Soft Gels in Food Systems: Recent Advances, Applications, and Technological Innovations
Abstract
1. Introduction
2. Types of Soft Gels in the Food Industry
2.1. Hydrogels
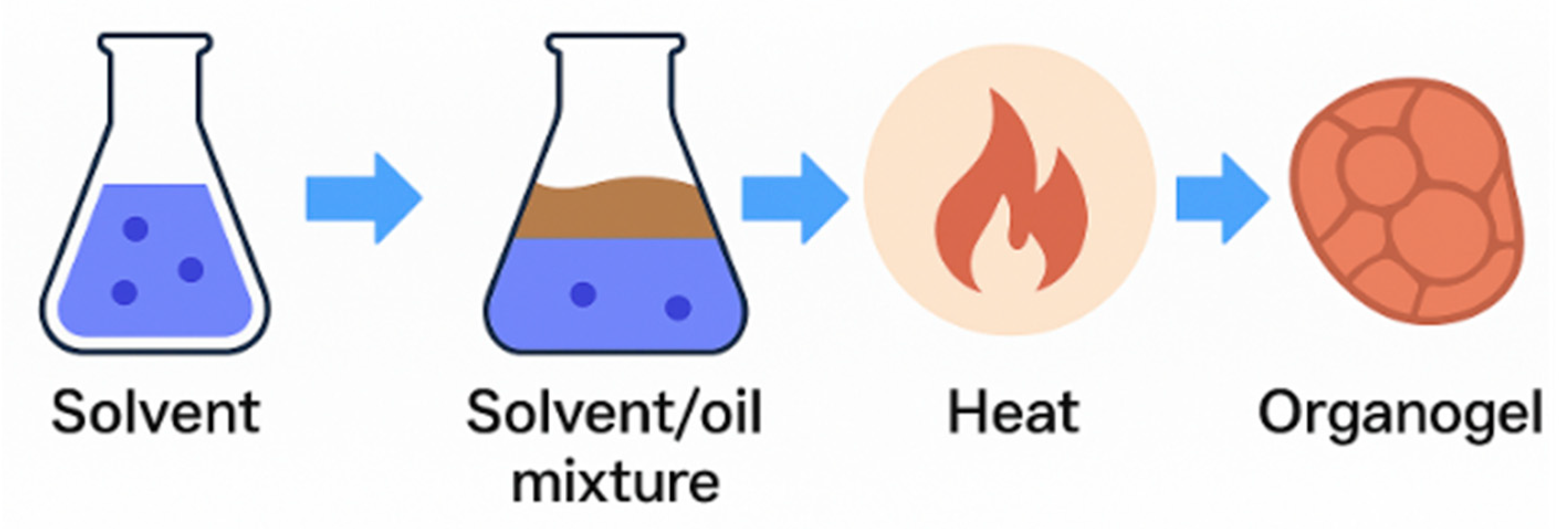
2.2. Organogel
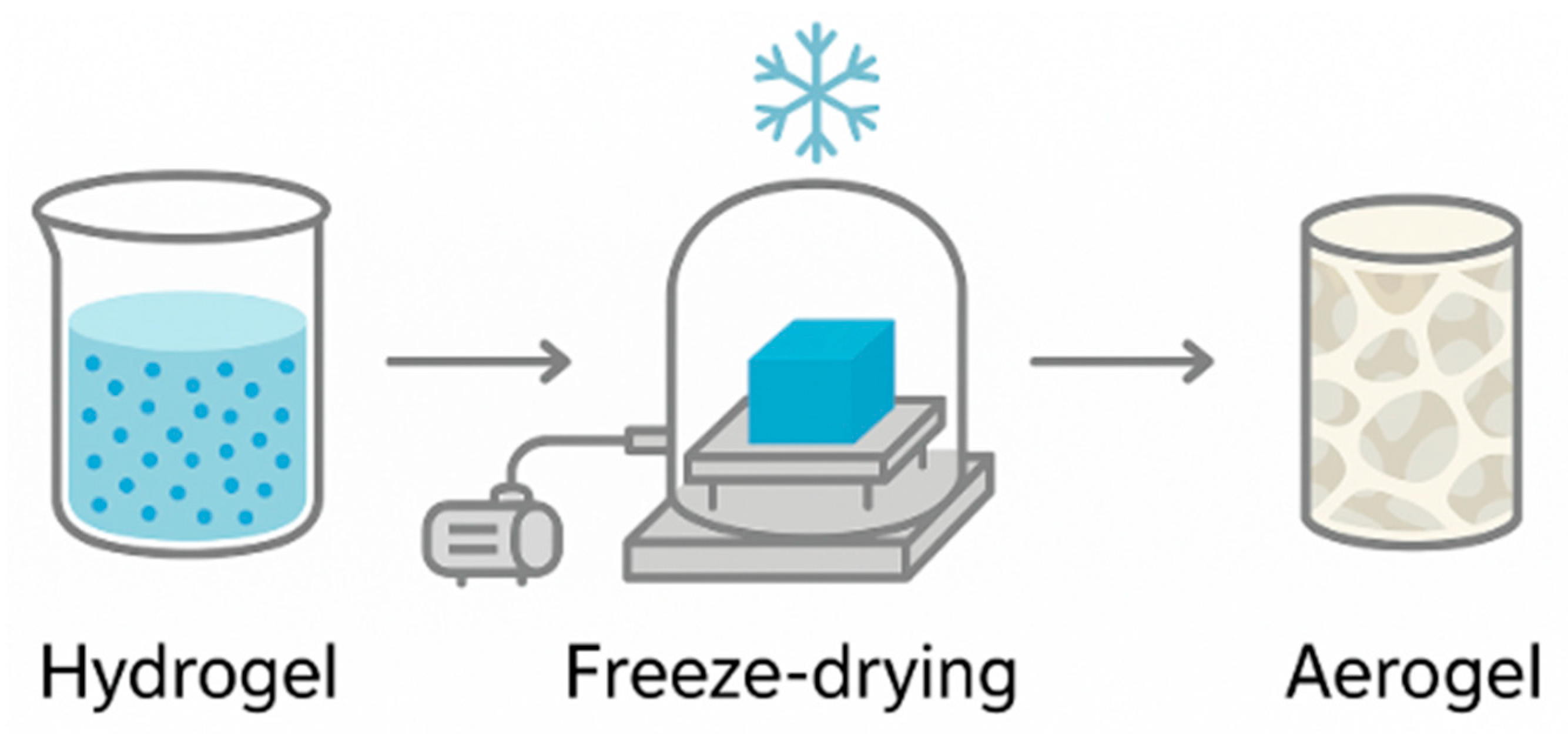
2.3. Aerogels
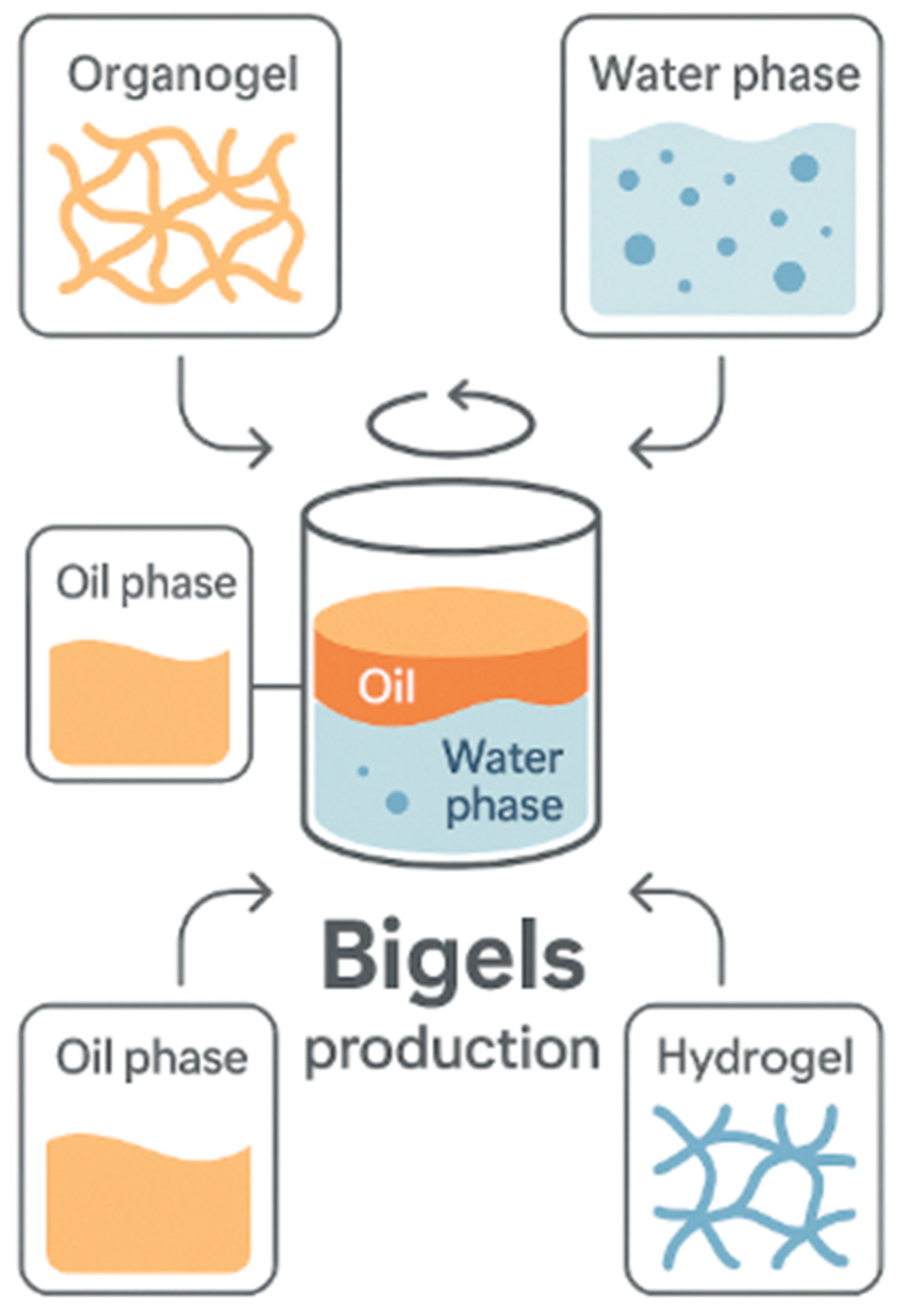
2.4. Bigels
3. Functional Roles of Soft Gels in the Food Industry
3.1. Texture Modification
3.2. Gels as Deep Frying Media
3.3. Encapsulation and Controlled Release
3.4. Fat Replacement and Caloric Reduction
3.5. Stabilization and Shelf-Life Enhancement
4. Innovative Technologies in Soft Gel Production
4.1. Three-Dimensional Printing of Soft Gels
4.2. Nanotechnology Approaches
5. Regulatory and Safety Aspects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, N.; Hamid; Choudhary, P.; Jaiswal, A.K. Recent Progress in Bioactive Loaded Hydrogels for Food Applications. J. Agric. Food Res. 2025, 20, 101756. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-Based Systems for the Delivery of Bioactive Compounds in Foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef]
- Leite, A.C.; Pereira, R.N.; Rodrigues, R.M. Protein Aerogels as Food-Grade Delivery Systems—A Comprehensive Review. Food Hydrocoll. 2025, 163, 111138. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A Unique Class of Materials for Drug Delivery Applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Zampouni, K.; Dimakopoulou-Papazoglou, D.; Katsanidis, E. Food-Grade Bigel Systems: Formulation, Characterization, and Applications for Novel Food Product Development. Gels 2024, 10, 712. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and Multi-Component Organogels: An Overview from Rheological Perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, L.; McClements, D.J.; Qiu, C.; Li, C.; Zhang, Z.; Miao, M.; Tian, Y.; Zhu, K.; Jin, Z. Stimulus-Responsive Hydrogels in Food Science: A Review. Food Hydrocoll. 2022, 124, 107218. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Padhiary, M.; Hoque, A.; Prasad, G. 3D Printing Technology for Valorization of Food Processing Wastes and Byproducts: A Systematic Review. Waste Manag. Bull. 2025, 3, 100192. [Google Scholar] [CrossRef]
- Neamah, H.A.; Tandio, J. Towards the Development of Foods 3D Printer: Trends and Technologies for Foods Printing. Heliyon 2024, 10, e33882. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2022, 9, 1. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Tong, Q.; Decker, E.A.; McClements, D.J. Food-Grade Filled Hydrogels for Oral Delivery of Lipophilic Active Ingredients: Temperature-Triggered Release Microgels. Food Res. Int. 2015, 69, 274–280. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and Application as Delivery Systems of Phenolic and Aroma Compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wanniarachchi, P.C.; Paranagama, I.T.; Idangodage, P.A.; Nallaperuma, B.; Samarasinghe, T.T.; Jayathilake, C. Natural Polymer-based Hydrogels: Types, Functionality, Food Applications, Environmental Significance and Future Perspectives: An Updated Review. Food Biomacromol. 2025, 2, 84–105. [Google Scholar] [CrossRef]
- May, C.D. Industrial Pectins: Sources, Production and Applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Machado, M.; Silva, S.; Costa, E. Uses of Gellan Gum for Nutrient Delivery. In Application of Gellan Gum as a Biomedical Polymer; Elsevier: Amsterdam, The Netherlands, 2024; pp. 309–321. [Google Scholar]
- Gomes, D.; Batista-Silva, J.P.; Sousa, A.; Passarinha, L.A. Progress and Opportunities in Gellan Gum-Based Materials: A Review of Preparation, Characterization and Emerging Applications. Carbohydr. Polym. 2023, 311, 120782. [Google Scholar] [CrossRef]
- Bradbeer, J.F.; Hancocks, R.; Spyropoulos, F.; Norton, I.T. Low Acyl Gellan Gum Fluid Gel Formation and Their Subsequent Response with Acid to Impact on Satiety. Food Hydrocoll. 2015, 43, 501–509. [Google Scholar] [CrossRef]
- Li, D.; Chen, E.; Chen, H.; Zhou, H.; Li, B.; Li, Y. Impact of Whey Protein Isolates and Concentrates on the Formation of Protein Nanoparticles-Stabilised Pickering Emulsions. Int. J. Food Sci. Technol. 2018, 53, 644–653. [Google Scholar] [CrossRef]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Pereira, C.J.D.; Gil, M.H.M. Effects of Liquid Whey Protein Concentrate on Functional and Sensorial Properties of Set Yogurts and Fresh Cheese. Food Bioprocess Technol. 2013, 6, 952–963. [Google Scholar] [CrossRef]
- Shiroodi, S.G.; Lo, Y.M. The Effect of PH on the Rheology of Mixed Gels Containing Whey Protein Isolate and Xanthan-Curdlan Hydrogel. J. Dairy Res. 2015, 82, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and Soy Protein-Based Hydrogels and Nano-Hydrogels as Bioactive Delivery Systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Malik, B.; Chawla, R.; Khatkar, S.K. Protein Hydrogels: A Concise Review of Properties and Applications. Int. J. Pept. Res. Ther. 2023, 29, 94. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design Principles of Food Gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Pascuta, M.S.; Varvara, R.-A.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemeş, S.-A.; Mitrea, L.; Martău, G.A.; Ciont, C.; Călinoiu, L.F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients: Characterization, Applicability, and Human Health Benefits. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Sandhya, R.; Nisha, P. Advances and Prospects in the Food Applications of Pectin Hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Zhu, C.; Xiao, J.; Cao, H.; Simal-Gandara, J.; Li, Y.; Fan, D.; Deng, J. A Comprehensive Review of Food Gels: Formation Mechanisms, Functions, Applications, and Challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 760–782. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and Textural Properties of Organogels Developed by Candelilla Wax in Safflower Oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Marangoni, A.G. Organogels: An Alternative Edible Oil-Structuring Method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible Applications of Shellac Oleogels: Spreads, Chocolate Paste and Cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Rice Bran Wax in Bulk and Organogels. J. Am. Oil Chem. Soc. 2009, 86, 1163–1173. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Development, Characterization, and Utilization of Food-Grade Polymer Oleogels. Annu. Rev. Food Sci. Technol. 2016, 7, 65–91. [Google Scholar] [CrossRef]
- Okuro, P.K.; Malfatti-Gasperini, A.A.; Vicente, A.A.; Cunha, R.L. Lecithin and Phytosterols-Based Mixtures as Hybrid Structuring Agents in Different Organic Phases. Food Res. Int. 2018, 111, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Banaś, K. Lecithin’s Roles in Oleogelation. Gels 2024, 10, 169. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Banerjee, I.; Pramanik, K.; Basak, P.; Pal, K. Core-Shell-Type Organogel-Alginate Hybrid Microparticles: A Controlled Delivery Vehicle. Chem. Eng. J. 2015, 264, 134–145. [Google Scholar] [CrossRef]
- de Oliveira, G.M.; Stahl, M.A.; Ribeiro, A.P.B.; Grimaldi, R.; Cardoso, L.P.; Kieckbusch, T.G. Development of Zero Trans/Low Sat Fat Systems Structured with Sorbitan Monostearate and Fully Hydrogenated Canola Oil. Eur. J. Lipid Sci. Technol. 2015, 117, 1762–1771. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Effraimopoulou, E.; Jaxel, J.; Budtova, T.; Rigacci, A. Hydrophobic Modification of Pectin Aerogels via Chemical Vapor Deposition. Polymers 2024, 16, 1628. [Google Scholar] [CrossRef]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-Based Aerogel Particles for Drug Delivery: Effect of Pectin Composition on Aerogel Structure and Release Properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef]
- Panda, D.; Gangawane, K.M. Recycled Cellulose-Silica Hybrid Aerogel for Effective Oil Adsorption: Optimization and Kinetics Study. Sādhanā 2023, 48, 110. [Google Scholar] [CrossRef]
- Gibowsky, L.; De Berardinis, L.; Plazzotta, S.; Manke, E.; Jung, I.; Méndez, D.A.; Heidorn, F.; Liese, G.; Husung, J.; Liese, A.; et al. Conversion of Natural Tissues and Food Waste into Aerogels and Their Application in Oleogelation. Green Chem. 2025, 27, 4713–4731. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Construction of Three-Dimensional Aerogels from Electrospun Cellulose Fibers as Highly Efficient and Reusable Oil Absorbents. Sep. Purif. Technol. 2025, 353, 128604. [Google Scholar] [CrossRef]
- Manzocco, L.; Mikkonen, K.S.; García-González, C.A. Aerogels as Porous Structures for Food Applications: Smart Ingredients and Novel Packaging Materials. Food Struct. 2021, 28, 100188. [Google Scholar] [CrossRef]
- Kaster, J.B.; Cruz, E.P.; da Silva, F.T.; dos Santos Hackbart, H.C.; Siebeneichler, T.J.; Camargo, T.M.; Radünz, M.; Fonseca, L.M.; da Rosa Zavareze, E. Bioactive Aerogels Based on Native and Phosphorylated Potato (Solanum tuberosum L.) Starches Incorporated with Star Fruit Extract (Averrhoa carambola L.). Int. J. Biol. Macromol. 2024, 272, 132907. [Google Scholar] [CrossRef]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and Characterization of Chitosan-Coated Pectin Aerogels: Curcumin Case Study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef]
- Zanotti, A.; Baldino, L.; Reverchon, E.; Cardea, S. Alginate/k-Carrageenan Interpenetrated Biopolymeric Aerogels for Nutraceutical Drug Delivery. Gels 2025, 11, 393. [Google Scholar] [CrossRef]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of κ-Carrageenan Aerogels as Template for Edible Oleogel Preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and Characterization of Novel Bigels Based on Monoglyceride-Beeswax Oleogel and High Acyl Gellan Gum Hydrogel for Lycopene Delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Bollom, M.A.; Clark, S.; Acevedo, N.C. Edible Lecithin, Stearic Acid, and Whey Protein Bigels Enhance Survival of Probiotics during in Vitro Digestion. Food Biosci. 2021, 39, 100813. [Google Scholar] [CrossRef]
- Kaimal, A.M.; Singhal, R.S. Bigels for Controlled Gastric Release of Ascorbic Acid: Impact on Rheology, Texture, Thermal Stability and Antioxidant Activity. Food Hydrocoll. Health 2023, 4, 100171. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Zhao, R.; Shi, Q.; Nian, Y.; Hu, B. Hydrogels as Promising Carriers for the Delivery of Food Bioactive Ingredients. Front. Nutr. 2022, 9, 1006520. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Oleogel-based Emulsions: Concepts, Structuring Agents, and Applications in Food. J. Food Sci. 2021, 86, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Gani, A. Alginate-Based PH-Sensitive Hydrogels Encoated with Chitosan as a Bioactive Cargo Carrier with Caffeic Acid as a Model Biomolecule. ACS Food Sci. Technol. 2022, 2, 667–672. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Valoppi, F.; Pal, K. Oleogels and Organogels: A Promising Tool for New Functionalities. Gels 2022, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Apostolides, D.E.; Patrickios, C.S. Dynamic Covalent Polymer Hydrogels and Organogels Crosslinked through Acylhydrazone Bonds: Synthesis, Characterization and Applications. Polym. Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Tang, C.; Wan, Z.; Chen, Y.; Tang, Y.; Fan, W.; Cao, Y.; Song, M.; Qin, J.; Xiao, H.; Guo, S.; et al. Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol. Foods 2022, 11, 939. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef]
- Silva, R.C.d.; Ferdaus, M.J.; Foguel, A.; da Silva, T.L.T. Oleogels as a Fat Substitute in Food: A Current Review. Gels 2023, 9, 180. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising Alternatives to Solid Fats for Food Applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Naqash, F.; Ahmad, M. Oleogels for the Development of Healthy Meat Products: A Review. Appl. Food Res. 2022, 2, 100212. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Arshad, R.; Mazhar, F.B.; Arshad, K.; Xu, B. Unlocking the Potential of Oleogels in Edible Applications and Health Impacts. Appl. Food Res. 2024, 4, 100620. [Google Scholar] [CrossRef]
- Lopez-Martínez, A.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Monoglyceride Organogels Developed in Vegetable Oil with and without Ethylcellulose. Food Res. Int. 2015, 72, 37–46. [Google Scholar] [CrossRef]
- Gaggero, G.; Subrahmanyam, R.P.; Schroeter, B.; Gurikov, P.; Delucchi, M. Organic Bio-Based Aerogel from Food Waste: Preparation and Hydrophobization. Gels 2022, 8, 691. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U. Hybrid Materials of Bio-Based Aerogels for Sustainable Packaging Solutions. Gels 2023, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Jarms, J.; Borzęcka, N.H.; Serrador Goncalves, B.; Ganesan, K.; Milow, B.; Rege, A. Modeling of the Gelation Process in Cellulose Aerogels. Biomacromolecules 2025, 26, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Patel, R.; Patel, M. Biopolymer-Based Biomimetic Aerogel for Biomedical Applications. Biomimetics 2024, 9, 397. [Google Scholar] [CrossRef]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef]
- Lovskaya, D.; Bezchasnyuk, A.; Mochalova, M.; Tsygankov, P.; Lebedev, A.; Zorkina, Y.; Zubkov, E.; Ochneva, A.; Gurina, O.; Silantyev, A.; et al. Preparation of Protein Aerogel Particles for the Development of Innovative Drug Delivery Systems. Gels 2022, 8, 765. [Google Scholar] [CrossRef]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Feasibility Study of Hydrophilic and Hydrophobic Silica Aerogels as Drug Delivery Systems. J. Non-Cryst. Solids 2004, 350, 54–60. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Bioaerogels as Food Materials: A State-of-the-Art on Production and Application in Micronutrient Fortification and Active Packaging of Foods. Food Hydrocoll. 2022, 131, 107760. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic Nanocellulose Aerogels with Ordered Structures Fabricated by Directional Freeze-Drying for Fast Liquid Transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Caminero-Huertas, S.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Effects of Freeze-Drying Conditions on Aerogel Properties. J. Mater. Sci. 2016, 51, 8977–8985. [Google Scholar] [CrossRef]
- Xiao, H.; Lv, J.; Tan, W.; He, X.; Chen, M.; Zeng, K.; Hu, J.; Yang, G. Ultrasound-Assisted Freeze-Drying Process for Polyimide Aerogels. Chem. Eng. J. 2022, 450, 138344. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Filipič, M.; Jose Frutos, M.; Galtier, P.; Gott, D.; et al. Re-evaluation of Celluloses E 460(i), E 460(Ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as Food Additives. EFSA J. 2018, 16, e05047. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of Alginic Acid and Its Sodium, Potassium, Ammonium and Calcium Salts (E 400–E 404) as Food Additives. EFSA J. 2017, 15, e05049. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Manco, M.; et al. Opinion on the Re-evaluation of Pectin (E 440i) and Amidated Pectin (E 440ii) as Food Additives in Foods for Infants below 16 Weeks of Age and Follow-up of Their Re-evaluation as Food Additives for Uses in Foods for All Population Groups. EFSA J. 2021, 19, e06387. [Google Scholar] [CrossRef]
- Martins, A.J.; Guimarães, A.; Fuciños, P.; Sousa, P.; Venâncio, A.; Pastrana, L.M.; Cerqueira, M.A. Food-Grade Bigels: Evaluation of Hydrogel: Oleogel Ratio and Gelator Concentration on Their Physicochemical Properties. Food Hydrocoll. 2023, 143, 108893. [Google Scholar] [CrossRef]
- Francavilla, A.; Corradini, M.G.; Joye, I.J. Bigels as Delivery Systems: Potential Uses and Applicability in Food. Gels 2023, 9, 648. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, V.K.; Uvanesh, K.; Biswal, D.; Anis, A.; Rana, U.A.; Al-Zahrani, S.M.; Pal, K. Development of Ionic and Non-ionic Natural Gum-based Bigels: Prospects for Drug Delivery Application. J. Appl. Polym. Sci. 2015, 132, 42561. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of Food-Grade Bigels Based on κ-Carrageenan Hydrogel and Monoglyceride Oleogels as Carriers for β-Carotene: Roles of Oleogel Fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid Gels: Influence of Oleogel/Hydrogel Ratio on Rheological and Textural Properties. Food Res. Int. 2019, 116, 1298–1305. [Google Scholar] [CrossRef]
- Chao, E.; Li, J.; Fan, L. Design of Bigel-Based Co-Delivery Systems for Lipophilic/Hydrophilic Nutraceuticals: Focus on Rheological Properties and in Vitro Digestive Performance. Food Hydrocoll. 2025, 162, 111017. [Google Scholar] [CrossRef]
- Eisinaitė, V.; Jasutienė, I.; Vinauskienė, R.; Leskauskaitė, D. Development of Bigel Based Dysphagia-oriented Products, Structured with Collagen and Carnauba Wax: Characterisation and Rheological Behaviour. Int. J. Food Sci. Technol. 2023, 58, 145–153. [Google Scholar] [CrossRef]
- Qiu, R.; Liu, X.; Tian, H.; Hu, Z.; Wang, K.; Zhao, L. Exploration of Bigel 4D Printing with Spontaneous Colour Change for Monitoring Bio-Actives Kinetic Behaviour Based on the Dual-Units 3D Printer. J. Food Eng. 2024, 367, 111861. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Zhang, Y.; Shen, Y.; Deng, X.; Wang, F. Novel Bigels Based on Walnut Oil Oleogel and Chitosan Hydrogel: Preparation, Characterization, and Application as Food Spread. Int. J. Biol. Macromol. 2024, 260, 129530. [Google Scholar] [CrossRef]
- Cui, H.; Tang, C.; Wu, S.; Julian McClements, D.; Liu, S.; Li, B.; Li, Y. Fabrication of Chitosan-Cinnamaldehyde-Glycerol Monolaurate Bigels with Dual Gelling Effects and Application as Cream Analogs. Food Chem. 2022, 384, 132589. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lu, Y.; Cui, M.; Miao, S.; Gao, Y. Design of Gel Structures in Water and Oil Phases for Improved Delivery of Bioactive Food Ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef]
- Coria-Hernández, J.; Méndez-Albores, A.; Meléndez-Pérez, R.; Rosas-Mendoza, M.; Arjona-Román, J. Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing. Polymers 2018, 10, 359. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Xiong, G.; Wu, W.; Wang, J.; Qiao, Y.; Liao, L. The Effect of Freeze-Thaw Cycles on Microstructure and Physicochemical Properties of Four Starch Gels. Food Hydrocoll. 2013, 31, 61–67. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qin, S.; Pei, Y.; Zheng, X.; Tang, K. High Mechanical Strength Gelatin Composite Hydrogels Reinforced by Cellulose Nanofibrils with Unique Beads-on-a-String Morphology. Int. J. Biol. Macromol. 2020, 164, 1776–1784. [Google Scholar] [CrossRef]
- Luthfianti, H.R.; Waresindo, W.X.; Rodhiyah, M.; Edikresnha, D.; Noor, F.A.; Elfahmi, E.; Khairurrijal, K. Tunable Physical Properties of Starch-Based Hydrogels Synthesized by Freeze-Thaw Technique. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, Q.; Liu, X.; Liu, F.; McClements, D.J. Double Network Hydrogels: Design, Fabrication, and Application in Biomedicines and Foods. Adv. Colloid Interface Sci. 2023, 320, 102999. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, Y.; Hu, M.; Wang, Y.; Dai, D.; Ma, L.; Zhang, Y.; Dai, H. Recent Advances in Nanocellulose Based Hydrogels: Preparation Strategy, Typical Properties and Food Application. Int. J. Biol. Macromol. 2024, 277, 134015. [Google Scholar] [CrossRef]
- Gan, X.; Li, C.; Sun, J.; Zhang, X.; Zhou, M.; Deng, Y.; Xiao, A. GelMA/κ-Carrageenan Double-Network Hydrogels with Superior Mechanics and Biocompatibility. RSC Adv. 2023, 13, 1558–1566. [Google Scholar] [CrossRef]
- Zubairee, K.; Yalcin, H.; Dursun Capar, T. Sunflower Oil-soybean Wax Oleogel: An Oxidation Stable Alternative to Traditional Frying Methods for Doughnut. J. Am. Oil Chem. Soc. 2025, 102, 239–250. [Google Scholar] [CrossRef]
- Aydeniz Guneser, B.; Yılmaz, E.; Uslu, E.K. Sunflower Oil-Beeswax Oleogels Are Promising Frying Medium for Potato Strips. Eur. J. Lipid Sci. Technol. 2021, 123, 2100063. [Google Scholar] [CrossRef]
- Ankaraligil, P.; Aydeniz-Guneser, B. Oleogel-Based Frying Medium: Influence of Rice Bran Wax-Canola Oil Oleogel on Volatile Profile in Fried Fish Fillets. Discov. Food 2024, 4, 90. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Oyom, W.; Adrah, K.; Adegoke, S.C.; Tahergorabi, R. A Review of Different Frying Oils and Oleogels as Alternative Frying Media for Fat-Uptake Reduction in Deep-Fat Fried Foods. Heliyon 2023, 9, e21500. [Google Scholar] [CrossRef]
- Yousef Ajo, R. Application of Hydrocolloids as Coating Films to Reduce Oil Absorption in Fried Potato Chip-Based Pellets. Pak. J. Nutr. 2017, 16, 805–812. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Cunha, R.L.; Vicente, A.A. Fortified Beeswax Oleogels: Effect of β-Carotene on the Gel Structure and Oxidative Stability. Food Funct. 2017, 8, 4241–4250. [Google Scholar] [CrossRef]
- Cui, M.; Mao, L.; Lu, Y.; Yuan, F.; Gao, Y. Effect of Monoglyceride Content on the Solubility and Chemical Stability of β-Carotene in Organogels. LWT 2019, 106, 83–91. [Google Scholar] [CrossRef]
- Machado, M.; Costa, E.M.; Silva, S.; Sousa, S.C.; Gomes, A.M.; Pintado, M. Enhancing Medium-Chain Fatty Acid Delivery Through Bigel Technology. Gels 2024, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Magalhães, W.V.; Sufi, B.d.S.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; Lannes, S.C.d.S.; Baby, A.R. Vitamin E-Loaded Bigels and Emulsions: Physicochemical Characterization and Potential Biological Application. Colloids Surf. B Biointerfaces 2021, 201, 111651. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Bigels as Delivery Systems of Bioactive Fatty Acids Present in Functional Edible Oils: Coconut, Avocado, and Pomegranate. Gels 2023, 9, 349. [Google Scholar] [CrossRef]
- Chen, L.; Lin, S.; Sun, N. Food Gel-Based Systems for Efficient Delivery of Bioactive Ingredients: Design to Application. Crit. Rev. Food Sci. Nutr. 2024, 64, 13193–13211. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Barbut, S.; Marangoni, A.G.; Thode, U.; Tiensa, B.E. Using Canola Oil Organogels as Fat Replacement in Liver Pâté. J. Food Sci. 2019, 84, 2646–2651. [Google Scholar] [CrossRef]
- Santos, P.H.d.S.; Lannes, S.C.d.S. Application of Organogel-like Structured System as an Alternative for Reducing Saturated Fatty Acid and Replacing Fat in Milk Ice Cream. J. Food Process. Preserv. 2022, 46, e16932. [Google Scholar] [CrossRef]
- Farzana, W.; Mahesh, S.; Sharma, S.; Syed, I.; Abdi, G.; Upadhyay, R. A Comprehensive Review on Bigels as a Potential Replacement to Solid Fat in Food Applications. J. Food Qual. 2025, 2025, 2483241. [Google Scholar] [CrossRef]
- Fonseca, L.M.; da Silva, F.T.; Bruni, G.P.; Borges, C.D.; Zavareze, E.d.R.; Dias, A.R.G. Aerogels Based on Corn Starch as Carriers for Pinhão Coat Extract (Araucaria angustifolia) Rich in Phenolic Compounds for Active Packaging. Int. J. Biol. Macromol. 2021, 169, 362–370. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Q.; Song, T.; Zhao, H.; Zhang, Y.; Ren, A.; Yang, W. Facile Fabrication of Anthocyanin-Nanocellulose Hydrogel Indicator Label for Intelligent Evaluation of Minced Pork Freshness. Foods 2023, 12, 2602. [Google Scholar] [CrossRef]
- Siribunbandal, P.; Osotchan, T.; Kim, Y.-H.; Jaisutti, R. Highly Sensitive Colorimetric Ammonia Sensors Based on Polydiacetylene/Zinc Oxide Nanopellet-Embedded PDMS Films for Meat Spoilage Detection. ACS Appl. Polym. Mater. 2023, 5, 7786–7794. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kim, J.; Kim, H.; Lim, J.; Seo, S.B.; Kang, B.; Kang, T.; Jung, J.; Seo, S.; et al. Polydiacetylene-Based Hydrogel Beads as Colorimetric Sensors for the Detection of Biogenic Amines in Spoiled Meat. Food Chem. 2023, 403, 134317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, W.; Min, C.; Qi, Y.; Chen, X.; Zhang, H. PH-Responsive Color Indicator Film Based on Gelatin/Chitosan Cross-Linking with Anthocyanin-Fe2+ Chelate for Pork Freshness Monitoring. Food Hydrocoll. 2025, 162, 110895. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent Developments in Intelligent Packaging for Enhancing Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y. Advances in Bio-Based Smart Food Packaging for Enhanced Food Safety. Trends Food Sci. Technol. 2025, 159, 104960. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Ruzicka, B. Swelling of Responsive-Microgels: Experiments versus Models. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 389–396. [Google Scholar] [CrossRef]
- Dhillon, A.; Khan, S.A.; Sonika. Smart, Responsive and Innovative Packaging. In Sustainable Packaging Strengthened by Biomass; Elsevier: Amsterdam, The Netherlands, 2025; pp. 193–224. [Google Scholar]
- Zhang, J.; Tan, J.; Chen, X.; Yin, Y.; Wang, C. High Humidity-Sensitive Discoloration Materials Fabricated with PH Indicator Ingredients. Dyes Pigment. 2021, 195, 109740. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.-Y.; Lou, C.-W.; Lin, J.-H.; Li, T.-T. Recent Advances of Cellulose-Based Hydrogels Combined with Natural Colorants in Smart Food Packaging. Gels 2024, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Sattayapanich, K.; Chaiwat, W.; Boonmark, S.; Bureekaew, S.; Sutthasupa, S. Alginate-Based Hydrogels Embedded with ZnO Nanoparticles as Highly Responsive Colorimetric Oxygen Indicators. New J. Chem. 2022, 46, 19322–19334. [Google Scholar] [CrossRef]
- Parhi, A.; Sonar, C.R.; Zhang, C.; Rasco, B.; Sankaran, S.; Tang, J.; Sablani, S.S. Agar Gel-Based Oxygen Indicator for Detection of Defects in Metal-Oxide-Coated Multilayered Food Packaging Films. In Proceedings of the 2020 ASABE Annual International Virtual Meeting, Online, 13–15 July 2020; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2020. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Z.; Huang, X.; Du, L.; Li, W.; Gao, P.; Chen, Z.; Zhang, J.; Guo, Z.; Li, Z.; et al. Advances in 3D and 4D Printing of Gel-Based Foods: Mechanisms, Applications, and Future Directions. Gels 2025, 11, 94. [Google Scholar] [CrossRef]
- Varvara, R.-A.; Szabo, K.; Vodnar, D.C. 3D Food Printing: Principles of Obtaining Digitally-Designed Nourishment. Nutrients 2021, 13, 3617. [Google Scholar] [CrossRef]
- Zhu, W.; Iskandar, M.M.; Baeghbali, V.; Kubow, S. Three-Dimensional Printing of Foods: A Critical Review of the Present State in Healthcare Applications, and Potential Risks and Benefits. Foods 2023, 12, 3287. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, M.; Mujumdar, A.S.; Li, C. Organic Nanomaterials Applied to the Manufacturing of Personalized Future 3D-Printed Foods: A Review. Trends Food Sci. Technol. 2025, 156, 104835. [Google Scholar] [CrossRef]
- Sharma, R.; Chandra Nath, P.; Kumar Hazarika, T.; Ojha, A.; Kumar Nayak, P.; Sridhar, K. Recent Advances in 3D Printing Properties of Natural Food Gels: Application of Innovative Food Additives. Food Chem. 2024, 432, 137196. [Google Scholar] [CrossRef]
- Pantić, M.; Nowak, M.; Lavrič, G.; Knez, Ž.; Novak, Z.; Zizovic, I. Enhancing the Properties and Morphology of Starch Aerogels with Nanocellulose. Food Hydrocoll. 2024, 156, 110345. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.T.J. Enhancement of the Mechanical Properties of Hydrogels with Continuous Fibrous Reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef]
- Shweta; Brindhav, A.M.; Sharma, S.; Azizi, S.; Rana, V.S. Unveiling the Cutting-Edge Applications of Nanotechnology in the Food Industry—From Lab to Table—A Comprehensive Review. J. Agric. Food Res. 2025, 21, 101831. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Hou, H.; Deng, J.; Lin, Q.; Li, W.; Bai, J. A Review on Migration Behavior and Safety Regulation of Inorganic Nanoparticles in Food Packaging: Multi-Scale Mechanisms, Risk Assessment, and Innovative Strategies. Food Control 2025, 178, 111490. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Hasanova, U.A.; Suleymanova, I.A.; Osmanova, G.E.; Hajiyeva, N.E. The Engineered Nanoparticles in Food Chain: Potential Toxicity and Effects. SN Appl. Sci. 2019, 1, 1362. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. In Official Journal of the European Union; The Publications Office of the European Union: Luxembourg, 2008.
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. In Official Journal of the European Union; The Publications Office of the European Union: Luxembourg, 2011.
- Title 21—Food and Drugs, Code of Federal Regulations, Parts 170–186; U.S. Food and Drug Administration: Tampa, FL, USA, 2025.
- Guidance for Industry: Preparation of Food Contact Notifications (FCNs) for Food Contact Substances (FCSs); U.S. Food and Drug Administration: Tampa, FL, USA, 2022.
- Health Canada. Lists of Permitted Food Additives; Health Canada: Ottawa, ON, Canada, 2024.
- Health Canada. Food and Drug Regulations; Health Canada: Ottawa, ON, Canada, 2025.
- Australia New Zealand Food Standards Code, Standard 1.4.3—Articles and Materials in Contact with Food; Food Standards Australia New Zealand (FSANZ): Wellington, New Zealand, 2023.
- Food Sanitation Act; Ministry of Health, Labour and Welfare (MHLW): Tokyo, Japan, 2025.
- Positive List System for Food Utensils, Containers, and Packaging; Ministry of Health, Labour and Welfare (MHLW): Tokyo, Japan, 2025.
- Food Safety and Standards; (Food Products Standards & Food Additives) Regulations, 2011; Food Safety and Standards Authority of India (FSSAI): New Delhi, India, 2024.
- Food Safety and Standards; (Packaging) Regulations, 2018; Version II (2022); Food Safety and Standards Authority of India (FSSAI): New Delhi, India, 2022.
- GB 2760; National Food Safety Standard: Standards for Uses of Food Additives. National Health Commission of the PRC: Beijing, China, 2024.
- GB 4806; National Food Safety Standards for Food Contact Materials. National Health Commission: Beijing, China, 2024.
- A.N. de V.S. RDC No. 326, of 3 December 2019—Positive List of Additives for Plastic Materials in Food Contact; Brazilian Health Regulatory Agency: Brasília, Brazil, 2019.
- A.N. de V.S. RDC No. 778, of 1 March 2023—General List of Food Additives and Technology Adjuvants Authorized for Use; Brazilian Health Regulatory Agency: Brasília, Brazil, 2023.
- Edo, G.I.; Mafe, A.N.; Akpoghelie, P.O.; Gaaz, T.S.; Yousif, E.; Yusuf, O.S.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Ayinla, J.L.; et al. The Utilization of Biopolymer Hydrogels to Encapsulate and Protect Probiotics in Foods. Process Biochem. 2025, 153, 66–91. [Google Scholar] [CrossRef]
- Chen, C.; Yu, W.; Kou, X.; Niu, Y.; Ji, J.; Shao, Y.; Wu, S.; Liu, M.; Xue, Z. Recent Advances in the Effect of Simulated Gastrointestinal Digestion and Encapsulation on Peptide Bioactivity and Stability. Food Funct. 2025, 16, 1634–1655. [Google Scholar] [CrossRef]
- Bannikova, A.; Rasumova, L.; Evteev, A.; Evdokimov, I.; Kasapis, S. Protein-loaded Sodium Alginate and Carboxymethyl Cellulose Beads for Controlled Release under Simulated Gastrointestinal Conditions. Int. J. Food Sci. Technol. 2017, 52, 2171–2179. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Brownlie, K. Marketing Perspective of Encapsulation Technologies in Food Applications. In Encapsulation and Controlled Release Technologies in Food Systems; Wiley: Hoboken, NJ, USA, 2007; pp. 213–233. [Google Scholar]

| Gel | Gel Type | Examples of Use | Types of Texture | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Gelatin Gel | Hydrogel | Gummy vitamins | Elastic, soft | Good encapsulation, widely used | Animal-derived, heat-sensitive | [30] |
| Pectin Gel | Hydrogel | Fruit jelly, vegan gummies | Soft, melts in mouth | Vegan, fruit-compatible | pH and calcium-sensitive | [31] |
| Agar Gel | Hydrogel | Asian jelly desserts | Firm, brittle | Stable at room temp. | Brittle texture | [32] |
| Carrageenan Gel | Hydrogel | Dairy-based desserts | Elastic, creamy | Plant-based, good gelling | Interactions with ions | [33] |
| Starch Gel | Hydrogel | Candy, jellybeans | Chewy, dense | Cost-effective | Low thermal resistance | [34] |
| Gelatin-Starch Blend | Hydrogel | Gummy bears | Chewy, resilient | Texture customization | Still animal-derived | [35] |
| Pullulan/HPMC Gel | Other (polysaccharide film) | Vegan soft capsules | Smooth, soft shell | Vegan, clean label | Expensive | [36] |
| Alginate Gel | Hydrogel | Spherification, filled candies | Soft outer, liquid core | Unique mouthfeel | Calcium-sensitive | [32] |
| Gum Arabic Gel | Hydrogel | Flavor encapsulation | Film-like | Natural emulsifier | Low strength | [33] |
| Monoglyceride Organogel | Organogel | Margarine, shortening | Creamy, firm | Replaces saturated fats; stable | High melting point, slow digestion | [37] |
| Phytosterol Oleogels | Organogel | Functional spreads, cholesterol-lowering foods | Smooth, semi-solid | Lowers LDL cholesterol, plant-based | Limited thermal stability | [38] |
| Beeswax Organogel | Organogel | Fat replacer in bakery and meat products | Firm, brittle | Natural, stable, widely available | Brittle at low temp. | [39] |
| Rice Bran Wax Oleogel | Organogel | Low-fat cookies, meat analogs | Waxy, spreadable | Vegan, sustainable, neutral flavor | Requires precise processing | [40] |
| Ethylcellulose Organogel | Organogel | Controlled lipid delivery in processed food | Elastic, spreadable | High oil-binding capacity | Needs high temp. to dissolve EC in oil | [41] |
| Lecithin-Based Organogel | Organogel | Nutrient carriers in oil-rich foods | Fluid–gel | Food-grade emulsifier, bioavailable | Weak mechanical stability | [42,43] |
| Sorbitan Monostearate Gel | Organogel | Confectionery fat substitutes | Smooth, semi-solid | Food-safe, good structuring agent | Not vegan, limited consumer appeal | [44,45,46] |
| Apple Pectin Aerogel | Aerogel | Encapsulation of bioactives, food foams | Light, crispy | Biodegradable, high porosity | Fragile under humidity | [47,48] |
| Cellulose-Based Aerogel | Aerogel | Oil absorption in low-fat frying | Dry, fibrous | High oil retention capacity, biodegradable | Expensive processing | [49,50,51,52] |
| Starch-Based Aerogel | Aerogel | Flavor and nutrient delivery systems | Porous, melts in mouth | Low-cost, digestible | Less thermal stability | [53] |
| Chitosan Aerogel | Aerogel | Controlled release in functional food | Lightweight, porous | Antimicrobial, excellent carrier | Allergen potential | [54] |
| Carrageenan Aerogel | Aerogel | Bioactive compound carrier in beverages | Brittle, friable | Water-soluble, renewable marine source | Sensitive to moisture | [55,56] |
| Monoglyceride–Beeswax Oleogel and High Acyl Gellan Gum Hydrogel | Bigel | Lycopene delivery | Semi-solid | High encapsulation ability | pH sensitive | [57] |
| Lecithin, Stearic and Whey Protein Bigel | Bigel | Probiotic survival | Semi-solid | Enhance probiotic resistance | Enzyme sensitive | [58] |
| Sunflower Oil and Xanthan–Guar Gum Mixture Bigels | Bigel | Ascorbic acid delivery | Semi-solid | High encapsulation efficiency | Less thermal stability | [59] |
| Jurisdiction | Food Additive Approval | Food-Contact Material Regulation | Regulatory Body | Key Safety Assessment Steps | References |
|---|---|---|---|---|---|
| European Union (EU) | Regulation (EC) No. 1333/2008 | Regulation (EU) No. 10/2011 | EFSA, European Commission | Toxicological testing; migration limits; long-term exposure studies; bioaccumulation risk assessment; post-market monitoring | [150,151] |
| United States (USA) | GRAS list (21 CFR Parts 170–186) | FDA Food Contact Notifications (FCNs) | U.S. FDA | GRAS determination; chronic toxicity evaluation; interaction testing with food matrices; surveillance for adverse effects | [152,153] |
| Canada | Lists of Permitted Food Additives | Food and Drug Act and Regulations | Health Canada | Maximum permissible levels; degradation product safety; cumulative exposure analysis; consumer safety reporting | [154,155] |
| Australia/New Zealand | FSANZ Food Standards Code | FSANZ Standard 1.4.3 | FSANZ | Composition compliance; stability under processing/storage; bioavailability and metabolism studies; continuous compliance audits | [156] |
| Japan | Food Sanitation Act | Positive List System for food-contact materials | MHLW | Migration testing; biodegradability evaluation; chronic and reproductive toxicity tests; periodic re-evaluation of new materials | [157,158] |
| India | Food Safety and Standards (Food Product Standards and Food Additives) Regulations, 2011 (and compendium updates) | Food Safety and Standards (Packaging) Regulations, 2018 (Version II 2022); updates to plastic migration limits | FSSAI | Additive permissions by category; migration limits for packaging; toxicology; labeling and surveillance | [159,160] |
| China | GB 2760 National Food Safety Standard—Standard for Uses of Food Additives (updated; replacing GB 2760-2014) | GB 4806 series for FCMs (material-specific requirements) | NHC (with CFSA), SAMR | Lists of approved or conditionally permitted items; usage categories and scopes; migration considerations and exposure levels; Specific Migration Limits (SMLs) for individual products | [161,162] |
| Brazil | ANVISA consolidated additive framework (e.g., RDC 778/2023 and amendments), aligned with MERCOSUR | RDC 326/2019 (positive list for additives in food-contact plastics and coatings); transposition of MERCOSUR GMC resolutions | ANVISA | Compliance with positive lists; SMLs such as phthalates; interaction testing; national implementation of GMC regulations | [163,164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Costa, E.M.A.d.; Silva, S. Soft Gels in Food Systems: Recent Advances, Applications, and Technological Innovations. Gels 2025, 11, 667. https://doi.org/10.3390/gels11080667
Machado M, Costa EMAd, Silva S. Soft Gels in Food Systems: Recent Advances, Applications, and Technological Innovations. Gels. 2025; 11(8):667. https://doi.org/10.3390/gels11080667
Chicago/Turabian StyleMachado, Manuela, Eduardo Manuel Aguiar da Costa, and Sara Silva. 2025. "Soft Gels in Food Systems: Recent Advances, Applications, and Technological Innovations" Gels 11, no. 8: 667. https://doi.org/10.3390/gels11080667
APA StyleMachado, M., Costa, E. M. A. d., & Silva, S. (2025). Soft Gels in Food Systems: Recent Advances, Applications, and Technological Innovations. Gels, 11(8), 667. https://doi.org/10.3390/gels11080667







