New Antimicrobial Gels Based on Clove Essential Oil–Cyclodextrin Complex and Plant Extracts for Topical Use
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterisation of the Plant-Derived Ingredients
2.1.1. The Inclusion Complex’s Chemical Composition
2.1.2. Tincture Chemical Characterisation
2.1.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis Results of Laurus nobilis Essential Oil
2.2. Physical Characterisation of the New Gels
2.2.1. Macroscopic Characteristics
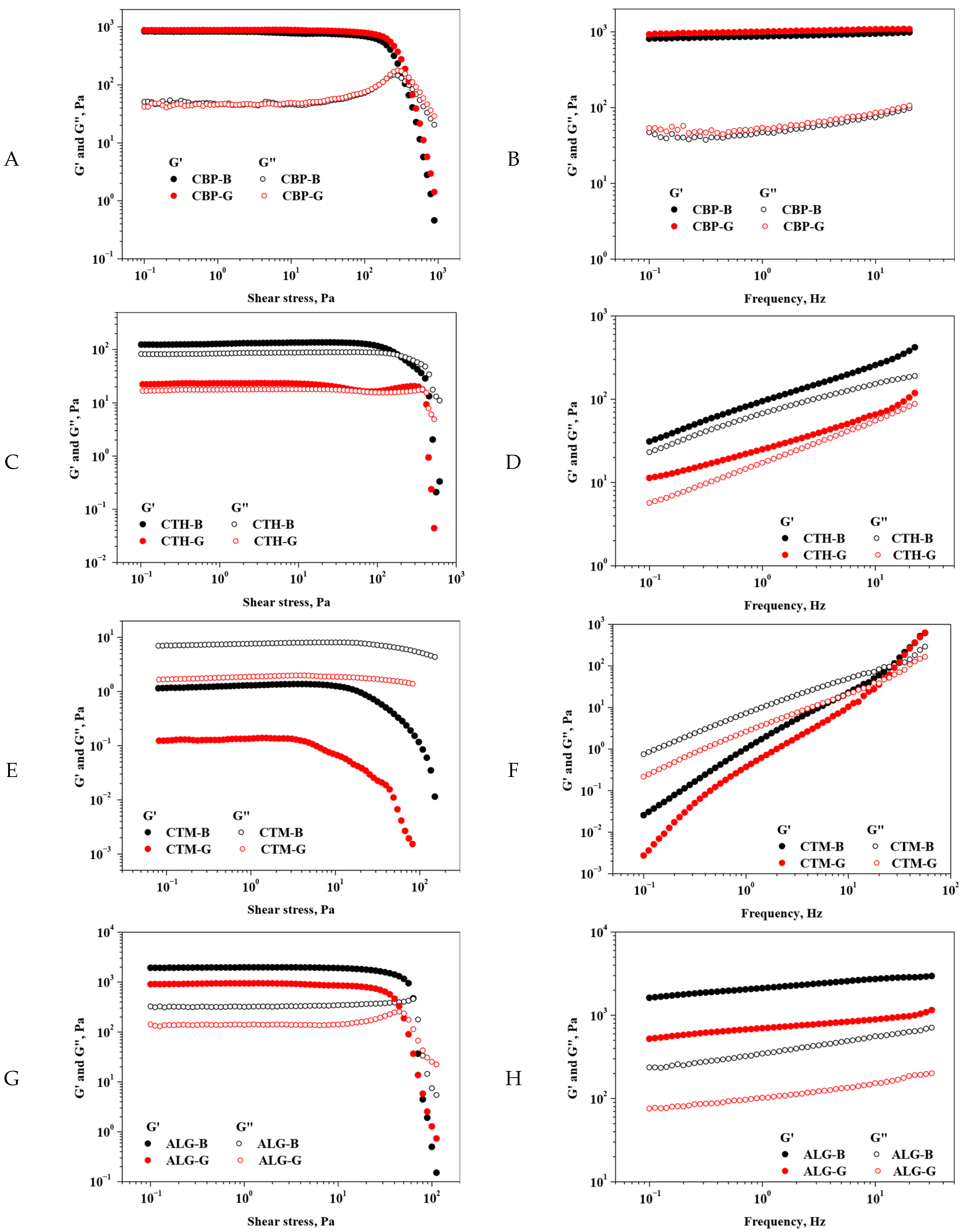
2.2.2. Rheological Data
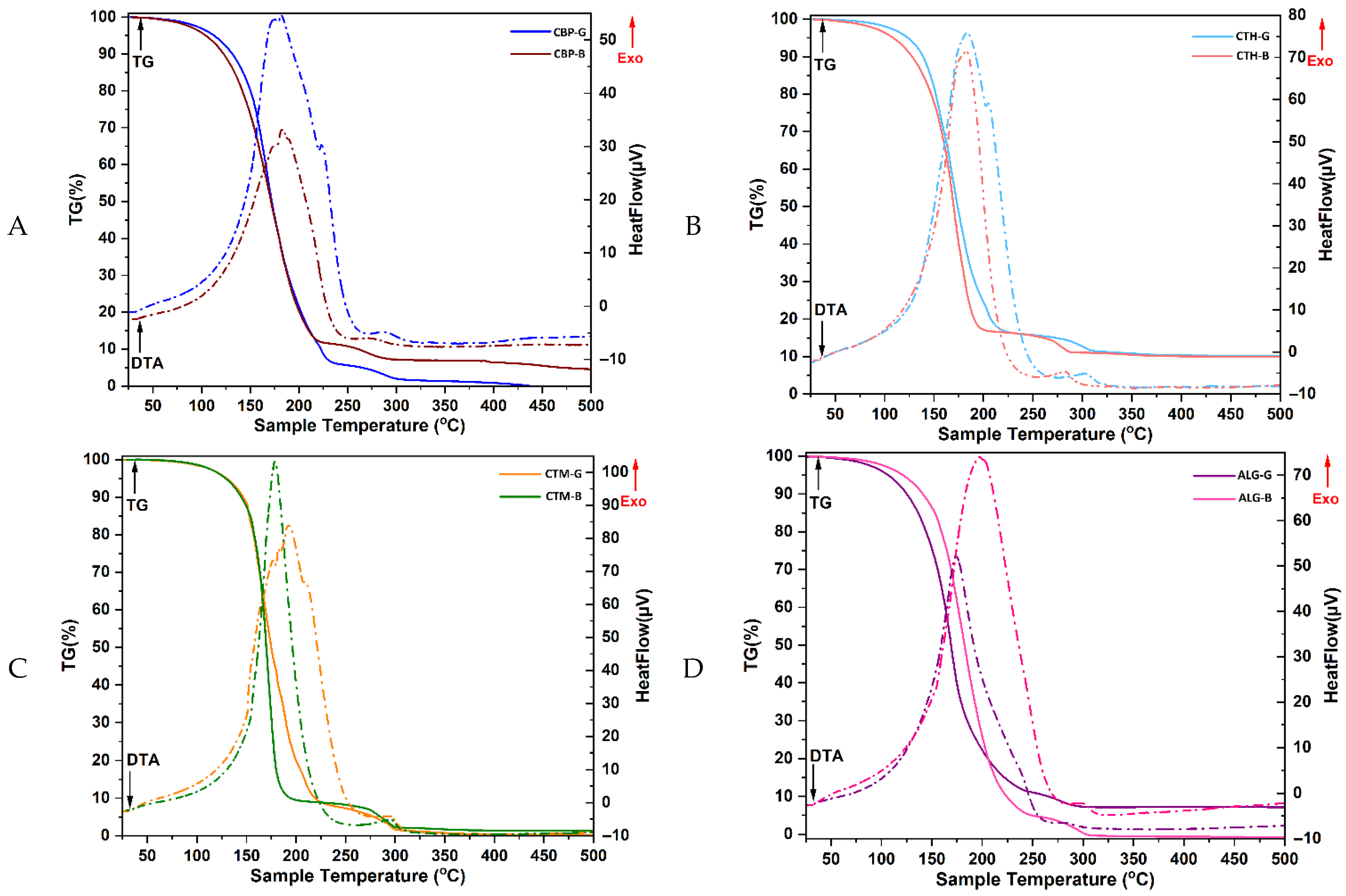
2.2.3. Thermogravimetric Differential Thermal Analysis (TG-DTA)
2.2.4. Scanning Electron Microscopy (SEM) Analysis
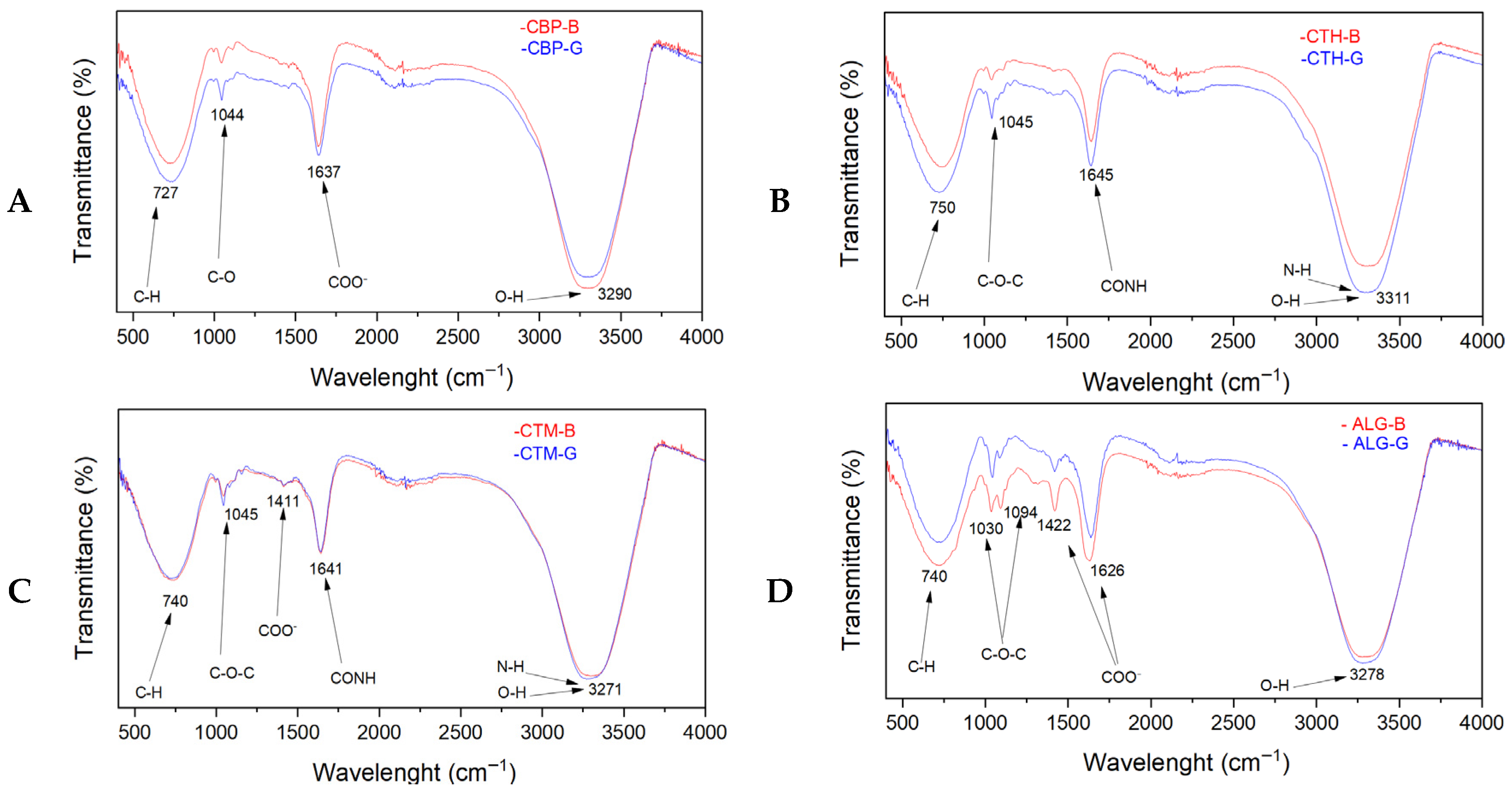
2.2.5. FTIR Analysis Results
2.3. Analysis of Antimicrobial Activity
2.3.1. Qualitative Assessment of the Antimicrobial Activity
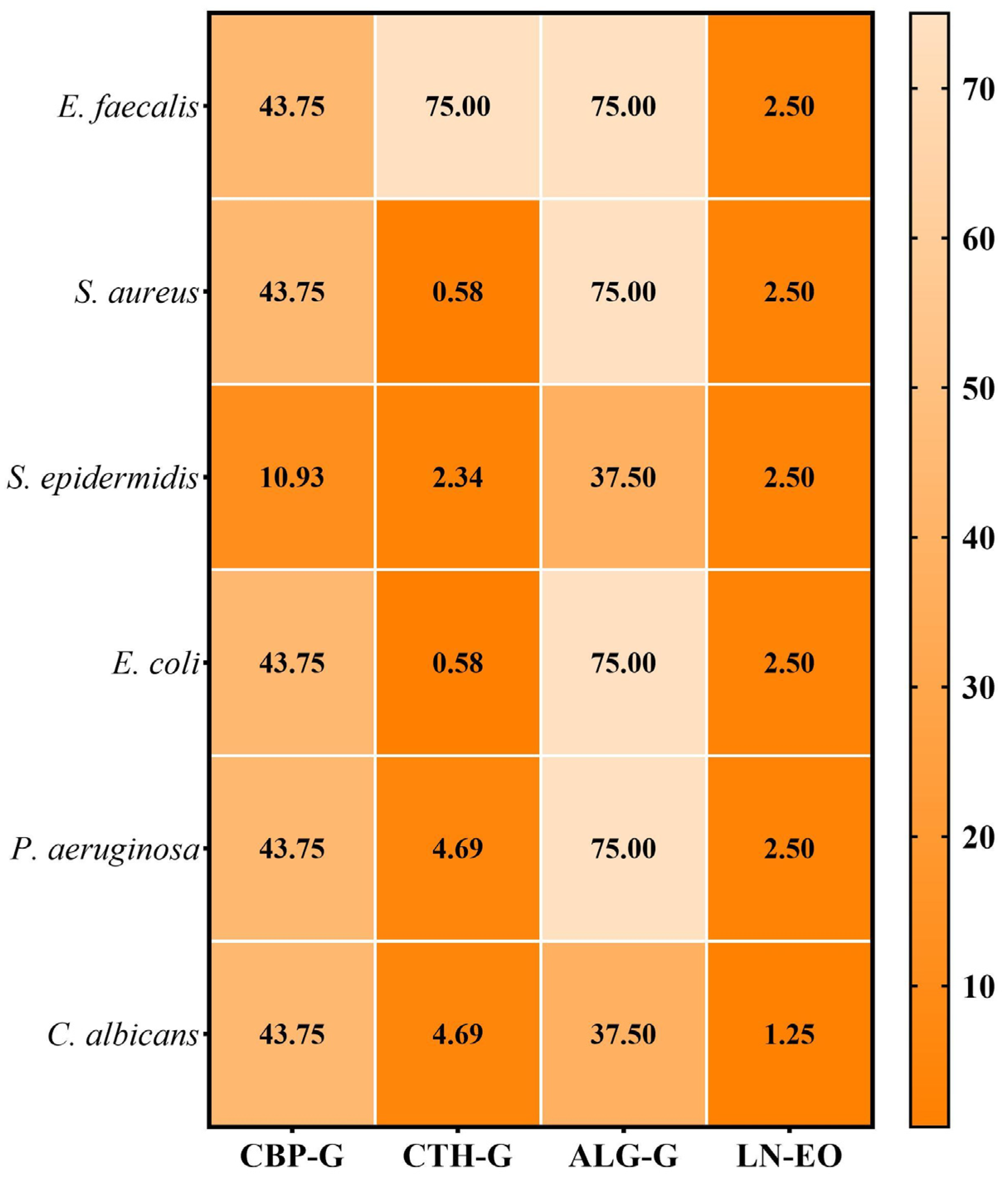
2.3.2. Quantitative Assessment of the Antimicrobial Activity
2.3.3. Semiquantitative Assessment of the Microbial Adherence to the Inert Substratum
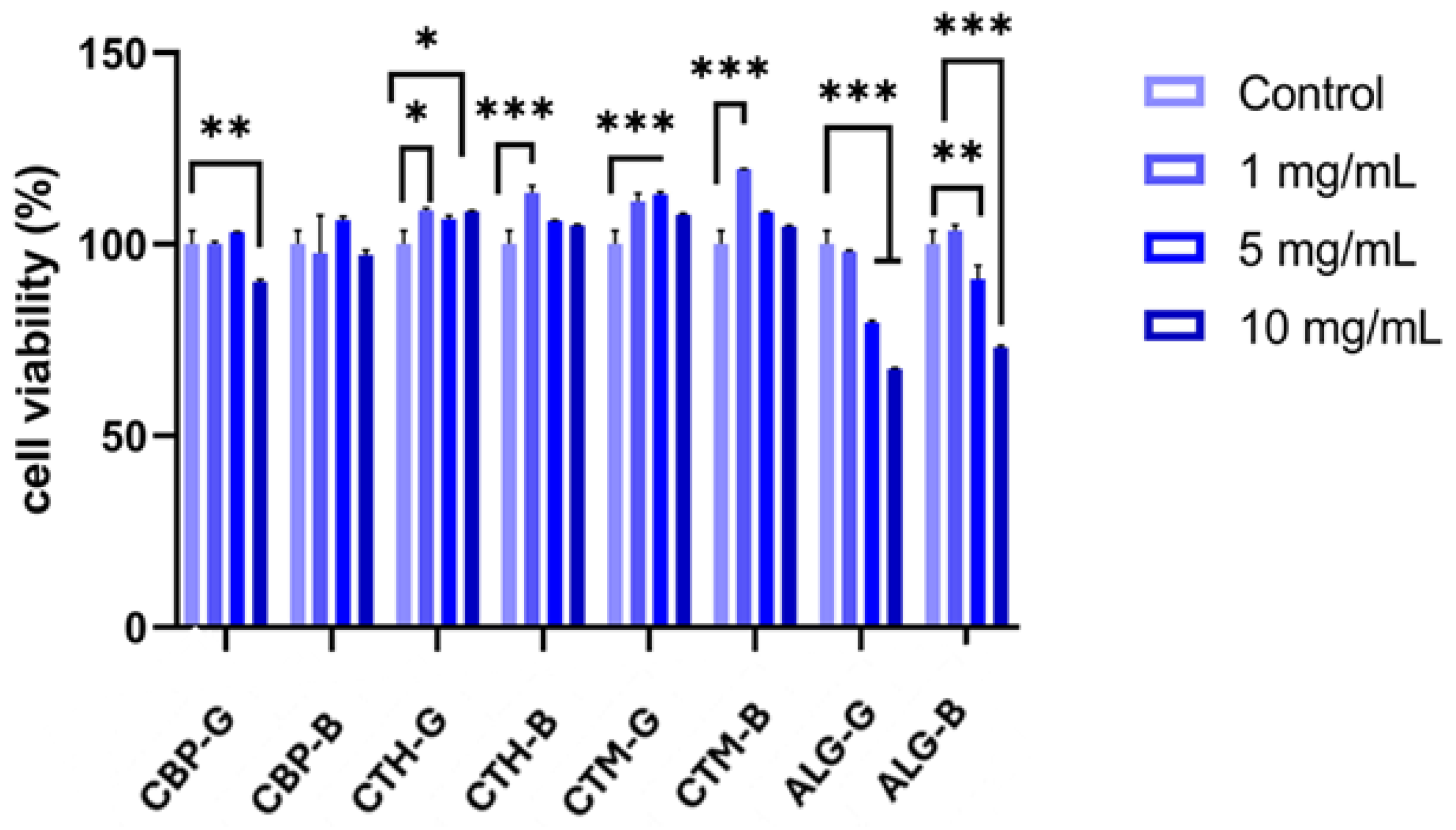
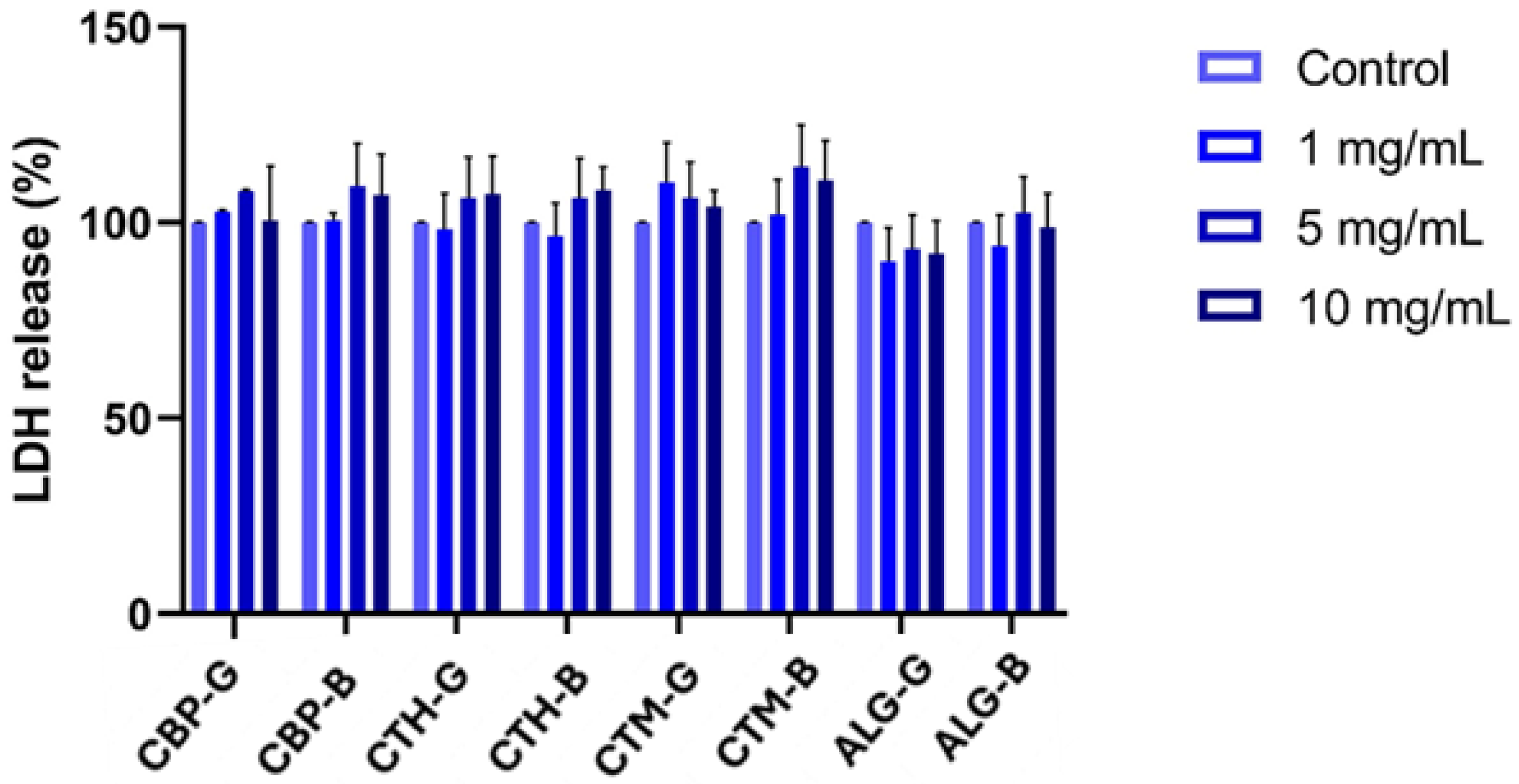
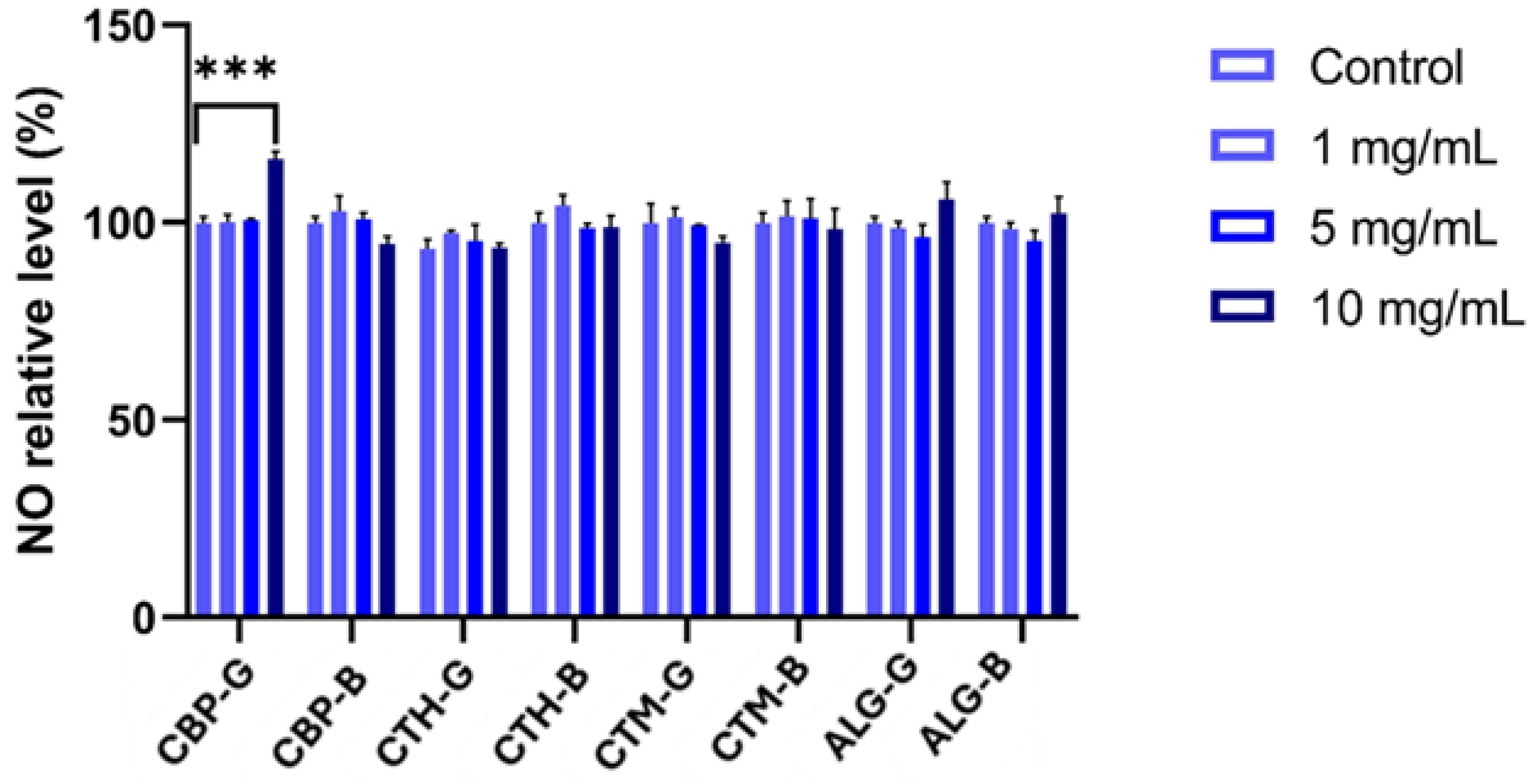
2.4. Cell Viability Assessment
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Plant-Derived Ingredients
4.2.1. The Inclusion Complex’s Preparation and Characterisation
4.2.2. Tincture Preparation and Characterisation
4.2.3. GC-MS Analysis of Laurus nobilis Essential Oil
4.3. Formulation Design
4.4. Physical Characterisation Methods
4.4.1. Rheological Analysis
4.4.2. Thermal Analysis Procedure (TG-DTA)
4.4.3. Scanning Electron Microscopy Procedure
4.4.4. FTIR Characterisation
4.5. Antimicrobial Activity
4.5.1. Qualitative Assay of the Antimicrobial Activity
4.5.2. Quantitative Assay of the Antimicrobial Activity
4.5.3. Semiquantitative Assay of the Microbial Adherence to the Inert Substratum
4.6. Experimental Protocol for Viability Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Sudheesh Kumar, P.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R. Exploration of Alginate Hydrogel/Nano Zinc Oxide Composite Bandages for Infected Wounds. Int. J. Nanomed. 2015, 10 (Suppl. S1), 53–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, H.; Zhang, Z.; Chen, K.; Zhang, Q.; Zhang, C.; Gao, W.; Zheng, Y. Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels 2024, 10, 495. [Google Scholar] [CrossRef]
- Osmokrovic, A.; Stojkovska, J.; Krunic, T.; Petrovic, P.; Lazic, V.; Zvicer, J. Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives. Int. J. Mol. Sci. 2025, 26, 4381. [Google Scholar] [CrossRef]
- Goh, M.; Du, M.; Peng, W.R.; Saw, P.E.; Chen, Z. Advancing Burn Wound Treatment: Exploring Hydrogel as a Transdermal Drug Delivery System. Drug Deliv. 2024, 31, 2300945. [Google Scholar] [CrossRef]
- Teng, Y.; Li, S.; Tang, H.; Tao, X.; Fan, Y.; Huang, Y. Medical Applications of Hydrogels in Skin Infections: A Review. Infect. Drug Resist. 2023, 16, 391–401. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Shahrour, H.; Ferreira, D.A.; Sheridan, L.; Fitzgerald-Hughes, D.; O’Gara, J.P.; Devocelle, M.; Kelly, H.; O’Neill, E. Potent Antimicrobial Activity of Hydrogel Loaded with the Antimicrobial Peptide, D-Bac8c2,5 Leu, against Monospecies and Polymicrobial Biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. Front. Microbiol. 2025, 16, 1571649. [Google Scholar] [CrossRef]
- Gou, Y.; Hu, L.; Liao, X.; He, J.; Liu, F. Advances of Antimicrobial Dressings Loaded with Antimicrobial Agents in Infected Wounds. Front. Bioeng. Biotechnol. 2024, 12, 1431949. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-Based Hydrogels Mediated Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Siemiradzka, W.; Dolińska, B. Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application. Appl. Sci. 2023, 13, 7826. [Google Scholar] [CrossRef]
- Tahir, D.; Ardiansyah, A.; Heryanto, H.; Noor, E.E.M.; Mohamed, M.A. Chitosan-Based Hydrogels: A Comprehensive Review of Transdermal Drug Delivery. Int. J. Biol. Macromol. 2025, 298, 140010. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Rahayu, P.; Munde, S.; Ade, N.; Chidrawar, V.R.; Singh, S.; Jayeoye, T.J.; Prajapati, B.G.; Bhattacharya, S.; Patel, R.J. Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review. Gels 2023, 9, 594. [Google Scholar] [CrossRef]
- Ozon, E.A.; Anastasescu, M.; Musuc, A.M.; Burloiu, A.M.; Socoteanu, R.P.; Atkinson, I.; Mitran, R.-A.; Culita, D.C.; Lupuliasa, D.; Mihai, D.P.; et al. Formulation and Characterization of Carbopol-Based Porphyrin Gels for Targeted Dermato-Oncological Therapy: Physicochemical and Pharmacotechnical Insights. Int. J. Mol. Sci. 2025, 26, 3641. [Google Scholar] [CrossRef]
- Francis, P.M.; Vinod, V.; Kumar, V.A.; Mohan, C.G.; Biswas, R. Development and Evaluation of Amikacin-Loaded Carbopol Hydrogel for Topical Treatment of Mycobacterium Marinum Skin Infections. Lett. Appl. Microbiol. 2025, 78, ovae134. [Google Scholar] [CrossRef]
- Rao, G.C.S.; Ramadevi, K.; Sirisha, K. Effect of β-Cyclodextrin on Rheological Properties of Some Viscosity Modifiers. Indian J. Pharm. Sci. 2014, 76, 545–548. [Google Scholar]
- Liu, J.; Tian, B.; Liu, Y.; Wan, J.-B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. Int. J. Mol. Sci. 2021, 22, 13516. [Google Scholar] [CrossRef]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 30 June 2025).
- Benzarti, S. Allelopathic and Antimicrobial Activities of Aqueous and Methanolic Extract of Two Verbena Species (Verbena officinalis L. and Aloysia citrodora L.) Leaves. Med. Aromat. Plants 2016, 5, 6. [Google Scholar] [CrossRef]
- Rezig, L.; Saada, M.; Trabelsi, N.; Tammar, S.; Limam, H.; Bettaieb Rebey, I.; Smaoui, A.; Sghaier, G.; Del Re, G.; Ksouri, R.; et al. Chemical Composition, Antioxidant and Antimicrobial Activities of Aloysia triphylla L. Essential Oils and Methanolic Extract. Ital. J. Food Sci. 2019, 31, 556. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Exploring Reserve Lots of Cymbopogon citratus, Aloysia citrodora and Thymus × citriodorus as Improved Sources of Phenolic Compounds. Food Chem. 2018, 257, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon Verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- de las Mercedes Oliva, M.; Carezzano, M.E.; Gallucci, M.N.; Demo, M.S. Antimycotic Effect of the Essential Oil of Aloysia triphylla against Candida Species Obtained from Human Pathologies. Nat. Prod. Commun. 2011, 6, 1039–1043. [Google Scholar] [CrossRef]
- Nabila, B.; Piras, A.; Fouzia, B.; Falconieri, D.; Kheira, G.; Fedoul, F.-F.; Majda, S.-R. Chemical Composition and Antibacterial Activity of the Essential Oil of Laurus nobilis Leaves. Nat. Prod. Res. 2022, 36, 989–993. [Google Scholar] [CrossRef]
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant Properties of Essential Oils from Salvia officinalis L. Cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef]
- Carla, M.M.F.-A.; Maurício, F.d.R.; Édela, B.; Fabiana, B.; Camila, C.I.; José, E.G.; Diógenes, A.G.C.; Cleide, V.B.M.; Giani, A.L.; Márcia, R.S.; et al. Chemical Composition and Antifungal Activity of Essential Oil and Fractions Extracted from the Leaves of Laurus nobilis L. Cultivated in Southern Brazil. J. Med. Plants Res. 2016, 10, 865–871. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal Activity, Mode of Action and Anti-Biofilm Effects of Laurus nobilis Linnaeus Essential Oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Stancu, A.I.; Mititelu, M.; Ficai, A.; Ditu, L.-M.; Buleandră, M.; Badea, I.A.; Pincu, E.; Stoian, M.C.; Brîncoveanu, O.; Boldeiu, A.; et al. Comparative Evaluation of β-Cyclodextrin Inclusion Complexes with Eugenol, Eucalyptol, and Clove Essential Oil: Characterisation and Antimicrobial Activity Assessment for Pharmaceutical Applications. Pharmaceutics 2025, 17, 852. [Google Scholar] [CrossRef]
- Stancu, A.-I.; Geană, E.I.; Ditu, L.-M.; Ficai, A.; Nagoda, E.; Oprea, E. Chemical Composition and Antimicrobial Activity of Verbena officinalis and Aloysia citrodora Extracts Obtained by Traditional and Laboratory Methods. U.P.B. Sci. Bull. Ser. B 2024, 86, 111–122. [Google Scholar]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. Essential Oil and Its Main Components on α-Glucosidase and Reactive Oxygen Species Scavenging Activity. Iran. J. Pharm. Res. 2013, 12, 367–379. [Google Scholar]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.M.; Fresno, M.J.; Ramírez, A. Rheological Study of Binary Gels with Carbopol UltrezTM 10 and Hyaluronic Acid. Chem. Pharm. Bull. 2007, 55, 1157–1163. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- El-hefian, E.A.; Yahaya, A.H. Rheological Study of Chitosan and Its Blends: An Overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A Poloxamer/Hyaluronic Acid/Chitosan-Based Thermosensitive Hydrogel That Releases Dihydromyricetin to Promote Wound Healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular Weight Dependence of Structure and Properties of Chitosan Oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- Klučáková, M. How the Addition of Chitosan Affects the Transport and Rheological Properties of Agarose Hydrogels. Gels 2023, 9, 99. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Vityazev, F.; Markov, P.A.; Erokhin, K.S. Effect of Chitosan on Rheological, Mechanical, and Adhesive Properties of Pectin–Calcium Gel. Mar. Drugs 2023, 21, 375. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of External and Internal Gelation and Their Impact on the Functions of Alginate as a Coat and Delivery System. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Chan, L.; Lee, H.; Heng, P.W. Production of Alginate Microspheres by Internal Gelation Using an Emulsification Method. Int. J. Pharm. 2002, 242, 259–262. [Google Scholar] [CrossRef]
- Sarfraz, M.; Iqbal, R.; Khan, K.U.; Minhas, M.U. Carbopol Based Hydrogels for ITOPRIDE Hydrochloride Delivery; Synthesis, Characterization and Comparative Assessment with Various Monomers. J. Funct. Biomater. 2022, 13, 295. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-Isothermal Kinetics of Thermal Degradation of Chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef]
- Pieróg, M.; Ostrowska-Czubenko, J.; Gierszewska-Druzyńska, M. Thermal Degradation of Double Crosslinked Hydrogel Chitosan Membranes. Prog. Chem. Appl. Chitin Its Deriv. 2012, 2012, 67–74. [Google Scholar]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and Thermal Properties of Calcium Alginate/Reduced Graphene Oxide Composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Said, A.A.; Hassan, R.M. Thermal Decomposition of Some Divalent Metal Alginate Gel Compounds. Polym. Degrad. Stab. 1993, 39, 393–397. [Google Scholar] [CrossRef]
- Sıçramaz, H.; Dönmez, A.B.; Güven, B.; Ünal, D.; Aşbay, E. Microstructure and Release Behavior of Alginate–Natural Hydrocolloid Composites: A Comparative Study. Polymers 2025, 17, 531. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Kaźmierski, Ł.; Bartniak, M.; Bajek, A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers 2024, 16, 2560. [Google Scholar] [CrossRef] [PubMed]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Chelu, M.; Popa, M.; Calderon Moreno, J.; Leonties, A.R.; Ozon, E.A.; Pandele Cusu, J.; Surdu, V.A.; Aricov, L.; Musuc, A.M. Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater. Gels 2023, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-Acetylation Degree in Chitosan Using Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum Turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Hadizadeh, A.; Dehghan, S.; Hadizadeh, S. Enhanced Antibacterial Activity of Porous Chitosan-Based Hydrogels Crosslinked with Gelatin and Metal Ions. Sci. Rep. 2024, 14, 7505. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. coli and S. aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Vaca Meza, E.T.; Vasquez-Kool, J.; Costilla Sánchez, N.I.; Vieira, A.; Rodrigues, R.A.F.; Sartoratto, A.; Flores Granados, A.d.P.; Marin Tello, C.L.; Ruiz, A.L.T.G. Chemical Composition and Anti-Proliferative Activity of Essential Oils from Some Medicinal Plants from Cachicadán, Región La Libertad, Perú. Nat. Prod. Res. 2024, 38, 2145–2150. [Google Scholar] [CrossRef]
- Ahmed, D.; Ahmed Qasim, K.; Ashraf, C.M.; Maab, H. Verbena officinalis a Herb with Promising Broad Spectrum Antimicrobial Potential. Cogent Chem. 2017, 3, 1363342. [Google Scholar] [CrossRef]
- Kuok, C.-F.; Hoi, S.-O.; Hoi, C.-F.; Chan, C.-H.; Fong, I.-H.; Ngok, C.-K.; Meng, L.-R.; Fong, P. Synergistic Antibacterial Effects of Herbal Extracts and Antibiotics on Methicillin-Resistant Staphylococcus Aureus: A Computational and Experimental Study. Exp. Biol. Med. 2017, 242, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Bussa, N.; Gashaw, T.; Mengistu, G. Investigating In Vitro Antibacterial Activities of Medicinal Plants Having Folkloric Repute in Ethiopian Traditional Medicine. J. Evid.-Based Integr. Med. 2019, 24, 2515690X1988627. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial Effect of Laurel Essential Oil Nanoemulsion on Food-Borne Pathogens and Fish Spoilage Bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef] [PubMed]
- Mkaddem Guedri, M.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Chemical Composition and Antimicrobial and Antioxidant Activities of Tunisian, France and Austrian Laurus nobilis (Lauraceae) Essential Oils. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 1929–1940. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; El Ghouizi, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical Composition, Antioxidant Activity, and Antifungal Effects of Essential Oil from Laurus nobilis L. Flowers Growing in Morocco. J. Food Qual. 2020, 2020, 8819311. [Google Scholar] [CrossRef]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of Wound Healing Effect of Chitosan-Based Gel Formulation Containing Vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Suhail, M.; Liu, J.-Y.; Hung, M.-C.; Chiu, I.-H.; Minhas, M.U.; Wu, P.-C. Preparation, In Vitro Characterization, and Cytotoxicity Evaluation of Polymeric PH-Responsive Hydrogels for Controlled Drug Release. Pharmaceutics 2022, 14, 1864. [Google Scholar] [CrossRef]
- Stancu, A.I.; Oprea, E.; Dițu, L.M.; Ficai, A.; Ilie, C.-I.; Badea, I.A.; Buleandra, M.; Brîncoveanu, O.; Ghica, M.V.; Avram, I.; et al. Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity. Gels 2024, 10, 645. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tuñón-Molina, A.; Bakshi, H.; Sabater i Serra, R.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, Á. Biocompatible Alginate Film Crosslinked with Ca2+ and Zn2+ Possesses Antibacterial, Antiviral, and Anticancer Activities. ACS Omega 2023, 8, 24396–24405. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, Calcium-Free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Yihan, W.; Jinjin, D.; Yingqi, W.; Guanai, M.; Xiwu, Z. Advances in plant essential oils and drug delivery systems for skincare. Front. Pharmacol. 2025, 16, 1578280. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Geana, E.; Chircov, C.; Ficai, A.; Ditu, L.; Oprea, E. Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features. Antioxidants 2024, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025.
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]


| Formulation | Polymer | Active Ingredients | Plasticizer | Gelling Agent | Solvent | ||
|---|---|---|---|---|---|---|---|
| Tinctures | Essential Oil | Other | |||||
| 2% | 2% | 1% | 0.50% | 5% | 1% | Until 100% | |
| CBP-G | Carbopol 940 | Verbena officinalis (VAT) Aloysia triphylla (ATT) | Laurus nobilis | β-CD-ECEO complex | Glycerin | NA | Purified water |
| CTH-G | Chitosan HMW | ||||||
| CTM-G | Chitosan MMW | ||||||
| ALG-G | Sodium alginate | CaCl2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancu, A.I.; Dițu, L.M.; Oprea, E.; Ficai, A.; Badea, I.A.; Buleandră, M.; Brîncoveanu, O.; Mirea, A.G.; Voicu, S.N.; Musuc, A.M.; et al. New Antimicrobial Gels Based on Clove Essential Oil–Cyclodextrin Complex and Plant Extracts for Topical Use. Gels 2025, 11, 653. https://doi.org/10.3390/gels11080653
Stancu AI, Dițu LM, Oprea E, Ficai A, Badea IA, Buleandră M, Brîncoveanu O, Mirea AG, Voicu SN, Musuc AM, et al. New Antimicrobial Gels Based on Clove Essential Oil–Cyclodextrin Complex and Plant Extracts for Topical Use. Gels. 2025; 11(8):653. https://doi.org/10.3390/gels11080653
Chicago/Turabian StyleStancu, Alina Ionela, Lia Mara Dițu, Eliza Oprea, Anton Ficai, Irinel Adriana Badea, Mihaela Buleandră, Oana Brîncoveanu, Anca Gabriela Mirea, Sorina Nicoleta Voicu, Adina Magdalena Musuc, and et al. 2025. "New Antimicrobial Gels Based on Clove Essential Oil–Cyclodextrin Complex and Plant Extracts for Topical Use" Gels 11, no. 8: 653. https://doi.org/10.3390/gels11080653
APA StyleStancu, A. I., Dițu, L. M., Oprea, E., Ficai, A., Badea, I. A., Buleandră, M., Brîncoveanu, O., Mirea, A. G., Voicu, S. N., Musuc, A. M., Aricov, L., Culita, D. C., & Mititelu, M. (2025). New Antimicrobial Gels Based on Clove Essential Oil–Cyclodextrin Complex and Plant Extracts for Topical Use. Gels, 11(8), 653. https://doi.org/10.3390/gels11080653













