CMCSMA-Citric Acid Hydrogel-Coated Pancreatic Duct Stent Used for Pancreatic Calculi
Abstract
1. Introduction
2. Results and Discussion
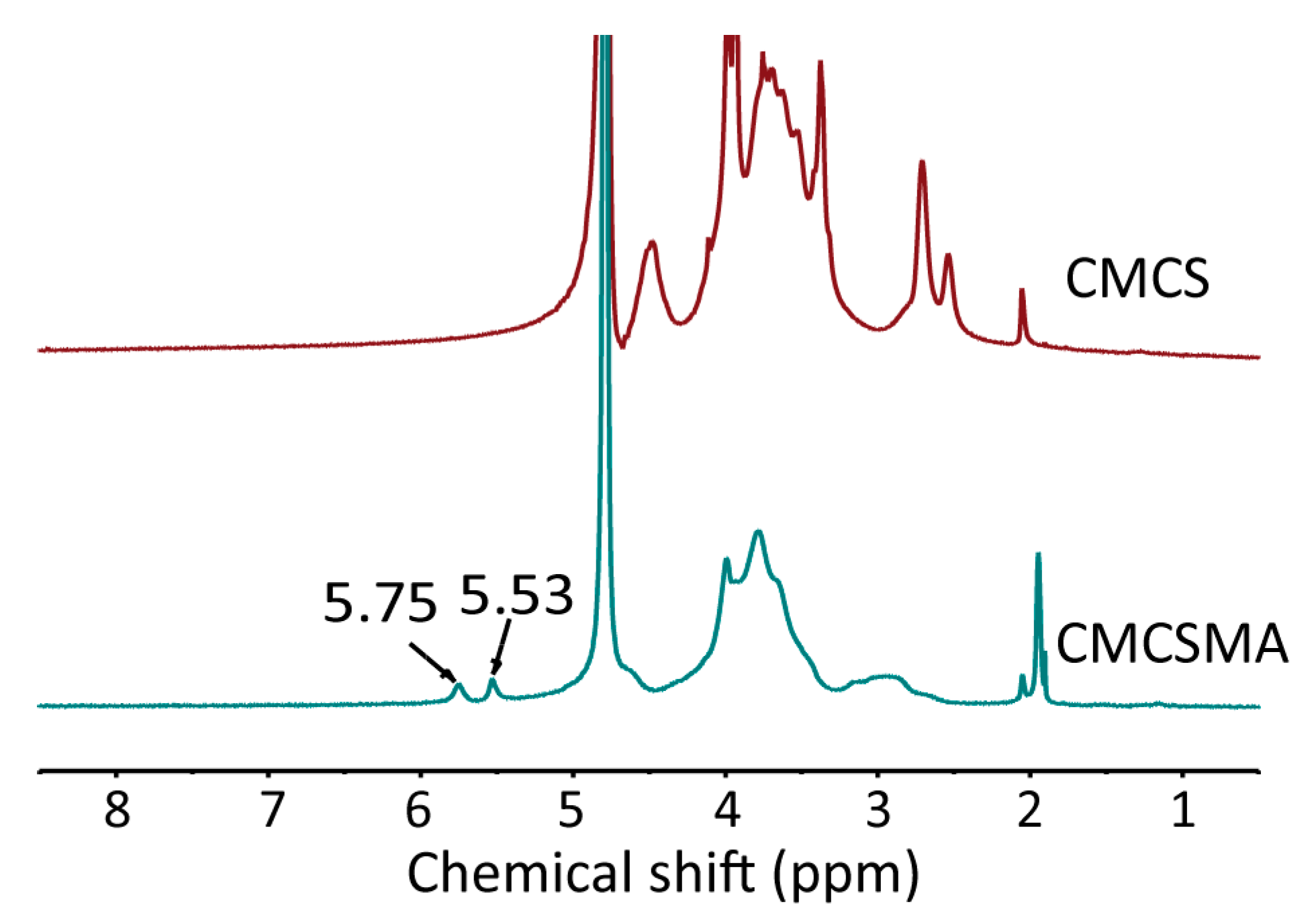
2.1. Characterization of CMCSMA
2.2. PDA Modification and In Situ Synthesis of CMCSMA-CA Hydrogel on Pancreatic Duct Stent
2.3. Characterization of Hydrogels
2.4. Rheological and Mechanical Properties of Hydrogel Coating
2.5. Swelling and In Vitro Degradation of CMCSMA Hydrogel Coating
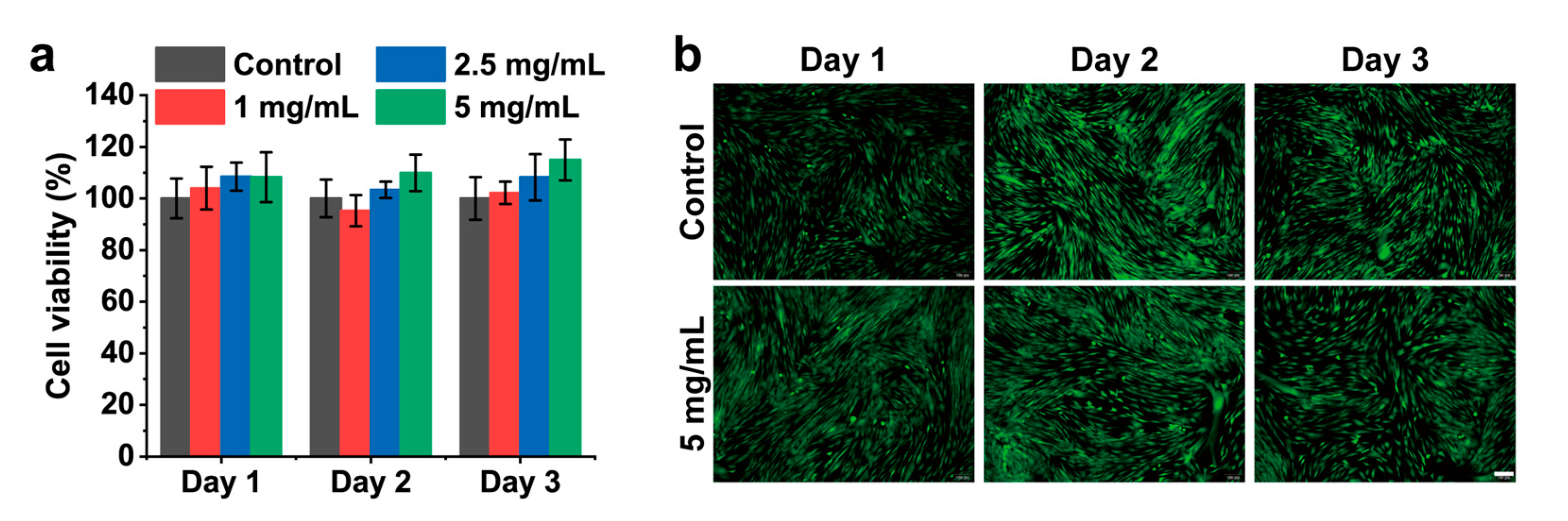
2.6. Hemolysis Assay and Cytocompatibility of CMCSMA Hydrogel Coating
2.7. In Vitro Release of CA and In Vitro Litholytic Effect of CMCSMA Hydrogel-Coated Pancreatic Duct Stent
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of CMCSMA
4.3. Preparation and Characterization of the Hydrogel-Coated Stents
4.4. Rheological Properties and Mechanical Properties of the Hydrogel
4.5. Swelling and Degradation Tests
4.6. Hemolysis Assay
4.7. In Vitro Cytocompatibility
4.8. Citric Acid Loading Content and Release Behavior Study
4.9. In Vitro Calculi Dissolving Effect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitchumoni, C.S.; Viswanathan, K.V.; GeeVarghese, P.J.; Banks, P.A. Ultrastructure and Elemental Composition of Human Pancreatic Calculi. Pancreas 1987, 2, 152–158. [Google Scholar] [CrossRef]
- Kaushik, N.; Dasari, V.; Jain, D. Management of Pancreatic Calculi in Chronic Pancreatitis: A Review Article. Cureus J. Med. Sci. 2023, 15, e35788. [Google Scholar] [CrossRef]
- Ouyang, J.M. Chemical basis in inhibition of urinary stones by citric acid and its salts. Chin. J. Inorg. Chem. 2004, 20, 1377–1382. [Google Scholar]
- Rao, T.; Bano, S. In-vitro chemodissolution of urinary stones by some chelating natural acids. Asian J. Chem. 2004, 16, 59–66. [Google Scholar]
- Joseph, K.C.; Parekh, B.B.; Joshi, M.J. Inhibition of growth of urinary type calcium hydrogen phosphate dihydrate crystals by tartaric acid and tamarind. Curr. Sci. India 2005, 88, 1232–1238. [Google Scholar]
- Sarles, H.; Verine, H.; Lohse, J.; Sahel, J.; de Caro, A.; Zerolo, J. Dissolution of pancreatic calculi during prolonged oral administration of citrate. Presse Med. 1979, 8, 1767–1768. [Google Scholar]
- Uscanga, L.; Galvan Guerra, E.; Robles-Diaz, G.; Campuzano Fernandez, M. Dissolution of pancreatic calcifications with oral citrates in a woman with chronic idiopathic pancreatitis. Rev. Investig. Clin. 1992, 44, 249–254. [Google Scholar]
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966. [Google Scholar] [CrossRef]
- Gerges, C.; Beyna, T.; Neuhaus, H. Management of Pancreatic Duct Stones: Nonextracorporeal Approach. Gastroenterol. Clin. N. Am. 2023, 33, 821–829. [Google Scholar]
- Holt, N.; Craig, P.I. Pancreatoscopy-guided laser lithotripsy to manage obstructing intraductal pancreatic calculi. VideoGIE 2023, 8, 450–453. [Google Scholar] [CrossRef]
- Tandan, M.; Nageshwar Reddy, D.; Talukdar, R.; Vinod, K.; Kiran, S.V.V.S.; Santosh, D.; Gupta, R.; Ramchandani, M.; Lakhtakia, S.; Rakesh, K.; et al. ESWL for large pancreatic calculi: Report of over 5000 patients. Pancreatology 2019, 19, 916–921. [Google Scholar] [CrossRef]
- Vedamurthy, A.; Krishnamoorthi, R.; Irani, S.; Kozarek, R. Endoscopic Management of Benign Pancreaticobiliary Disorders. J. Clin. Med. 2025, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Ryozawa, S.; Fujita, N.; Irisawa, A.; Hirooka, Y.; Mine, T. Current status of interventional endoscopic ultrasound. Dig. Endosc. 2017, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, N.; Zhang, X.; Xu, W.; Zhang, W.; Ding, Y.; Li, L.; Liu, T.; Xia, S. A review on pancreatic duct stents: Materials and emerging trends. Biomed. Mater. 2025, 20, 032004. [Google Scholar] [CrossRef] [PubMed]
- Testoni, P.A. Endoscopic pancreatic duct stent placement for inflammatory pancreatic diseases. World J. Gastroenterol. 2007, 13, 5971–5978. [Google Scholar] [CrossRef]
- Gupta, R.; Reddy, D.N. Stent selection for both biliary and pancreatic strictures caused by chronic pancreatitis: Multiple plastic stents or metallic stents? J. Hepato-Bil-Pan Sci. 2011, 18, 636–639. [Google Scholar] [CrossRef]
- Siddappa, P.K.; Hawa, F.; Prokop, L.J.; Murad, M.H.; Abu Dayyeh, B.K.; Chandrasekhara, V.; Topazian, M.D.; Bazerbachi, F. Endoscopic pancreatic duct stenting for pain palliation in selected pancreatic cancer patients: A systematic review and meta-analysis. Gastroenterol. Rep. 2021, 9, 105–114. [Google Scholar] [CrossRef]
- Yi, J.H.; Li, Z.S.; Hu, L.H. Pancreatic duct stents. J. Dig. Dis. 2022, 23, 675–686. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, C.; Sun, B.; Aqeel, B.M.; Wang, J.; Chen, W.; Mo, X.; Wan, X. Fabrication and characterization of metal stent coating with drug-loaded nanofiber film for gallstone dissolution. J. Biomater. Appl. 2016, 31, 784–796. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Chen, Z.; Zhao, J.; Wang, S. Citric Acid Loaded Hydrogel-Coated Stent for Dissolving Pancreatic Duct Calculi. Gels 2024, 10, 125. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid. Interfac. 2022, 305, 102689. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Zou, Y.; Lu, C.; Li, N.; Shi, Z.; Li, X.; Lai, X. Ultra-sensitive pH responsive hydrogels with injectable and self-healing performance for controlled drug delivery. Int. J. Pharm.-X 2025, 9, 100334. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Li, H.; Shuai, Q.; Zhang, X.; Pich, A. Sprayed Aqueous Microdroplets for Spontaneous Synthesis of Functional Microgels. Angew. Chem. Int. Ed. 2025, 64, e202420926. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mat. Sci. Eng. C-Mater. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, P.; Naghib, S.M.; Mozafari, M.R. Carboxymethyl Chitosan-based Nanomaterials for Biomedical Applications: An Overview of Recent Advances and Prospects. Mini-Rev. Org. Chem. 2024, 22, 479–504. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Chen, K.; Tong, C.; Yang, J.; Cong, P.; Liu, Y.; Shi, X.; Liu, X.; Zhang, J.; Zou, R.; Xiao, K.; et al. Injectable melatonin-loaded carboxymethyl chitosan (CMCS)-based hydrogel accelerates wound healing by reducing inflammation and promoting angiogenesis and collagen deposition. J. Mater. Sci. Technol. 2021, 63, 236–245. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Kruczkowska, W.; Klosinski, K.K.; Grabowska, K.H.; Galeziewska, J.; Gromek, P.; Kc iuk, M.; Kaluzinska-Kolat, Z.; Kolat, D.; Wach, R.A. Medical Applications and Cellular Mechanisms of Action of Carboxymethyl Chitosan Hydrogels. Molecules 2024, 29, 4360. [Google Scholar] [CrossRef]
- Gan, S.; Zheng, Z.; Zhang, M.; Long, L.; Zhang, X.; Tan, B.; Zhu, Z.; Liao, J.; Chen, W. Lyophilized Platelet-Rich Fibrin Exudate-Loaded Carboxymethyl Chitosan/GelMA Hydrogel for Efficient Bone Defect Repair. ACS Appl. Mater. Interfaces 2023, 15, 26349–26362. [Google Scholar] [CrossRef]
- Chichiricco, P.M.; Riva, R.; Thomassin, J.-M.; Lesoeur, J.; Struillou, X.; Le Visage, C.; Jérôme, C.; Weiss, P. In situ photochemical crosslinking of hydrogel membrane for Guided Tissue Regeneration. Dent. Mater. 2018, 34, 1769–1782. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Zhang, L.; Fan, P.; Zhao, J.; Zheng, X.; Lai, Y.; Liu, H.; Wang, S. Injectable hemostatic hydrogel adhesive with antioxidant, antibacterial and procoagulant properties for hemorrhage wound management. J. Colloid Interface Sci. 2024, 673, 395–410. [Google Scholar]
- Obiweluozor, F.O.; Maharjan, B.; Emechebe, A.G.; Park, C.H.; Kim, C.S. Mussel-inspired elastic interpenetrated network hydrogel as an alternative for anti-thrombotic stent coating membrane. Chem. Eng. J. 2018, 347, 932–943. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Chen, M.; Tan, H.; Xu, W.; Wang, Z.; Zhang, J.; Li, S.; Zhou, T.; Li, J.; Niu, X. A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair. Acta Biomater. 2022, 153, 159–177. [Google Scholar] [CrossRef]
- De Piano, R.; Caccavo, D.; Barba, A.A.; Lamberti, G. Swelling Behavior of Anionic Hydrogels: Experiments and Modeling. Gels 2024, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.; Zhao, Z. Facile fabrication of self-healing, injectable and antimicrobial cationic guar gum hydrogel dressings driven by hydrogen bonds. Carbohydr. Polym. 2023, 310, 120723. [Google Scholar] [CrossRef] [PubMed]
- Pansuriya, R.; Patel, T.; Singh, K.; Al Ghamdi, A.; Kasoju, N.; Kumar, A.; Kailasa, S.K.; Malek, N.I. Self-healable, stimuli-responsive bio-ionic liquid and sodium alginate conjugated hydrogel with tunable Injectability and mechanical properties for the treatment of breast cancer. Int. J. Biol. Macromol. 2024, 277, 134112. [Google Scholar] [CrossRef] [PubMed]
- Van Tienderen, G.S.; Berthel, M.; Yue, Z.; Cook, M.; Liu, X.; Beirne, S.; Wallace, G.G. Advanced fabrication approaches to controlled delivery systems for epilepsy treatment. Expert. Opin. Drug Del. 2018, 15, 915–925. [Google Scholar] [CrossRef]
- Risangud, N.; Lertwimol, T.; Sitthisang, S.; Wongvitvichot, W.; Uppanan, P.; Tanodekaew, S. The preparation of 3D-printed self-healing hydrogels composed of carboxymethyl chitosan and oxidized dextran via stereolithography for biomedical applications. Int. J. Biol. Macromol. 2025, 292, 139251. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, J.; Wang, S. CMCSMA-Citric Acid Hydrogel-Coated Pancreatic Duct Stent Used for Pancreatic Calculi. Gels 2025, 11, 651. https://doi.org/10.3390/gels11080651
Li J, Yang J, Wang S. CMCSMA-Citric Acid Hydrogel-Coated Pancreatic Duct Stent Used for Pancreatic Calculi. Gels. 2025; 11(8):651. https://doi.org/10.3390/gels11080651
Chicago/Turabian StyleLi, Jing, Jiahao Yang, and Shige Wang. 2025. "CMCSMA-Citric Acid Hydrogel-Coated Pancreatic Duct Stent Used for Pancreatic Calculi" Gels 11, no. 8: 651. https://doi.org/10.3390/gels11080651
APA StyleLi, J., Yang, J., & Wang, S. (2025). CMCSMA-Citric Acid Hydrogel-Coated Pancreatic Duct Stent Used for Pancreatic Calculi. Gels, 11(8), 651. https://doi.org/10.3390/gels11080651






