] [CrossRef]
- Yang, G.; Ren, H.; Li, Q.; Bi, F.; Chen, G.; Yu, D.; Chen, M.; Wang, Z.; Wang, Y.; Wang, J. Enhancing environment resistance and bioactivity of pesticide enabled by structure-controllable polymer nanocarriers: Emphasizing the role of morphology. Small 2025, 21, e2409537. [Google Scholar] [CrossRef]
- Wong, C.; Qiang, X.; Müller, A.H.E.; Gröschel, A.H. Self-assembly of block copolymers into internally ordered microparticles. Prog. Polym. Sci. 2020, 102, 101211. [Google Scholar] [CrossRef]
- Hurst, P.J.; Rakowski, A.M.; Patterson, J.P. Ring-opening polymerization-induced crystallization-driven self-assembly of poly-L-lactide-block-polyethylene glycol block copolymers (ROPI-CDSA). Nat. Commun. 2020, 11, 4690. [Google Scholar] [CrossRef]
- Han, J.; Lou, Q.; Ding, Z.; Zheng, G.; Ni, Q.; Song, R.; Liu, K.; Zang, J.; Dong, L.; Shen, C.; et al. Chemiluminescent carbon nanodots for dynamic and guided antibacteria. Light Sci. Appl. 2023, 12, 104. [Google Scholar] [CrossRef]
- Ma, K.; Dong, J.; Yan, D.; Wang, D.; Wang, Y.; Wang, J.; Wang, D.; Tan, H.; Tang, B.Z. Molecular engineering of aie-active photosensitizers with high biosafety for effect extracellular antibacteria. Small 2024, 15, e2403937. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Yang, T.; Li, L.; Liang, F.; Yang, Z. Temperature-sensitive anti-inflammatory organohydrogels containing janus particle stabilized phase-change microinclusions. ACS Nano. 2022, 16, 9859–9870. [Google Scholar] [CrossRef]
- Fu, J.; Wang, C.; Liu, X.; Zhu, S.; Zheng, Y.; Li, Z.; Cui, Z.; Zhang, Y.; Jiang, H.; Cao, Y.; et al. Smart responsive materials for antibacterial therapy: Progress, opportunities, and challenges. Prog. Mater. Sci. 2025, 155, 101532. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Shalaeva, D.N. A bacterial coat that is not pure cotton. Science 2018, 359, 276–277. [Google Scholar] [CrossRef]
- Billaudeau, C.; Chastanet, A.; Yao, Z.; Cornilleau, C.; Mirouze, N.; Fromion, V.; Carballido-López, R. Contrasting mechanisms of growth in two model rod-shaped bacteria. Nat. Commun. 2017, 8, 15730. [Google Scholar] [CrossRef]
- Wei, H.; Yang, C.; Bi, F.; Li, B.; Xie, R.; Yu, D.; Fang, S.; Hua, Z.; Wang, Q.; Yang, G. Structure-controllable and mass-produced glycopolymersomes as a template of the carbohydrate@Ag nanobiohybrid with inherent antibacteria and biofilm eradication. Biomacromolecules 2023, 25, 315–327. [Google Scholar] [CrossRef]
- Yang, G.; Bi, F.; Yu, D.; Wang, Y.; Ren, H.; Wei, H.; Wang, Z.; Huang, B. Engineering entomopathogenic fungi using thermal-responsive polymer to boost their resilience against abiotic Stresses. J. Agric. Food Chem. 2024, 72, 20308–20320. [Google Scholar] [CrossRef]
- Bi, F.; Yu, D.; Wei, Z.; Wei, H.; Ren, H.; Wang, Y.; Ren, D.; Hua, Z.; Huang, B.; Yang, G. Core-shell polymeric nanostructures with Intracellular ATP-fueled dsRNA delivery toward genetic control of insect pests. J. Agric. Food Chem. 2023, 71, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, C.; Xu, Y.; Wen, K.; Song, J.; Bai, S.; Wu, C.; Huang, W.; Cai, Q.; Zhou, K.; et al. An alternatingly amphiphilic, resistance-resistant antimicrobial oligoguanidine with dual mechanisms of action. Biomaterials 2021, 275, 120858. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.; Zhou, L.; An, j.; Feng, S.; Wu, M.; Yu, X. H2S donor functionalized molecular machine for combating multidrug-resistant bacteria infected chronic wounds. Angew. Chem. Int. Ed. 2025, 64, e202507833. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, Y.; Zhu, Z.; Su, C.; Ye, S.; Wang, J.; Liu, X.; Shao, W. Construction of an efficient nonleaching graphene nanocomposites with enhanced contact antibacterial performance. Chem. Eng. J. Adv. 2020, 382, 122906. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of nanozyme-hydrogel for enhanced capture and Elieination of bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Shi, X.; Lv, J.; Deng, S.; Zhou, F.; Mei, J.; Zheng, L.; Zhang, J. Construction of interlayer coupling diatomic nanozyme with peroxidase-like and photothermal activities for efficient synergistic antibacteria. Adv. Sci. 2024, 11, e2305823. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, P.; Zhang, L.; Wang, Z.; Wang, F.; Dong, K.; Liu, Z.; Ren, J.; Qu, X. Silver-infused porphyrinic metal-organic framework: Surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection. Adv. Funct. Mater. 2019, 29, 1808594. [Google Scholar] [CrossRef]
- Song, J.; Chen, H.; Wei, Y.; Liu, J. Synthesis of carboxymethylated β-glucan from naked barley bran and its antibacterial activity and mechanism against Staphylococcus aureus. Carbohydr. Polym. 2020, 242, 116418. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Gao, X.; Liu, K.; Yu, D.; Li, Q.; Yang, G.; Bi, F. Phenylethyl Alcohol-Based Polymeric Nanogels Obtained Through Polymerization-Induced Self-Assembly Toward Achieving Broad-Spectrum Antibacterial Activity. Gels 2025, 11, 690. https://doi.org/10.3390/gels11090690
Xie R, Gao X, Liu K, Yu D, Li Q, Yang G, Bi F. Phenylethyl Alcohol-Based Polymeric Nanogels Obtained Through Polymerization-Induced Self-Assembly Toward Achieving Broad-Spectrum Antibacterial Activity. Gels. 2025; 11(9):690. https://doi.org/10.3390/gels11090690
Chicago/Turabian StyleXie, Rui, Xinru Gao, Ketao Liu, Deshui Yu, Qiaoran Li, Guang Yang, and Feihu Bi. 2025. "Phenylethyl Alcohol-Based Polymeric Nanogels Obtained Through Polymerization-Induced Self-Assembly Toward Achieving Broad-Spectrum Antibacterial Activity" Gels 11, no. 9: 690. https://doi.org/10.3390/gels11090690
APA StyleXie, R., Gao, X., Liu, K., Yu, D., Li, Q., Yang, G., & Bi, F. (2025). Phenylethyl Alcohol-Based Polymeric Nanogels Obtained Through Polymerization-Induced Self-Assembly Toward Achieving Broad-Spectrum Antibacterial Activity. Gels, 11(9), 690. https://doi.org/10.3390/gels11090690




