Modulating CT Attenuation of Polyvinyl Alcohol Cryogels for Individualized Training Phantoms in Interventional Radiology: A Proof-of-Concept Study
Abstract
1. Introduction
2. Results and Discussion
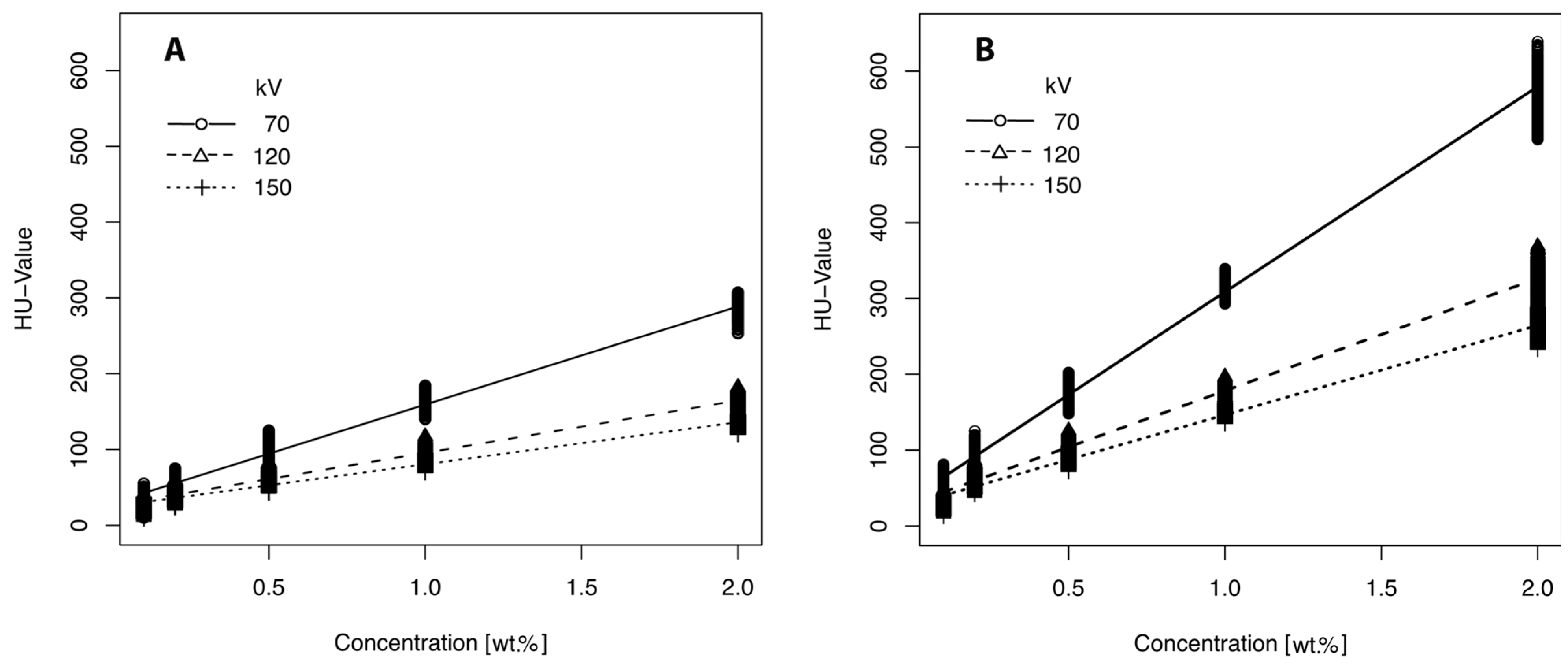
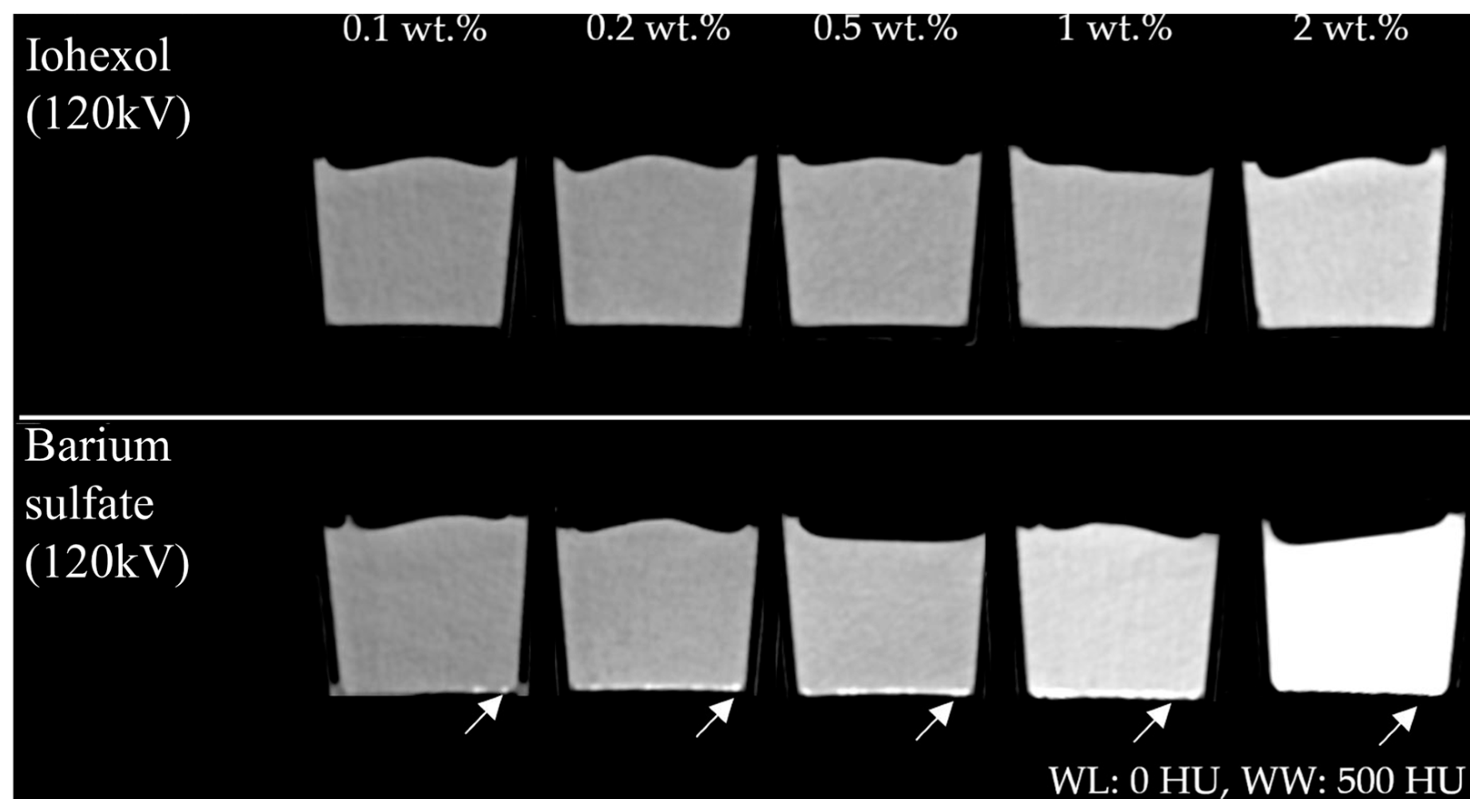
2.1. HU Modification by Positive Contrast Agents
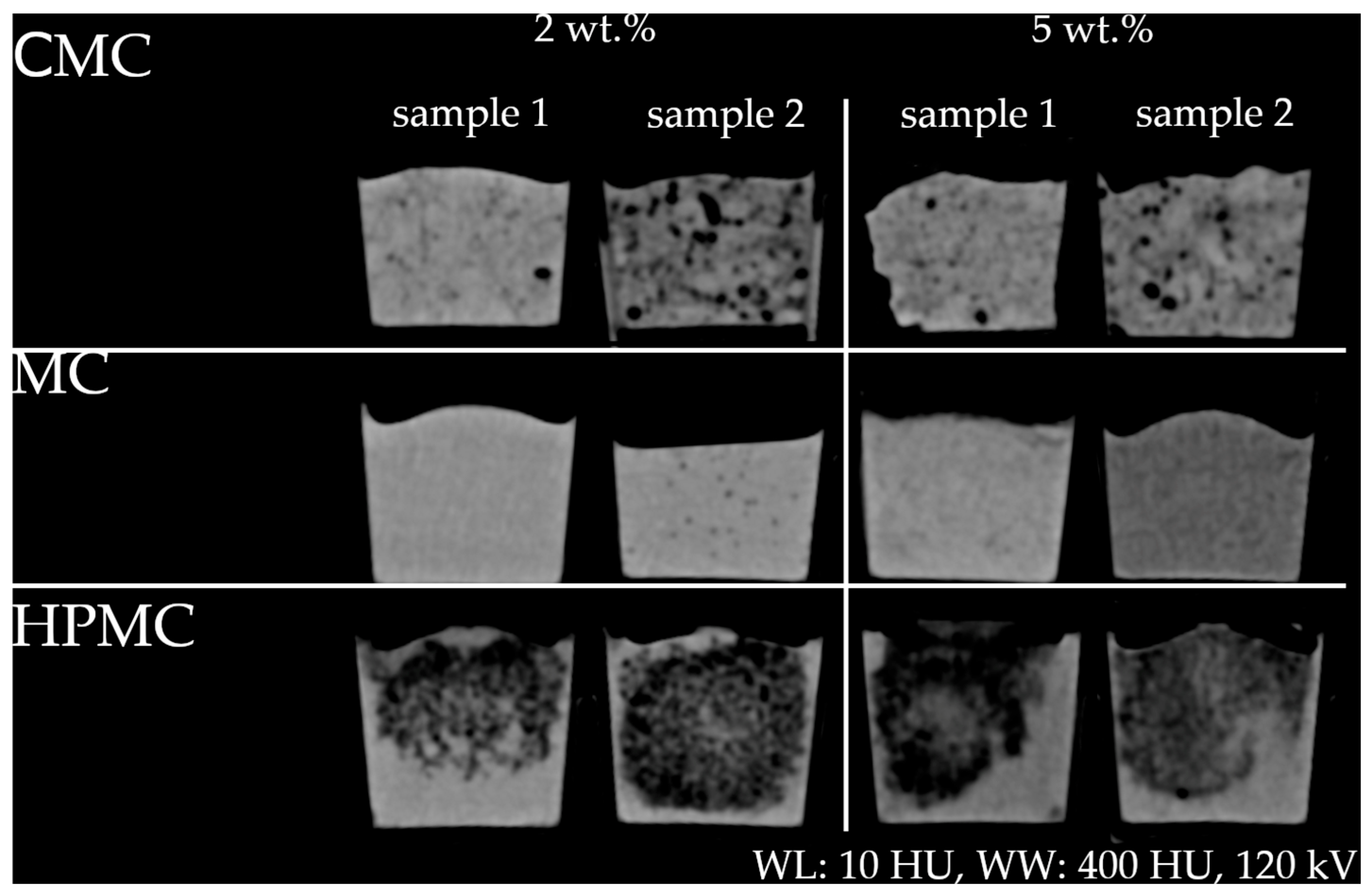
2.2. HU Modification by Cellulosic Substances
2.3. CT Calibration
2.4. Further Material Characterization and Analytical Methods
3. Conclusions
4. Materials and Methods
4.1. General Processing of PVA Probes
4.2. Modulation of X-Ray Attenuation
4.3. CT Imaging
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Concentration | 2 wt.% | 5 wt.% | ||||
|---|---|---|---|---|---|---|
| Cellulose | kV | Sample | Mean | IQR | Mean | IQR |
| CMC | 70 | 1 | 10.03 ± 14.23 | 16 | −9.22 ± 18.72 | 21 |
| 2 | −46.76 ± 48.68 | 60 | −31.63 ± 58.46 | 41 | ||
| 120 | 1 | 10.66 ± 13.85 | 15 | −10.19 ± 17.43 | 20 | |
| 2 | −58.57 ± 46.04 | 47 | −30.68 ± 51.54 | 36 | ||
| 150 | 1 | 10.57 ± 14.34 | 14 | −8.60 ± 17.91 | 20 | |
| 2 | −42.84 ± 47.02 | 56 | −36.47 ± 63.11 | 43 | ||
| MC | 70 | 1 | 29.79 ± 6.54 | 8 | 19.94 ± 6.15 | 8 |
| 2 | 21.23 ± 9.23 | 7 | −49.22 ± 7.14 | 10 | ||
| 120 | 1 | 28.43 ± 4.67 | 7 | 18.29 ± 6.24 | 8 | |
| 2 | 23.07 ± 9.33 | 7 | −46.49 ± 6.09 | 8 | ||
| 150 | 1 | 28.14 ± 3.90 | 5 | 18.08 ± 6.29 | 9 | |
| 2 | 23.04 ± 8.81 | 7 | −47.88 ± 5.67 | 8 | ||
| HPMC | 70 | 1 | −65.49 ± 38.76 | 54 | −96.36 ± 34.89 | 54 |
| 2 | −74.64 ± 29.12 | 43 | −84.98 ± 29.24 | 37 | ||
| 120 | 1 | −67.04 ± 43.25 | 56 | −94.96 ± 26.75 | 37 | |
| 2 | −74.56 ± 31.44 | 46 | −86.86 ± 29.52 | 37 | ||
| 150 | 1 | −68.12 ± 39.82 | 51 | −98.06 ± 30.50 | 43 | |
| 2 | −76.60 ± 29.07 | 42 | −82.23 ± 30.33 | 39 | ||
| Concentration | 2 wt.% | 5 wt.% | ||
|---|---|---|---|---|
| Cellulose | Radiologist | Sample | Description | Description |
| CMC | 1 | 1 | Liver parenchyma in cirrhosis | Granular intestinal contents |
| 2 | Granular intestinal contents | |||
| 2 | 1 | Fatty muscles | Fibrosis | |
| 2 | Corpostasis | Corpostasis | ||
| MC | 1 | In general
| ||
| 1 | Tumor, cerebral cortex | Lobular parenchyma (pancreas) | ||
| 2 | - | Steatosis hepatis or parenchyma without contrast medium | ||
| 2 | 1 2 | Liver/spleen Cysts/metastases in the liver | Metastases in the liver, fatty liver | |
| HPMC | 1 | In general
| ||
| 2 | 1 2 | Fatty muscles Honeycomb lung | - Osteolysis | |
References
- Murphy, T.P.; Soares, G.M. The evolution of interventional radiology. Semin. Interv. Radiol. 2005, 22, 6–9. [Google Scholar] [CrossRef]
- Mashar, M.; Nanapragasam, A.; Haslam, P. Interventional radiology training: Where will technology take us? BJR Open 2019, 1, 20190002. [Google Scholar] [CrossRef]
- Summary of the proceedings of the International Forum 2017: “Position of interventional radiology within radiology”. Insights Imaging 2018, 9, 189–197. [CrossRef] [PubMed]
- Chikwe, J.; de Souza, A.C.; Pepper, J.R. No time to train the surgeons. BMJ 2004, 328, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Pradella, M.; Trumm, C.; Stieltjes, B.; Boll, D.T.; Zech, C.J.; Huegli, R.W. Impact factors for safety, success, duration and radiation exposure in CT-guided interventions. Br. J. Radiol. 2019, 92, 20180937. [Google Scholar] [CrossRef] [PubMed]
- Elsholtz, F.H.J.; Vahldiek, J.L.; Wyschkon, S.; de Bucourt, M.; Koletzko, G.; Hamm, B.; Niehues, S.M. Radiation exposure of radiologists during different types of CT-guided interventions: An evaluation using dosimeters placed above and under lead protection. Acta Radiol. 2020, 61, 110–116. [Google Scholar] [CrossRef]
- Mandal, I.; Ojha, U. Training in Interventional Radiology: A Simulation-Based Approach. J. Med. Educ. Curric. Dev. 2020, 7, 2382120520912744. [Google Scholar] [CrossRef]
- Pacioni, A.; Carbone, M.; Freschi, C.; Viglialoro, R.; Ferrari, V.; Ferrari, M. Patient-specific ultrasound liver phantom: Materials and fabrication method. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1065–1075. [Google Scholar] [CrossRef]
- Hunt, A.; Ristolainen, A.; Ross, P.; Opik, R.; Krumme, A.; Kruusmaa, M. Low cost anatomically realistic renal biopsy phantoms for interventional radiology trainees. Eur. J. Radiol. 2013, 82, 594–600. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef]
- Babu, J.; Murthy, Z. Treatment of textile dyes containing wastewaters with PES/PVA thin film composite nanofiltration membranes. Sep. Purif. Technol. 2017, 183, 66–72. [Google Scholar] [CrossRef]
- Tan, B.; Ching, Y.; Poh, S.; Abdullah, L.; Gan, S. A Review of Natural Fiber Reinforced Poly(Vinyl Alcohol) Based Composites: Application and Opportunity. Polymers 2015, 7, 2205–2222. [Google Scholar] [CrossRef]
- Mano, I.; Goshima, H.; Nambu, M.; Iio, M. New polyvinyl alcohol gel material for MRI phantoms. Magn. Reson. Med. 1986, 3, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.C.; Rutt, B.K. Polyvinyl alcohol cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef]
- Arteaga-Marrero, N.; Villa, E.; Llanos González, A.B.; Gómez Gil, M.E.; Fernández, O.A.; Ruiz-Alzola, J.; González-Fernández, J. Low-Cost Pseudo-Anthropomorphic PVA-C and Cellulose Lung Phantom for Ultrasound-Guided Interventions. Gels 2023, 9, 74. [Google Scholar] [CrossRef]
- Vogt, I.; Volk, M.; Kulzer, E.-L.; Seibt, J.; Pech, M.; Rose, G.; Grosser, O.S. Microwave-Assisted Optimization of Polyvinyl Alcohol Cryogel (PVA-C) Manufacturing for MRI Phantom Production. Bioengineering 2025, 12, 171. [Google Scholar] [CrossRef]
- Volk, M. Optimization of manufacturing process for polyvinyl alcohol cryogels (PVA-C) intended for anthropomorphic training phantoms in CT-interventions. Eur. Congr. Radiol. EPOS 2024. [Google Scholar] [CrossRef]
- Surry, K.J.M.; Austin, H.J.B.; Fenster, A.; Peters, T.M. Poly(vinyl alcohol) cryogel phantoms for use in ultrasound and MR imaging. Phys. Med. Biol. 2004, 49, 5529–5546. [Google Scholar] [CrossRef]
- Mackle, E.C.; Shapey, J.; Maneas, E.; Saeed, S.R.; Bradford, R.; Ourselin, S.; Vercauteren, T.; Desjardins, A.E. Patient-Specific Polyvinyl Alcohol Phantom Fabrication with Ultrasound and X-Ray Contrast for Brain Tumor Surgery Planning. J. Vis. Exp. 2020, 161, e61344. [Google Scholar] [CrossRef]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.P.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef]
- Galvis-García, E.S.; Sobrino-Cossío, S.; Reding-Bernal, A.; Contreras-Marín, Y.; Solórzano-Acevedo, K.; González-Zavala, P.; Quispe-Siccha, R.M. Experimental model standardizing polyvinyl alcohol hydrogel to simulate endoscopic ultrasound and endoscopic ultrasound-elastography. World J. Gastroenterol. 2020, 26, 5169–5180. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, D.W.; Ott, D.J. Barium sulfate suspensions: An evaluation of available products. AJR Am. J. Roentgenol. 1982, 138, 935–941. [Google Scholar] [CrossRef] [PubMed]
- El Salmawi, K.M. Application of Polyvinyl Alcohol (PVA)/Carboxymethyl Cellulose (CMC) Hydrogel Produced by Conventional Crosslinking or by Freezing and Thawing. J. Macromol. Sci. Part A 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, M.-J. Removal of Heavy Metal Ions by Poly(vinyl alcohol) and Carboxymethyl Cellulose Composite Hydrogels Prepared by a Freeze–Thaw Method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Namkaew, J.; Laowpanitchakorn, P.; Sawaddee, N.; Jirajessada, S.; Honsawek, S.; Yodmuang, S. Carboxymethyl Cellulose Entrapped in a Poly(vinyl) Alcohol Network: Plant-Based Scaffolds for Cartilage Tissue Engineering. Molecules 2021, 26, 578. [Google Scholar] [CrossRef]
- BeMiller, J.N. Cellulose and Cellulose-Based Hydrocolloids. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing: Sawston, UK, 2019; pp. 223–240. ISBN 9780128120699. [Google Scholar]
- Crețu, B.-E.-B.; Nita, L.E.; Șerban, A.-M.; Rusu, A.G.; Doroftei, F.; Chiriac, A.P. New Cryogels Based on Poly(vinyl alcohol) and a Copolymacrolactone System: I-Synthesis and Characterization. Nanomaterials 2022, 12, 2420. [Google Scholar] [CrossRef]
- Górska, A.; Baran, E.; Knapik-Kowalczuk, J.; Szafraniec-Szczęsny, J.; Paluch, M.; Kulinowski, P.; Mendyk, A. Physically Cross-Linked PVA Hydrogels as Potential Wound Dressings: How Freezing Conditions and Formulation Composition Define Cryogel Structure and Performance. Pharmaceutics 2024, 16, 1388. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Study on the Preparation and Lipophilic Properties of Polyvinyl Alcohol (PVA) Nanofiber Membranes via Green Electrospinning. Nanomaterials 2021, 11, 2514. [Google Scholar] [CrossRef]
- Wagner, M.G.; Hinshaw, J.L.; Li, Y.; Szczykutowicz, T.P.; Laeseke, P.; Mistretta, C.A.; Lee, F.T. Ultra-Low Radiation Dose CT Fluoroscopy for Percutaneous Interventions: A Porcine Feasibility Study. Radiology 2019, 291, 241–249. [Google Scholar] [CrossRef]
- Peppas, N.A. Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Makromol. Chem. 1975, 176, 3433–3440. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Peppas, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(vinyl alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. ISBN 978-3-540-67313-2. [Google Scholar]
- Chatelin, S.; Bernal, M.; Deffieux, T.; Papadacci, C.; Flaud, P.; Nahas, A.; Boccara, C.; Gennisson, J.-L.; Tanter, M.; Pernot, M. Anisotropic polyvinyl alcohol hydrogel phantom for shear wave elastography in fibrous biological soft tissue: A multimodality characterization. Phys. Med. Biol. 2014, 59, 6923–6940. [Google Scholar] [CrossRef] [PubMed]
- Nyman, U.; Ekberg, O.; Aspelin, P. Torsten Almén (1931–2016): The father of non-ionic iodine contrast media. Acta Radiol. 2016, 57, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.L.; Arenson, R.L.; Cross, A.P. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: Comparison of adverse effects. AJR Am. J. Roentgenol. 1989, 152, 939–944. [Google Scholar] [CrossRef]
- Levine, M.S.; Rubesin, S.E. History and Evolution of the Barium Swallow for Evaluation of the Pharynx and Esophagus. Dysphagia 2017, 32, 55–72. [Google Scholar] [CrossRef]
- Hecht, G. Röntgenkontrastmittel. In Handbuch der Experimentellen Pharmakologie; Heubner, W., Schüller, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1939; pp. 79–163. ISBN 978-3-642-88938-7. [Google Scholar]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose Cryogels as Promising Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Khaleel Basha, S.; Sugantha Kumari, V. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 484–491. [Google Scholar] [CrossRef]
- Sakellariou, P.; Hassan, A.; Rowe, R.C. Phase separation and polymer interactions in aqueous poly(vinyl alcohol)/hydroxypropyl methylcellulose blends. Polymer 1993, 34, 1240–1248. [Google Scholar] [CrossRef]
- Würzteufel GmbH. Produktspezifikation Hydroxypropylmethylcellulose. Available online: https://wuerzteufel.de/Unterlagen/Spezifikationen/HPMC.pdf (accessed on 28 August 2023).
- Würzteufel GmbH. Produktspezifikation Methylcellulose. Available online: https://wuerzteufel.de/Unterlagen/Spezifikationen/MC.pdf (accessed on 28 August 2023).
- Würzteufel GmbH. Produktspezifikation Carboxymethylcellulose. Available online: https://wuerzteufel.de/Unterlagen/Spezifikationen/CMC.pdf (accessed on 28 August 2023).
| Contrast Agent | Contrast Media Concentration | kV | Intercept | R2 |
|---|---|---|---|---|
| Barium sulfate | 179.16 (178.23–180.09) p < 0.001 | −1.61 (from −1.63 to −1.59) p < 0.001 | 214.74 (212.33–217.15) p < 0.001 | 0.886 |
| Iohexol | 84.63 (84.17–85.09) p < 0.001 | −0.78 (from −0.79 to −0.77) p < 0.001 | 115.84 (114.64–117.04) p < 0.001 | 0.876 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volk, M.; Vogt, I.; Georgiades, M.; Menhorn, J.; Becker, M.; Rose, G.; Pech, M.; Grosser, O.S. Modulating CT Attenuation of Polyvinyl Alcohol Cryogels for Individualized Training Phantoms in Interventional Radiology: A Proof-of-Concept Study. Gels 2025, 11, 664. https://doi.org/10.3390/gels11080664
Volk M, Vogt I, Georgiades M, Menhorn J, Becker M, Rose G, Pech M, Grosser OS. Modulating CT Attenuation of Polyvinyl Alcohol Cryogels for Individualized Training Phantoms in Interventional Radiology: A Proof-of-Concept Study. Gels. 2025; 11(8):664. https://doi.org/10.3390/gels11080664
Chicago/Turabian StyleVolk, Martin, Ivan Vogt, Marilena Georgiades, Johanna Menhorn, Mathias Becker, Georg Rose, Maciej Pech, and Oliver S. Grosser. 2025. "Modulating CT Attenuation of Polyvinyl Alcohol Cryogels for Individualized Training Phantoms in Interventional Radiology: A Proof-of-Concept Study" Gels 11, no. 8: 664. https://doi.org/10.3390/gels11080664
APA StyleVolk, M., Vogt, I., Georgiades, M., Menhorn, J., Becker, M., Rose, G., Pech, M., & Grosser, O. S. (2025). Modulating CT Attenuation of Polyvinyl Alcohol Cryogels for Individualized Training Phantoms in Interventional Radiology: A Proof-of-Concept Study. Gels, 11(8), 664. https://doi.org/10.3390/gels11080664