Degradation of Polymers and Heavy Metals in Waste Drilling Fluid by Sulfur-Doped BiOBr0.5Cl0.5 Photocatalysts
Abstract
1. Introduction
2. Results and Discussion
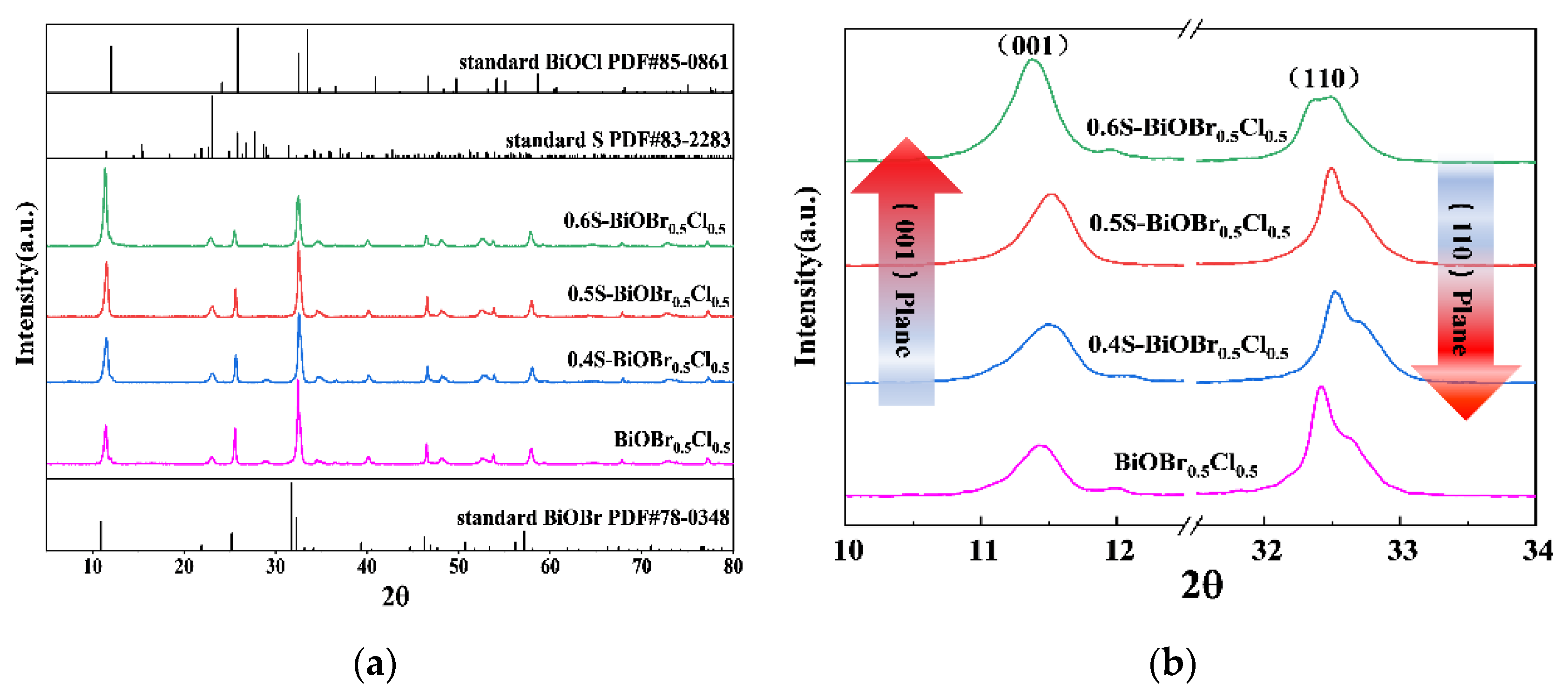
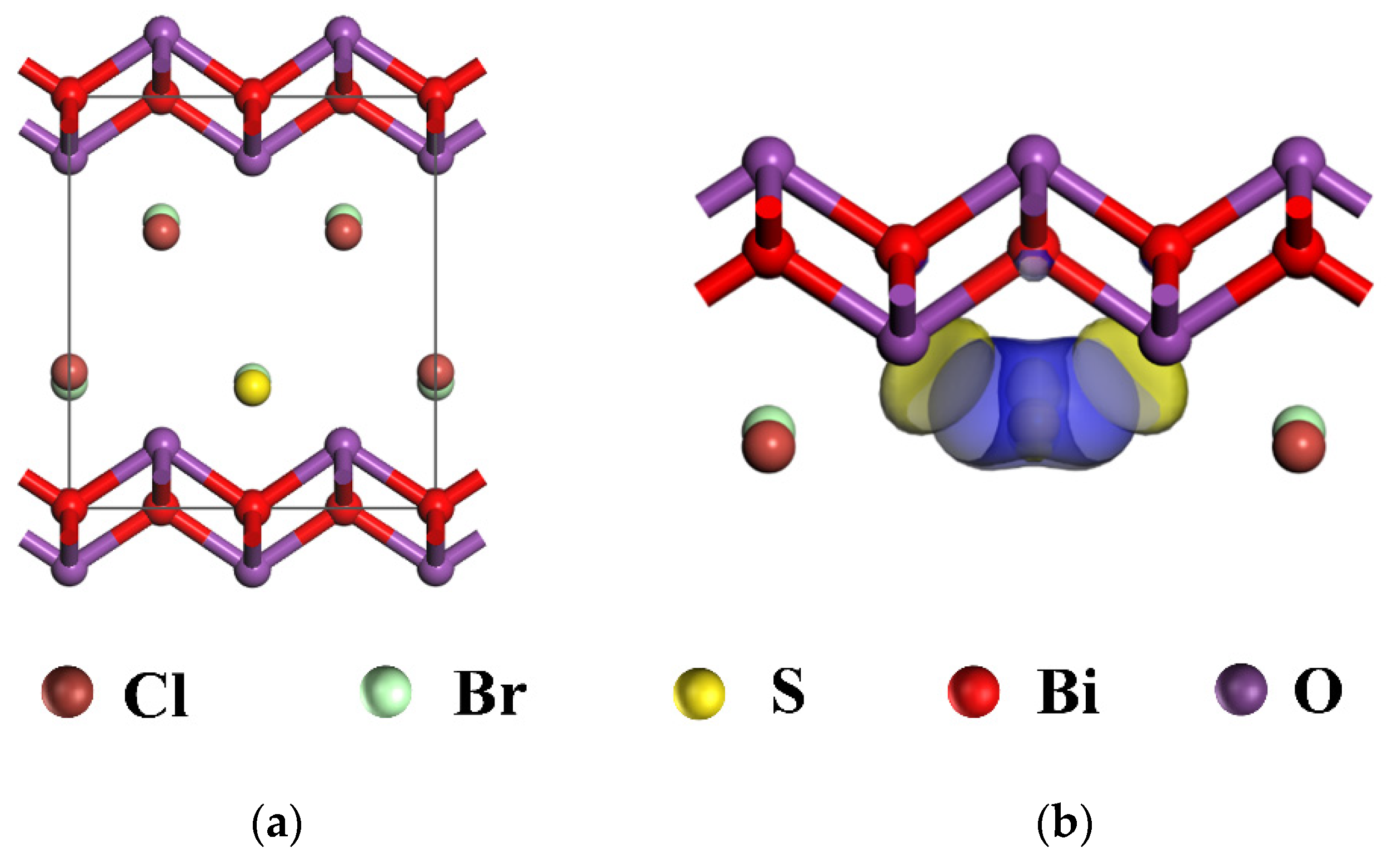
2.1. The Crystal Structure of xS-BiOBr0.5Cl0.5 Materials
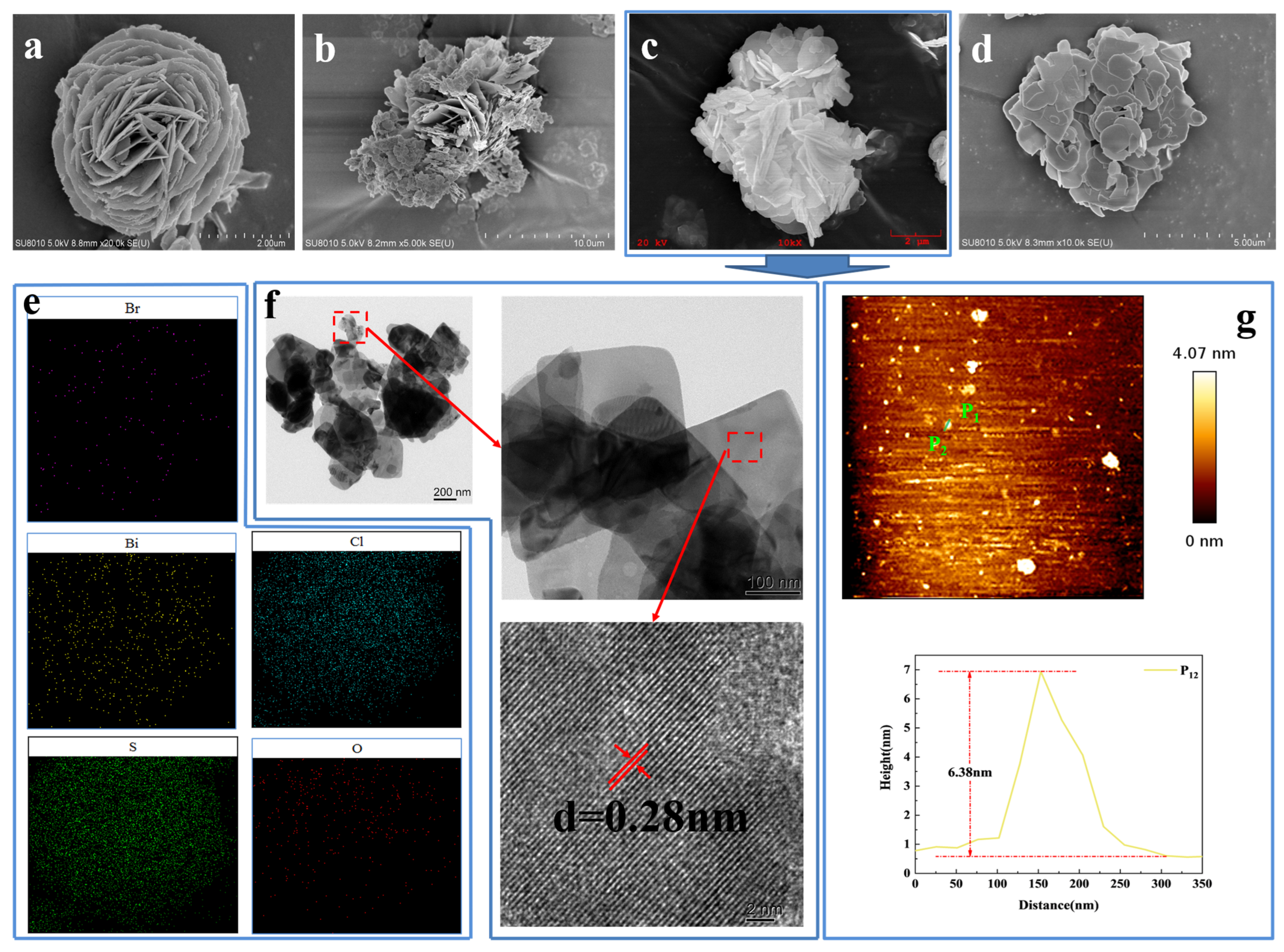
2.2. The Morphology and Thickness of the xS-BiOBr0.5Cl0.5 Materials
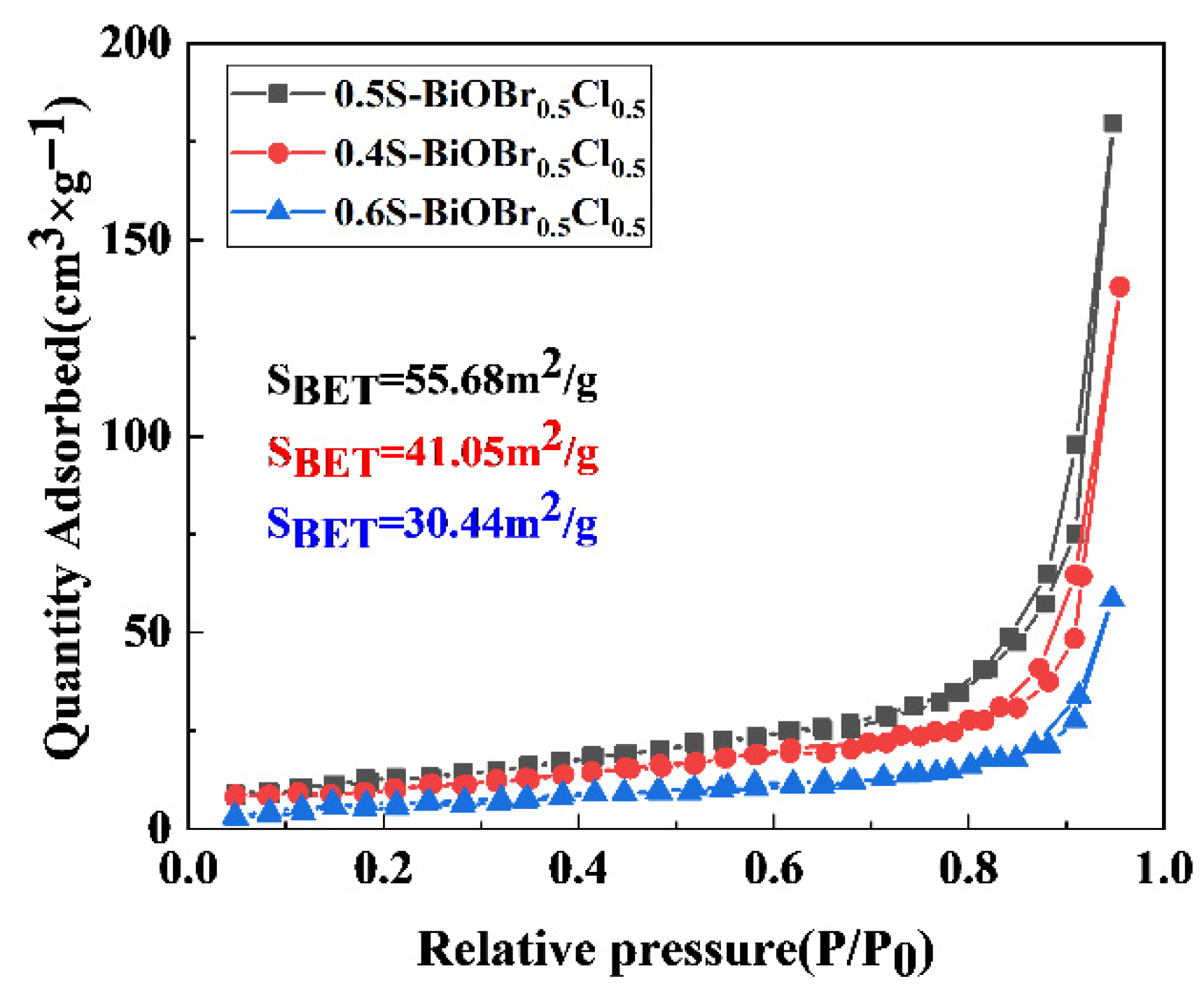
2.3. Specific Surface Areas of the xS-BiOBr0.5Cl0.5 Materials
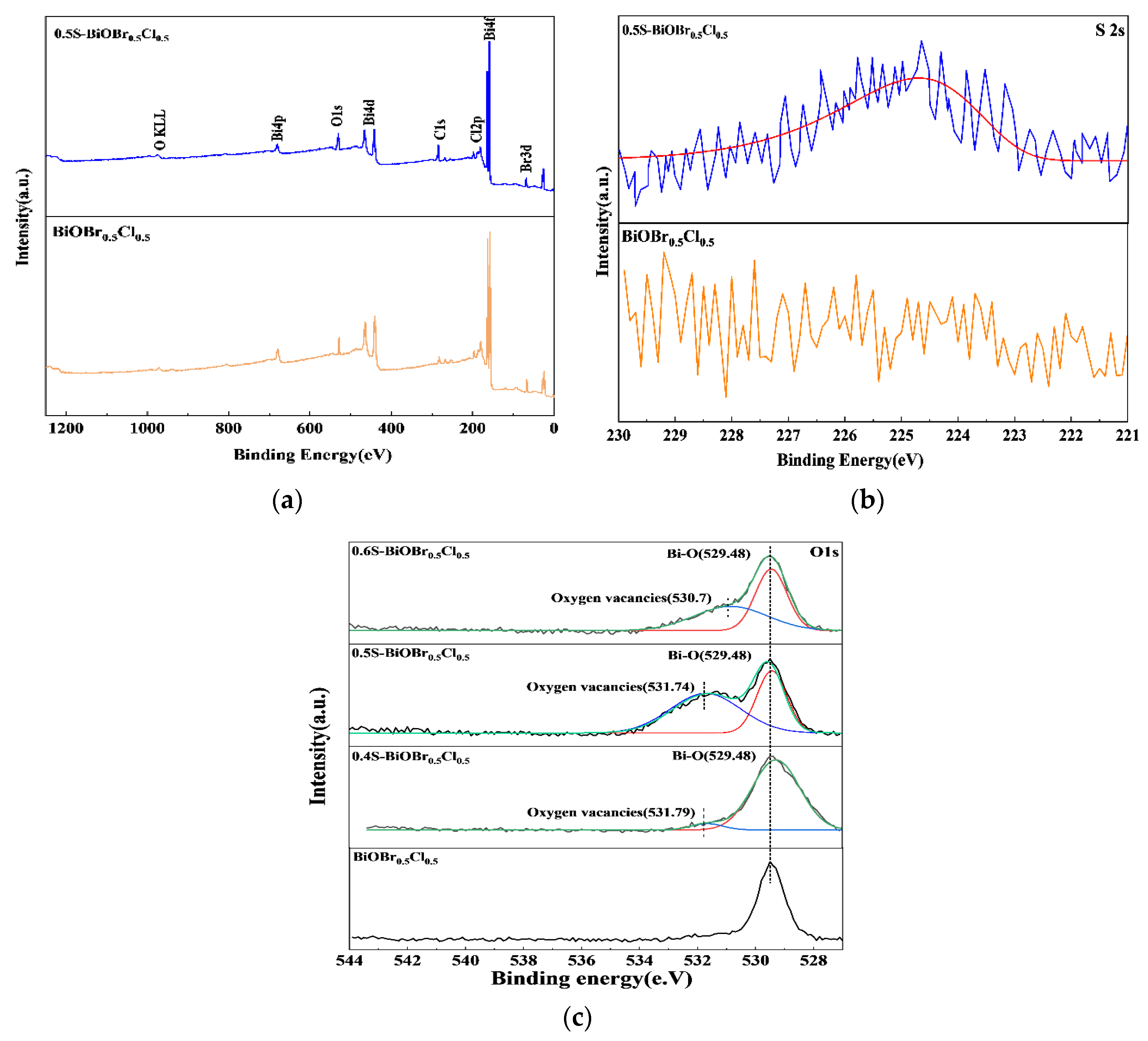
2.4. Surface Chemical Composition and Surface-Chemical-Element Valence States of the xS-BiOBr0.5Cl0.5 Materials
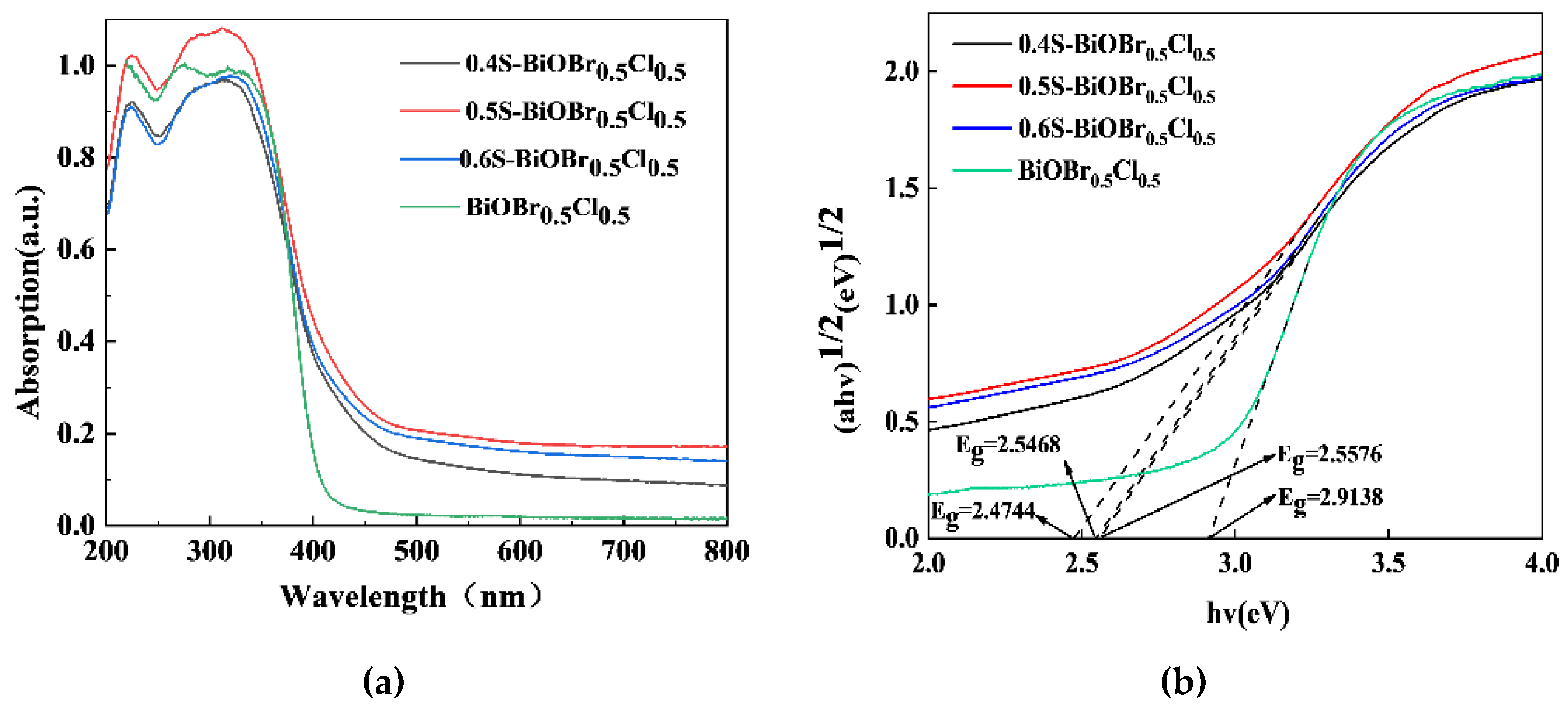
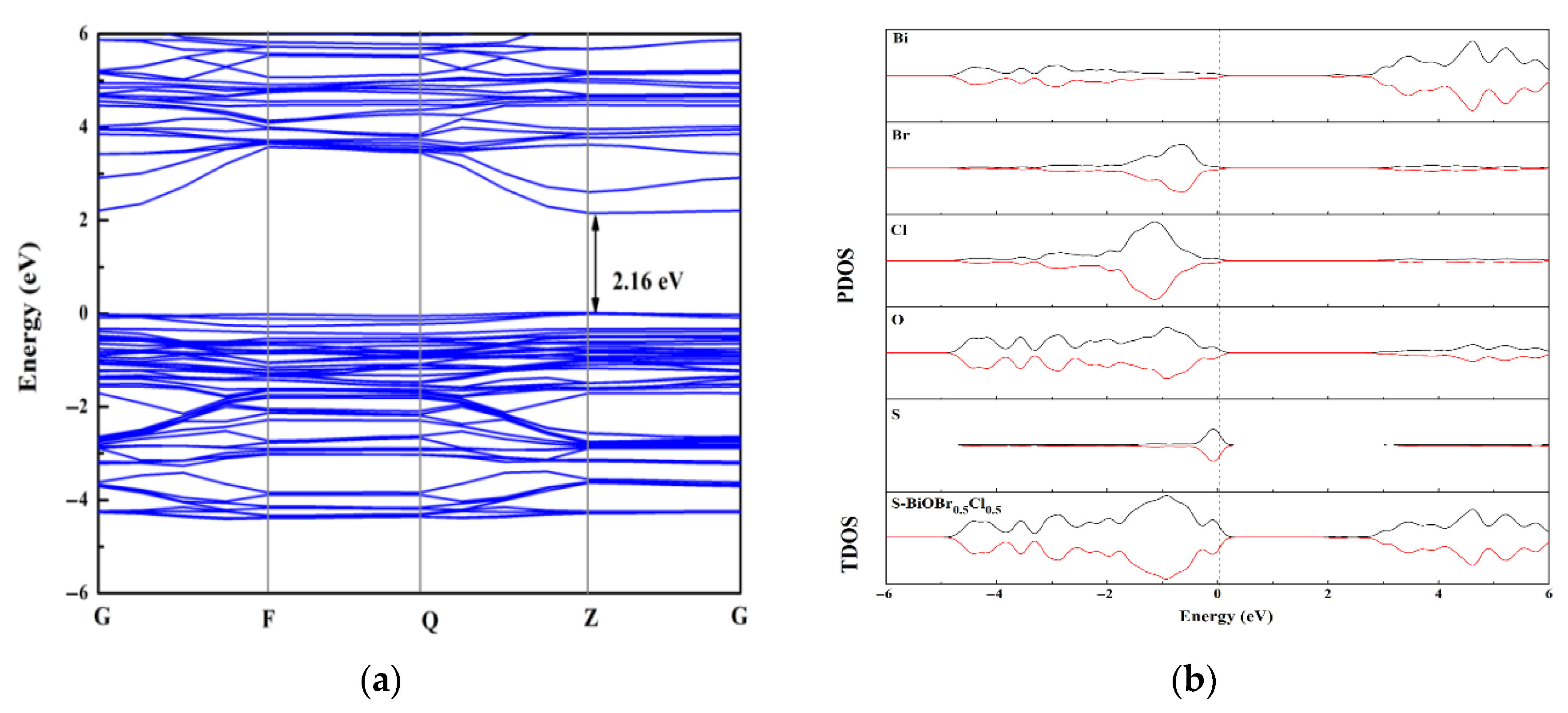
2.5. The Optical Properties of the xS-BiOBr0.5Cl0.5 Materials
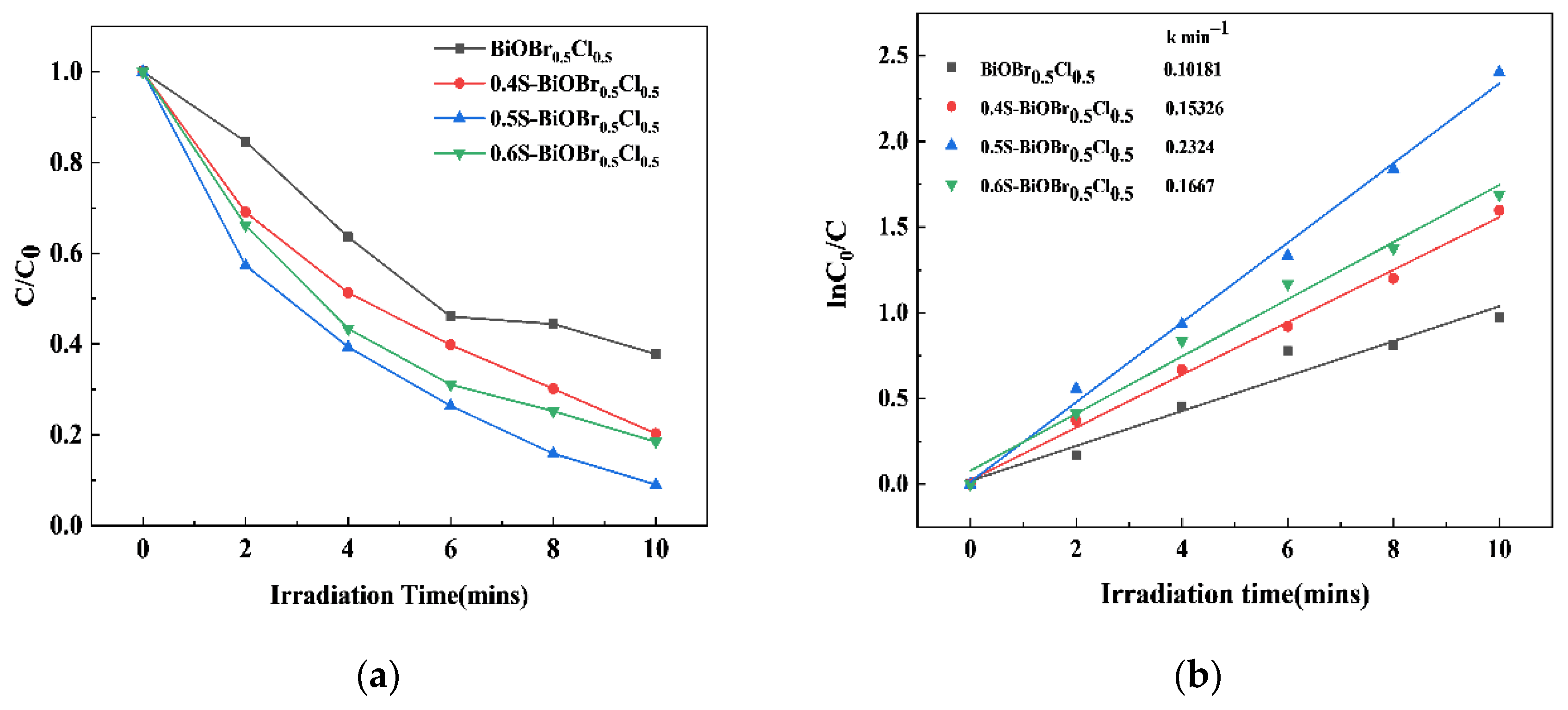
2.6. The Photocatalytic Activity of the xS-BiOBr0.5Cl0.5 Materials
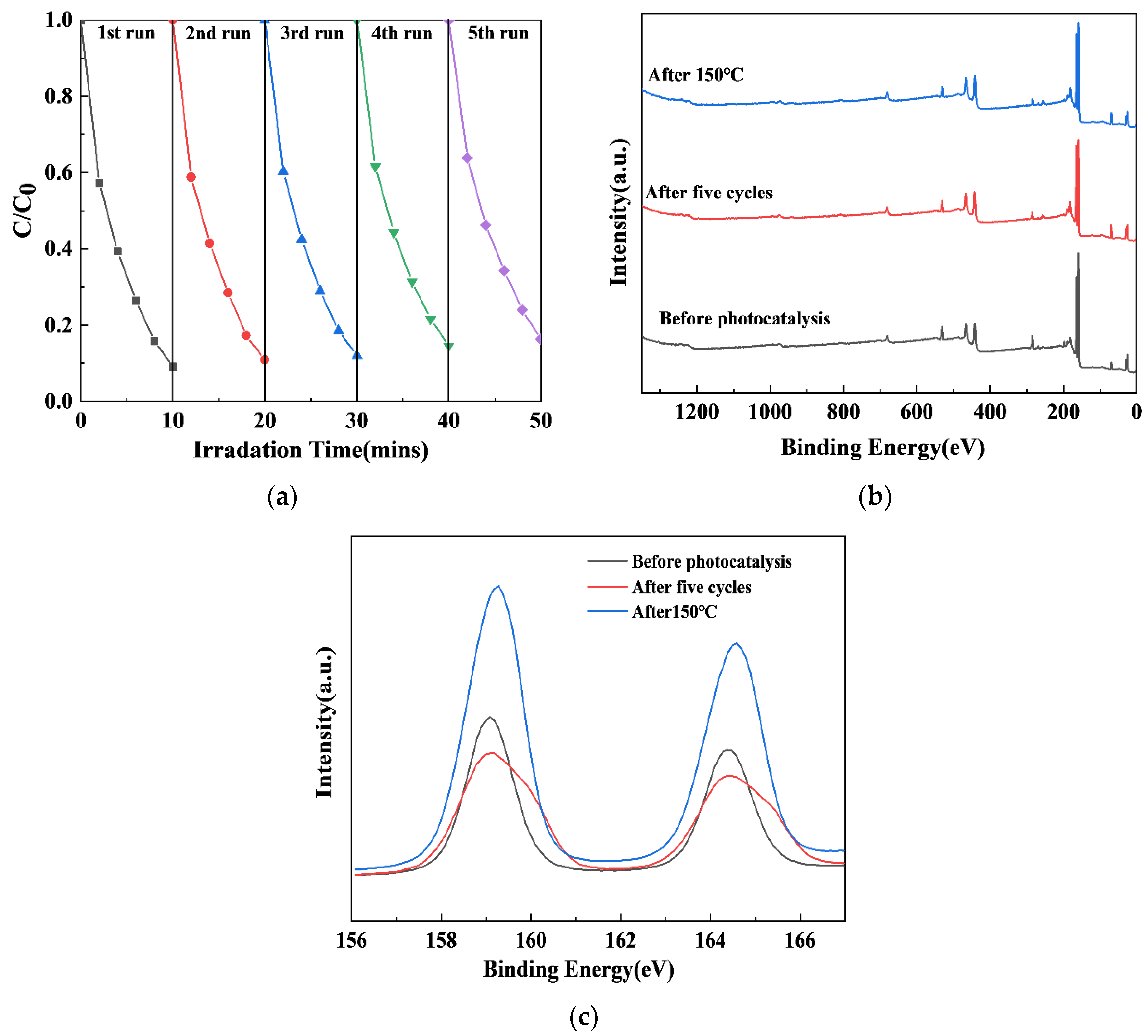
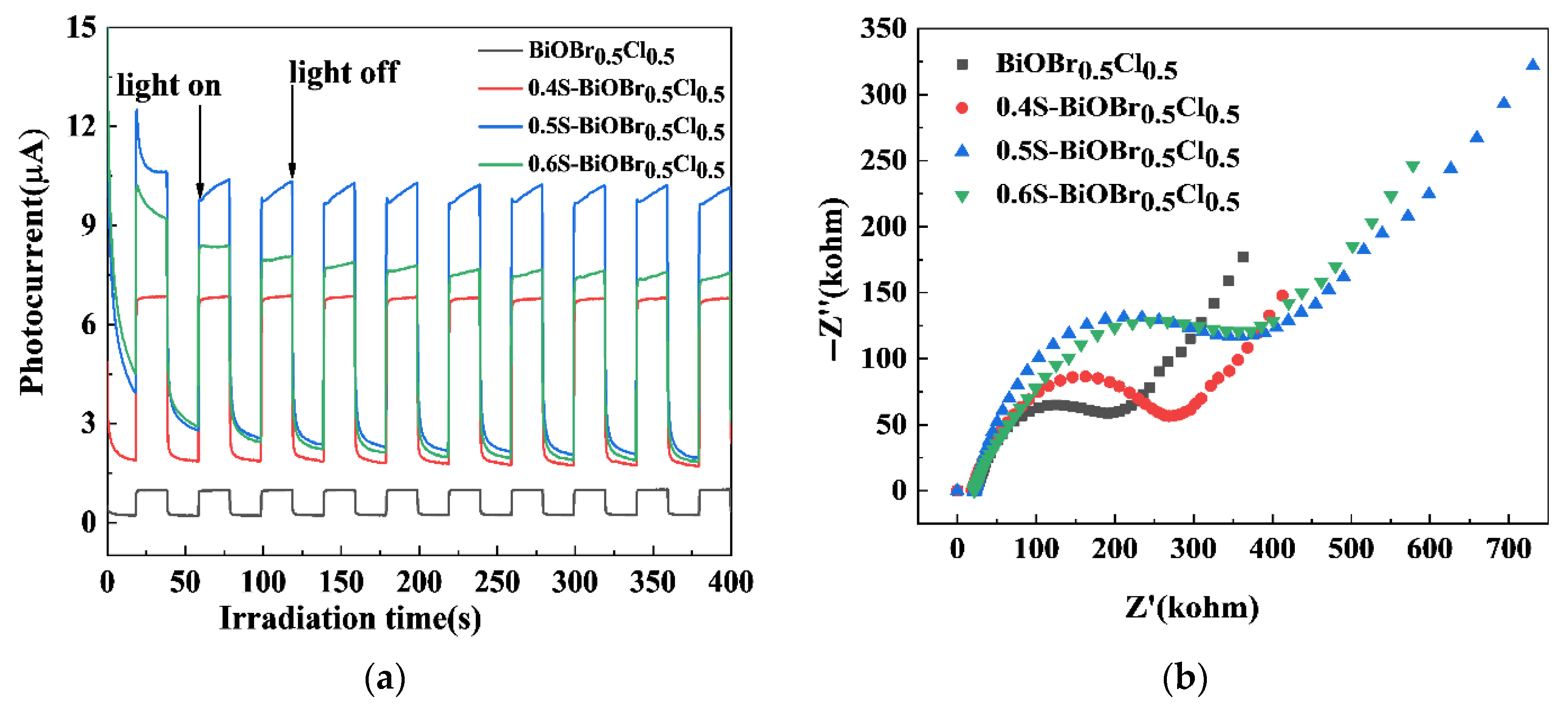
2.7. The Cyclic Stability of the xS-BiOBr0.5Cl0.5 Materials
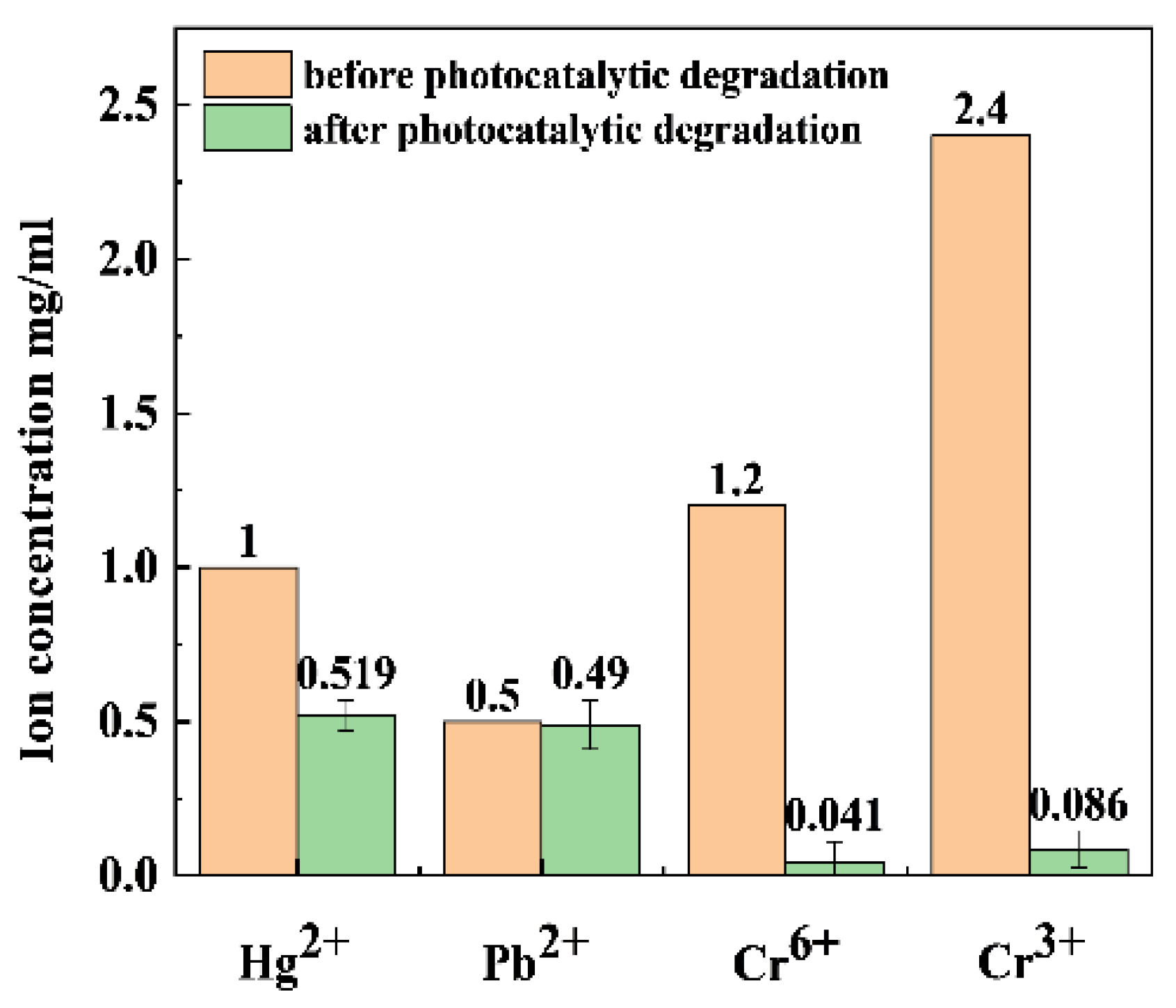
2.8. The Photocatalytic Reduction of Heavy-Metal Ions by xS-BiOBr0.5Cl0.5 Materials
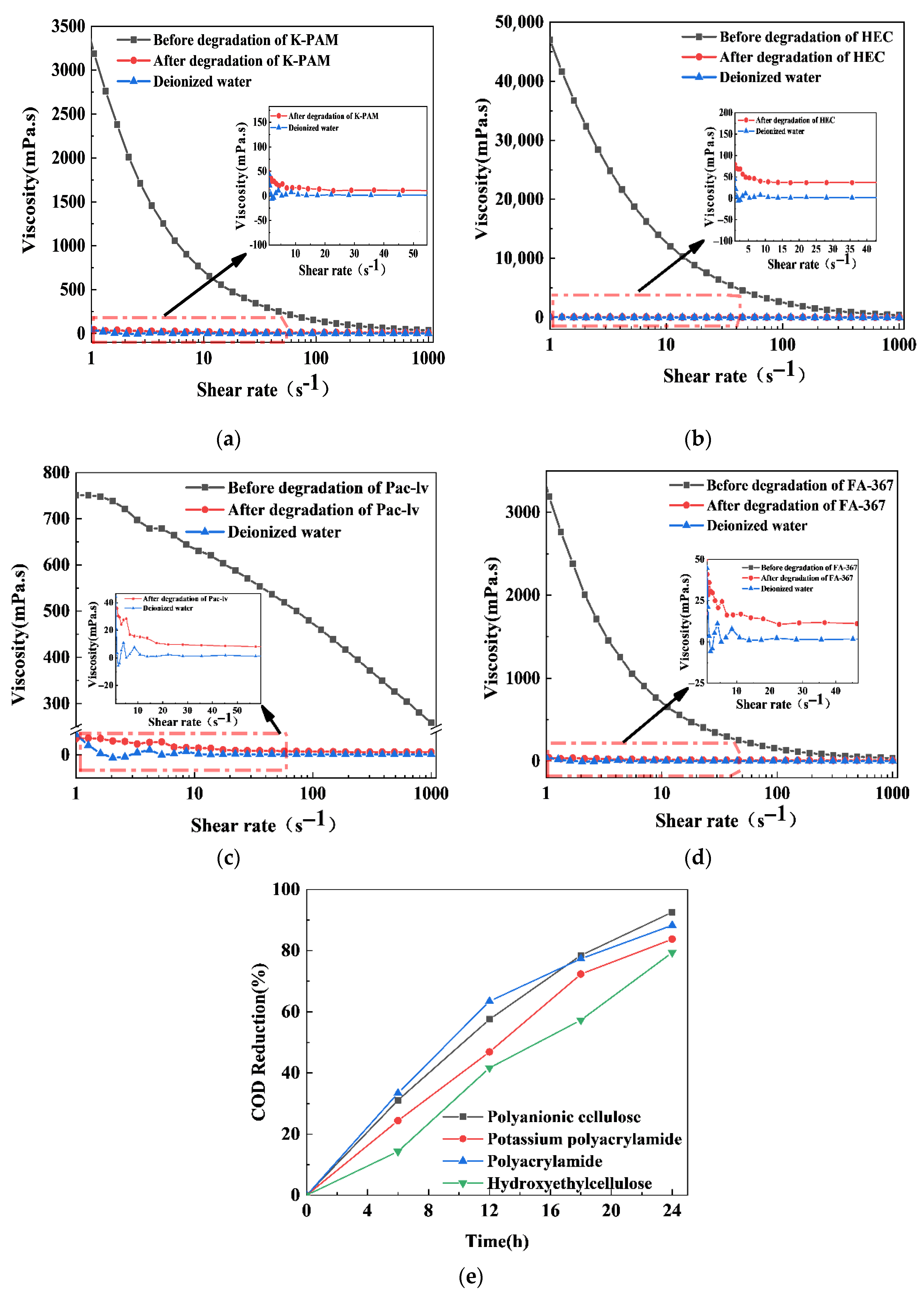
2.9. Photocatalytic Degradation of Polymers by the xS-BiOBr0.5Cl0.5 Materials
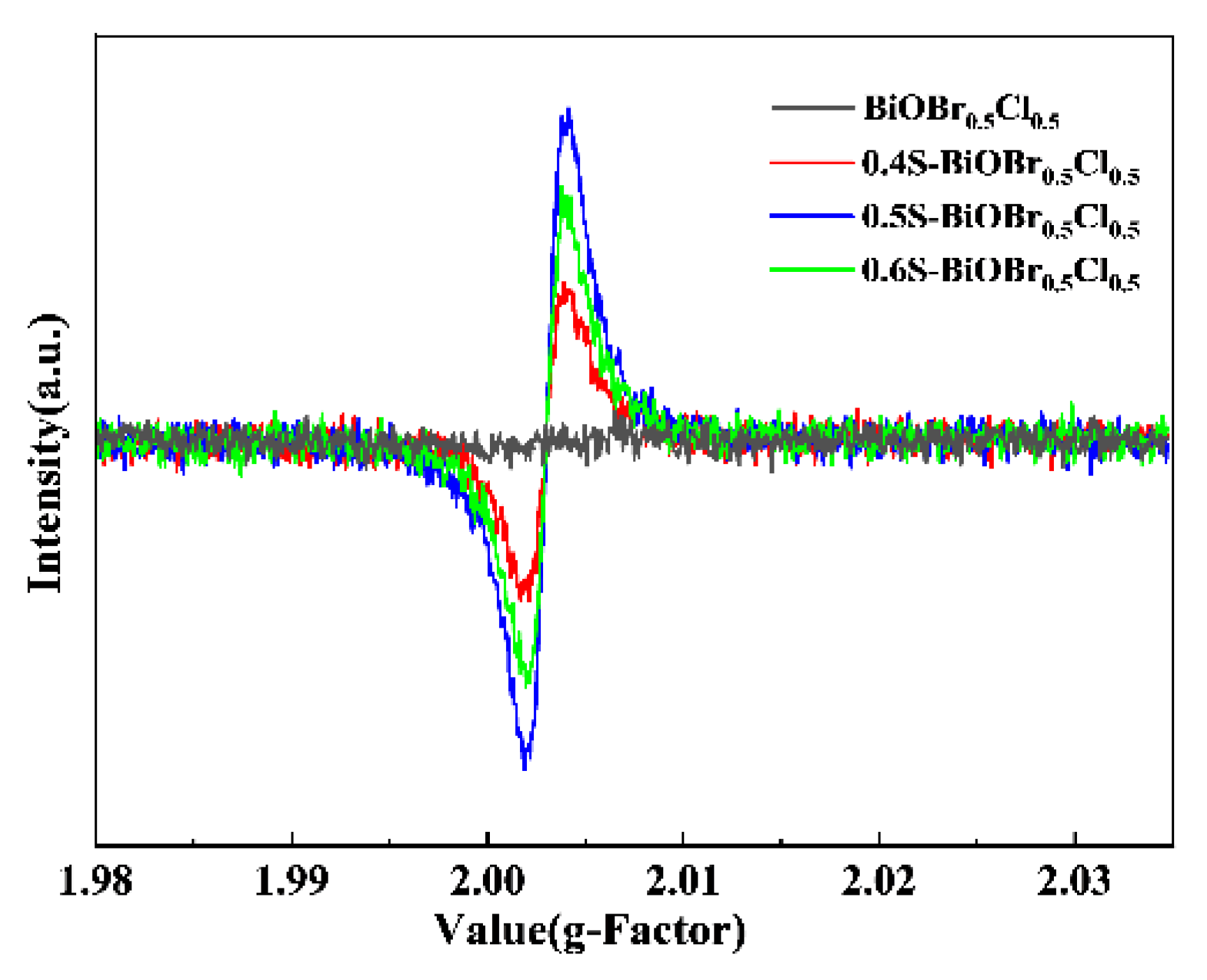
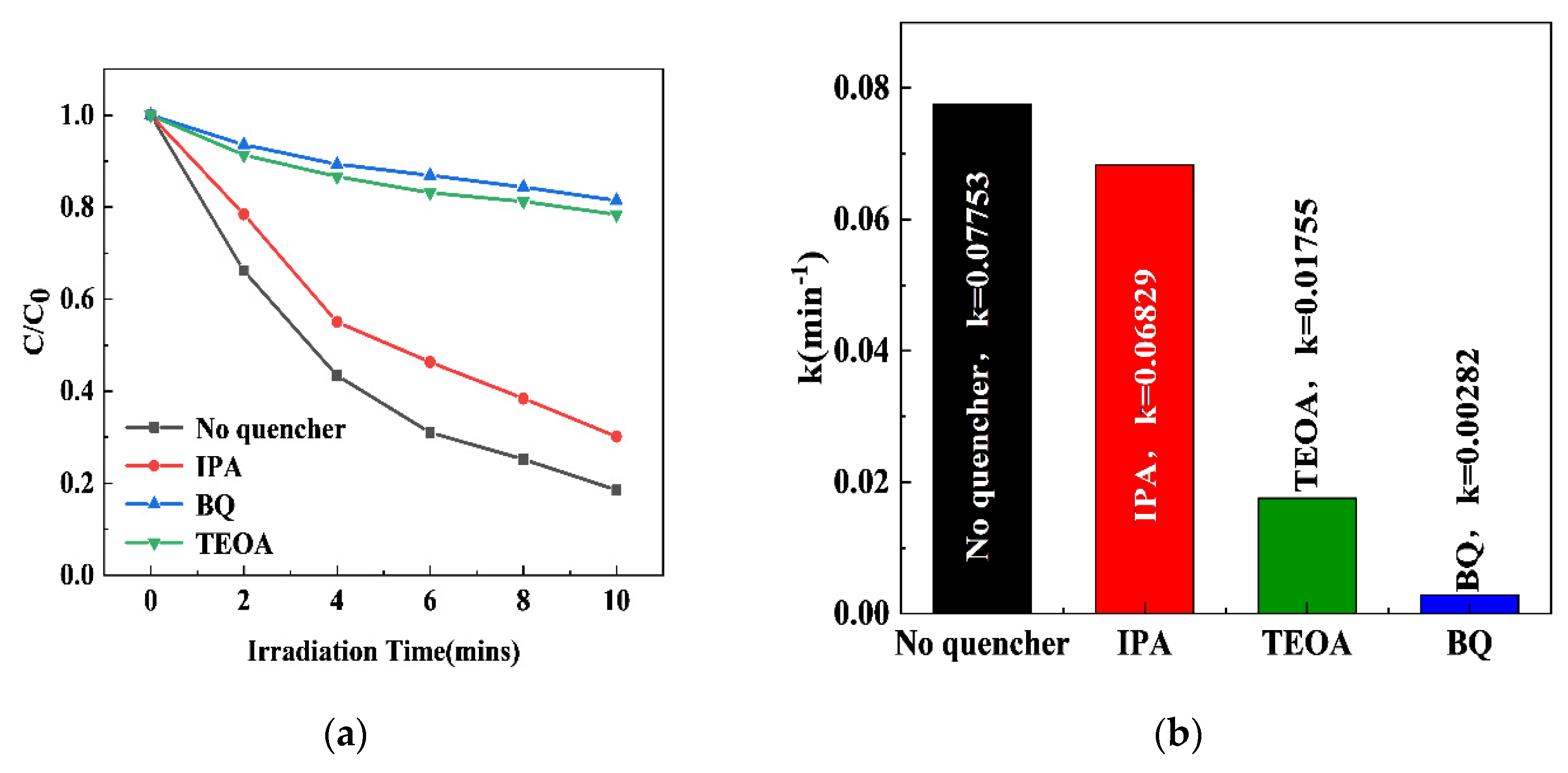
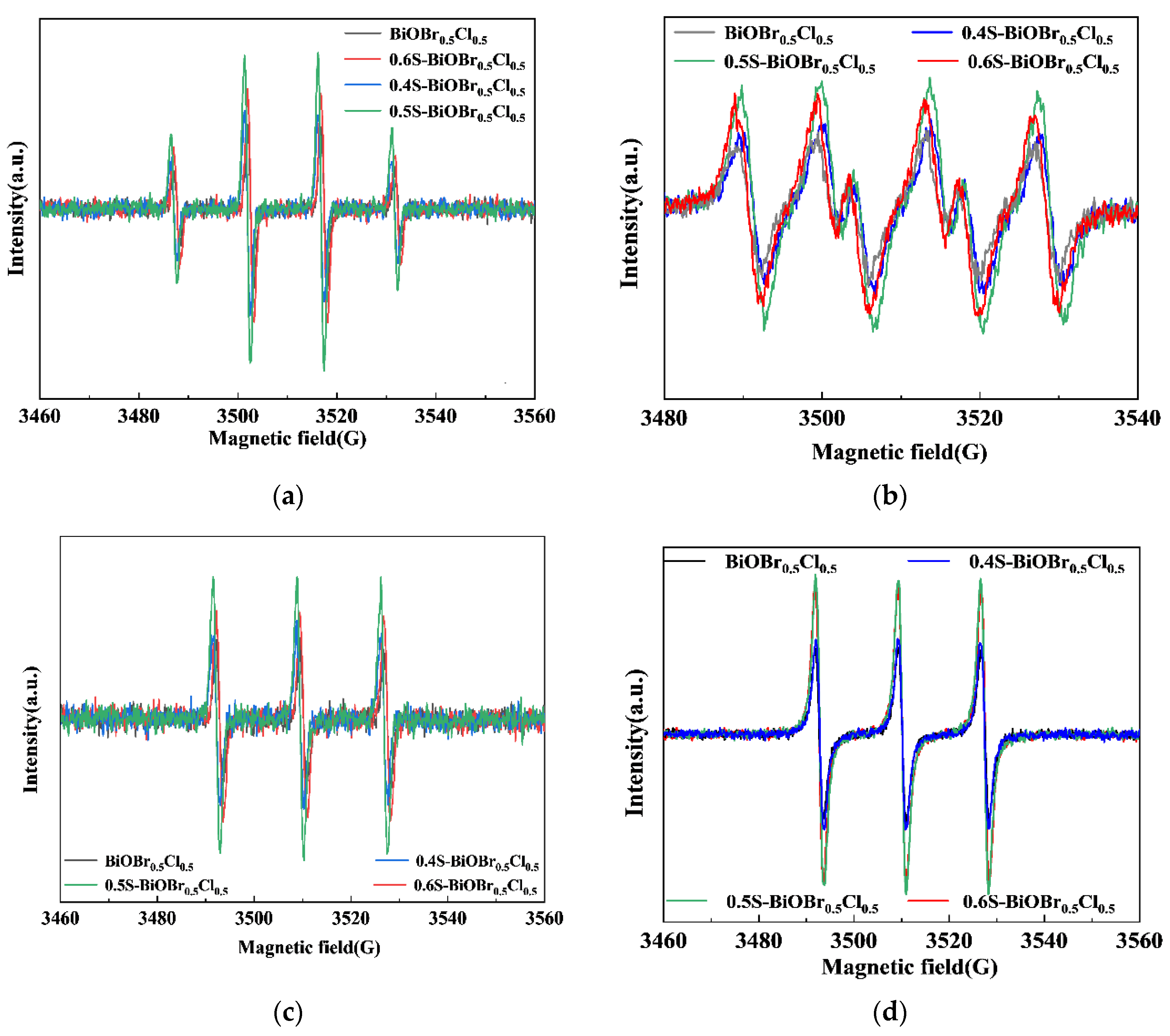
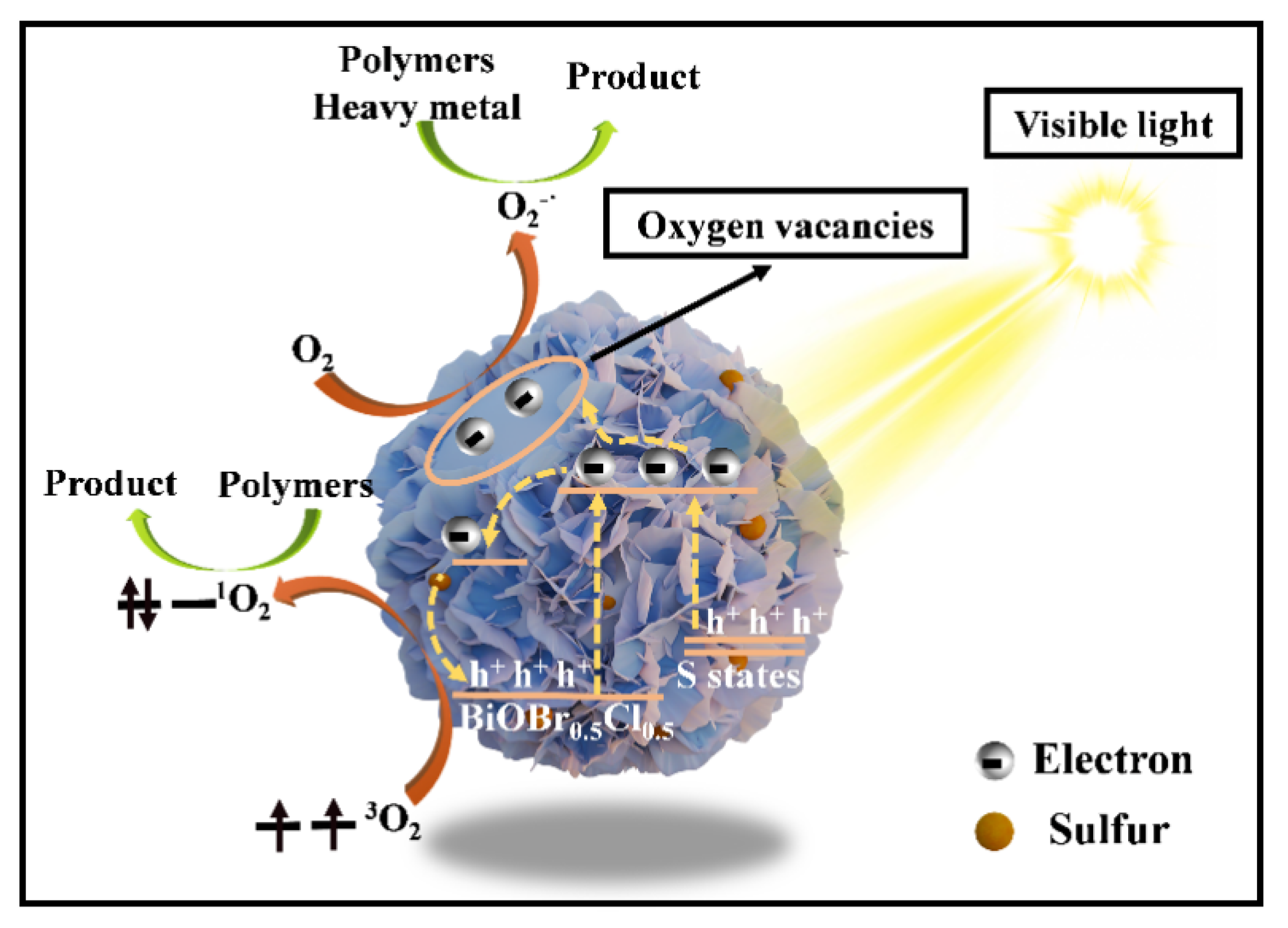
2.10. The Photocatalytic Mechanism of xS-BiOBr0.5Cl0.5 Materials
3. Conclusions
4. Materials and Methods
4.1. Materials
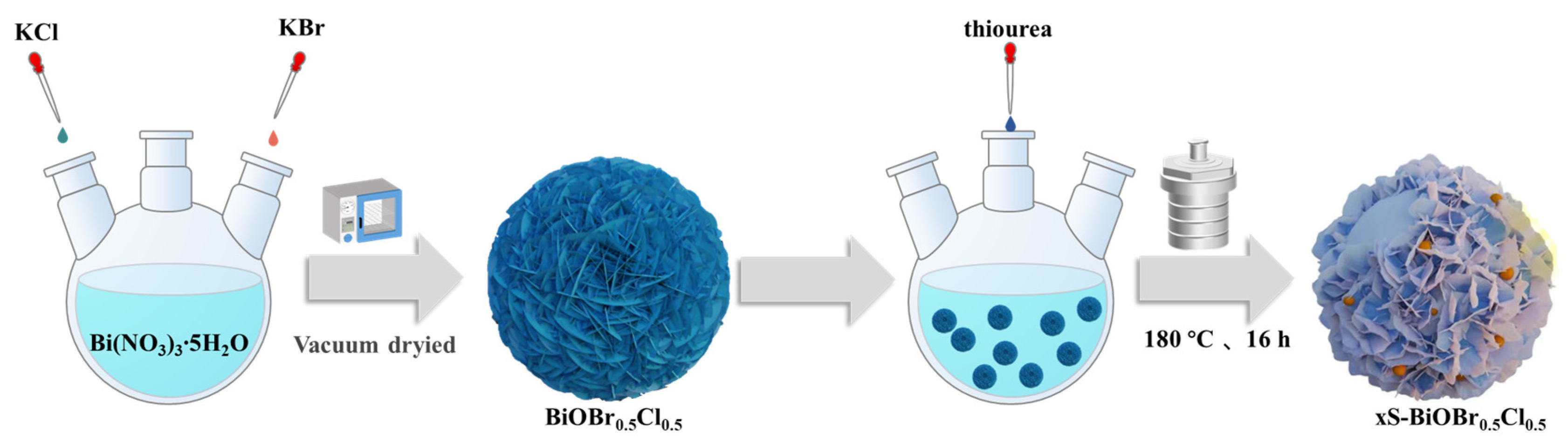
4.2. Synthesis of the xS-BiOBr0.5Cl0.5 Materials
4.3. Characterization of the xS-BiOBr0.5Cl0.5 Materials
4.4. The Photocatalytic Degradation of RhB, Drilling Fluid Polymer Additives, and Heavy Metals
4.5. The Density Functional Theory (DFT) Computations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Jiang, G.; He, Y.; Shi, H.; Du, M.L.; Dong, P.F.; Yang, L.L. Technical difficulties and challenges faced by oil-based drilling fluid. J. China Univ. Pet. Ed. Nat. Sci. 2023, 47, 76–89. [Google Scholar]
- Zou, J.; Zhu, H.; Wang, F.; Sui, H.; Fan, J. Preparation of a new inorganic–organic composite flocculant used in solid–liquid separation for waste drilling fluid. Chem. Eng. J. 2011, 171, 350–356. [Google Scholar] [CrossRef]
- Ozbayoglu, E.M.; Osgouei, R.E.; Ozbayoglu, A.M.; Yuksel, E. Hole-cleaning performance of gasified drilling fluids in horizontal well sections. SPE J. 2012, 17, 912–923. [Google Scholar] [CrossRef]
- Mody, F.K.; Hale, A.H. Borehole-stability model to couple the mechanics and chemistry of drilling-fluid/shale interactions. J. Pet. Technol. 1993, 45, 1093–1101. [Google Scholar] [CrossRef]
- Kausor, M.A.; Gupta, S.S.; Chakrabortty, D. Ag3po4-based nanocomposites and their applications in photodegradation of toxic organic dye contaminated wastewater: Review on material design to performance enhancement. J. Saudi Chem. Soc. 2020, 24, 20–41. [Google Scholar] [CrossRef]
- Zhu, J.; Chakarov, D.; Zch, M. Nanostructured materials for photolytic hydrogen production. In Energy Efficiency and Renewable Energy Through Nanotechnology; Green Energy and Technology; Zang, L., Ed.; Springer: London, UK, 2011; pp. 441–486. [Google Scholar]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants—Sciencedirect. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B. Environ. Int. J. Devoted Catal. Sci. Its Appl. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Casalongue, H.S.; Zhuo, C.; Dai, H. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Shang, N. Photocatalytic degradation of dimethyl phthalate in an aqueous solution with Pt-doped TiO2-coated magnetic PMMA microspheres. J. Hazard. Mater. 2009, 172, 20–29. [Google Scholar] [CrossRef]
- Park, H.A.; Liu, S.; Salvador, P.A.; Rohrer, G.; Islam, M.F. High Visible-Light Photochemical Activity of Titania Decorated on Single-wall Carbon Nanotube Aerogels. RSC Adv. 2016, 6, 22285–22294. [Google Scholar] [CrossRef]
- Mao, L.; Cai, X.; Yang, S.; Han, K.; Zhang, J. Black phosphorus-cds-la2ti2o7 ternary composite: Effective noble metal-free photocatalyst for full solar spectrum activated h2 production. Appl. Catal. B Environ. 2019, 242, 441–448. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Subhiksha, V.; Kokilavani, S.; Khan, S.S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2021, 290, 133228. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, C. A review of bipo4, a highly efficient oxyacid-type photocatalyst, used for environmental applications. Catal. Sci. Technol. 2015, 5, 3071–3079. [Google Scholar]
- Henle, J.; Simon, P.; Frenzel, A.; Scholz, S.; Kaskel, S. Nanosized biox (x = cl, br, i) particles synthesized in reverse microemulsions. Chem. Mater. 2007, 19, 366–373. [Google Scholar] [CrossRef]
- Mi, L.; Feng, Y.; Cao, L.; Xue, M.; Qin, C.; Huang, Y.; Qin, L.; Seo, H.J. Photocatalytic ability of bi6ti3wo18 nanoparticles with a mix-layered aurivillius structure. J. Nanoparticle Res. 2018, 20, 2. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Yu, J.C.; Leung, M.K.H. An efficient bismuth tungstate visible-light-driven photocatalyst for breaking down nitric oxide. Environ. Sci. Technol. 2010, 44, 4276–4281. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Teller, R.G.; Grasselli, R.K.; And, E.K. Structural and Thermodynamic Basis for Catalytic Behavior of Bismuth-Cerium Molybdate Selective Oxidation Catalysts. ACS Symp. Ser. 1985, 279, 57–74. [Google Scholar]
- Cheng, H.; Huang, B.; Dai, Y. Engineering biox (x = cl, br, i) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, R.; Xiong, M.; Yuan, Q.; He, J.; Jia, J.; Yao, M.Y.; Luo, S.L.; Au, C.T.; Yin, S.F. Room-temperature synthesis of flower-like biox (x = cl, br, i) hierarchical structures and their visible-light photocatalytic activity. Inorg. Chem. 2013, 52, 11118–11125. [Google Scholar] [CrossRef]
- Bardos, E.; Kiraly, A.K.; Pap, Z.; Baia, L.; Garg, S.; Hernadi, K. The effect of the synthesis temperature and duration on the morphology and photocatalytic activity of biox (x = cl, br, i) materials. Appl. Surf. Sci. 2019, 479, 745–756. [Google Scholar] [CrossRef]
- Lee, G.J.; Zheng, Y.C.; Wu, J.J. Fabrication of hierarchical bismuth oxyhalides (biox, x = cl, br, i) materials and application of photocatalytic hydrogen production from water splitting. Catal. Today 2017, 39, 698–701. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Yu, C.; Fan, Q.; Liu, X. Solvothermal fabrication of Bi2MoO6 nanocrystals with tunable oxygen vacancies and excellent photocatalytic oxidation performance in quinoline production and antibiotics degradation. Chin. J. Catal. 2022, 43, 472–484. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 1872–2067. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Peng, J.; Yu, X.; Tang, H.; Zhang, J. Hot-electron-assisted s-scheme heterojunction of tungsten oxide/graphitic carbon nitride for broad-spectrum photocatalytic h 2 generation. Chin. J. Catal. 2021, 42, 1478–1487. [Google Scholar] [CrossRef]
- Hou, W.; Xu, H.; Cai, Y.; Zou, Z.; Xia, D. Precisely control interface ovs concentration for enhance 0D/2D Bi2O2CO3/BiOCl photocatalytic performance. Appl. Surf. Sci. 2020, 530, 147218. [Google Scholar] [CrossRef]
- Peng, J.; Shen, J.; Yu, X.; Tang, H.; Liu, Q. Construction of lspr-enhanced 0d/2d cds/moo3 s-scheme heterojunctions for visible-light-driven photocatalytic h2 evolution. Chin. J. Catal. 2021, 42, 87–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ self-doped blue TiO2(b) single-crystalline nanorods for efficient solar-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic performance of bipo4 nanorods adjusted via defects. Appl. Catal. B Environ. Int. J. Devoted Catal. Sci. Its Appl. 2016, 187, 204–211. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Li, J.F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes. Angew. Chem. 2017, 57, 5278–5282. [Google Scholar] [CrossRef]
- Ma, Z.; Li, P.; Ye, L.; Zhou, Y.; Su, F.; Ding, C.; Xie, H.; Bai, Y.; Wong, P.K. Oxygen vacancies induced exciton dissociation of flexible biocl nanosheets for effective photocatalytic CO2 conversion. J. Mater. Chem. A 2017, 5, 24995–25004. [Google Scholar] [CrossRef]
- Zheng, J.; Lyu, Y.; Wang, R.; Xie, C.; Zhou, H.; Jiang, S.P.; Wang, S. Crystalline TiO2 protective layer with graded oxygen defects for efficient and stable silicon-based photocathode. Nat. Commun. 2018, 9, 3572. [Google Scholar] [CrossRef]
- Zeng, L.; Zhe, F.; Wang, Y.; Zhang, Q.; Zhao, X.; Hu, X.; Wu, Y.; He, Y. Preparation of interstitial carbon doped BiOI for enhanced performance in photocatalytic nitrogen fixation and methyl orange degradation. J. Colloid Interface Sci. 2019, 539, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Gao, X.; Zhu, W.; Wang, C.; Li, Q. The hierarchical layered microsphere of bioixbr1-x solid solution decorated with n-doped cqds with enhanced visible light photocatalytic oxidation pollutants. Chem. Eng. J. 2021, 406, 127155. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, X.; Guo, N.; Xu, M.; Jia, D.; Wang, L.; Zha, M. Br-Doped BiOCl Nanosheet Exposed (001) Facet: Surface Oxygen Vacancy and Directed Electron Flow Boosting the Photocatalytic Performance. Langmuir 2023, 39, 18073–18081. [Google Scholar] [CrossRef]
- Liu, Y.; Son, W.J.; Lu, J.; Huang, B.; Dai, Y.; Whangbo, M.H. Cheminform abstract: Composition dependence of the photocatalytic activities of biocl1-xbrx solid solutions under visible light. ChemInform 2011, 42, 45. [Google Scholar] [CrossRef]
- Liang, J.; Deng, J.; Li, M.; Tong, M. Bactericidal activity and mechanism of agi/agbr/biobr0.75i0.25 under visible light irradiation. Colloids Surf. B Biointerfaces 2016, 138, 102–109. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Li, T.; Xing, X.; Fan, W.; Zhang, L.; Hu, C. Enhanced internal electric field in s-doped biobr for intercalation, adsorption and degradation of ciprofloxacin by photoinitiation. Appl. Catal. B Environ. 2021, 302, 120824. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Jimmy, C.Y. Photocatalytic degradation of ibuprofen on S-doped BiOBr. Chemosphere 2021, 278, 130376. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Liu, D.; Wang, W.; Zhao, Z.; Cui, F.; Li, G. Oxygen vacancy-rich ultrathin sulfur-doped bismuth oxybromide nanosheet as a highly efficient visible-light responsive photocatalyst for environmental remediation. Chem. Eng. J. 2018, 360, 838–847. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C. Rapid synthesis of hierarchical biocl microspheres for efficient photocatalytic degradation of carbamazepine under simulated solar irradiation. Chem. Eng. J. 2015, 263, 419–426. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Wang, Q.; Wu, Q. High 001 facets dominated biobr lamellas: Facile hydrolysis preparation and selective visible-light photocatalytic activity. J. Mater. Chem. A 2013, 1, 8622. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/zno heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Liu, Z.Y.J.C. Liquid bismuth initiated growth of phosphorus microbelts with efficient charge polarization for photocatalysis. Appl. Catal. B. Environ. Int. J. Devoted Catal. Sci. Its Appl. 2019, 247, 100–106. [Google Scholar] [CrossRef]
- Rezaei, M.; Rastegari Mehr, M.; Keshavarzi, B.; Moore, F.; Sorooshian, A.; Soltani, N.; Sadegh, M. Heavy metal content and potential health risk of geogenic and anthropogenic soils close to oil fields in southern Iran. Environ. Geochem. Health 2020, 42, 2409–2428. [Google Scholar]
- Al-Hameedi, A.T.T.; Alkinani, H.H.; Dunn-Norman, S.; Al-Alwani, M.A.; Alshammari, A.F.; Alkhamis, M.M. Experimental Investigation of Environmentally Friendly Drilling Fluid Additives. SPE Drill. Complet. 2019, 34, 352–366. [Google Scholar]
- Lv, Y.; Pan, C.; Ma, X.; Zong, R.; Bai, X.; Zhu, Y. Production of visible activity and uv performance enhancement of zno photocatalyst via vacuum deoxidation. Appl. Catal. B Environ. 2013, 138–139, 26–32. [Google Scholar] [CrossRef]
- Jia, T.; Popczun, E.J.; Lekse, J.W.; Duan, Y.; Yan, J. Effective Ca2+-doping in Sr1-xCaxFeO3-δ oxygen carriers for chemical looping air separation: A theoretical and experimental investigation. Appl. Energy 2021, 281, 116040. [Google Scholar] [CrossRef]
- Shah, V.; Cheng, Z.; Baser, D.S.; Fan, J.A.; Fan, L.S. Highly selective production of syngas from chemical looping reforming of methane with CO2 utilization on mgo-supported calcium ferrite redox materials. Appl. Energy 2021, 282, 116111. [Google Scholar] [CrossRef]
- Ohashi, N.; Nakata, T.; Sekiguchi, T.; Hosono, H.; Haneda, H. Yellow emission from zinc oxide giving an electron spin resonance signal at g = 1.96. Jpn. J. Appl. Phys. 1999, 38 Pt 2, L113–L115. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Ahmed, S.A.; Khairou, K.S.; Mokhtar, M. Carbon nanotube/titanium nanotube composites loaded platinum nanoparticles as high performance photocatalysts. Appl. Catal. A Gen. 2014, 475, 90–97. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Xie, B. Ultrathin two-dimensional biocl with oxygen vacancies anchored in three-dimensional porous g-C3N4 to construct a hierarchical z-scheme heterojunction for the photocatalytic degradation of no. Ind. Eng. Chem. Res. 2022, 61, 317–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, T.; Jiang, G.; Jiang, S.; He, Y.; Yang, L. Degradation of Polymers and Heavy Metals in Waste Drilling Fluid by Sulfur-Doped BiOBr0.5Cl0.5 Photocatalysts. Gels 2025, 11, 684. https://doi.org/10.3390/gels11090684
Dong T, Jiang G, Jiang S, He Y, Yang L. Degradation of Polymers and Heavy Metals in Waste Drilling Fluid by Sulfur-Doped BiOBr0.5Cl0.5 Photocatalysts. Gels. 2025; 11(9):684. https://doi.org/10.3390/gels11090684
Chicago/Turabian StyleDong, Tengfei, Guancheng Jiang, Sihe Jiang, Yinbo He, and Lili Yang. 2025. "Degradation of Polymers and Heavy Metals in Waste Drilling Fluid by Sulfur-Doped BiOBr0.5Cl0.5 Photocatalysts" Gels 11, no. 9: 684. https://doi.org/10.3390/gels11090684
APA StyleDong, T., Jiang, G., Jiang, S., He, Y., & Yang, L. (2025). Degradation of Polymers and Heavy Metals in Waste Drilling Fluid by Sulfur-Doped BiOBr0.5Cl0.5 Photocatalysts. Gels, 11(9), 684. https://doi.org/10.3390/gels11090684




