Research Progress of Supramolecular Gels in the Field of Petroleum Engineering
Abstract
1. Introduction
2. Classification of Supramolecular Gels
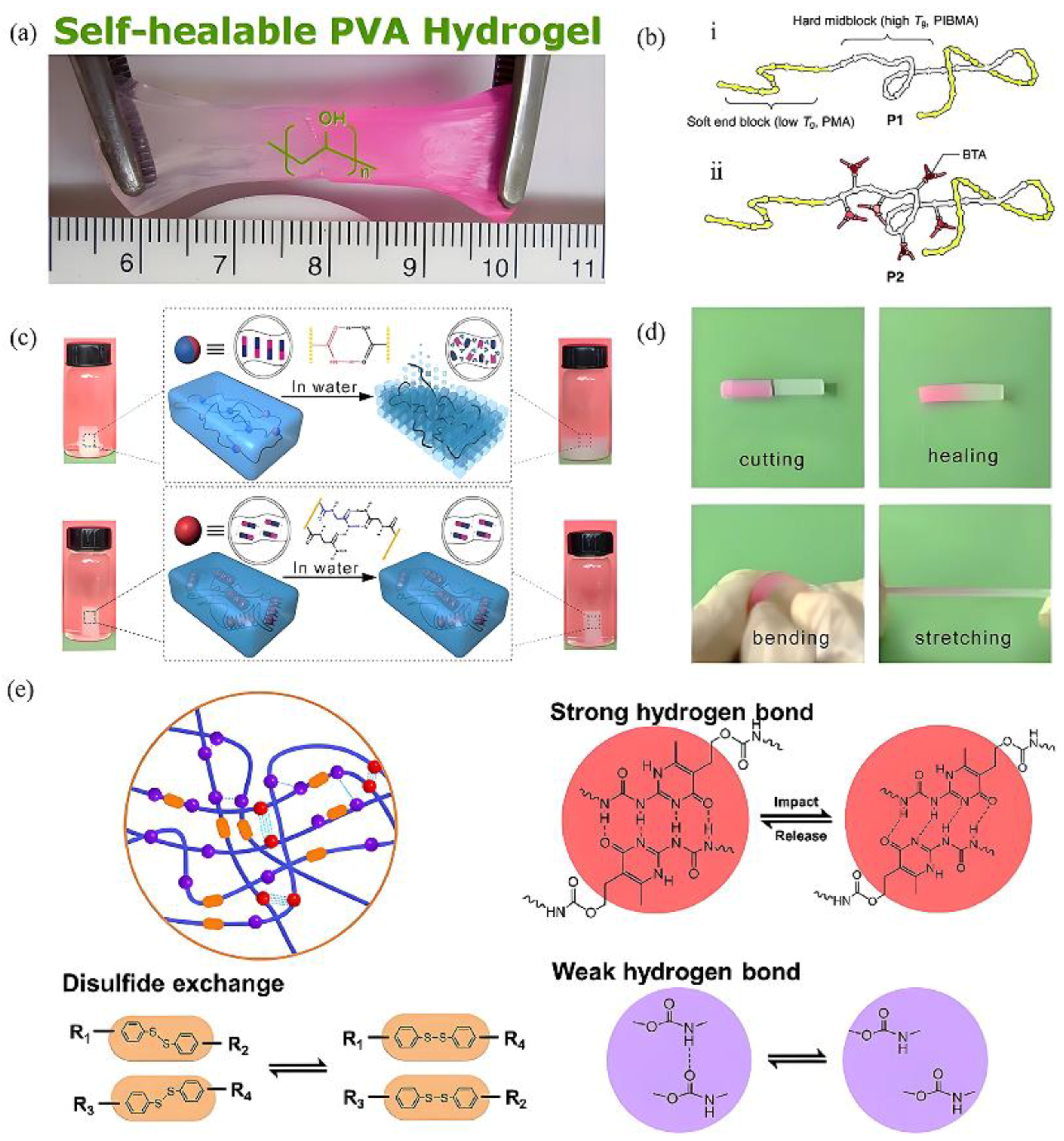
2.1. Hydrogen Bond
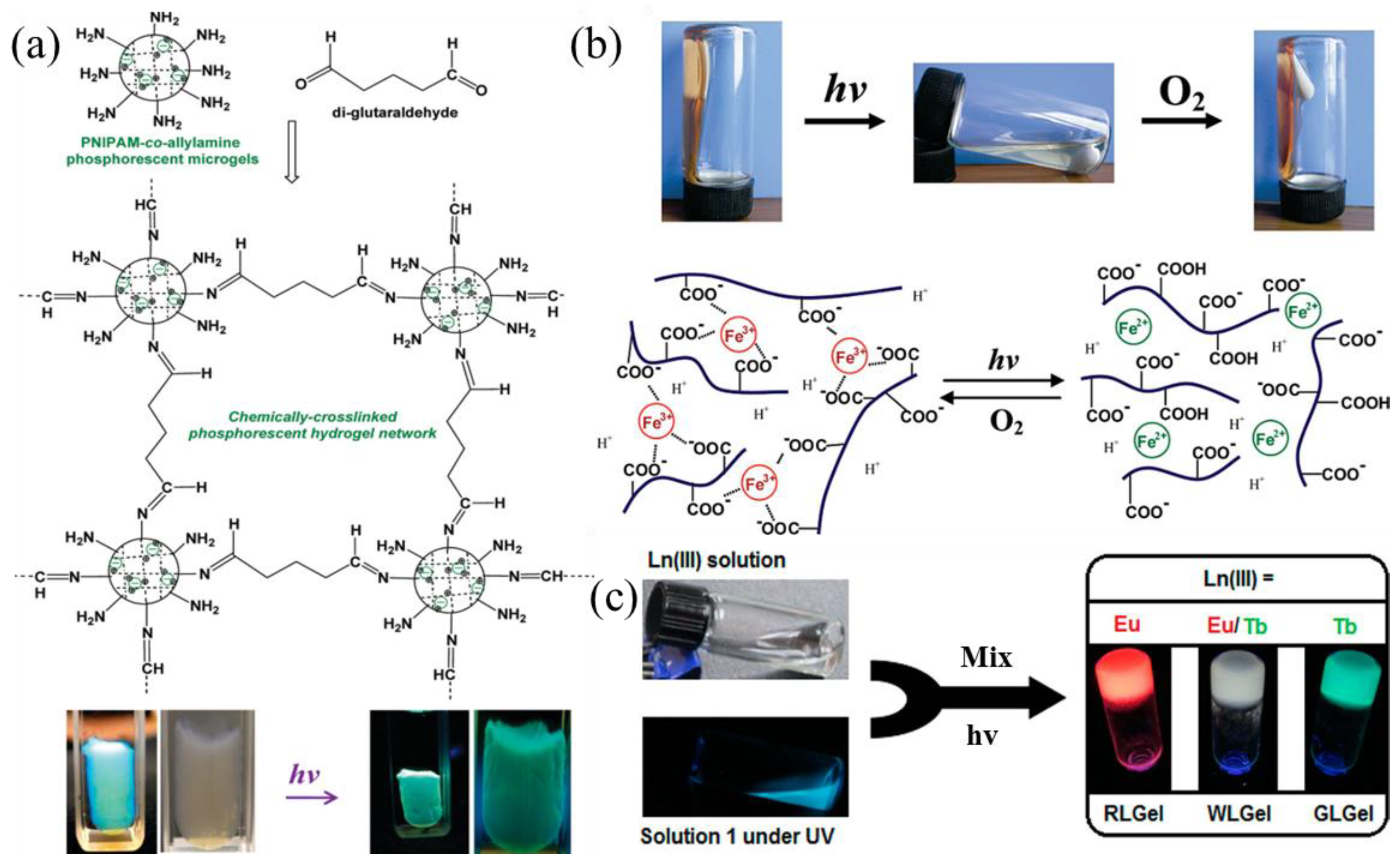
2.2. Metal Coordination
2.3. Host–Guest Interaction
2.4. Hydrophobic Interaction
2.5. Electrostatic Interaction
3. The Application of Supramolecular Gels in the Oil Industry
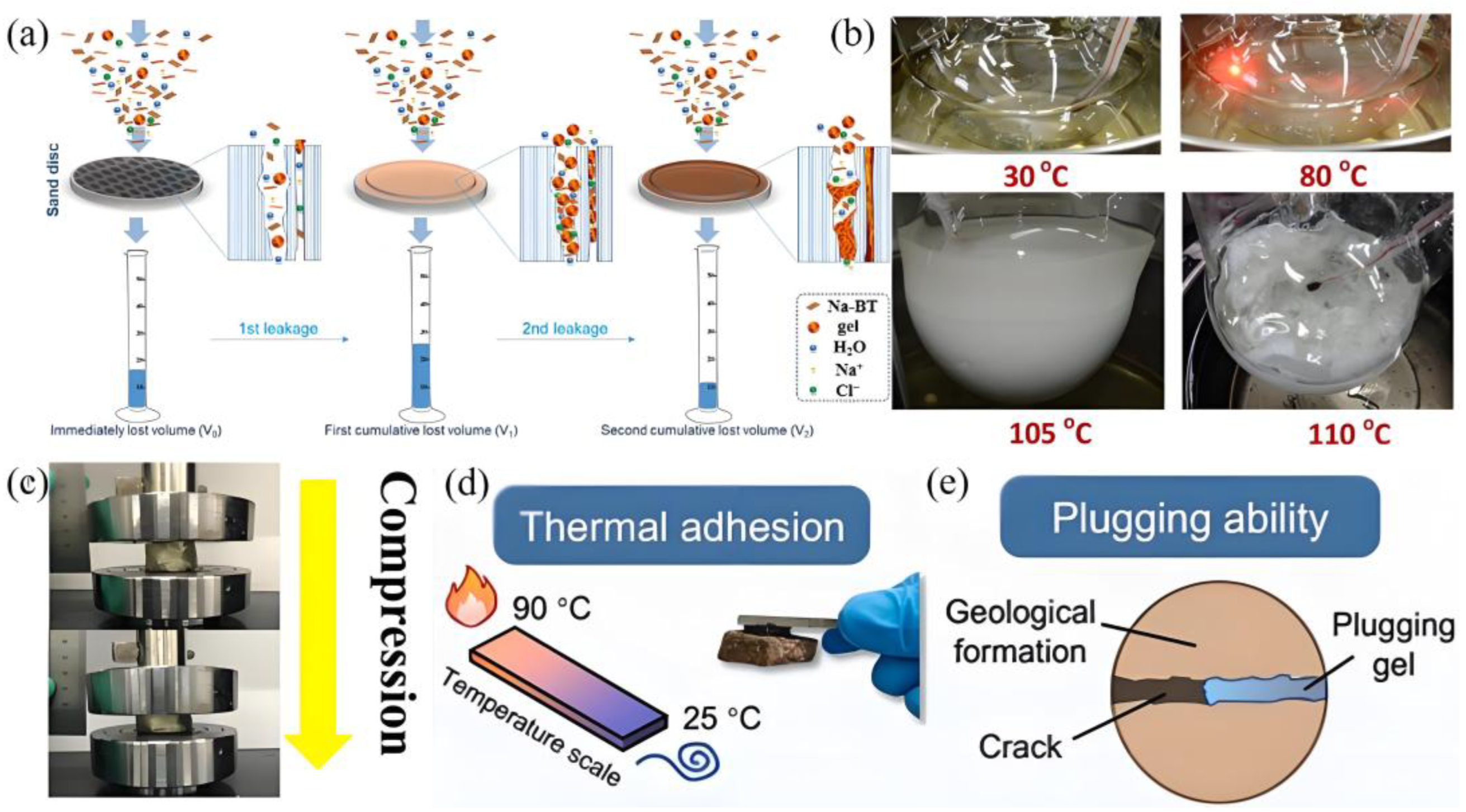
3.1. Lost Circulation Control in Drilling
| Regarding the Failure Mode | Material Type | Trigger Mechanism | Shear Behavior | Mechanical Properties | Key Performance | Laboratory/Field Application | Reference |
|---|---|---|---|---|---|---|---|
| The lack of sufficient interactions between material interfaces | Self-healing polyampholyte gel particles-H-bonding, cation-anion, and dipole-dipole interactions | Temperature (70–150 °C) and salinity (2–15% NaCl) | G′ increased to 14.6 MPa from 0.24 MPa (dimensions of 11.6 mm × 7.5 mm × 2 mm, frequency of 1 Hz, heating rate of 3 °C/min) | Compressive strength and tensile strength decreased from 7.3 to 1.9 MPa and 80.0–16.0 KPa, respectively, at 0–20% NaCl, 600% of strain after soaking in 20.0 wt % NaCl solution | Sand disk of 180 D: average porosity dropped from 9.86% to 1.06% (90 °C), 63.5% of the leakage volume reduction rate (LVRR); 63.4% LVRR at 15% NaCl and CaCl2 (90 °C); it can withstand a pressure of 6 MPa at 150 °C, 41.8% of LVRR | Laboratory stage | [58] |
| The lack of sufficient interactions between material interfaces | Zwitterionic polymer/nano-silica microgels-the strong hydrogen bonds, electrostatic interaction and cation-π | N/A | N/A | 1.03 MPa of elongation, 217% of tensile deformation (120 mm/min of tensile speed) | Sand disks loss volume (5D): 17 mL at 80 °C, 29 mL at 150 °C, 37 mL at 7.5% NaCl (2% microgel after aging at 120 °C for 16 h) | Laboratory stage | [59] |
| The problem of the difficulty in degrading the temporary sealing materials | The reversible heat-set supramolecular gels-host–guest interactions and hydrogen bonding | Temperature(90 °C gel–110 °C sol) | G′ = 0.00001 Pa, 800 pa, and 100 pa at 30 °C, 95 °C, and 110 °C (0.01–10 Hz of frequency) | N/A | Fractured core test (0.5 mm): 6.8 MPa (105 °C) | Laboratory stage | [60] |
| The problem of the difficulty in degrading the temporary sealing materials | Cellulose nanofiber-reinforced supramolecular polymer gels- hydrogen bonding | N/A | G′ = ~20,000 Pa at 84 rad/s (10% of strain, 1–15 0.5–84 rad/s, 25 °C) | Tensile stress and elongation at break were 442 ± 35 kPa and 3212 ± 266%, 0.69 MPa of compressive strength at a fixed strain of 75% (50 mm/min tensile speed and a 20 mm/min compression speed, room temperature) | Sealing capacity (40–120 mesh, diameter of 45 mm and a length of 25 cm): 12.16 MPa (120 °C); Stability: the storage moduli adding brine was 6396 Pa (70,000 mg/L Na+ and 16,000 mg/L Ca2+) | Pilot field test | [61] |
| Insufficient adhesive capacity of the gel | Polydopamine embedded hydrogel-hydrogen bonding | N/A | N/A | 65.3 kPa of the tensile strength, 570% of elongation; 9.9 kPa and 130% of tensile strength and elongation after adding 20 × 104 mg/L (20 × 10 × 2 mm3, 100 mm/min of speed, room temperature) | Plugging test (1 mm): 7.6 MPa (90 °C); adhesion test: ~12 kPa, 8 kPa of PAM | Laboratory stages | [63] |
| Poor retention capability | Thixotropic polymer gel–hydrogen bonds, hydrophobic interactions, electrostatic interactions | N/A | G′ = 1000 Pa at 10 Hz (0.053 mm in thickness, 35 mm of diameter, room temperature) | N/A | Adhesion test: 0.62 ± 0.05 MPa; snad bed test (20–40 mesh): 27 mL (6 MPa and 150 °C); plugging test (8–16 mesh quartz sand): 5.3 MPa, 2.7 MPa of PAM (150 °C) | Laboratory stages | [64] |
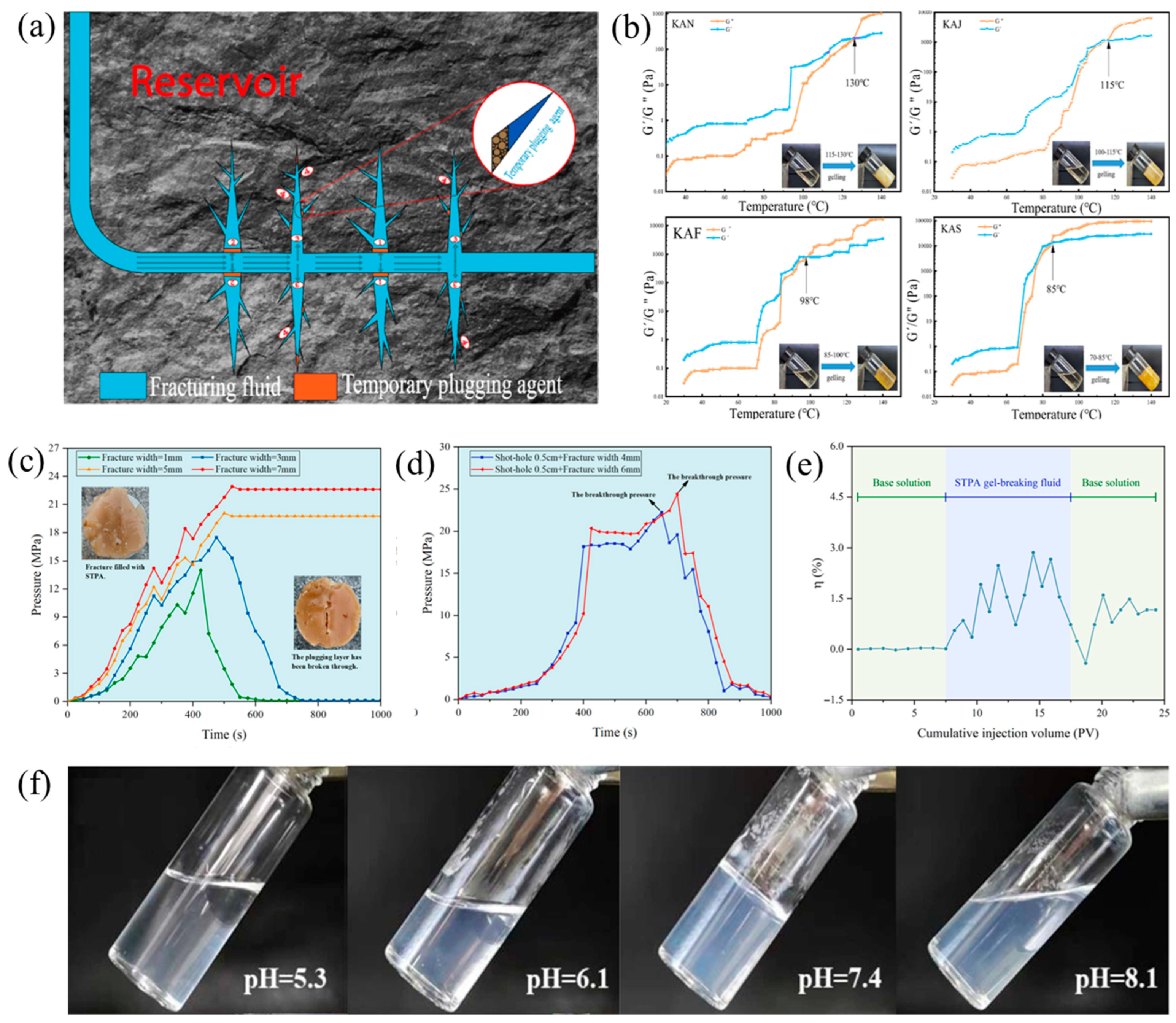
3.2. Temporary Plugging in Fracturing
| Regarding the Failure Mode | Material Type | Trigger Mechanism | Shear Behavior | Mechanical Properties | Key Performance | Laboratory/Field Application | Reference |
|---|---|---|---|---|---|---|---|
| Narrow gel transition temperature | β-cyclodextrin/benzene derivatives gel (KAN without adding any benzene derivates, KAJ adding toluene, KAF adding phenol and KAS adding benzoic acid)—host–guest interactions | Temperature (gelling at 70–130 °C), degradation time (1.2, 1.7, 2.6 and 3.2 h for KAS, KAF, KAJ, KAN) | N/A | N/A | Degradation rate: 98, 97.5, 97.6, 97.7% for KAS, KAF, KAJ, KAN; Plugging test: 5.6 MPa (~45 × 10−3 μm2), 6.9 MPa (~40 × 10−3 μm2), 7.4 MPa (~37 × 10−3 μm2) and 7.6 MPa (~36.5 × 10−3 μm2) for KAN, KAJ, KAF and KAS (KAN gelling at 130 °C, KAJ gelling at 115 °C, KAS gelling at 98 °C, KAF gelling at 85 °C) | Laboratory stages | [67] |
| Conventional gels are difficult to remove blockages | Phase change fracturing—β-CD-aliphatic alcohols and fatty acids | Gelatinizing temperature (83–122 °C) | N/A | N/A | Plugging test: 8.45 MPa (50 mm of core length, 0.5 mm of fracture width) | Laboratory stages | [68] |
| Poor adaptability, insufficient pressure-bearing capacity, and low efficiency of returning to the ground | Low-damage temperature-controlled phase change temporary plugging agent—hydrogen bonds | Gel formation time (10–40 min at 60–80 °C), Gel-breaking time (100–420 min at 120–160 °C) | 20,000 Pa at 1 Hz (0.1 of shear stress, 80 °C) | N/A | Plugging performance tests: the fractures of 1 mm, 3 mm, 5 mm, and 7 mm all have a width greater than 14.2 MPa; the shot-hole of 0.5 mm size and 4 mm/6 mm sizes are all greater than 18 MPa (120 °C), damage performance: 1.2%. | Field application | [69] |
| Poor adaptability and low efficiency of returning to the ground | Thermoresponsive in situ-generated proppant based on liquid solid transition of a supramolecular self-propping fracturing fluids | Temperature (clear liquid at 30 °C, solid at 60, 90, and 120 °C) | G″ was always higher than G′ at 30 °C, (the stress and frequency were set as 1 Pa and 0.01–10 Hz) | N/A | Dynamic leak off tests: 0.0007 of matrix, 0.3 of fractures | Laboratory stages | [70] |
| Break-up in presence of slats and high temperature | Supramolecular assembly of maleic acid and an amino–amide charge interaction (2 wt%) | pH (4–10), 10.2 mPa·s at pH = 4, 2500 mPa·s at pH = 8.5 | Viscosity: no total break-up or degradation at 5 wt% salt | N/A | 52 kJ/mol supramolecular solution, polyacrylamide (~16 kJ/mol) | Laboratory stages | [71] |
| Low values of polymer injectivity and pumping efficiencies | Responsive Amphiphilic systems as displacement fluids—long-chain amino–amide and maleic acid | pH (3.7 × 104 mPa·s at pH = 4, 4.5 × 105 mPa·s at pH = 8) | N/A | N/A | 2 wt% of adaptable amphiphile/maleic acid into water increased the viscosity of water by a factor of 4.5 × 105 | Laboratory stages | [72] |
| The gel breaking time and degree of gel breaking are still difficult to control | Quaternary ammonium-based supramolecular gel-hydrogen bonds | pH (solution at pH < 7.4, gel at pH = 7.4, sol at pH > 8.1) and temperatures | N/A | N/A | Viscosity (mPa·s): 1.63 × 105, 4.35 × 105, 5.02 × 105, 6.12 × 105 of SDA (azelaic acid derived TPA), SDT (tartaric acid derived TPA), SDC (citric acid derived TPA), SDM (maleic acid derived TPA); plugging rate (%): 29.05, 68.73, 74.95, and 90.43 of SDA, SDT, SDC, and SDM. (70 °C) | Laboratory stages | [73] |
3.3. Profile Control in Enhanced Oil Recovery
| Regarding the Failure Mode | Material Type | Trigger Mechanism | Shear Behavior | Mechanical Properties | Key Performance | Laboratory/Field Application | Reference |
|---|---|---|---|---|---|---|---|
| Migration distance and plugging strength | Self-growing hydrogel particles—hydrogen bonds | N/A | N/A | N/A | Median size of hydrogel particles: increased from 3.5 to 18.0 μm (aging for 15 days at 80 °C and 0.5 M NaCl solution) | Laboratory stages | [75] |
| Durability in harsh reservoir conditions | Hyperbranched, nanowire-prepared weak gels-hydrogen bonding and electrostatic interactions | N/A | 0.8 wt% of crosslinker, 5637 mPa·s at 0.1 rad/s, G′ > G″ at frequency range (110 °C, 1–100 rad/s) | N/A | Core plugging test: permeability reduction from 313 mD to 9.9 mD, yielding a resistance factor of 31.3; (110 °C, 48 h, 217, 501 of TDS), oil recovery rate: 89.9% (110 °C, 217, 501 of TDS) | Laboratory stages | [76] |
| Environmental pollution problems | Urea-containing supramolecular polymer gel–hydrogen bonding | N/A | G′ > G″ at 0.1–100 Hz (1 mm of thickness, 17.5 mm of the radius, room temperature) | N/A | Pressure-bearing properties: 250 N (8.75% monomer gel) and 500 N (17.5% polymer gel) (150 °C) | Laboratory stages | [77] |
| Some deficiencies of gel in-depth profile control | Self-lubricating supramolecular hydrogel/π-π stacking, hydrogen bond | Shear rate | Gel–sol transition frequency of FPP-0.5 displayed a first stable and then significant increase trend with shear cycle rise (20–60 Hz at frequency, 25 °C) | N/A | Core flooding experiments (30 cm × 4.5 cm × 4.5 cm of size): 86.6 of the plugging rate, 83.5 of water flooding volumetric sweep efficiency, 71.3 of oil recovery (80 °C). | Laboratory stages | [78] |
| Gas channeling and mobility control | CO2-responsive and smart mobility control agent | CO2 | 33,000 mPa·s at 0.01 1/s (35 mm of d, 25 °C) | N/A | Plugging performance: 171 kPa, 99.2% of plugging efficiency (50 °C, 10,985 mg/L salinity) | Laboratory stages | [79] |
| Acidic and thermal hydrolysis of acrylamide/polyacrylamide | Smart polymer | CO2 and temperatures (solution in 25 °C, gel in 90 °C) | N/A | N/A | Core flooding test: increase 21–22% total oil recovery than the conventional a | Laboratory stages | [80] |
| Environmental problem | Alkyl bicarbamates supramolecular organogelators–H bonding, π-π stacking and van der Waals interactions | N/A | N/A | N/A | Oil removal rates are always higher than 95% and the oil retention rates can be close to 100% | Laboratory stages | [81] |

- (1)
- Through molecular structure design, non-covalent interactions (e.g., hydrophobic association, hydrogen bonding, electrostatic interactions) are combined with covalent crosslinking to construct a composite crosslinked network, thereby enhancing the long-term stability of supramolecular gels.
- (2)
- The incorporation of solid fillers (e.g., SiO2, CaCO3, asphalt nanoparticles) into supramolecular gel systems enhances material strength and reduces production costs through the synergistic effect of non-covalent interactions between the fillers.
- (3)
- The development of a gel system capable of coordinating with multivalent metal ions (e.g., Ca2+ and Mg2+) in formation water is essential, as is the enhancement of the material’s mechanical properties through the coordination crosslinking effect of metal ions. This adaptation to complex reservoir environments is crucial.
3.4. Field Applications
4. Conclusions
- (1)
- Supramolecular gels constructed based on non-covalent interactions (hydrogen bonds, metal coordination, host–guest recognition, hydrophobic interactions, and electrostatic interactions) combine high structural strength, dynamic reversibility, and functional designability, effectively addressing key technical challenges, such as lost circulation, temporary plugging during fracturing, and enhanced oil recovery in complex geological conditions.
- (2)
- Different crosslinking mechanisms confer differentiated properties on gel materials. Hydrogen bonding and metal coordination significantly enhance mechanical strength, while host–guest interactions enable dynamic reversible regulation. Hydrophobic interactions ensure stability in high-temperature, high-salt environments, and electrostatic interactions provide self-healing and functionalization properties.
- (3)
- In the current study, such as the lost circulation, the host–guest gels achieve dynamic fracture sealing through temperature-triggered hydrophobic crosslinking, while hydrogen bond gels enhance fracture interface bonding strength through high adhesion (10 kPa). In fracturing fluid, the host–guest supramolecular gel formed by cyclodextrin and benzene derivatives exhibits excellent temperature control properties, meeting the dynamic requirements of “sol–gel–sol” in fracturing operations. In profile control, hydrogen bonds and π-π bonds enhance the interparticle interaction forces, with an average interparticle adhesion force of 1.21 ± 0.04 nN. The synergistic effects of hydrogen bonds, π-π bonds, and van der Waals forces provide more options for profile control.
Funding
Data Availability Statement
Conflicts of Interest
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry. Science 1974, 183, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007, 36, 151–160. [Google Scholar] [CrossRef]
- Lehn, J.M. Cryptates: The chemistry of macropolycyclic inclusion complexes. Acc. Chem. Res. 1978, 11, 49–57. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Wu, B.-B.; Xu, Y.-Y.; Gao, F.; Wu, Y.-J.; Xi, L.-H.; Kang, Z.; He, H.-K.; Yang, Z.-G.; Xu, Z.-X. Low temperature hydrochar from sewage sludge enhancing polyacrylamide-based preformed particle gels with high temperature and salinity tolerance. J. Water Process Eng. 2025, 76, 108282. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Liu, J.; Zhang, Y.; Gao, M.; Song, X.; Sun, N.; Li, L.; Wu, Y.; Dai, C. Study on formation process and reservoir damage mechanism of blockages caused by polyacrylamide fracturing fluid in production wells. Fuel 2024, 358, 130154. [Google Scholar] [CrossRef]
- Telin, A.; Lenchenkova, L.; Yakubov, R.; Poteshkina, K.; Krisanova, P.; Filatov, A.; Stefantsev, A. Application of Hydrogels and Hydrocarbon-Based Gels in Oil Production Processes and Well Drilling. Gels 2023, 9, 609. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, K.; Lu, J. Novel modification method for inorganic geopolymer by using water soluble organic polymers. Mater. Lett. 2004, 58, 1292–1296. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wu, K.; Qian, L.-P.; Wang, Y.-S.; Guo, D. Effect of polyethylene glycol (PEG) modification on strength and microstructure of geopolymer paste. Constr. Build. Mater. 2025, 489, 142378. [Google Scholar] [CrossRef]
- Akhiyarov, D. Gel Preparation and Properties Control for Operations in Pipeline Transport. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007; p. SPE–113785-STU. [Google Scholar]
- Mohammadi, S.; Ghazanfari, M.H.; Masihi, M.; Vossoughi, S. Effect of small scale flow barriers heterogeneities and connate water on displacement efficiency of polymer floods to heavy oil reservoirs. Can. J. Chem. Eng. 2013, 91, 1729–1740. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Review of gel systems for CO2 geological storage leakage and conformance control for enhanced oil recovery: Mechanisms, recent advances, and future perspectives. J. Pet. Sci. Eng. 2022, 219, 111110. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Wei, Q.; Wu, X.; Bai, B. Terpolymer Gel System Formed by Resorcinol–Hexamethylenetetramine for Water Management in Extremely High-Temperature Reservoirs. Energy Fuels 2017, 31, 1519–1528. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, X.-G.; Du, K.; Liu, H.-K. Development of a new high-temperature and high-strength polymer gel for plugging fractured reservoirs. Upstream Oil Gas Technol. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Liu, J.; Yang, L.; Zhang, L.; Wang, J.; Fan, H. Enhancing thermal stability of preformed particle gels (PPGs) under high temperature: The role of crosslinkers. J. Mol. Liq. 2025, 425, 127208. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D. The effects of pH and ionic strength on the volume phase transition temperature of thermo-responsive anionic copolymer gels. Polymer 2021, 221, 123637. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, X.; Rui, Z.; Ding, Y. A novel dual-network CO2-responsive particle gel for mitigating CO2 channeling and leakage in hydrocarbon recovery and carbon storage. Chem. Eng. J. 2024, 498, 155187. [Google Scholar] [CrossRef]
- Pu, W.-F.; Du, D.-J.; Fan, H.-C.; Chen, B.-W.; Yuan, C.-D.; Varfolomeev, M.A. CO2-responsive preformed gel particles with interpenetrating networks for controlling CO2 breakthrough in tight reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126065. [Google Scholar] [CrossRef]
- Kovalchuk, V.; Guo, Z.; Cheremisin, A.; Plank, J. Evaluation of a low carbon cement containing calcined clay for oil well cementing. Geoenergy Sci. Eng. 2025, 254, 214061. [Google Scholar] [CrossRef]
- Oglesby, K.D.; D’Souza, D.; Roller, C.; Logsdon, R.; Burns, L.D.; Felber, B.J. Field Test Results of a New Silicate Gel System that is Effective in Carbon Dioxide Enhanced Recovery and Waterfloods. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016; p. SPE–179615-MS. [Google Scholar]
- Hatami, S.; Hughes, T.J.; Sun, H.; Roshan, H.; Walsh, S.D.C. On the application of silica gel for mitigating CO2 leakage in CCS projects: Rheological properties and chemical stability. J. Pet. Sci. Eng. 2021, 207, 109155. [Google Scholar] [CrossRef]
- Yang, J.-B.; Sun, J.-S.; Bai, Y.-R.; Lv, K.-H.; Li, J.; Li, M.-C.; Zhu, Y.-C. Preparation and characterization of supramolecular gel suitable for fractured formations. Pet. Sci. 2023, 20, 2324–2342. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, L.; Zhang, Y.; Sun, H.; Fan, K.; Zhang, B.; Song, J. High toughness, ultra-sensitive, and extreme environment resistant fabric temperature sensors based on supramolecular-polymer dual network ionic conductive elastomers. Chem. Eng. J. 2024, 487, 150688. [Google Scholar] [CrossRef]
- Cao, X.; Guo, W.; Zhu, Q.; Ge, H.; Yang, H.; Ke, Y.; Shi, X.; Lu, X.; Feng, Y.; Yin, H. Supramolecular self-assembly of robust, ultra-stable, and high-temperature-resistant viscoelastic worm-like micelles. J. Colloid Interface Sci. 2023, 649, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, H.; Ning, C.; Peng, L.; Zhang, S.; Chen, X.; Shi, H.; Wang, R.; Sarsenbekuly, B.; Kang, W. Amphiphilic polymer with ultra-high salt resistance and emulsification for enhanced oil recovery in heavy oil cold recovery production. Geoenergy Sci. Eng. 2025, 252, 213920. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Yang, Z.; Wang, P.; Liu, J.; Xie, Y.; Wu, Y.; Gao, M.; Li, L.; Song, X.; et al. Novel Mussel-Inspired High-Temperature Resistant Gel with Delayed Crosslinking Property for Ultra-Deep Reservoir Fracturing. Adv. Funct. Mater. 2024, 34, 2405111. [Google Scholar] [CrossRef]
- Zheng, H.; Shi, Y.; Tian, Y.; Yan, X.; Zhao, S.; Zhang, R. Synthesis and performance evaluation of amphiphilic hyperbranched hydrophobically linked polymer thickeners. Colloids Surf. A Physicochem. Eng. Asp. 2025, 715, 136667. [Google Scholar] [CrossRef]
- Li, Z.; Hao, J.; Lu, Y.; Yu, Z.; Yang, H.; Zhou, B.; Jiang, H.; Sarsenbekuly, B.; Kang, W. Host-Guest recognition strengthened supramolecular preformed particle gel for conformance control in low-permeability fractured reservoirs. J. Mol. Liq. 2024, 412, 125893. [Google Scholar] [CrossRef]
- Zuo, C.; Liu, P.; Du, J.; Wu, G.; Chen, X.; Liu, J. A review: Supramolecular polymers used in high-temperature fracturing fluids research status and prospects on the coupling relationship between molecular conformation and temperature resistance mechanism. Geoenergy Sci. Eng. 2025, 247, 213699. [Google Scholar] [CrossRef]
- Moore, T.S.; Winmill, T.F. CLXXVII.—The state of amines in aqueous solution. J. Chem. Soc. Trans. 1912, 101, 1635–1676. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(vinyl alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Wang, H.; Song, X.; Sun, Y.; Zhao, J.; Cui, P.; Hu, R.; Zhu, Q. A robust-switchable poly(vinyl alcohol) gel with adjustable multiple hydrogen bonding interactions. Eur. Polym. J. 2023, 195, 112192. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. [Google Scholar] [CrossRef]
- Hosono, N.; Pitet, L.M.; Palmans, A.R.A.; Meijer, E.W. The effect of pendant benzene-1,3,5-tricarboxamides in the middle block of ABA triblock copolymers: Synthesis and mechanical properties. Polym. Chem. 2014, 5, 1463–1470. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Deng, Y.; Ngai, T.; Wang, C. Dynamic Supramolecular Hydrogels: Regulating Hydrogel Properties through Self-Complementary Quadruple Hydrogen Bonds and Thermo-Switch. ACS Macro Lett. 2017, 6, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yang, Y.; Tian, Q.; Mi, H.-Y.; Li, H.; Guo, R.; Wang, Y.; Liu, C.; Shen, C. Strain-Hardening, impact protective and Self-Healing supramolecular polyurethane nanocomposites enabled by quadruple H-Bonding, disulfide bonds and nanoparticles. Chem. Eng. J. 2023, 467, 143434. [Google Scholar] [CrossRef]
- Marpu, S.; Hu, Z.; Omary, M.A. Brightly Phosphorescent, Environmentally Responsive Hydrogels Containing a Water-Soluble Three-Coordinate Gold(I) Complex. Langmuir 2010, 26, 15523–15531. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-Responsive Gel−Sol/Sol−Gel Transition in Poly(acrylic acid) Aqueous Solution Containing Fe(III) Ions Switched by Light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef]
- Chen, P.; Li, Q.; Grindy, S.; Holten-Andersen, N. White-Light-Emitting Lanthanide Metallogels with Tunable Luminescence and Reversible Stimuli-Responsive Properties. J. Am. Chem. Soc. 2015, 137, 11590–11593. [Google Scholar] [CrossRef]
- Peng, Z.W.; Yuan, D.; Jiang, Z.W.; Li, Y.F. Novel metal-organic gels of bis(benzimidazole)-based ligands with copper(II) for electrochemical selectively sensing of nitrite. Electrochim. Acta 2017, 238, 1–8. [Google Scholar] [CrossRef]
- Qi, Z.; Wu, C.; Malo de Molina, P.; Sun, H.; Schulz, A.; Griesinger, C.; Gradzielski, M.; Haag, R.; Ansorge-Schumacher, M.B.; Schalley, C.A. Fibrous Networks with Incorporated Macrocycles: A Chiral Stimuli-Responsive Supramolecular Supergelator and Its Application to Biocatalysis in Organic Media. Chem.–A Eur. J. 2013, 19, 10150–10159. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, X.; Dong, L.; Huang, Y.; Li, Y.; Chen, H.; Lan, R.; Hao, A.; Xie, Z.; Shang, W. Construction of β-Cyclodextrin supramolecular gel and the effect of metal ions on its formation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134504. [Google Scholar] [CrossRef]
- Liubimtsev, N.; Kösterke, T.; Che, Y.; Appelhans, D.; Gaitzsch, J.; Voit, B. Redox-sensitive ferrocene functionalised double cross-linked supramolecular hydrogels. Polym. Chem. 2022, 13, 427–438. [Google Scholar] [CrossRef]
- Chen, S.; Bai, M.; Wang, Q.; Li, X.; Shao, J.; Shi, S.Q.; Zhou, W.; Cao, J.; Li, J. A strong and tough supramolecular assembled β-cyclodextrin and chitin nanocrystals protein adhesive: Synthesis, characterization, bonding performance on three-layer plywood. Carbohydr. Polym. 2024, 333, 121971. [Google Scholar] [CrossRef]
- Granata, G.; Petralia, S.; Forte, G.; Conoci, S.; Consoli, G.M.L. Injectable supramolecular nanohydrogel from a micellar self-assembling calix[4]arene derivative and curcumin for a sustained drug release. Mater. Sci. Eng. C 2020, 111, 110842. [Google Scholar] [CrossRef]
- Rauwald, U.; Scherman, O.A. Supramolecular Block Copolymers with Cucurbit[8]uril in Water. Angew. Chem. Int. Ed. 2008, 47, 3950–3953. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Iliopoulos, I.; Ducouret, G. An Associative Polyelectrolyte End-Capped with Short Polystyrene Chains. Synthesis and Rheological Behavior. Macromolecules 2000, 33, 2936–2943. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Peng, J.; Tan, Y.; Li, C.; Boey, F.Y.C.; Long, Y. Liquid Thermo-Responsive Smart Window Derived from Hydrogel. Joule 2020, 4, 2458–2474. [Google Scholar] [CrossRef]
- Klymenko, A.; Nicolai, T.; Benyahia, L.; Chassenieux, C.; Colombani, O.; Nicol, E. Multiresponsive Hydrogels Formed by Interpenetrated Self-Assembled Polymer Networks. Macromolecules 2014, 47, 8386–8393. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; van Steenbergen, M.J.; De Smedt, S.C.; van Nostrum, C.F.; Hennink, W.E. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials 2005, 26, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wei, L.; Mingyue, W.; Guansheng, Q.; Zhenglong, H.; Jinliang, L. Preparation and properties of self-assembled gels based on electrostatic interactions: Applications for N2/CO2 adsorption and inhibition. Fuel 2021, 302, 121053. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Ao, T.; Cui, K.; Jiang, G.; Bai, B.; Zhang, Y.; Yang, J.; Wang, X.; Tian, W. Comprehensive evaluation of self-healing polyampholyte gel particles for the severe leakoff control of drilling fluids. J. Pet. Sci. Eng. 2022, 212, 110249. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Zhang, Y.; Jiang, G.; Wu, Y.; Liu, H.; Dong, T.; Guo, C. Performance of Self-healing microgel incorporating Nano-Silica as plugging material for drilling fluid. J. Mol. Liq. 2023, 386, 122392. [Google Scholar] [CrossRef]
- Du, G.; Peng, Y.; Pei, Y.; Zhao, L.; Wen, Z.; Hu, Z. Thermo-responsive Temporary Plugging Agent Based on Multiple Phase Transition Supramolecular Gel. Energy Fuels 2017, 31, 9283–9289. [Google Scholar] [CrossRef]
- Dai, L.; Sun, J.; Lv, K.; Liu, F.; Bai, Y.; Liao, B.; Yang, D.; Liu, C.; Li, M.-C. Cellulose nanofiber-reinforced supramolecular polymer gels for temporary plugging of fractured oil and gas reservoirs. Carbohydr. Polym. 2025, 356, 123370. [Google Scholar] [CrossRef]
- Dai, L.; Sun, J.; Lv, K.; Bai, Y.; Wang, J.; Liu, C.; Li, M.-C. Influences of Reservoir Conditions on the Performance of Cellulose Nanofiber/Laponite-Reinforced Supramolecular Polymer Gel-Based Lost Circulation Materials. Gels 2025, 11, 472. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, X.; Wang, S.; Feng, Y.; Yin, H. Designing polydopamine embedded hydrogel with high-stable adhesion and long-term stability at high temperature. Polymer 2025, 317, 127914. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, G.; Guan, J.; Huang, S.; Guo, Y.; He, Y.; Yang, L.; Dong, T. Preparation and performance evaluation of a thixotropic polymer gel for loss circulation control. Fuel 2024, 371, 132148. [Google Scholar] [CrossRef]
- Jia, H.; Niu, C.; Liang, W.; He, W.; Sun, J. High-Density Solid-Free Flexible Microgel Fluid Loss Pill in High-Temperature and High-Pressure Reservoirs: Curing Mechanism and Working Performance. SPE J. 2023, 28, 917–933. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, Z.; Fu, H.; Chen, K.; Guo, Y.; Lu, J. Research and application progress of temporary plugging agent for acidification fracturing: A review. Geoenergy Sci. Eng. 2025, 247, 213600. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Yang, H.; Zuo, T.; Tan, H.-R.; Li, J.-P.; Yu, X.-R.; Su, G.-S.; Zheng, Y.-C. A thermally induced β-cyclodextrin/benzene derivatives gel and the potential application in fracturing temporary plugging. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131738. [Google Scholar] [CrossRef]
- Lai, N.; Chen, Y.; Wang, J.; Wu, C.; Zhang, X. Temporary Plugging Agent System for Thermally Induced Phase Change Fracturing in Shale Gas Reservoirs. Energy Fuels 2022, 36, 6270–6279. [Google Scholar] [CrossRef]
- Li, J.-B.; Luo, Z.-F.; Fu, H.-R.; Zhang, N.-L.; He, J.; Yan, C.-Z.; Ren, D.-F.; Peng, F.; Liu, J.-Y. Research and application of the low-damage temperature-controlled phase change temporary plugging agent. Geoenergy Sci. Eng. 2024, 241, 213122. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, N.; Zhao, L.; Pei, Y.; Liu, P.; Li, N. Thermoresponsive in Situ Generated Proppant Based on Liquid–Solid Transition of a Supramolecular Self-Propping Fracturing Fluid. Energy Fuels 2019, 33, 10659–10666. [Google Scholar] [CrossRef]
- Yegin, C.; Temizel, C.; Yegin, Y.; Sari, M.M.; Jia, B.; Alklih, M.Y. pH-Responsive Supramolecular Gelling Agents Used in EOR and Their Potential as Fracking Fluids. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017; p. D011S018R001. [Google Scholar]
- Chen, I.C.; Yegin, C.; Zhang, M.; Akbulut, M. Use of pH-Responsive Amphiphilic Systems as Displacement Fluids in Enhanced Oil Recovery. SPE J. 2014, 19, 1035–1046. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.; Chen, P.; Jiang, W.; Chen, F.; Yu, X.; Su, G.; Xu, Z. Quaternary-ammonium-based supramolecular gel for temporary plugging diversion fracturing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131174. [Google Scholar] [CrossRef]
- Gao, G.Q.; Sun, L.M.; Wang, L.S.; Tian, Z.Q.; Tang, H.T.; Wang, J. Phenolic Resin Based Supramolecular Aggregates-Novel In-Depth Profile Modifier for Oil Reservoir. Adv. Mater. Res. 2012, 554–556, 319–322. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Xu, Z.; Chen, J.; Dai, C. Self-growing Hydrogel Particles with Applications for Reservoir Control: Growth Behaviors and Influencing Factors. J. Phys. Chem. B 2021, 125, 9870–9878. [Google Scholar] [CrossRef]
- Ma, Q.; Li, J.; Wu, Y.; Wu, B.; Qin, X.; Gou, R.; Xi, L.; Pu, W.; Liu, R. New approaches in enhanced oil recovery (EOR): A beneficial research of hyperbranched nanowire-prepared weak gels for profile control in high-temperature and high-salinity environments. Geoenergy Sci. Eng. 2025, 254, 214032. [Google Scholar] [CrossRef]
- Zhang, J.; Bo, S.; Wang, R.; Fang, J.; Wang, X.-g.; Bai, Y.; Ma, Z.; Liang, Y.; Zhang, M.; Yu, Q.; et al. Supramolecular Polymer Gel Lubricant with Excellent Mechanical Stability and Tribological Performances. ACS Appl. Mater. Interfaces 2022, 14, 45934–45944. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; He, S.; Lian, Y.; Yu, T.; Dai, C.; Zhao, J.; Zhang, H. Self-Lubricating Supramolecular Hydrogel for In-Depth Profile Control in Fractured Reservoirs. ACS Omega 2020, 5, 7244–7253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, M.; You, Q.; Fan, H.; Li, W.; Liu, Y.; Fang, J.; Zhao, G.; Jin, Z.; Dai, C. Smart mobility control agent for enhanced oil recovery during CO2 flooding in ultra-low permeability reservoirs. Fuel 2019, 241, 442–450. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, P.; Gao, K.; Wei, B.; Feng, Y. Thermo- and CO2-triggered viscosifying of aqueous copolymer solutions for gas channeling control during water-alternating-CO2 flooding. Fuel 2021, 291, 120171. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Yan, X.; Ma, T.; Shao, L.; Liu, Y.; Guo, Z. Alkyl bicarbamates supramolecular organogelators with effective selective gelation and high oil recovery from oil/water mixtures. Chemosphere 2017, 167, 178–187. [Google Scholar] [CrossRef]
- Yong, W.; Guanchen, J.; Qingfu, D.; Minsheng, X. Research and application of supramolecular chemical plugging technology. Drill. Fluid Complet. Fluid 2018, 35, 48–53. [Google Scholar] [CrossRef]
- Lubin, Y. Research and application of supramolecular gel plugging agent gelseal. China Pet. Chem. Stand. Qual. 2025, 45, 138–141. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, J.; Zhang, X.; Feng, R.; Li, H. Flow Behavior Through Porous Media and Microdisplacement Performances of Hydrophobically Modified Partially Hydrolyzed Polyacrylamide. SPE J. 2016, 21, 688–705. [Google Scholar] [CrossRef]
- Temizel, C.; Putra, D.; Zhang, M.; Moreno, R. Smart Nanoparticles for Conformance Improvement in Waterflooding. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017; p. D031S029R005. [Google Scholar]
- Ai, L.; Yang, H.; Wang, S.; Zheng, L.; Yuan, H.; Yu, X.; Su, G. Acrylamide preformed particle gels with movable-crosslinking for conformance control. J. Mol. Liq. 2023, 384, 122217. [Google Scholar] [CrossRef]
- Lai, N.; Chen, S.; Tang, L.; Huang, Y.; Xu, H. Migration characteristics and profile control capabilities of preformed particle gel in porous media. Petroleum 2022, 8, 483–498. [Google Scholar] [CrossRef]
- Maury Fernandez, D.; Emadi, H.; Hussain, A.; Thiyagarajan, S.R.; Ispas, I.; Watson, M. Experimental investigation of the impact of short-term hydrogen exposure on cement sheath’s mechanical and sealing integrity. Geoenergy Sci. Eng. 2025, 251, 213885. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Nasr–El–Din, H.A.; Sokhanvarian, K.; AlKhaldi, M. Dual-Polymer Hydraulic-Fracturing Fluids: A Synergy Between Polysaccharides and Polyacrylamides. SPE J. 2019, 24, 2635–2652. [Google Scholar] [CrossRef]
- Yegin, C.; Singh, B.P.; Zhang, M.; Biopharm, F.; Balaji, K.; Suhag, A.; Ranjith, R.; Peksaglam, Z.; Wijaya, Z.; Putra, D.; et al. Next-Generation Displacement Fluids for Enhanced Oil Recovery. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 4–6 April 2017; p. D021S010R001. [Google Scholar]
- Yang, H.; Wang, K.; Lu, J.; Zhang, S. Structuraldesign of a supergelator: Influence of the alkyl chain isomers on gelation behaviors, thermal stabilities, and supramolecular structures. Colloids Surf. A Physicochem. Eng. Asp. 2024, 689, 133726. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Hu, Y.; Liu, C.-L.; Wu, Y.-N.; Zou, C.-W.; Zhang, L.-Y.; Zhao, M.-W.; Dai, C.-L. Supramolecular polymer-based gel fracturing fluid with a double network applied in ultra-deep hydraulic fracturing. Pet. Sci. 2024, 21, 1875–1888. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, Y.; Gao, K.; Zhang, L.; Zhou, J.; Zhang, D.; Liao, R.; Li, Z. Experimental Investigation of a Novel Nanocomposite Particle Gel for Water Shutoff Treatment in Mature Oilfields. ACS Omega 2022, 7, 8887–8895. [Google Scholar] [CrossRef]
- Pereira, K.A.B.; Pereira, K.A.B.; Oliveira, P.F.; Mansur, C.R.E. Behavior of partially hydrolyzed polyacrylamide/polyethyleneimine reinforced with coal fly ash for preformed particle hydrogels. J. Appl. Polym. Sci. 2020, 137, 49423. [Google Scholar] [CrossRef]
- Khoshkar, P.A.; Fatemi, M.; Ghazanfari, M.H. Static and dynamic evaluation of the effect of nanomaterials on the performance of a novel synthesized PPG for water shut-off and improved oil recovery in fractured reservoirs. J. Pet. Sci. Eng. 2020, 189, 107019. [Google Scholar] [CrossRef]
- Deng, J.; Lian, H.; Zhuang, Y.; Zhao, H.; Wang, Z.; Tian, Y.; Lin, C.; Yuan, H.; Han, M.; Lu, G.; et al. Synthesis and performance evaluation of multi-crosslinked preformed particle gels with ultra-high strength and high-temperature and high-salinity resistance for conformance control. Fuel 2024, 357, 130027. [Google Scholar] [CrossRef]
- Yang, H.-B.; Jiang, H.-Z.; Xu, Z.; Zhang, X.; Wang, T.; Liu, H.-N.; Ma, X.; Zhu, J.-J.; Zhang, X.-F.; Kang, W.-L. Development and evaluation of organic/metal ion double crosslinking polymer gel for anti-CO2 gas channeling in high temperature and low permeability reservoirs. Pet. Sci. 2025, 22, 724–738. [Google Scholar] [CrossRef]
- van Haaften, E.E.; Duijvelshoff, R.; Ippel, B.D.; Söntjens, S.H.M.; van Houtem, M.H.C.J.; Janssen, H.M.; Smits, A.I.P.M.; Kurniawan, N.A.; Dankers, P.Y.W.; Bouten, C.V.C. The degradation and performance of electrospun supramolecular vascular scaffolds examined upon in vitro enzymatic exposure. Acta Biomater. 2019, 92, 48–59. [Google Scholar] [CrossRef]
| Composition | Preparation Method | Testing Condition | Performance | Application | Reference |
|---|---|---|---|---|---|
| Hydrogen bonds/PVA (15%) | 8 h freezing at −20 °C and 4 h thawing at +25 °C, 12 h gelation | Swelling studies were conducted in deionized water at 25 °C | 19% after 26 Day | Biomedical applications | [32] |
| Hydrogen bonds/PVA (35%) | Freezing/thawing method, 13 h gelation | Room temperature, tensile rate of 1 mm/s | 1.0 × 105 Pa (fracture stress) | biomedical applications | [33] |
| Hydrogen bonds/PVA/tannic acid (TA) | Freezing/thawing method, 6 h gelation | Strain rate is 50 mm/min, gels shape (30 mm × 5 mm × 1 mm), room temperature | Tensile strength and elongation achieve 4.9 MPa and 950%. | Intelligent devices, energy storage, tissue engineering | [34] |
| Hydrogen bonds/N-acryloyl glycinamide (NAGA) | Photoinitiated aqueous-phase polymerization | Gels with thickness of 0.5 mm, 50 mm in length and 6 mm in width, the extension of 50 mm/min, room temperature | Tensile strength and elongation achieve 1.1 MPa and 1400%. (room temperature) | Biomedical field | [35] |
| Hydrogen bonds/ABA triblock copolymers containing benzene-1,3,5-tricarboxamide | At 40 °C for 12 h | 20 °C | Young’s modulus: 360 MPa, fracture strain 203%. (room temperature) | Engineering thermoplastics | [36] |
| Hydrogen bonds/four cooperative hydrogen bonds | Units of 2-ureido–4-pyrimidone | N/A | N/A | N/A | [37] |
| Hydrogen bonds/a central poly(ethylene oxide) block and terminal poly(N-isopropylacrylamide) (PNIPAm) block with ureido pyrimidinone (UPy) (10 wt%) | Heated to 37 °C for hydrogelation | 40 mm of diameter, 1 rad/s of frequency, 10% of polymer concentration (37 °C) | γ = 0.5%, G′ = 3000 Pa. | Biomedical applications | [38] |
| Hydrogen bonds/supramolecular polyurethane | Mixing at 55 °C for 3 h | Mechanical and self-healing tests: dimension of 10 × 5 × 0.6 mm, room temperature | Mechanical and self-healing tests: dimension of 10 × 5 × 0.6 mm, room temperature | Protective materials | [39] |
| Composition | Preparation Method | Testing Condition | Performance | Application | Reference |
|---|---|---|---|---|---|
| Au (I)-ligand | A water-soluble phosphorescent Au(I) and poly(N-isopropylacrylamide) (PNIPAM), 4 °C for chemical crosslinking | N/A | Temperature-dependent photoluminescence enhancement of the hybrid microgel upon heating from room temperature (RT) to 37 °C, only PNIPAM-co-allylamine microgels at acidic pH showed efficient loading of the Au phosphor | Various biological and environmental applications | [40] |
| Fe-ligand | Aqueous PAA solution containing an Fe (III)-citrate complex, the gel–sol transition in the PAA + Fe (III)-citrate aqueous system switched by photoreduction and oxidation | N/A | N/A | N/A | [41] |
| Ln-ligand | lanthanide metal–ligand coordination complexes via a terpyridyl-end-capped four-arm poly(ethylene glycol) polymer | Room temperature | Variety of reversible stimuli-responsive properties (mechano-, vapo-, thermo-, and chemochromism) of both sol–gel systems | Smart coatings | [42] |
| Copper (II)-ligand | Organic ligand 2,6-Bis (2-benzimidazolyl) pyridine and copper (II) | N/A | N/A | Electrochemistry material | [43] |
| Composition | Preparation Method | Testing Condition | Performance | Application | Reference |
|---|---|---|---|---|---|
| The crown ether represents a molecular recognition unit | Benzo-21-crown-7 ether | 25 °C | Three different chemical stimuli that induce gel–sol transitions: K binding, pseudorotaxane formation, and anion binding, 10 kPa of G′ | N/A | [44] |
| β-Cyclodextrin and metal ions | The CD was mixed with deionized water containing metal ions and then ultrasonicated for 15 min before being left to stand | 0.105 of thickness, 35 mm diameter (room temperature) | Yield stress values of GelCaCl2, GelZnCl2, GelBaCl2, GelMgSO4, GelCuCl2 and GelFeCl3 are 466 Pa, 469 Pa, 573 Pa, 833 Pa, 936 Pa and 460 Pa, respectively | Intelligent materials, drug delivery | [45] |
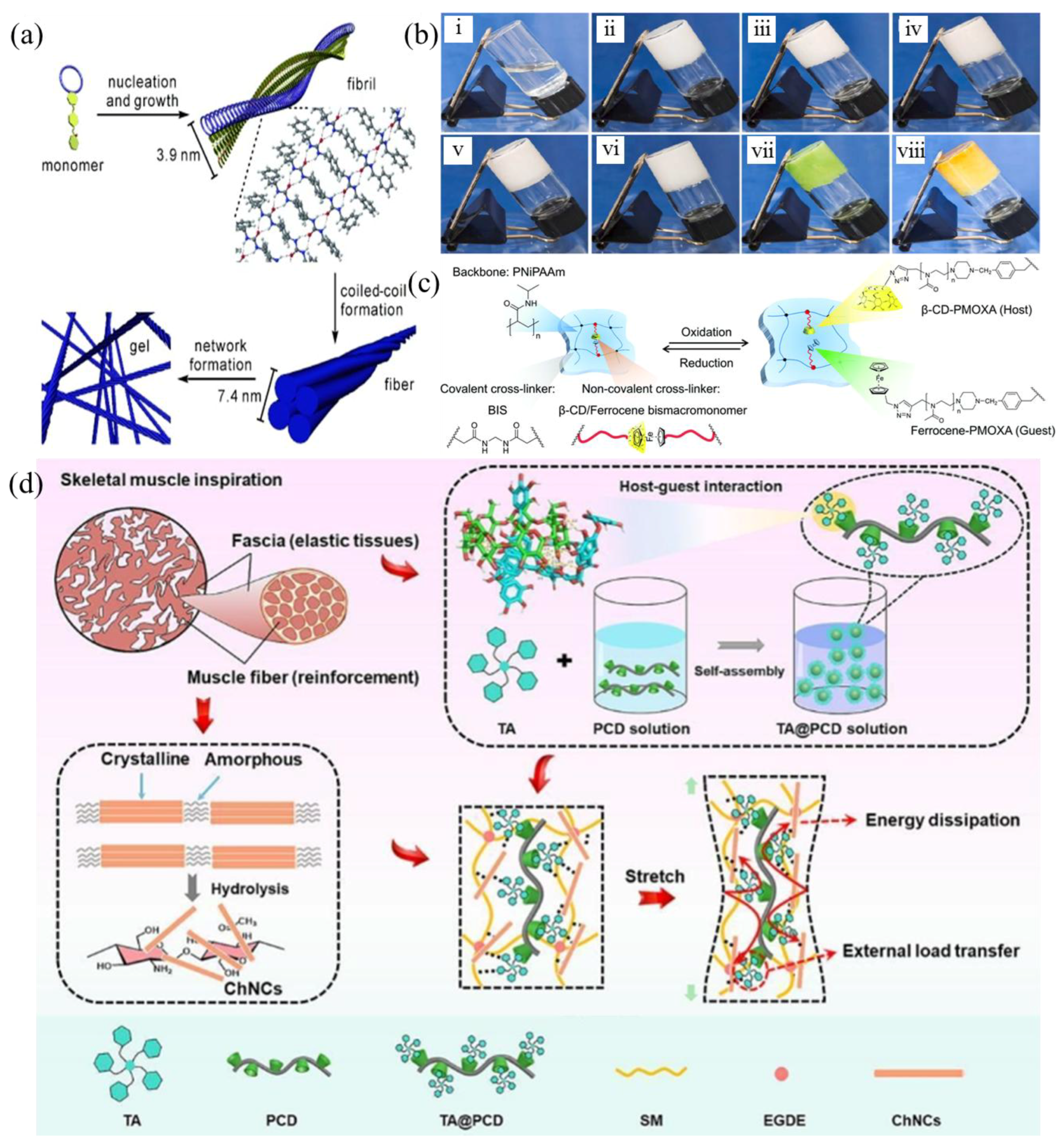
| β-cyclodextrin and ferrocene | Poly (N-isopropylacrylamide) (PNiPAAm) backbone crosslinked with N,N′-methylenebis (acrylamide) (BIS) as a permanent crosslinker, and β-CD/ferrocene host–guest complexes as reversible crosslinking points | 5 mm of thick slice (room temperature) | 15% of swelling degree | Microfluidics and diagnostics | [46] |
| Chitin nanocrystal (ChNCs), soybean meal (SM), and β-cyclodextrin | Tannin acid (TA)-functionalized poly-β-cyclodextrin (PCD) (TA@PCD) and chitin nanocrystals (ChNCs) into the SM matrix | Bonding performance: at 25 °C for 10 min to test the wet strength, 25 mm × 25 mm of bonding area, the adhesive endured 500 g and was maintained for 80 days (25 °C) | 2.57 and 1.25 MPa of dry and wet shear strength (dry for 3 h at 120 °C), 0.69 J of adhesive, the cost of SM/TA@PCD/ChNCs-2 adhesive was approximately 2290 RMB (or 322 US dollars)/ton, which was comparable to that of the commercial urea-formaldehyde resins (around 2100 RMB (or 295 US dollars)/ton), thus showing great promise for wood adhesive applications | Biomass adhesives | [47] |
| Choline-calix arene derivative and curcumin | Choline-calix arene derivative and curcumin mixture was sonicated for 15 min, then stirred (500 rpm) at 37 °C | 40 mm of diameter, frequency sweep at 2% strain with varying angular frequency (1–25 rad/s), 25 °C | No significant changes in G′ and G″ within the applied range of angular frequency (1–10 rad/s) (25 °C) | Drug delivery | [48] |
| Cucurbit[8]uril | Poly (ethylene glycol) (PEG) and cis-1,4-poly(isoprene) (PI) were chosen as the polymers | N/A | N/A | Dynamic functional materials | [49] |
| Composition | Preparation Method | Testing Condition | Performance | Application | Reference |
|---|---|---|---|---|---|
| Strong hydrophobic associations between stearyl methacrylate (C18) and dococyl acrylate (C22) | C18, C22, acrylamide in a micellar solution of sodium dodecyl sulfate (SDS) | 4 mm diameter × 50 mm length, 50 mm/min of speed, 25 °C | The original and healed gel samples broke at elongation ratios of 3600 ± 630 and 3580 ± 520%, respectively, indicating a healing efficiency of about 100%, 12 ± 1 kPa of tensile strength | Biological materials | [50] |
| Polystyrene (PS) end-capped polyelectrolytes | Modification of polystyrene-poly (tert-butyl acrylate)-polystyrene triblock copolymers synthesized via anionic polymerization | 50 mm of diameter, 1% concentration, 25 °C | 1000 Pa at 100 rad/s | Paints, cosmetics, drilling fluids | [51] |
| Poly (N-isopropylacrylamide) | N-isopropylacrylamide, N,N-methylenebis, and N,N,N,N-tetramethylethylenediamine mixing at 600 rpm for 30 min, then the solution was left stirring for at least 24 h | N/A | N/A | Energy-saving buildings | [52] |
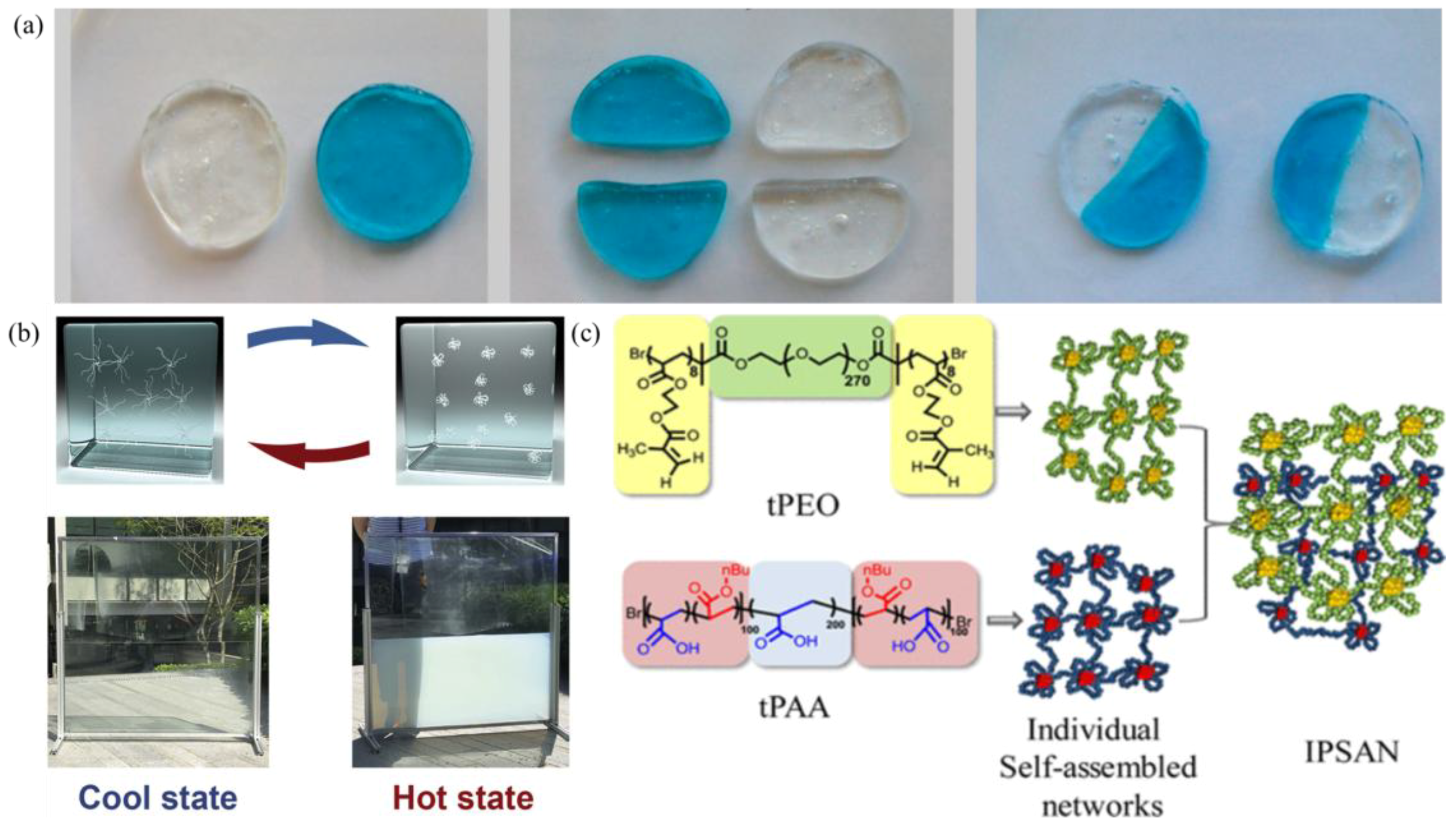
| Amphiphilic BAB triblock copolymers | Mixing the same volumes of the P (nBA50%-stat-AA50%)-b-PAA-b-P(nBA50%-stat-AA50%) (tPAA) solution and the poly(2-methacryloyloxyethyl acrylate)-b-poly(ethylene oxide)-b-poly(2-methacryloyloxyethyl acrylate) (tPEO) solution | 0.103 mm of thickness, 25 mm of diameter | 1000 Pa at 10 rad/s (after UV-irradiation, 20 °C) | N/A | [53] |
| Composition | Preparation Method | Testing Condition | Performance | Application | Reference |
|---|---|---|---|---|---|
| Oppositely charged crosslinked dextran microspheres | The dispersions (Hydroxyethyl methacrylate-derivatized dextran and methacrylic acid) were stored at 4 °C for 2 h | 500 μm of thickness, 20 mm of diameter | 6500 Pa of G′ with a solid content of 25%. (20 °C and 1 Hz) | Drug delivery | [54] |
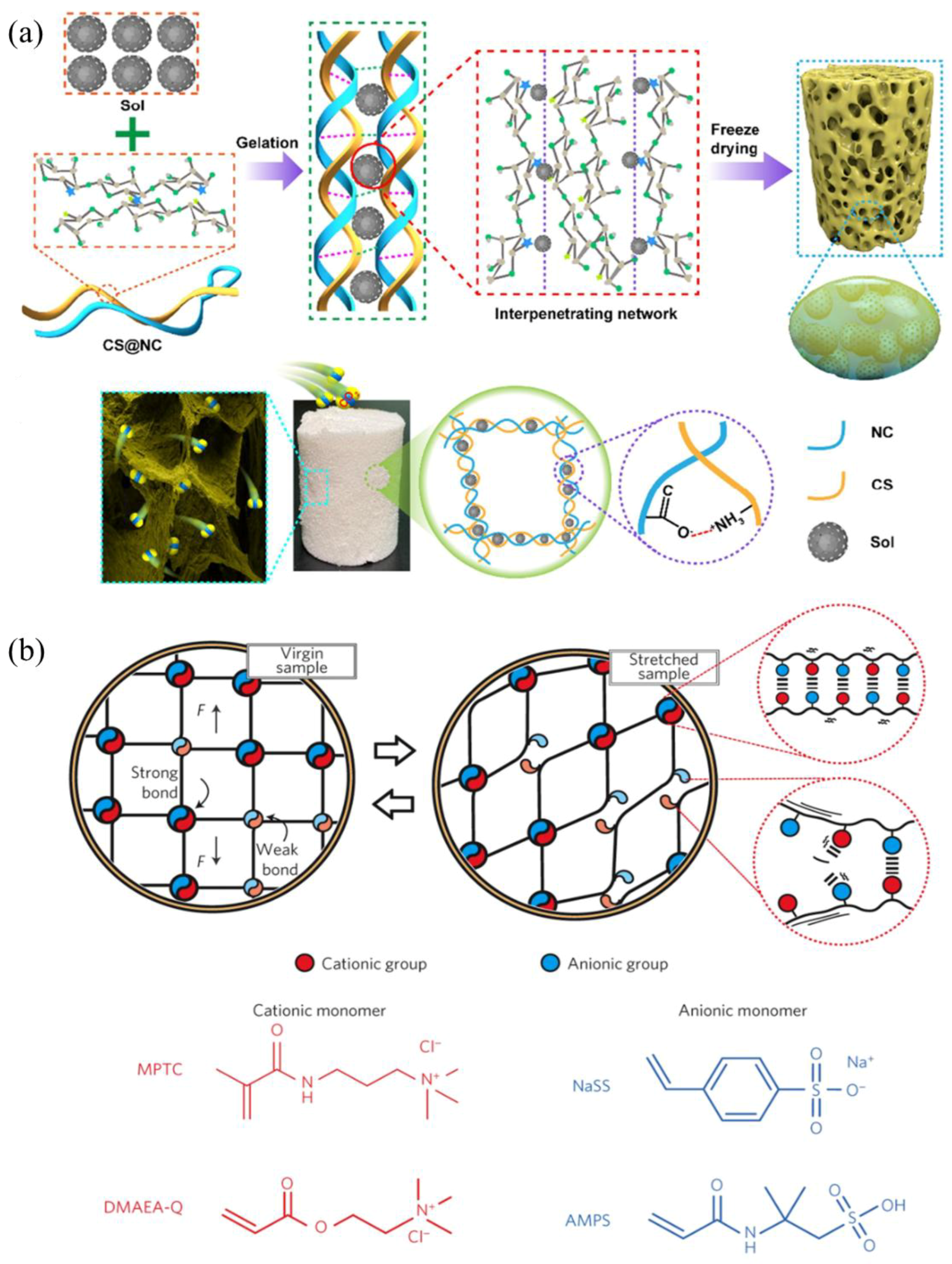
| Oxidized nanocellulose (NC) and Chitosan (CS) | NC, CS, and silica sol mix thoroughly for 2 min, then the samples were freeze-dried at 60 °C for 12 h | At room temperature (25 °C) for 5 h | The N2 adsorption capacity was 241.18 cm3/g STP, and the CO2 adsorption capacity was 23 mL/g | Inhibitor material | [55] |
| Polyampholytes | Cationic monomers: 3-(methacryloylamino)propyl-trimethylammonium chloride (MPTC), N,N-dimethylamino ethylacrylate (DMAEA-Q); anionic monomers: sodium p-styrenesulphonate (NaSS), 2-acrylamido-2-methylpropanesulphonic acid (AMPS). Polyampholyte hydrogels were synthesized using the one-step random copolymerization of an anionic monomer and a cationic monomer | 12 mm (L) × 2 mm (d) × 2–3 mm (w), 100 mm/min of rate, room temperature | 1.8 MPa and 750% of fracture stress σb and strain ɛb values, 4000 J/m2 of the tearing energy, reached ~30% after 1 h healing | Smart structural materials | [56] |
| Ca2+ and carboxyl groups | Hydrogels by mixing two types of crosslinked polymer (onically crosslinked alginate, and covalently crosslinked polyacrylamide) | 75.0 × 5.0 × 3.0 mm3 of size, 2 mm/min of rate, room temperature | 29 kPa of elastic modulus, ~2000% of strain rate, ~9000 J/m2 | Tissue engineering | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Sun, J.; Lv, K.; Bai, Y.; Wang, J.; Liu, C.; Li, M.-C. Research Progress of Supramolecular Gels in the Field of Petroleum Engineering. Gels 2025, 11, 661. https://doi.org/10.3390/gels11080661
Dai L, Sun J, Lv K, Bai Y, Wang J, Liu C, Li M-C. Research Progress of Supramolecular Gels in the Field of Petroleum Engineering. Gels. 2025; 11(8):661. https://doi.org/10.3390/gels11080661
Chicago/Turabian StyleDai, Liyao, Jinsheng Sun, Kaihe Lv, Yingrui Bai, Jianlong Wang, Chaozheng Liu, and Mei-Chun Li. 2025. "Research Progress of Supramolecular Gels in the Field of Petroleum Engineering" Gels 11, no. 8: 661. https://doi.org/10.3390/gels11080661
APA StyleDai, L., Sun, J., Lv, K., Bai, Y., Wang, J., Liu, C., & Li, M.-C. (2025). Research Progress of Supramolecular Gels in the Field of Petroleum Engineering. Gels, 11(8), 661. https://doi.org/10.3390/gels11080661






