Development and Mechanistic Evaluation of Polymeric Nanomicrogels Under High-Temperature and High-Salinity Conditions
Abstract
1. Introduction
2. Results and Discussion
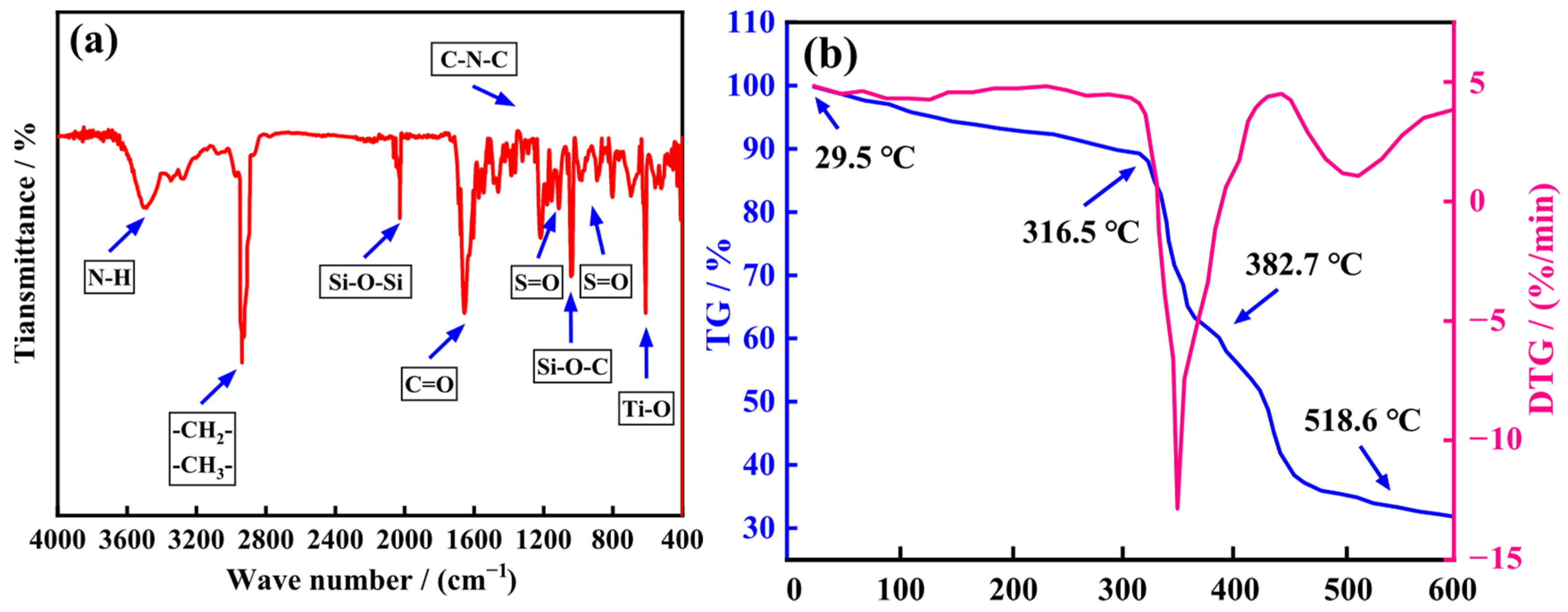
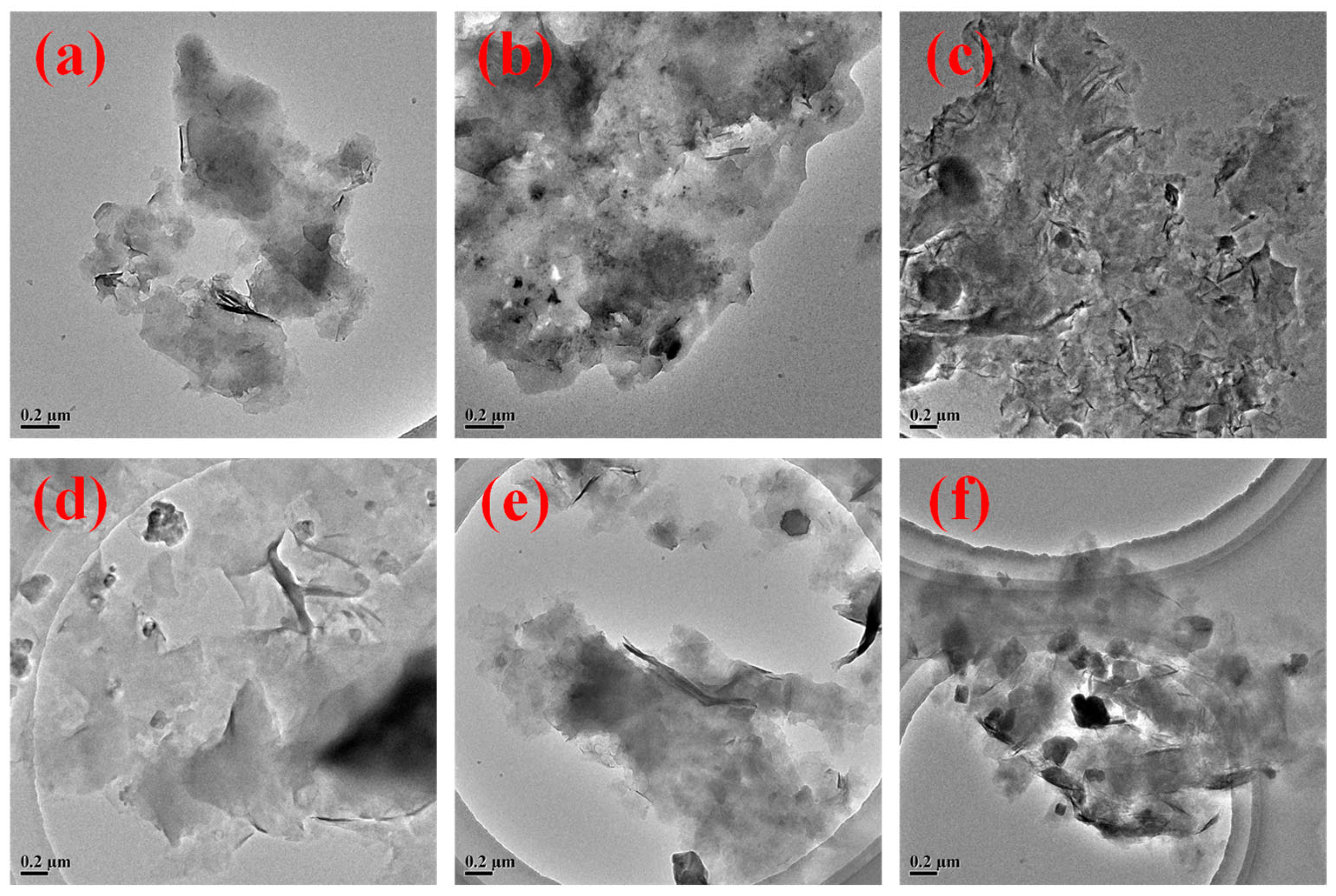
2.1. Characterization of ANDT-70
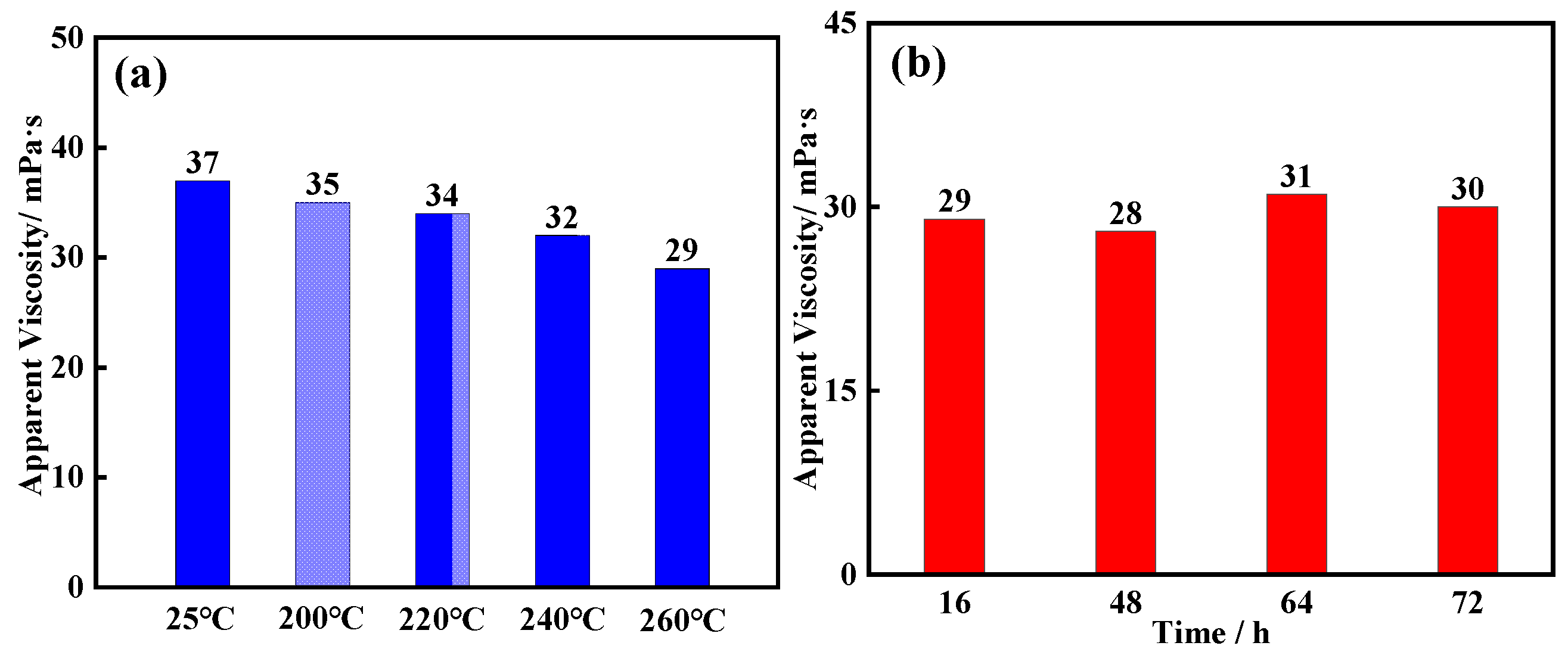
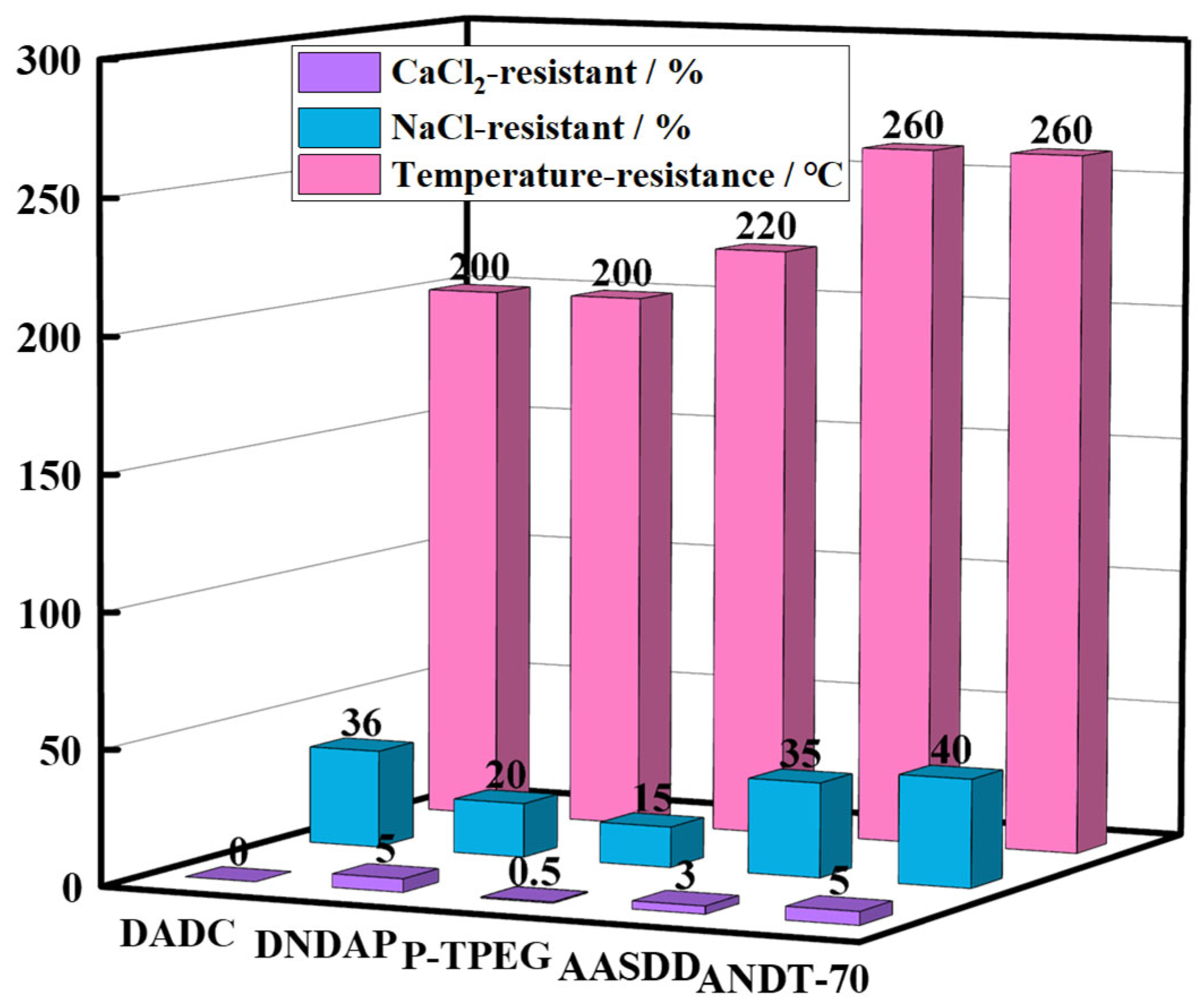
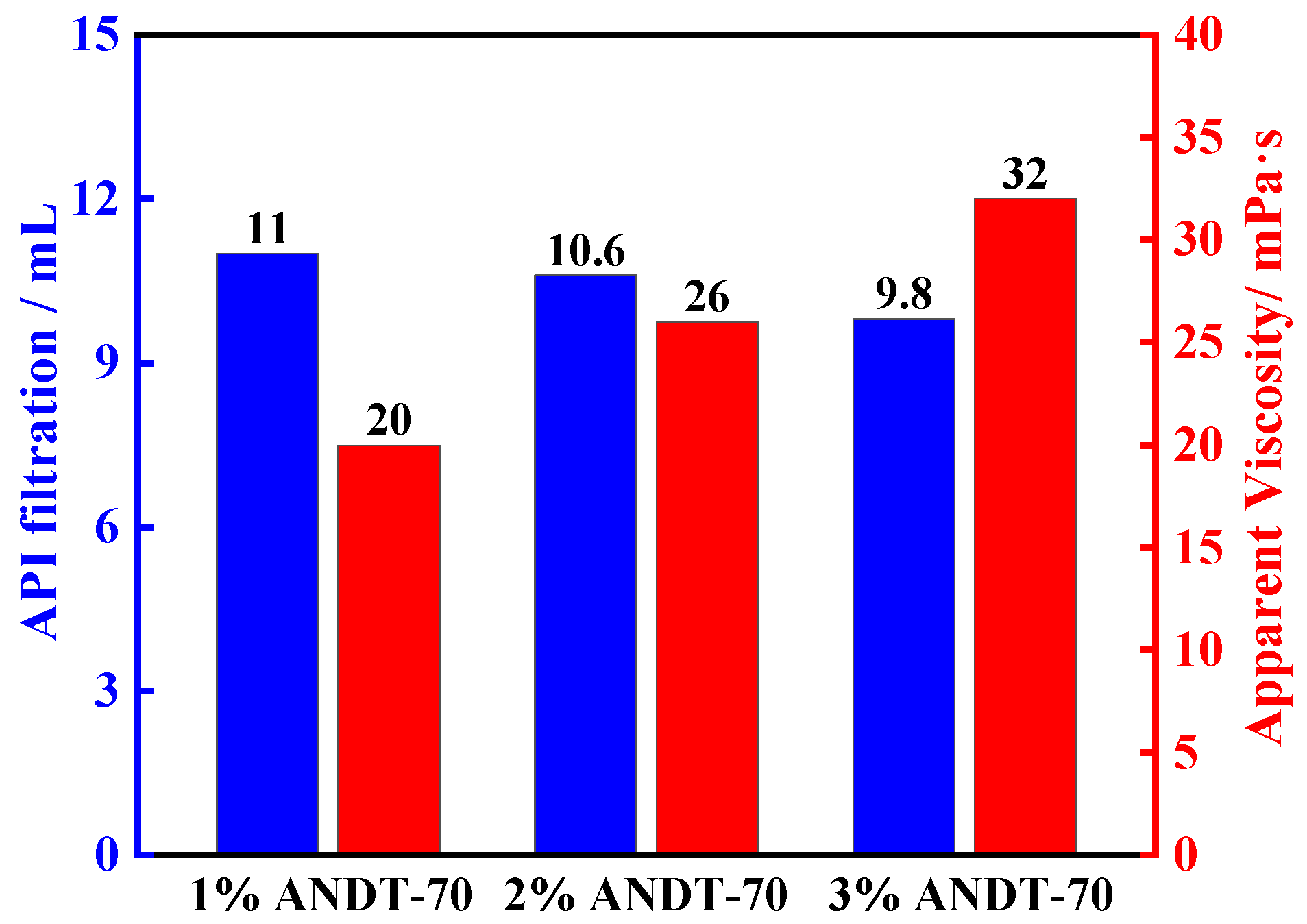
2.2. Thermal Stability Analysis
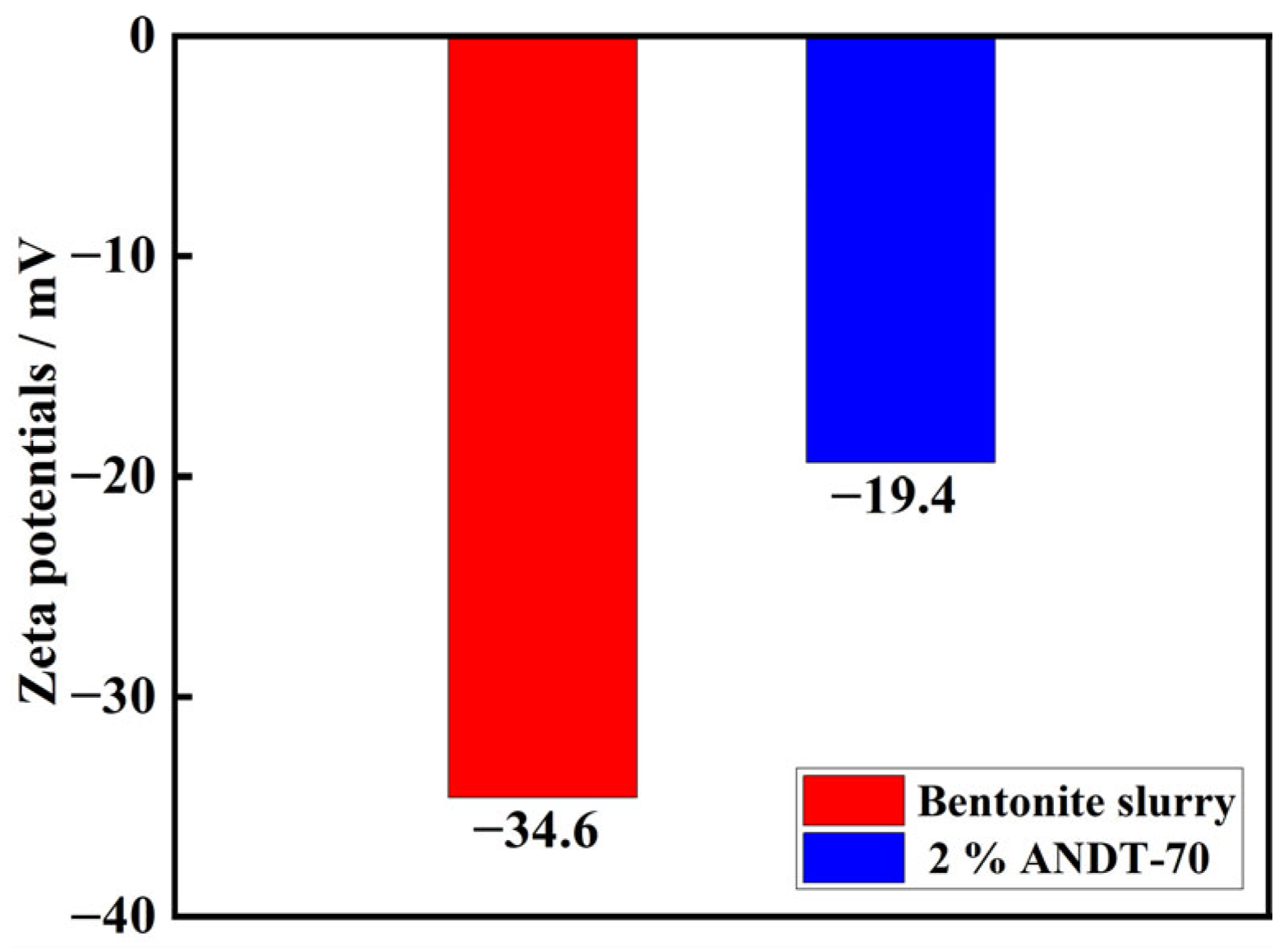
2.3. Salt Resistance Analysis
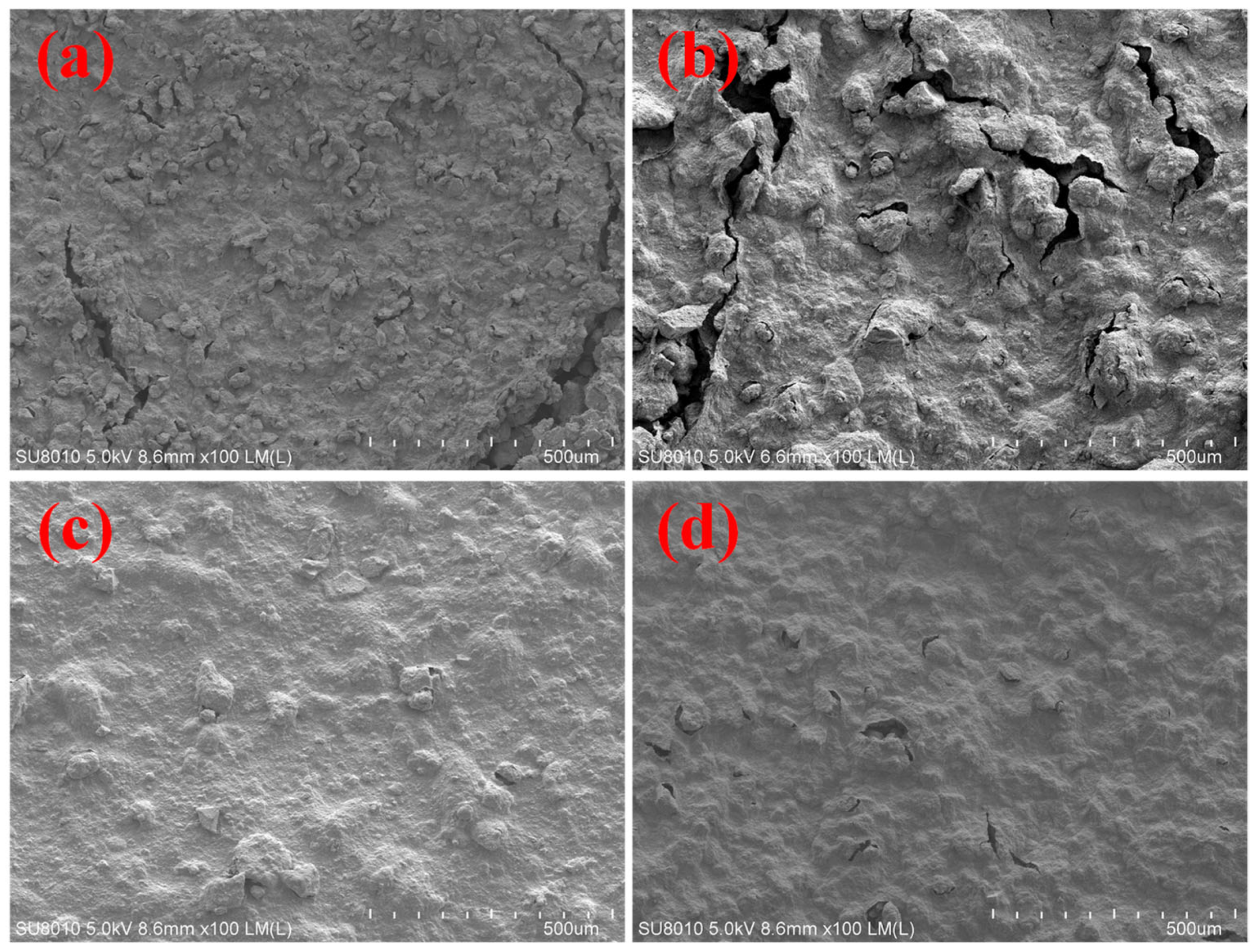
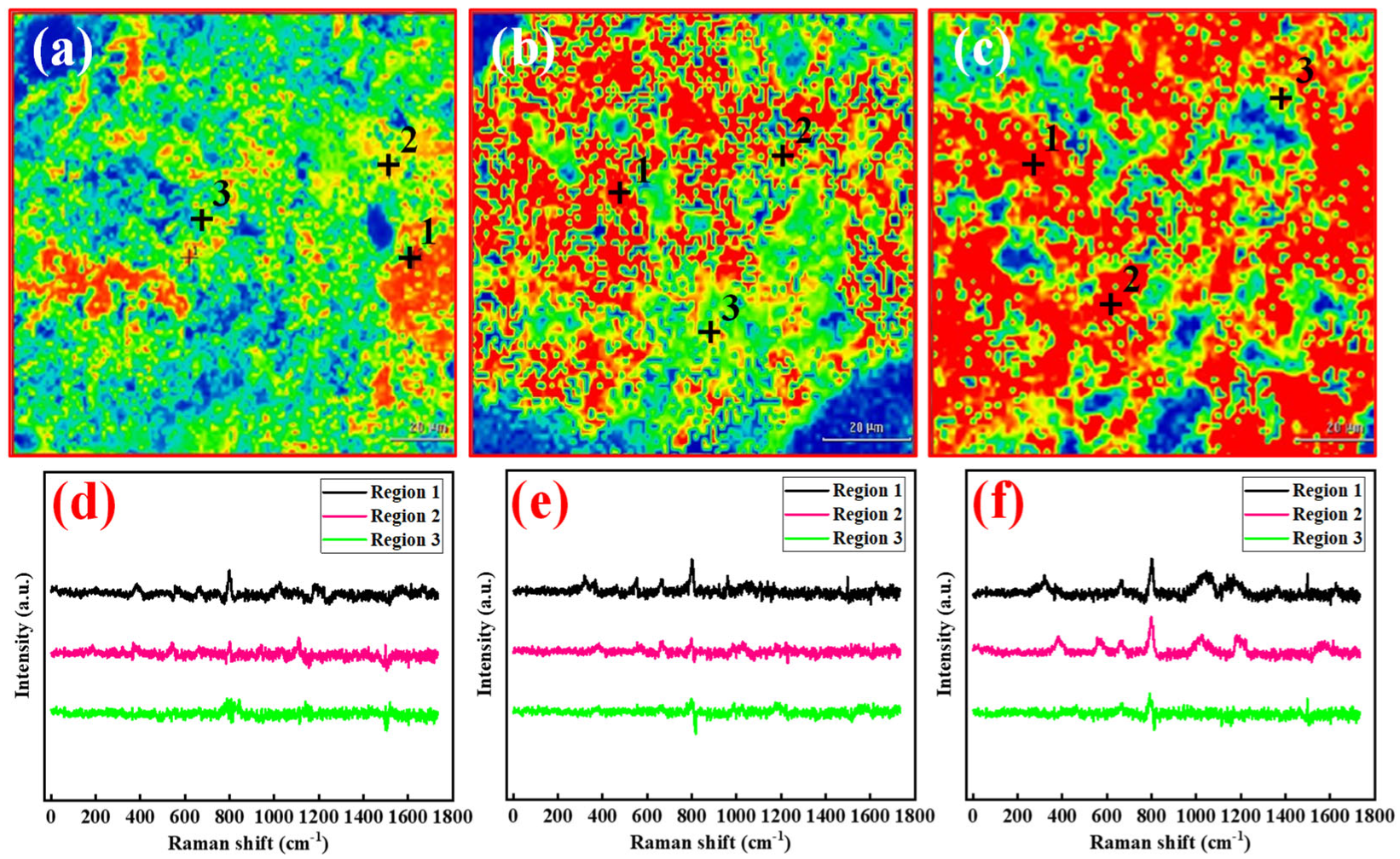
2.4. Bonding Adhesion Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. Characterizations
4.4. Thermal, Salinity, and Long-Term Aging Tests
4.5. Bonding Adhesion Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, H.; Chen, M.; Wang, H.; Jin, Y.; Lu, Y.; Li, J. Lost circulation pattern in the vug-fractured limestone formation. Energy Rep. 2023, 9, 941–954. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, Z.; Yang, D. Magnetic Responsive Pickering Emulsion Containing SiO2–Fe3O4 Particles with Highly Selective Water Plugging in High-Salt Reservoirs. Langmuir 2025, 41, 8740–8752. [Google Scholar] [CrossRef]
- Han, J.; Sun, J.; Lv, K.; Yang, J.; Li, Y. Polymer gels used in oil–gas drilling and production engineering. Gels 2022, 8, 637. [Google Scholar] [CrossRef]
- Pu, L.; Xu, P.; Xu, M.; Song, J.; He, M. Lost circulation materials for deep and ultra-deep wells: A review. J. Pet. Sci. Eng. 2022, 214, 110404. [Google Scholar] [CrossRef]
- Mirabbasi, S.; Ameri, M.; Alsaba, M.; Karami, M.; Zargarbashi, A. The evolution of lost circulation prevention and mitigationbased on wellbore strengthening theory: A review on experimental issues. J. Pet. Sci. Eng. 2022, 211, 110149. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Zhou, X.; Zhao, J. Salt-responsive zwitterionic copolymer as tackifier in brine drilling fluids. J. Mol. Liq. 2020, 319, 114345. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C. Optimal synthesis, characterization, and performance evaluation of high-pressure high-temperature polymer-based drilling fluid: The effect of viscoelasticity on cutting transport, filtration loss, and lubricity. SPE J. 2020, 25, 1333–1350. [Google Scholar] [CrossRef]
- Moreira, B.; De Oliveira Arouca, F.; Damasceno, J. Analysis of suspension sedimentation in fluids with rheological shear-thinning properties and thixotropic effects. Powder Technol. 2017, 308, 290–297. [Google Scholar] [CrossRef]
- Kalantariasl, A.; Zeinijahromi, A.; Bedrikovetsky, P. External Filter Cake Buildup in Dynamic Filtration: Mechanisms and Key Factors. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014. SPE-168144. [Google Scholar]
- Zhong, H.; Gao, X.; Qiu, Z.; Zhao, C.; Zhang, X.; Guo, B.; Li, G. Formulation and evaluation of β-cyclodextrin polymer microspheres for improved HTHP filtration control in water-based drilling fluids. J. Mol. Liq. 2020, 313, 113549. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Zhou, C.; Wen, X.; Zhang, J.; Mao, H. Preparation and gelation behavior evaluation of ultra-stable chitosan gel plugging agent. Oilfield Chem. 2023, 40, 284–290. [Google Scholar]
- Dai, L.; Sun, J.; Lv, K.; Liu, F.; Bai, Y.; Liao, B.; Li, M. Cellulose nanofiber-reinforced supramolecular polymer gels for temporary plugging of fractured oil and gas reservoirs. Carbohydr. Polym. 2025, 356, 123370. [Google Scholar] [CrossRef]
- Shen, H.; Sun, J.; Lyu, K.; Huang, X.; Liu, J.; Wang, J. Research Progress and Application prospects of Intelligent Organic Treatment Agent for Water-Based Drilling Fluid. Oilfield Chem. 2022, 39, 155–162. [Google Scholar]
- Kang, C.; Guo, J.; Kiyingi, W.; Li, J.; Xue, P. A New Self-Healing Green Polymer Gel with Dynamic Networks for Flow Control in Harsh Reservoirs. Adv. Funct. Mater. 2025, 35, 2423892. [Google Scholar] [CrossRef]
- Frenkel, P. Viscosifiers for Drilling Fluids. U.S. Patent 0336086, 13 November 2014. [Google Scholar]
- Yang, J.; Jiang, G.; Yi, J.; He, Y.; Yang, L.; Dong, T.; Wang, G. Natural rubber latex as a potential additive for water-based drilling fluids. Pet. Sci. 2024, 21, 2677–2687. [Google Scholar] [CrossRef]
- Yang, J.; Dong, T.; Yi, J.; Jiang, G. Development of Multiple Crosslinked Polymers and Its Application in Synthetic-Based Drilling Fluids. Gels 2024, 10, 120. [Google Scholar] [CrossRef]
- Xie, B.; Liu, X. Thermo-thickening behavior of LCST-based copolymer viscosifier for water-based drilling fluids. J. Pet. Sci. Eng. 2017, 154, 244–251. [Google Scholar] [CrossRef]
- Abdo, J.; Zaier, R.; Hassan, E.; Al-Sharji, H.; Al-Shabibi, A. ZnO–clay nanocomposites for enhance drilling at HTHP conditions. Surf. Interface Anal. 2014, 46, 970–974. [Google Scholar] [CrossRef]
- Bardhan, A.; Khan, F.; Kesarwani, H.; Vats, S.; Sharma, S.; Kumar, S. Performance evaluation of novel silane coated nanoparticles as an additive for high-performance drilling fluid applications. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 1–3 March 2023; p. D022S028R002. [Google Scholar]
- Yang, J.; Wang, R.; Sun, J.; Wang, J.; Liu, L.; Qu, Y.; Yang, Z. Nanolaponite/comb polymer composite as a rheological modifier for water-based drilling fluids. ACS Appl. Nano Mater. 2023, 6, 13453–13465. [Google Scholar] [CrossRef]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Madanchi, P.; Mahani, H. Pore-scale insights into the effect of surface-modified nanosilica on invert-emulsion drilling-fluid and formation damage. Phys. Fluids 2025, 37, 032109. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, G.; Wang, G.; Yang, L.; He, Y.; Dong, T.; Yuan, X. Performance evaluation of polymer nanolatex particles as fluid loss control additive in water-based drilling fluids. Geoenergy Sci. Eng. 2023, 223, 211462. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.J.; Zhu, D.Y.; Wang, Z.Y.; Qin, J.H.; Zhao, Q.; Hou, J.R. Effect of nano TiO2 and SiO2 on gelation performance of HPAM/PEI gels for high-temperature reservoir conformance improvement. Pet. Sci. 2023, 20, 3819–3829. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Sheng, W.; Shang, X.; Zhang, Z. Thixotropy and gelation characteristics of thermosensitive polymer nanofluids at high temperature. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136340. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.; Al Brahim, A.; Bai, B. Ultra-high temperature resistant preformed particle gels for enhanced oil recovery. Chem. Eng. J. 2021, 426, 130712. [Google Scholar] [CrossRef]
- Elaf, R.; Ben Ali, A.; Saad, M.; Hussein, I.A.; Nimir, H.; Bai, B. Biodegradable preformed particle gel (PPG) made of natural chitosan material for water shut-off application. Polymers 2023, 15, 1961. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, S.; Long, Y.; Bai, B.; Schuman, T. Comprehensive evaluation of a high-temperature resistant re-crosslinkable preformed particle gel for water management. Fuel 2022, 309, 122086. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Liu, Y.; Chen, L.; Zhang, J.; Wang, J.; Yu, Y. Performance evaluation and physical simulation experiment of SiO2 nanoparticle synergistic crosslinked polymer system. J. Phys. Conf. Ser. 2024, 2845, 012017. [Google Scholar] [CrossRef]
- Dehghani, F.; Kalantariasl, A.; Peyvandi, K. The Effect of a Novel Nanocomposite in Water-Based Drilling Fluid on Filtration and Rheology Factors. Trans. Indian Natl. Acad. Eng. 2023, 8, 639–645. [Google Scholar] [CrossRef]
- Guo, Y.; Du, M.; Lv, Y.; He, Y.; Jiang, G. Study of Three-Dimensional Reticulated Filtration Control Agent Formed by Condensation of A-151 in Long-Lasting, Ultra-High-Temperature-Resistant, Salt-Resistant, and Filtration-Loss-Reducing. Chem. Eng. J. 2024, 502, 158047. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.X.; Ni, X.X.; Lv, K.H.; Huang, X.B.; Sun, J.S. A micro-crosslinked amphoteric hydrophobic association copolymer as high temperature- and salt-resistance fluid loss reducer for water-based drilling fluids. Pet. Sci. 2024, 21, 1980–1991. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Wang, R.; Liu, F.; Gao, S.; Yang, J.; Feng, Z. New Zwitterionic Polymer as a Highly Effective Salt- and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. Gels 2022, 8, 735. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; He, Y.; Dong, T.; Mu, H.; Jiang, G.; Wang, Q. Development and Mechanistic Evaluation of Polymeric Nanomicrogels Under High-Temperature and High-Salinity Conditions. Gels 2025, 11, 689. https://doi.org/10.3390/gels11090689
Zhang W, He Y, Dong T, Mu H, Jiang G, Wang Q. Development and Mechanistic Evaluation of Polymeric Nanomicrogels Under High-Temperature and High-Salinity Conditions. Gels. 2025; 11(9):689. https://doi.org/10.3390/gels11090689
Chicago/Turabian StyleZhang, Wei, Yinbo He, Tengfei Dong, Huayan Mu, Guancheng Jiang, and Quande Wang. 2025. "Development and Mechanistic Evaluation of Polymeric Nanomicrogels Under High-Temperature and High-Salinity Conditions" Gels 11, no. 9: 689. https://doi.org/10.3390/gels11090689
APA StyleZhang, W., He, Y., Dong, T., Mu, H., Jiang, G., & Wang, Q. (2025). Development and Mechanistic Evaluation of Polymeric Nanomicrogels Under High-Temperature and High-Salinity Conditions. Gels, 11(9), 689. https://doi.org/10.3390/gels11090689





