Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films
Abstract
1. Introduction
2. Results and Discussion
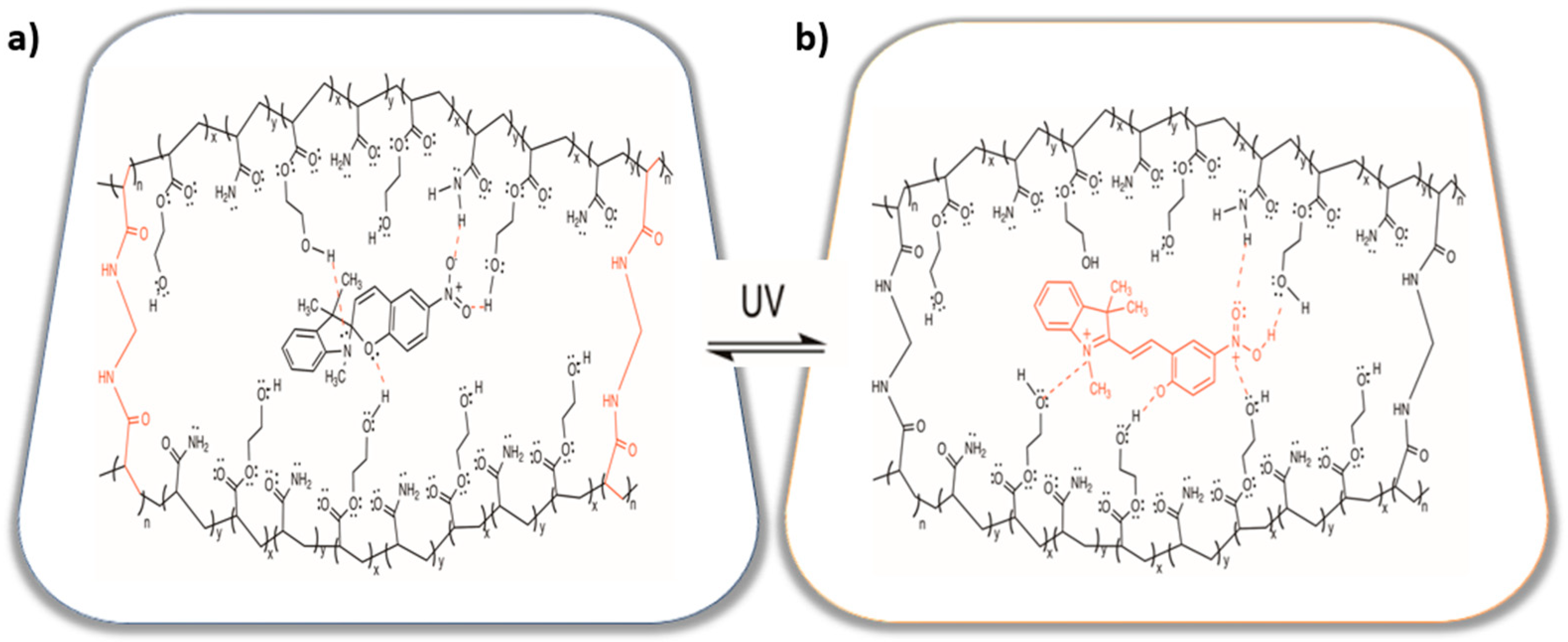
2.1. Synthesis and Characterization
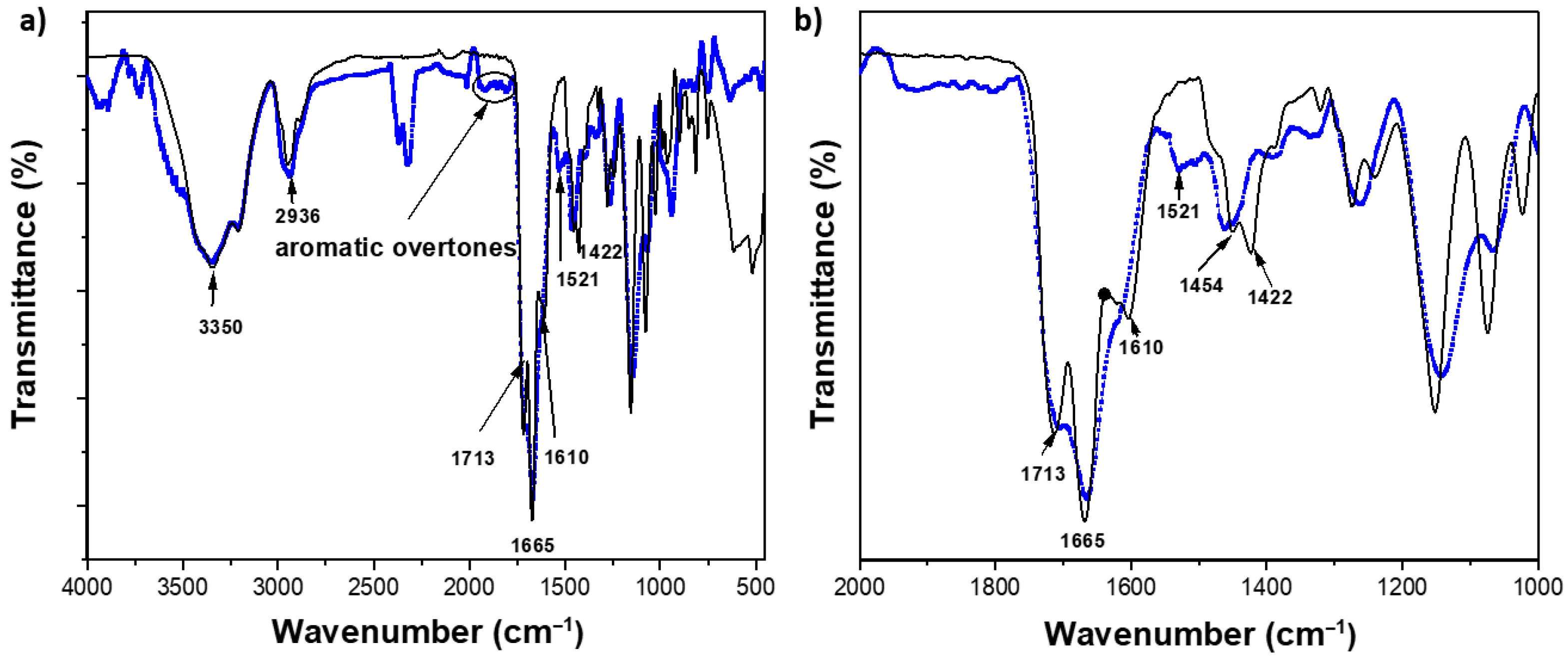
2.2. Structural Characterization by FTIR Studies of the Hydrogels
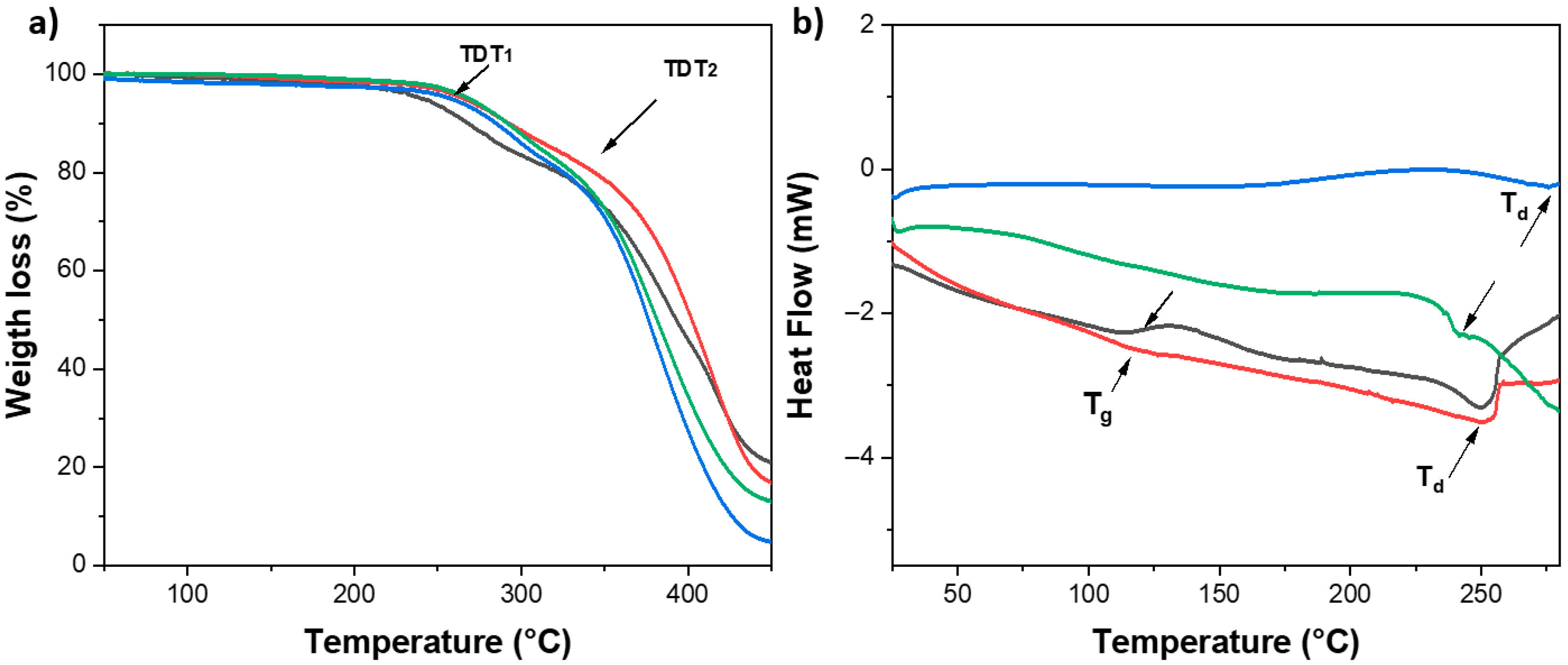
2.3. Characterization by TGA and DSC
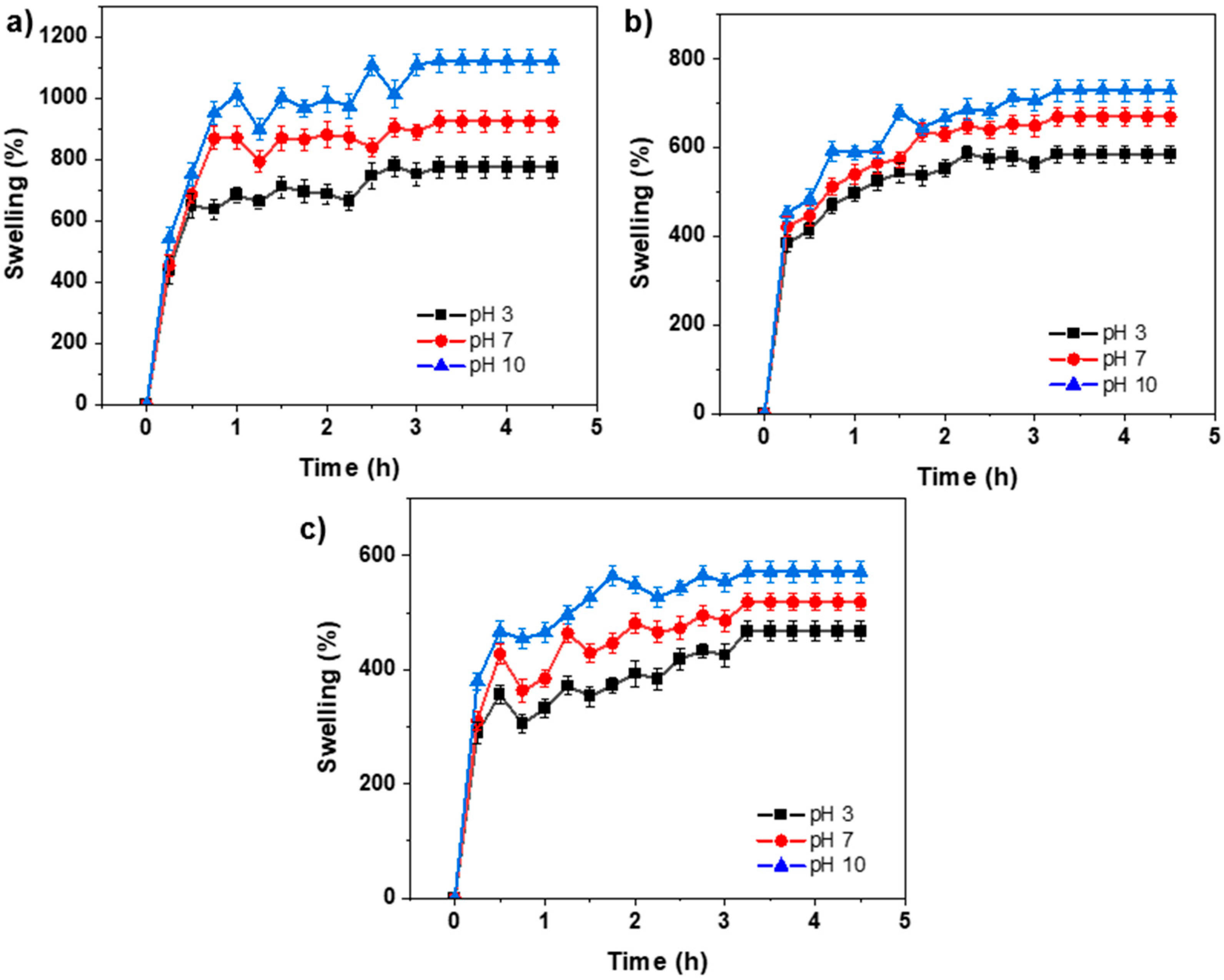
2.4. Swelling Behavior
2.5. Lap Shear Test of Polymeric Hydrogel
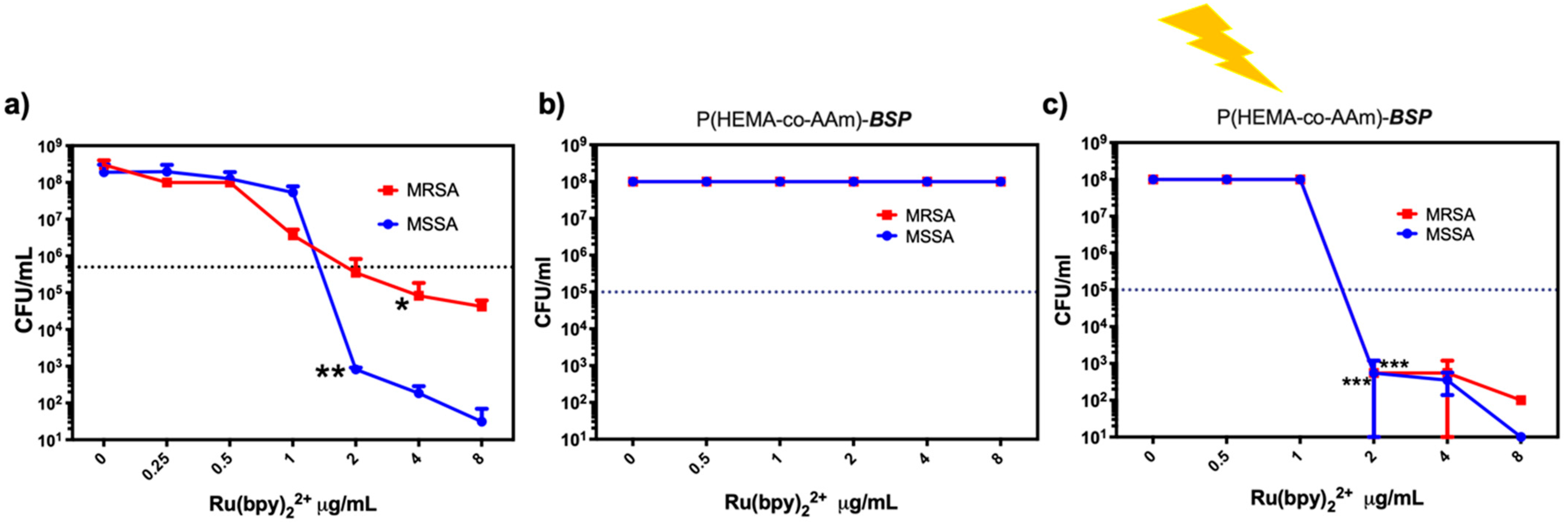
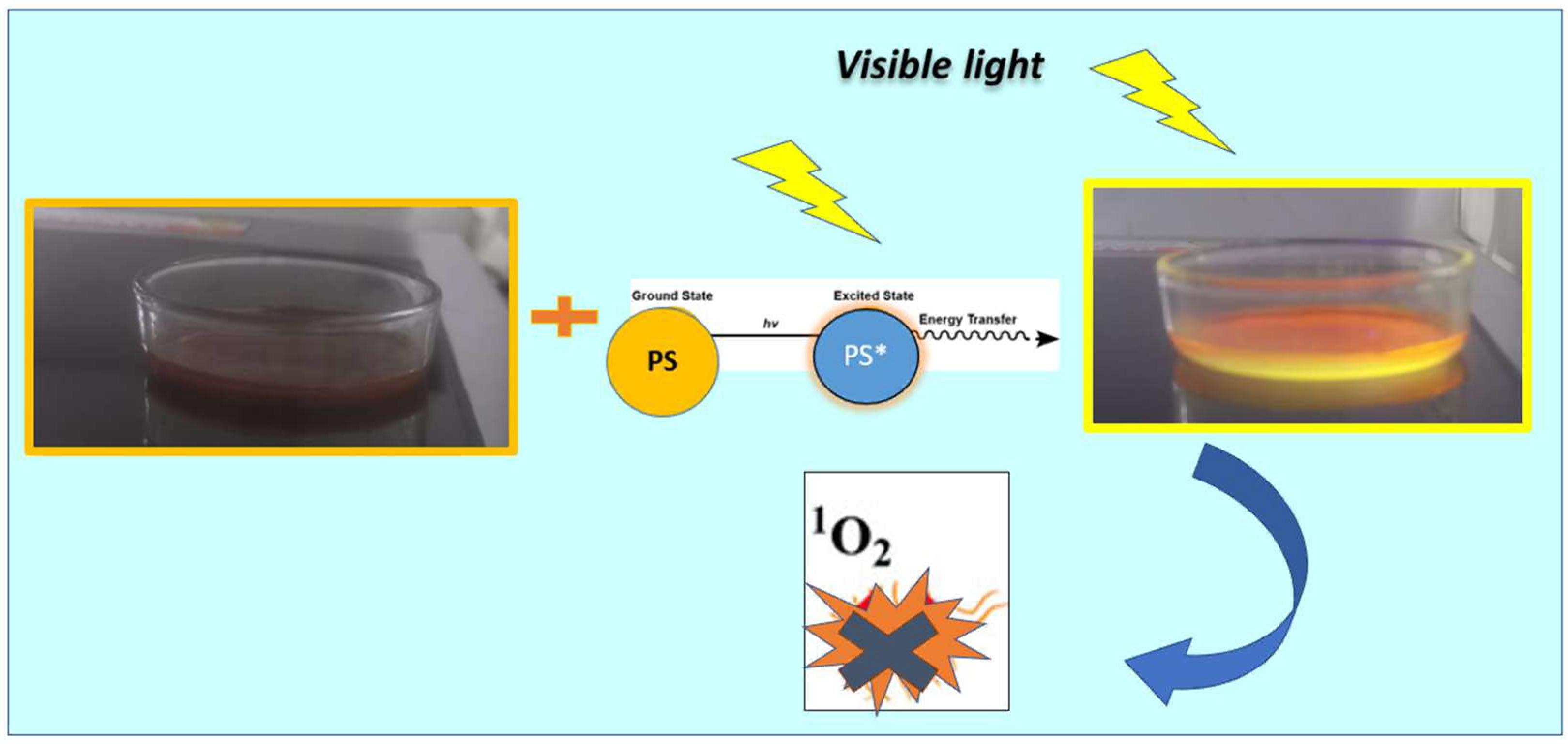
2.6. Antimicrobial Properties Based on Photodynamic Therapy (PDT)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Analytical Techniques
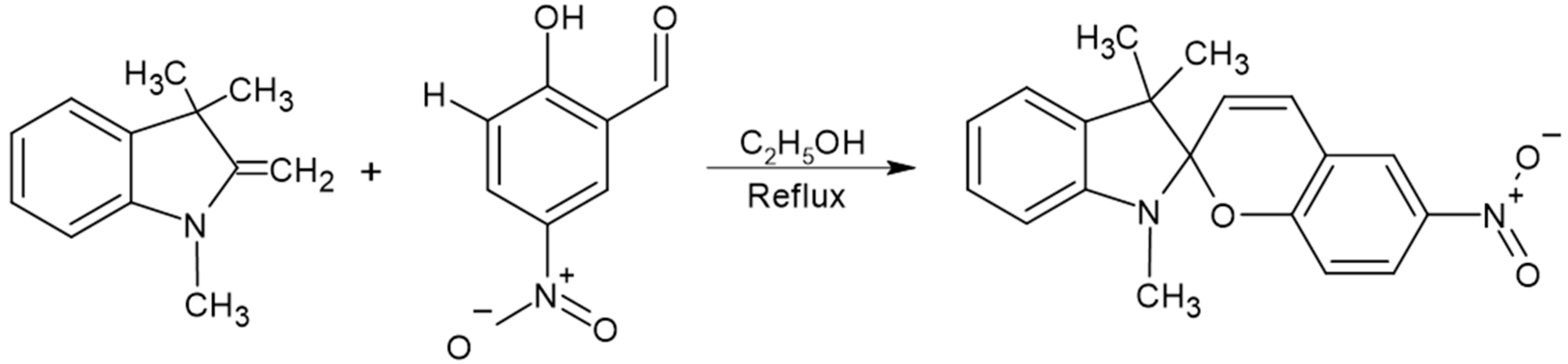
4.3. Synthesis of 3,3-Dimethylindoline-6′-Nitrobenzospiropyran (BSP)
4.4. Preparation of Hydrogel Films
4.5. Swelling Studies
4.6. Lap Shear Test
4.7. Determination of Antimicrobial Photodynamic Properties
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering Design and Molecular Dynamics of Mucoadhesive Drug Delivery Systems as Targeting Agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef]
- Mizrahi, B.; Weldon, C.; Kohane, D.S. Tissue Adhesives as Active Implants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–56. [Google Scholar] [CrossRef]
- Mandell, S.P.; Gibran, N.S. Fibrin Sealants: Surgical Hemostat, Sealant and Adhesive. Expert. Opin. Biol. Ther. 2014, 14, 821–830. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular Gels: Functions and Uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A. Study on Superabsorbent Composites. IX: Synthesis, Characterization and Swelling Behaviors of Polyacrylamide/Clay Composites Based on Various Clays. React. Funct. Polym. 2007, 67, 737–745. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M.; Liang, R. Preparation and Properties of a Double-Coated Slow-Release NPK Compound Fertilizer with Superabsorbent and Water-Retention. Bioresour. Technol. 2008, 99, 547–554. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, H.N.; Seong, M.; Lee, S.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional Smart Skin Adhesive Patches for Advanced Health Care. Adv. Healthc. Mater. 2018, 7, e1800275. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Zhang, W.; Wang, M.; Yan, L.; Wang, K.; Han, L.; Lu, X. Infant Skin Friendly Adhesive Hydrogel Patch Activated at Body Temperature for Bioelectronics Securing and Diabetic Wound Healing. ACS Nano 2022, 16, 8662–8676. [Google Scholar] [CrossRef]
- Lee, S.; An, S.; Ryu, Y.C.; Seo, S.H.; Park, S.; Lee, M.J.; Cho, S.; Choi, K. Adhesive Hydrogel Patch-Mediated Combination Drug Therapy Induces Regenerative Wound Healing through Reconstruction of Regenerative Microenvironment. Adv. Healthc. Mater. 2023, 12, e2203094. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, N.; Du, S.; Gao, Y.; Suo, H.; Yang, J.; Tao, J.; Zhu, J.; Zhang, L. Transparent Photothermal Hydrogels for Wound Visualization and Accelerated Healing. Fundam. Res. 2022, 2, 268–275. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently Adaptable Elastin-Like Protein–Hyaluronic Acid (ELP–HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery. Adv. Funct. Mater. 2017, 27, 1605609. [Google Scholar] [CrossRef]
- Shin, J.Y.; Yeo, Y.H.; Jeong, J.E.; Park, S.A.; Park, W.H. Dual-Crosslinked Methylcellulose Hydrogels for 3D Bioprinting Applications. Carbohydr. Polym. 2020, 238, 116192. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized Dynamic Hydrogels with Tunable Viscoelastic Properties through Thioester Exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized Maleilated Chitosan/Methacrylated Silk Fibroin Micro/Nanocomposite Hydrogels as Potential Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.; Kim, B.S.; An, Y.H.; Lee, U.J.; Lee, S.H.; Kim, S.L.; Kim, B.G.; Hwang, N.S. Fabrication of Polyphenol-Incorporated Anti-Inflammatory Hydrogel via High-Affinity Enzymatic Crosslinking for Wet Tissue Adhesion. Biomaterials 2020, 242, 119905. [Google Scholar] [CrossRef]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic Crosslinking to Fabricate Antioxidant Peptide-Based Supramolecular Hydrogel for Improving Cutaneous Wound Healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Pathak, S.K.; Tran, V.T.; Cui, J.; Jeon, I. Hydrogels with Superior Mechanical Properties from the Synergistic Effect in Hydrophobic–Hydrophilic Copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and Processable Hydrogels Based on Lignin and Hydrophilic Polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable Scaffolds: Preparation and Application in Dental and Craniofacial Regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Romero-Gilbert, S.; Castro-García, M.; Díaz-Chamorro, H.; Marambio, O.G.; Sánchez, J.; Martin-Trasancos, R.; Inostroza, M.; García-Herrera, C.; Pizarro, G.D.C. Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-Co-Acrylamide) Hydrogels. Polymers 2024, 16, 187. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Liu, K. Mechanical Characterisation of Hydrogels for Tissue Engineering Applications. Top. Tissue Eng. 2008, 4, 1–16. [Google Scholar]
- Oyen, M.L. Mechanical Characterisation of Hydrogel Materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- George, B.; Bhatia, N.; Kumar, A.; Gnanamani, A.; Thilagam, R.; Shanuja, S.K.; Vadakkadath Meethal, K.; Shiji, T.M.; Suchithra, T.V. Bioinspired Gelatin Based Sticky Hydrogel for Diverse Surfaces in Burn Wound Care. Sci. Rep. 2022, 12, 13735. [Google Scholar] [CrossRef]
- Swain, S.; Singh, A.P.; Yadav, R.K. A Review on Polymer Hydrogel and Polymer Microneedle Based Transdermal Drug Delivery System. Mater. Today Proc. 2022, 61, 1061–1066. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible Nanoparticles and Vesicular Systems in Transdermal Drug Delivery for Various Skin Diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Kamei, M.; Matsuo, K.; Imanishi, H.; Hara, Y.; Quen, Y.-S.; Kamiyama, F.; Oiso, N.; Kawada, A.; Okada, N.; Nakayama, T. Transcutaneous Immunization with a Highly Active Form of XCL1 as a Vaccine Adjuvant Using a Hydrophilic Gel Patch Elicits Long-Term CD8+ T Cell Responses. J. Pharmacol. Sci. 2020, 143, 182–187. [Google Scholar] [CrossRef]
- Chung, D.; Ito, Y.; Imanishi, Y. Preparation of Porous Membranes Grafted with Poly(Spiropyran-containing Methacrylate) and Photocontrol of Permeability. J. Appl. Polym. Sci. 1994, 51, 2027–2033. [Google Scholar] [CrossRef]
- Bardavid, Y.; Goykhman, I.; Nozaki, D.; Cuniberti, G.; Yitzchaik, S. Dipole Assisted Photogated Switch in Spiropyran Grafted Polyaniline Nanowires. J. Phys. Chem. C 2011, 115, 3123–3128. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sabey, C.J.; Fernando, G.F. Photoresponsive Polymers: An Investigation of Their Photoinduced Temperature Changes during Photoviscosity Measurements. Polymer 2007, 48, 255–263. [Google Scholar] [CrossRef]
- Warshawsky, A.; Kahana, N.; Buchholtz, F.; Zelichonok, A.; Ratner, J.; Krongauz, V. Photochromic Polysulfones. 1. Synthesis of Polymeric Polysulfone Carrying Pendant Spiropyran and Spirooxazine Groups. Ind. Eng. Chem. Res. 1995, 34, 2825–2832. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kim, C. Photochromic Polyphosphazenes with Spiropyran Units. Macromolecules 1991, 24, 2846–2851. [Google Scholar] [CrossRef]
- Raymo, F.M.; Tomasulo, M. Optical Processing with Photochromic Switches. Chem. A Eur. J. 2006, 12, 3186–3193. [Google Scholar] [CrossRef]
- Lewis, S.M.; Harbron, E.J. Photomodulated PPV Emission in a Photochromic Polymer Film. J. Phys. Chem. C 2007, 111, 4425–4430. [Google Scholar] [CrossRef]
- Wang, S.; Yu, C.; Choi, M.; Kim, S. A Switching Fluorescent Photochromic Carbazole–Spironaphthoxazine Copolymer. Dye. Pigment. 2008, 77, 245–248. [Google Scholar] [CrossRef]
- Tomasulo, M.; Yildiz, I.; Raymo, F.M. Nanoparticle-Induced Transition from Positive to Negative Photochromism. Inorganica Chim. Acta 2007, 360, 938–944. [Google Scholar] [CrossRef]
- Zhu, M.-Q.; Zhu, L.; Han, J.J.; Wu, W.; Hurst, J.K.; Li, A.D.Q. Spiropyran-Based Photochromic Polymer Nanoparticles with Optically Switchable Luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. [Google Scholar] [CrossRef]
- Shao, N.; Jin, J.; Wang, H.; Zheng, J.; Yang, R.; Chan, W.; Abliz, Z. Design of Bis-Spiropyran Ligands as Dipolar Molecule Receptors and Application to in Vivo Glutathione Fluorescent Probes. J. Am. Chem. Soc. 2010, 132, 725–736. [Google Scholar] [CrossRef]
- Heng, S.; Zhang, X.; Pei, J.; Abell, A. A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione. Biosensors 2017, 7, 36. [Google Scholar] [CrossRef]
- Song, Y.; Xu, C.; Wei, W.; Ren, J.; Qu, X. Light Regulation of Peroxidase Activity by Spiropyran Functionalized Carbon Nanotubes Used for Label-Free Colorimetric Detection of Lysozyme. Chem. Commun. 2011, 47, 9083. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Takahashi, S.H.; Rubira, A.F.; Feitosa, J.P.A.; Muniz, E.C. Synthesis of a Novel Superabsorbent Hydrogel by Copolymerization of Acrylamide and Cashew Gum Modified with Glycidyl Methacrylate. Carbohydr. Polym. 2005, 61, 464–471. [Google Scholar] [CrossRef]
- Patachia, S.; Valente, A.J.M.; Baciu, C. Effect of Non-Associated Electrolyte Solutions on the Behaviour of Poly(Vinyl Alcohol)-Based Hydrogels. Eur. Polym. J. 2007, 43, 460–467. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, H.; Xu, L.A.; Zhang, L.F.; Xiong, C.D.; Huang, X. Preparation and Properties of Thermo-Sensitive Hydrogels of Konjac Glucomannan Grafted n-Isopropylacrylamide for Controlled Drug Delivery. Iran. J. Polym. Sci. Technol. 2007, 16, 425–531. [Google Scholar]
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible Adsorption by a PH- and Temperature-Sensitive Acrylic Hydrogel. J. Control. Release 2002, 80, 247–257. [Google Scholar] [CrossRef]
- Lum, L.; Elisseeff, J. Injectable Hydrogels for Cartilage Tissue Engineering. Top. Tissue Eng. 2003, 3, 1–25. [Google Scholar]
- Ding, K.; Liao, M.; Wang, Y.; Lu, J.R. Advances in Composite Stimuli-Responsive Hydrogels for Wound Healing: Mechanisms and Applications. Gels 2025, 11, 420. [Google Scholar] [CrossRef]
- Wang, P.; Cai, F.; Li, Y.; Yang, X.; Feng, R.; Lu, H.; Bai, X.; Han, J. Emerging Trends in the Application of Hydrogel-Based Biomaterials for Enhanced Wound Healing: A Literature Review. Int. J. Biol. Macromol. 2024, 261, 129300. [Google Scholar] [CrossRef]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing Wound Healing and Minimizing Scarring: A Comprehensive Review of Nanofiber Technology in Wound Dressings. Prog. Mater. Sci. 2025, 147, 101350. [Google Scholar] [CrossRef]
- Yang, M.; Tian, J.; Zhang, K.; Fei, X.; Yin, F.; Xu, L.; Wang, Y.; Li, Y. Bioinspired Adhesive Antibacterial Hydrogel with Self-Healing and On-Demand Removability for Enhanced Full-Thickness Skin Wound Repair. Biomacromolecules 2023, 24, 4843–4853. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional Hydrogels for Diabetic Wound Management. APL Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.-Q.; Li, Z.-B.; Wu, Y.-L.; Li, D.-W. Research Advances in Smart Responsive-Hydrogel Dressings with Potential Clinical Diabetic Wound Healing Properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. PH Factors in Chronic Wound and PH-Responsive Polysaccharide-Based Hydrogel Dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Wang, C.; Xing, Y.; Liu, S.; Feng, L.; Zhang, X.; Chen, J. Advances in Smart-Response Hydrogels for Skin Wound Repair. Polymers 2024, 16, 2818. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. PH-responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, H.; Fang, Y.; Wu, J. Hydrogel Combined with Phototherapy in Wound Healing. Adv. Healthc. Mater. 2022, 11, e2200494. [Google Scholar] [CrossRef]
- He, Q.-T.; Qian, P.; Yang, X.-Y.; Kuang, Q.; Lin, Y.-T.; Yi, W.; Tian, T.; Cai, Y.-P.; Hong, X.-J. Rational Design of Bacteria-Targeted and Photo-Responsive MOF Gel with Antibacterial and Anti-Inflammatory Function for Infected Wound Healing. Chem. Eng. J. 2024, 493, 152760. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Adv. Funct. Mater. 2020, 30, 2003196. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Li, W.; Fan, Y.; Zhou, W.; Xiao, C.; Yu, P.; Liu, Y.; Liu, X.; Huang, Z.; et al. LSPR-Enhanced Photoresponsive Antibacterial Efficiency of Bi/MoS2-Loaded Fibrin Gel for Management of Diabetic Wounds. Int. J. Biol. Macromol. 2024, 277, 134430. [Google Scholar] [CrossRef]
- Lahsoune, M.; Boutayeb, H.; Zerouali, K.; Belabbes, H.; El Mdaghri, N. Prévalence et État de Sensibilité Aux Antibiotiques d’Acinetobacter Baumannii Dans Un CHU Marocain. Med. Mal. Infect. 2007, 37, 828–831. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy Using Methylene Blue, Combined or Not with Gentamicin, against Staphylococcus Aureus and Pseudomonas Aeruginosa. Photodiagn. Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Jabed, M.A.; Hetu, M.; Wang, C.; Monro, S.; Zhu, X.; Kilina, S.; McFarland, S.A.; Sun, W. π-Expansive Heteroleptic Ruthenium(II) Complexes as Reverse Saturable Absorbers and Photosensitizers for Photodynamic Therapy. Inorg. Chem. 2017, 56, 3245–3259. [Google Scholar] [CrossRef]
- Wu, J.; Pang, Y.; Liu, D.; Sun, J.; Bai, W. Photodynamic Inactivation of Staphylococcus Aureus Using Aloe-Emodin as Photosensitizer. Food Res. Int. 2024, 178, 113959. [Google Scholar] [CrossRef]
- Pan, X.; Xiao, S.; Wang, B.; Cai, Y.; Chen, X.; Wang, J. Curcumin Nanocapsules Based on Carbon Dots for Photodynamic Sterilization towards Listeria Monocytogenes and Staphylococcus Aureus. Food Chem. 2024, 458, 140295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nie, X.; Lv, Z.; Hao, Y.; Wang, Q.; Wei, Q. A Fast Hemostatic and Enhanced Photodynamic 2-Dimensional Metal-Organic Framework Loaded Aerogel Patch for Wound Management. J. Colloid. Interface Sci. 2024, 656, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, D.; Yu, H.; Zhong, Y.; Zhang, L.; Sui, X.; Wang, B.; Feng, X.; Xu, H.; Mao, Z. Composite Hydrogel Based Oxidated Sodium Carboxymethyl Cellulose and Gelatin Loaded Carboxymethylated Cotton Fabric for Hemostasis and Infected Wound Treatment. Int. J. Biol. Macromol. 2023, 224, 1382–1394. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, J.; Nie, X.; Li, Z.; Xia, X.; Hussain, T.; Wang, Q.; Wei, Q. Photodynamic Activity Enhanced by in Situ Biosynthetic BC/CQDs@PCN-224 Membranes through FRET Strategy. Carbohydr. Polym. 2023, 307, 120623. [Google Scholar] [CrossRef]
- Pérez, C.; Zúñiga, T.; Palavecino, C.E. Photodynamic Therapy for Treatment of Staphylococcus Aureus Infections. Photodiagnosis Photodyn. Ther. 2021, 34, 102285. [Google Scholar] [CrossRef]
- Yan, E.; Kwek, G.; Qing, N.S.; Lingesh, S.; Xing, B. Antimicrobial Photodynamic Therapy for the Remote Eradication of Bacteria. ChemPlusChem 2023, 88, e202300009. [Google Scholar] [CrossRef]
- Abdelkarim-Elafifi, H.; Parada-Avendaño, I.; Arnabat-Dominguez, J. Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics 2021, 10, 1106. [Google Scholar] [CrossRef]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial Photodynamic Therapy for Inactivation of Biofilms Formed by Oral Key Pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Ko, W.-C. Community-Acquired Klebsiella Pneumoniae Bacteremia: Global Differences in Clinical Patterns. Emerg. Infect. Dis. 2002, 8, 160–166. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus Aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of Infections Due to Staphylococcus Species: Frequency of Occurrence and Antimicrobial Susceptibility of Isolates Collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32, S114–S132. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella Spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Nadasy, K.A.; Domiati-Saad, R.; Tribble, M.A. Invasive Klebsiella Pneumoniae Syndrome in North America. Clin. Infect. Dis. 2007, 45, e25–e28. [Google Scholar] [CrossRef]
- Giulieri, S.G.; Tong, S.Y.C.; Williamson, D.A. Using Genomics to Understand Meticillin- and Vancomycin-Resistant Staphylococcus Aureus Infections. Microb. Genom. 2020, 6, e000324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, F.; Kang, J.; Wang, X.; Yin, D.; Dang, W.; Duan, J. Prevalence of Multidrug Resistant Gram-Positive Cocci in a Chinese Hospital over an 8-Year Period. Int. J. Clin. Exp. Med. 2015, 8, 9462. [Google Scholar]
- Ramakrishna, G.; Jose, D.A.; Kumar, D.K.; Das, A.; Palit, D.K.; Ghosh, H.N. Ultrafast Dynamics and Excited State Deactivation of [Ru(Bpy) 2 Sq] + and Its Derivatives. J. Phys. Chem. B 2006, 110, 10197–10203. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Tian, N.; Li, C.; Wang, X. Ru(II)-Complex-Based DNA Photocleaver Having Intense Absorption in the Phototherapeutic Window. Inorg. Chem. 2017, 56, 1865–1873. [Google Scholar] [CrossRef]
- Feringa, B.L.; Browne, W.R. Molecular Switches; Feringa, B.L., Browne, W.R., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Conger-Onder, E.; Gültekinoğlu, M.; Ulubayram, K. Hydrogels Used in Wound Healing; Springer: Berlin/Heidelberg, Germany, 2025; pp. 297–319. [Google Scholar] [CrossRef]
- Hurlow, J.; Wolcott, R.D.; Bowler, P.G. Clinical Management of Chronic Wound Infections: The Battle against Biofilm. Wound Repair. Regen. 2025, 33, e13241. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Morita, S.; Kitagawa, K.; Ozaki, Y. Hydrogen-Bond Structures in Poly(2-Hydroxyethyl Methacrylate): Infrared Spectroscopy and Quantum Chemical Calculations with Model Compounds. Vib. Spectrosc. 2009, 51, 28–33. [Google Scholar] [CrossRef]
- Morita, S.; Ye, S.; Li, G.; Osawa, M. Effect of Glass Transition Temperature (Tg) on the Absorption of Bisphenol A in Poly(Acrylate)s Thin Films. Vib. Spectrosc. 2004, 35, 15–19. [Google Scholar] [CrossRef]
- Huang, C.W.; Sun, Y.M.; Huang, W.F. Curing Kinetics of the Synthesis of Poly(2-Hydroxyethyl Methacrylate) (PHEMA) with Ethylene Glycol Dimethacrylate (EGDMA) as a Crosslinking Agent. J. Polym. Sci. A Polym. Chem. 1997, 35, 1873–1889. [Google Scholar] [CrossRef]
- Singh, N.K.; Lesser, A.J. A Physical and Mechanical Study of Prestressed Competitive Double Network Thermoplastic Elastomers. Macromolecules 2011, 44, 1480–1490. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New Insight into Agarose Gel Mechanical Properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Buckley, C.T.; Thorpe, S.D.; O’Brien, F.J.; Robinson, A.J.; Kelly, D.J. The Effect of Concentration, Thermal History and Cell Seeding Density on the Initial Mechanical Properties of Agarose Hydrogels. J. Mech. Behav. Biomed. Mater. 2009, 2, 512–521. [Google Scholar] [CrossRef]
- Chan, E.S.; Lim, T.K.; Voo, W.P.; Pogaku, R.; Tey, B.T.; Zhang, Z. Effect of Formulation of Alginate Beads on Their Mechanical Behavior and Stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Choi, S.S.; Hong, J.P.; Seo, Y.S.; Chung, S.M.; Nah, C. Fabrication and Characterization of Electrospun Polybutadiene Fibers Crosslinked by UV Irradiation. J. Appl. Polym. Sci. 2006, 101, 2333–2337. [Google Scholar] [CrossRef]
- Han, W.; Zhou, B.; Yang, K.; Xiong, X.; Luan, S.; Wang, Y.; Xu, Z.; Lei, P.; Luo, Z.; Gao, J.; et al. Biofilm-Inspired Adhesive and Antibacterial Hydrogel with Tough Tissue Integration Performance for Sealing Hemostasis and Wound Healing. Bioact. Mater. 2020, 5, 768–778. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2022, 15, 144. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Tang, Z.; Xiao, H.; Zhang, M.; Liu, K.; Chen, L.; Huang, L.; Ni, Y.; Wu, H. Mussel-Inspired Blue-Light-Activated Cellulose-Based Adhesive Hydrogel with Fast Gelation, Rapid Haemostasis and Antibacterial Property for Wound Healing. Chem. Eng. J. 2021, 417, 129329. [Google Scholar] [CrossRef]
- Romero-Gilbert, S.; Díaz-Chamorro, H.; Marambio, O.G.; Sánchez, J.; Martin-Trasancos, R.; Inostroza, M.; García-Herrera, C.; Pizarro, G.d.C. Influence of Different Experimental Conditions on Bond Strength of Self-Adhesive Synthetic Polymer System Hydrogels for Biological Applications. React. Funct. Polym. 2025, 213, 106264. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Zheng, Y.; Shariati, K.; Ghovvati, M.; Vo, S.; Origer, N.; Imahori, T.; Kaneko, N.; Annabi, N. Hemostatic Patch with Ultra-Strengthened Mechanical Properties for Efficient Adhesion to Wet Surfaces. Biomaterials 2023, 301, 122240. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Martinez, G.R.; Ravanat, J.-L.; Cadet, J.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Energy Transfer between Singlet (1Δg) and Triplet (3Σg-) Molecular Oxygen in Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 3056–3057. [Google Scholar] [CrossRef]
- Greer, A. Christopher Foote’s Discovery of the Role of Singlet Oxygen [1O2 (1Δg)] in Photosensitized Oxidation Reactions. Acc. Chem. Res. 2006, 39, 797–804. [Google Scholar] [CrossRef]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2007, 82, 1161–1177. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Hormazábal, D.B.; Reyes, Á.B.; Cuevas, M.F.; Bravo, A.R.; Costa, D.M.; González, I.A.; Navas, D.; Brito, I.; Dreyse, P.; Cabrera, A.R.; et al. Photodynamic Effectiveness of Copper-Iminopyridine Photosensitizers Coupled to Zinc Oxide Nanoparticles Against Klebsiella Pneumoniae and the Bacterial Response to Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 4178. [Google Scholar] [CrossRef] [PubMed]
| Feed Monomer Ratio | [AAm] (mol/g) | [HEMA] (mol/g) | [MBA] (mol.-%) | [BSP] (mol.-%) | Yield (%) |
|---|---|---|---|---|---|
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.0 | 0.0 | 95 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.1 | 0.0 | 90 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.3 | 0.0 | 90 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.5 | 0.0 | 92 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.1 | 0.1 | 90 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.3 | 0.3 | 90 |
| P(HEMA-co-AAm) 1:1 | 0.015/1.065 | 0.015/1.950 | 0.5 | 0.5 | 90 |
| Hydrogel | AAm | HEMA | Ester | -NO2 |
|---|---|---|---|---|
| (C = O) | (C = O) | (C = O) | -N-O | |
| P(HEMA-co-AAm) with 0.1 mol.% MBA | 1658 | 1713 (s) | (w) | --- |
| P(HEMA-co-AAm) with 0.1 mol.% MBA/BSP | 1659 | (w) | 1613 (s) | 1454 (s) |
| P(HEMA-co-AAm) with 0.5 mol.% MBA | 1656 | 1713 (s) | (w) | --- |
| P(HEMA-co-AAm) with 0.5 mol.% MBA/BSP | 1657 | (w) | 1610 (s) | 1454 (s) |
| Feed Monomer Ratio | MBA (mol.-%) | BSP (mol.-%) | Shear Stress (kPa) | Shear Modulus (kPa) |
|---|---|---|---|---|
| P(HEMA-co-AAm) without BSP | 0.1 0.5 | 0.0 0.0 | 0.67 ± 033 1.97 ± 0.25 | 1.69 ± 0.46 3.33 ± 1.27 |
| P(HEMA-co-AAm) with MBA and BSP | 0.1 0.5 | 0.1 0.5 | 1.81 ± 0.37 3.41 ± 0.17 | 4.34 ± 0.91 6.18 ± 1.02 |
| Polymeric Materials | Biomedical Application | Young’s Modulus (E) * | Reference |
|---|---|---|---|
| Hydroxyethyl methacrylate –co-acrylamide with Dopamine | Catechol-based bioinspired adhesive properties in a wet medium | 33.37 ± 2.74 − 58.86 ± 2.04 kPa | [23] |
| Maleic anhydride-modified β-cyclodextrin (CD), amantadine as a competitive guest | Adhesive antibacterial hydrogel | 37.4 − 85.8 37.6 kPa | [53] |
| Chitosan grafted with methacrylate, dopamine, and N-hydroxymethyl acrylamide | Biofilm-inspired adhesive antibacterial hydrogel | 34.0 kPa | [104] |
| Carboxymethyl chitosan hydrogel | Biodegradable carbohydrate polymers | 2.3 kPa to 13.3 kPa, 12.5 kPa | [105] |
| Allyl cellulose with Dopamine | Mussel-inspired cellulose-based adhesive hydrogel | 38.8 to 40.2 kPa | [106] |
| Hydroxyethyl methacrylate –co-acrylamide with cross-linker (MBA) | Biomimetic adhesive hydrogel | 1.39 ± 0.06 kPa, 3.85 ± 1.87 kPa | [107] |
| Hydroxyethyl methacrylate with acrylamide, N′N-methylene bis-acrylamide (MBA) and photochromic agent (BSP) | Photoactive hydrogels as materials for biological applications, such as antimicrobial patches | 4.34 ± 0.91 6.18 ± 1.02 kPa | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marambio, O.G.; Álvarez, L.; Díaz-Chamorro, H.; Sánchez, J.; Martin-Trasancos, R.; Palavecino, C.E.; Pizarro, G.d.C. Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films. Gels 2025, 11, 663. https://doi.org/10.3390/gels11080663
Marambio OG, Álvarez L, Díaz-Chamorro H, Sánchez J, Martin-Trasancos R, Palavecino CE, Pizarro GdC. Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films. Gels. 2025; 11(8):663. https://doi.org/10.3390/gels11080663
Chicago/Turabian StyleMarambio, Oscar G., Lidia Álvarez, Héctor Díaz-Chamorro, Julio Sánchez, Rudy Martin-Trasancos, Christian Erick Palavecino, and Guadalupe del C. Pizarro. 2025. "Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films" Gels 11, no. 8: 663. https://doi.org/10.3390/gels11080663
APA StyleMarambio, O. G., Álvarez, L., Díaz-Chamorro, H., Sánchez, J., Martin-Trasancos, R., Palavecino, C. E., & Pizarro, G. d. C. (2025). Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films. Gels, 11(8), 663. https://doi.org/10.3390/gels11080663











